Abstract
Objective This study aimed to determine the adequate resection margin in skull base surgery for head and neck sarcoma.
Design We retrospectively reviewed 22 sarcomas with skull base invasion. Induction chemotherapy, followed by surgery and postoperative radiotherapy and adjuvant chemotherapy, was performed in 18 patients with chemosensitive sarcomas, and surgery with or without postoperative radiotherapy was performed in four patients with chemoresistant sarcomas. Radical resection was performed in patients with chemosensitive sarcomas with a poor response to induction chemotherapy and in patients with chemoresistant sarcomas. Conservative resection with close surgical margin was performed in patients with chemosensitive sarcomas with a good response to induction chemotherapy.
Setting and Participants This single-centered retrospective study included patients from the National Cancer Center Hospital, Japan.
Results The response to induction chemotherapy was significantly associated with the 3-year local control rate (LCR; good response versus poor response: 100% versus 63%, p = 0.048). Patients with a good response to chemotherapy had a favorable local prognosis even when the local therapy was conservative resection. In radical skull base surgery, patients whose surgical margins were classified as “wide margin positive” had significantly poorer 3-year LCR than did patients with “margin negative” or “micro margin positive” margins (25% versus 83%, p = 0.014).
Conclusion Conservative resection with close surgical margins might be acceptable for chemosensitive sarcomas with a good response to chemotherapy. Resection margin status was an important predictive factor for local recurrence after radical skull base surgery. Microscopic microresidual tumor might be controlled by postoperative treatment.
Keywords: head and neck sarcoma, skull base surgery, surgical margin, induction chemotherapy, chemosensitivity
Introduction
Head and neck sarcomas are rare tumors that comprise less than 1% of head and neck malignancies. 1 They develop in the soft or osseous tissue of the head and neck region and are found in people of all ages and both sexes. Some head and neck sarcomas are specific to certain sites. Rhabdomyosarcoma, malignant peripheral nerve sheath tumor, angiosarcoma, and myxofibrosarcoma are found in the sinonasal tract. 2 In about 75% of the head and neck rhabdomyosarcomas, the primary lesion is located in the parameningeal region or the orbit. 3 These sites may be associated with skull base invasion.
In general, head and neck sarcomas with skull base invasion are associated with unfavorable local control rates. This is attributed to the difficulty of performing intensive treatment due to the complex cranial anatomy. Multidisciplinary therapy including surgery, radiotherapy, and chemotherapy is necessary. Certainly, surgery and radiotherapy are fundamental treatments for local management of sarcomas; however, some sarcomas such as rhabdomyosarcoma and Ewing sarcoma are reported to have an excellent response to chemotherapy. Because skull base surgery is extremely invasive, chemosensitivity should be considered to evaluate the resection margins. This study aimed to determine the adequate resection margin in skull base surgery for head and neck sarcoma.
Materials and Methods
Study Population
From January 2006 to December 2014, 22 patients with sarcoma invading the skull base underwent definitive treatment at the National Cancer Center Hospital of Japan. All patients were previously untreated. Patient characteristics are shown in Table 1 .
Table 1. Patient characteristics.
| Characteristic | Value |
|---|---|
| Sex | |
| Male/Female, No. | 11/11 |
| Median age, years (range) | 25 (9–60) |
| Follow-up period (alive patients), months (range) | 36 (16–112) |
| Primary tumor site, No. | |
| Paranasal sinus | 8 |
| Nasal cavity | 7 |
| Orbita | 2 |
| Temporal fossa | 2 |
| Nasopharynx | 1 |
| Oral cavity | 1 |
| Parapharyngeal space | 1 |
| Histopathological type, No. | |
| Rhabdomyosarcoma (Embryonal/Alveolar/Solid/Pleomorphic) | 15 (2/8/2/3) |
| Ewing's sarcoma | 3 |
| Chondrosarcoma Grade2 | 1 |
| Undifferentiated/unclassified sarcoma | 3 |
| Pattern of skill base invasion, No. | |
| Dura invasion | 5 |
| Cranial fossa invasion | 3 |
| Canopy of ethmoid sinus invasion | 8 |
| Orbital superior wall invasion | 2 |
| Infratemporal fossa invasion | 4 |
| Pathological neck metastasis, No. | |
| Positive/Negative | 2/20 |
| Distant metastasis, No. | |
| Positive/Negative | 0/22 |
| Induction chemotherapy, No. | |
| Positive/Negative | 18/4 |
| Free flap reconstruction, No. | |
| Positive/Negative | 5/17 |
| Postoperative radiotherapy, No. | |
| Positive/Negative | 16/6 |
Abbreviation: No., Number of patients.
The study population comprised 11 males and 11 females aged from 9 to 60 years (median, 25 years). The median follow-up period was 36 months (range, 16–112 months). We used the 2013 version of the World Health Organization criteria for the histological classification. We observed 15 rhabdomyosarcomas, 3 Ewing sarcomas, 1 chondrosarcoma (grade 2), and 3 undifferentiated/unclassified sarcomas. We classified rhabdomyosarcoma and Ewing sarcoma as chemosensitive sarcomas and chondrosarcoma and undifferentiated/unclassified sarcoma as chemoresistant sarcomas.
Skull base invasion was defined as the attachment of the tumor to the infratemporal fossa, the orbital superior wall, or the canopy of the ethmoid sinus without invading the cranial fossa. Five patients revealed invasion of the dura mater beyond the cranial fossa, three revealed invasion of the cranial fossa without invasion of the dura mater, eight revealed invasion of the canopy of the ethmoid sinus, two revealed invasion of the orbital superior wall, and four patients revealed invasion of the infratemporal fossa.
Treatment Strategies
Fig. 1 displays the main treatment strategies used in this study. For chemosensitive sarcomas, induction chemotherapy followed by surgery, postoperative radiotherapy (50.4Gy, in 1.8Gy fraction), and adjuvant chemotherapy was performed. As the standard induction regimen, four cycles of the VAC (vincristine, dactinomycin, and cyclophosphamide) chemotherapy were administered for rhabdomyosarcoma and four courses of the VDC/IE (vincristine, doxorubicin, and cyclophosphamide/ifosfamide and etoposide) chemotherapy were administered for Ewing sarcoma. VAC and VDC/IE chemotherapy were administrated according to previous studies. 4 5 Response after induction chemotherapy was defined as follows: good response was defined as a tumor reduction of >60%, and poor response was defined as a tumor reduction of ≤60% or progression of the disease. The extent of the resection margin depended on the tumor response to induction chemotherapy. In general, radical resection, that is, skull base surgery, based on the initial imaging study, was performed; however, conservative resection, that is, partial maxillectomy, based on the repeated imaging study, was performed in cases of good response to induction chemotherapy ( Fig. 2A ).
Fig. 1.
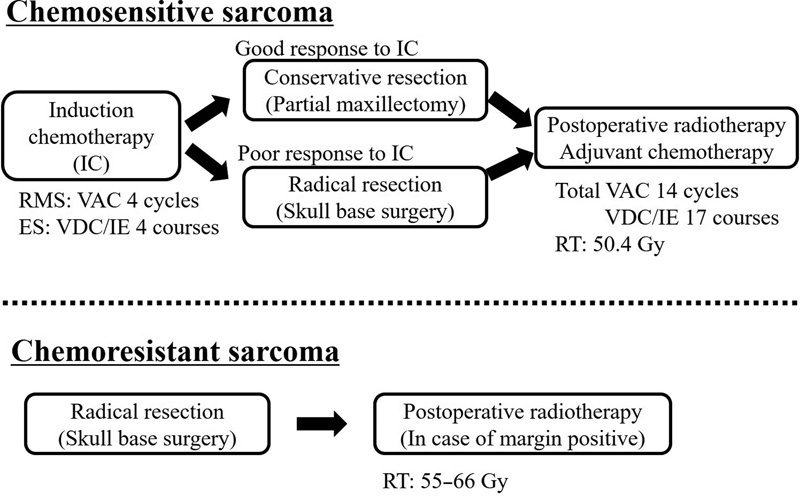
Treatment strategy. The main treatment strategies for the two groups are displayed. RMS, rhabdomyosarcoma; VDC, vincristine, dactinomycin, and cyclophosphamide; ES, Ewing sarcoma; VDC/IE, vincristine, doxorubicin and cyclophosphamide/ifosfamide and etoposide; RT, radiotherapy.
Fig. 2.
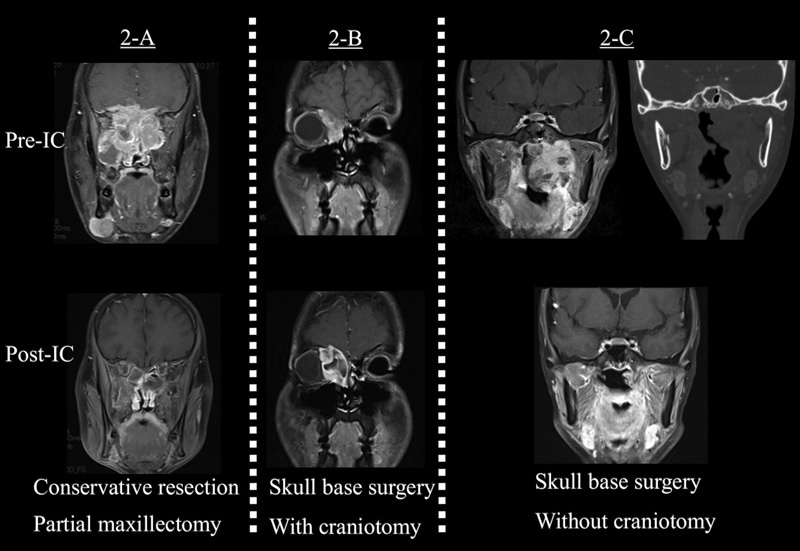
Case presentations. ( A ) A 25-year-old man with a nasal cavity primary rhabdomyosarcoma. The tumor invaded the dura mater. He underwent four cycles of VAC chemotherapy and achieved a clinical complete response. A conservative resection was performed in this case. ( B ) A 25-year-old man with an ethmoid sinus primary rhabdomyosarcoma. He underwent four cycles of VAC chemotherapy, and the tumor response revealed a stable disease. A radical resection with craniotomy was performed in this case. ( C ) A 21-year-old woman with a nasopharynx primary rhabdomyosarcoma. The tumor invaded the infratemporal fossa; however, computed tomography images reported absence of cranial base bony erosion. She underwent four cycles of VAC chemotherapy, and the tumor response was partial. A radical resection without craniotomy by the mandibular swing approach was performed in this case.
For chemoresistant sarcomas, radical resection was performed as an initial treatment. Postoperative radiotherapy (55–66 Gy) was performed in case of positive surgical margin.
Unresectability was defined as invasion of the cavernous sinus, carotid artery, wide dura mater, and cerebrum. The indication for craniotomy was based on the presence or absence of cranial base bony erosion and the range of infiltration of the infratemporal fossa ( Fig. 2B ). If the tumor showed no cranial base bony erosion and a limited range of invasion of the infratemporal fossa, we resected the tumor without craniotomy by approaches such as the mandibular swing approach or the orbitozygomatic approach ( Fig. 2C ). Frontal craniotomy, temporal craniotomy, or frontotemporal craniotomy was selected on the basis of the tumor location. For reconstruction, a pericranial flap was used to close the defect of the anterior craniofacial resection without orbital exenteration. Free flap reconstruction by a rectus abdominis flap or an anterolateral thigh flap was performed in case of a wide defect. For all cases requiring resection of the dura mater or orbital periosteum, we performed the reconstruction using the fascia late or abdominal fascia.
After surgery, we performed a pathological evaluation of the surgical specimens. Collectively, the surgeon and the pathologist discussed and evaluated all the histological sections for margin surveillance, to obtain an accurate result. We classified the pathological surgical margin status as “margin negative (MN),” “micro margin positive (MMP),”or “wide margin positive (WMP).” MN indicated pathologically complete resection, MMP indicated pathological residual tumor existing in only one subsite, and WMP indicated pathological residual tumor existing in multiple subsites.
The operative procedures are summarized in Fig. 3 . Conservative resection was performed in nine patients who displayed a good response to induction chemotherapy. Radical resection was performed in nine patients who reported a poor response to induction chemotherapy and in four patients with chemoresistant sarcomas. Of the 13 patients who underwent radical resection, 6 were subjected to skull base surgery with craniotomy and 7 to skull base surgery without craniotomy.
Fig. 3.
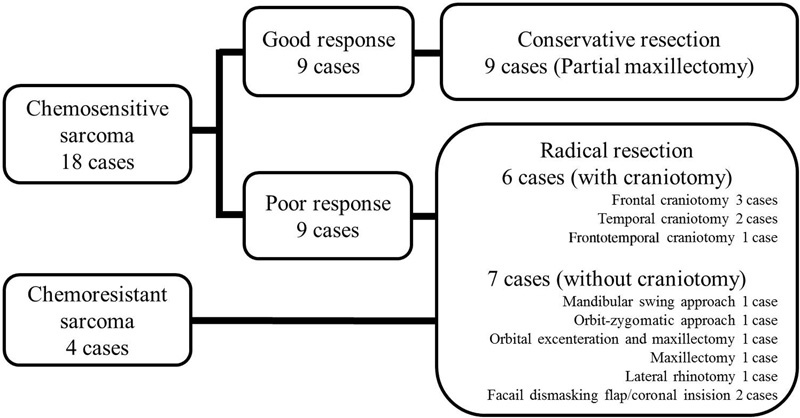
Surgery performed. The operative procedures are summarized.
Statistical Analysis
We retrospectively analyzed the recurrence pattern, risk factors for local recurrence, 3-year local control rate (LCR), and overall survival (OS). LCR and OS were calculated by the Kaplan–Meier method, and the differences were analyzed by log rank tests. A value of P < 0.05 was considered to be statistically significant. The data were analyzed using the Statmate Version 2 (GraphPad, La Jolla, California, United States).
Results
Overall Outcome
Among the nine patients with chemosensitive sarcomas who underwent conservative resection due to a good response to induction chemotherapy, no primary recurrences were observed. In contrast, among the nine patients with poor responses to induction chemotherapy, three developed primary recurrences despite radical resection. The response to induction chemotherapy was significantly associated with the 3-year LCR in univariate analysis (good response versus poor response: 100% versus 63%, p = 0.048; Fig. 4A ). Distant metastasis was detected in two patients with a good response to induction chemotherapy and three with a poor response to induction chemotherapy. Regardless of the recurrence patterns, salvage treatment was difficult. All patients with recurrence died of the disease. The 3-year OS among patients with chemosensitive sarcomas with good response and poor response to induction chemotherapy was 76% and 48%, respectively ( Fig. 4B ).
Fig. 4.
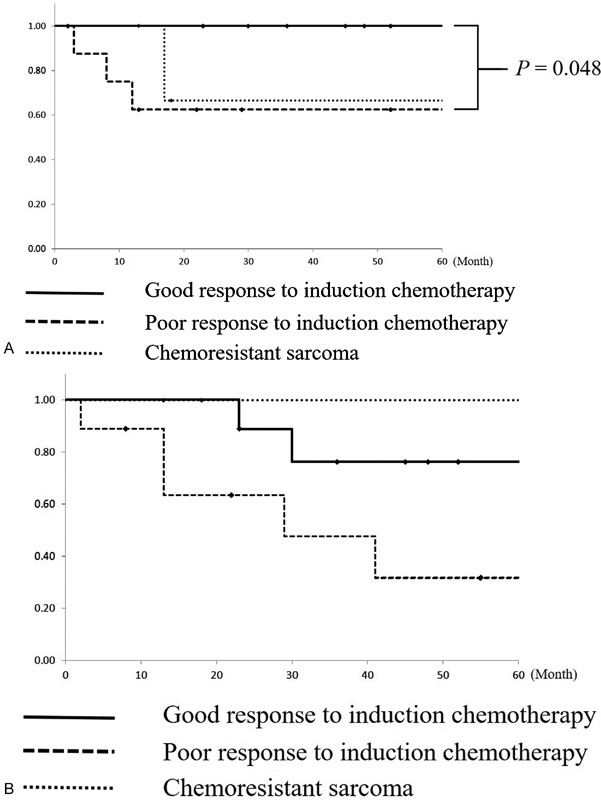
( A ) Local control rates of the three groups. Patients with a good response to induction chemotherapy had significantly better 3-year local control rate than patients with a poor response to induction chemotherapy. ( B ) Overall survival of the three groups. Chemosensitivity was not associated with overall survival.
Among the four patients with chemoresistant sarcomas, local recurrence was detected in one patient. Salvage surgery was successful. The 3-year LCR and OS were 67% and 100%, respectively ( Fig. 4A,B ).
Risk Factors for Local Recurrence after Radical Skull Base Surgery
We analyzed 13 patients who underwent radical skull base surgery. Tumor size, extent of skull base invasion, age distribution, and chemosensitivity were not associated with the 3-year LCR. Patients with WMP had significantly poorer 3-year LCR than the patients with MN or MMP (WMP versus MN/MMP: 25% versus 83%, p = 0.014; Table 2 ).
Table 2. Univariate analysis of risk factors of local recurrence after skull base surgery.
| 3 Years LCR | p value | ||
|---|---|---|---|
| Location of primary tumor | Nasal/Paranasal cavity | 53% | 0.84 |
| Orbitae/Others | 71% | ||
| Age | < 20 years old | 67% | 0.99 |
| ≥ 20 years old | 62% | ||
| Tumor size at the time of surgery | < 50 mm | 63% | 0.91 |
| ≥ 50 mm | 67% | ||
| Cranial fossa invasion | Negative | 67% | 0.87 |
| Positive | 67% | ||
| Dural invasion | Negative | 70% | 0.44 |
| Positive | 50% | ||
| Chemosensitivity | Chemosensitive sarcoma | 63% | 0.59 |
| Chemoresistant sarcoma | 67% | ||
| Type of skull base surgery | With craniotomy | 67% | 0.87 |
| Without craniotomy | 67% | ||
| Pathological surgical margin status | Margin negative/micro margin positive | 83% | 0.014 |
| Wide margin positive | 25% |
Abbreviation: LCR, local control rate.
Analysis of Positive Surgical Margin
After radical resection based on initial imaging study, pathologically complete resection was achieved in only three patients (23%). The other 10 patients (77%) reported positive margin resections (4 patients with WMP and 6 patients with MMP). Details of the positive margin location, pathological surgical margin status, and clinical outcomes in patients with positive margins are shown in Table 3 .
Table 3. Details of positive margin location and clinical outcome after radical skull base surgery.
| Pathological margin status | Histopathology | Primary location | Induction chemotherapy | Approach to the skull base | Location of positive margin | Postoperative treatment | Local recurrence | Outcome |
|---|---|---|---|---|---|---|---|---|
| Micro margin positive | RMS | Nasal cavity | Positive | Frontal craniotomy | Lateral nasal wall | CRT | Negative | DOD |
| Micro margin positive | ES | Paranasal sinus | Positive | Frontal craniotomy | Oribital apex | CRT | Negative | NED |
| Micro margin positive | OS | Oral cavity | Negative | temporal craniotomy | Mandibular bone | CRT/surgery | Negative | NED |
| Micro margin positive | UUS | Orbit | Negative | frontotemporal craniotomy | Dura (near sinus) | RT | Negative | NED |
| Micro margin positive | RMS | Paranasal sinus | Positive | Orbital exenteration, maxillectomy | Lateral nasal wall | CRT | Negative | NED |
| Micro margin positive | RMS | Parapharyngeal space | Positive | Zygomatic approach | Masseter | CRT | Negative | NED |
| Wide margin positive | RMS | Parapharyngeal space | Positive | Frontal craniotomy | Skull bone/Orbital apex | chemotherapy | Orbita | DOD |
| Wide margin positive | UUS | Temporal fossa | Positive | temporal craniotomy | Orbital periosteum/Dura | CRT | Oribta | AWD |
| Wide margin positive | RMS | Nasopharynx | Positive | Mandibular swing approach | Clivus/Nasopharynx | CRT | Nasopharynx | DOD |
| Wide margin positive | UUS | Temporal fossa | Negative | Coronal incision approach | Piece by piece resection | CRT | Negative | NED |
Abbreviations: DOD, die of disease; ES, Ewing's sarcoma; NED, no evidence of disease; OS, osteosarcoma; RMC, rhabdomyosarcoma; UUS, undifferentiated/unclassified sarcoma.
The location of the positive surgical margins varied. The predominant locations were the skull base and orbit (skull base 37%, orbit 27%, nasal cavity 18%, and oral cavity 18%). These locations were not only in the dura mater near the cavernous sinus or clivus, where additional resection was difficult, but also in the nasal mucosa or masseter, which were directly seen during surgery and were like normal tissue. Microscopically, the tumors invaded far beyond the grossly evaluated tumor contour.
As a result of postoperative treatment, patients with MMP did not have local recurrence. Many patients with WMP developed local recurrence, despite postoperative treatment.
Discussion
Soft tissue sarcoma comprises a heterogeneous group of tumors with varying histology and behavior. Accurate pathological diagnosis is essential, as certain sarcomas such as rhabdomyosarcoma and Ewing sarcoma are extremely sensitive to chemotherapy.
In general, the initial surgery is recommended for rhabdomyosarcoma in the Children's Oncology Group (COG) guideline 6 ; however, because the anatomy of the skull base is complicated and the skull base surgery is immensely invasive, we performed induction chemotherapy before surgery. The indication for induction chemotherapy for chemosensitive sarcomas is controversial. In the treatment strategy for rhabdomyosarcoma, surgery after induction chemotherapy is referred as delayed primary excision. 7 8 Fewer studies have evaluated the efficacy of delayed primary excision in skull base surgery. 9
In our analysis, patients with a good response to induction chemotherapy reported a favorable local prognosis even when the local therapy included conservative resection. In such patients, conservative resection with close surgical margins might be acceptable. However, there is a strategy of induction chemotherapy followed by chemoradiotherapy, that is, omitting the surgery due to the tumor's extreme sensitivity to induction chemotherapy. The long cessation of chemotherapy induced distant metastases. However, conservative surgery such as partial maxillectomy was minimally invasive, and immediate resumption of adjuvant chemotherapy was possible after surgery (median time for resumption in our study, 15 days; range, 7–33 days). We also received important pathological information from the surgical specimens. Therefore, the conservative surgery might be acceptable.
According to previous reports of skull base surgery, the risk factors for local recurrence are dural invasion, adverse histological findings, and positive margins. The importance of en bloc resection with a tumor-free margin was emphasized 10 11 ; however, a few reports of skull base surgery limited to sarcoma are available. In the present analysis, margin status was also considered as an important factor for local recurrence. Two problems may arise in evaluating a surgical margin after the skull base surgery for head and neck sarcoma: the difficulty of accurate margin surveillance and the discrepancy between the microscopic and macroscopic invasion.
Considering the first problem, the surgical specimens are very large and have a complicated three-dimensional structure. Moreover, to obtain a good surgical view and perform safe surgery, often we need to drill the skull bone close to the tumor. Thus, accurate surveillance of the pathological margin is difficult. To resolve these problems, in our institution, the surgeon and the pathologist discussed all histological sections considered for surveillance. We also labeled all resection margin planes with different colors of ink to identify the margin-positive locations during microscopic margin surveillance.
Considering the second problem, we observed discrepancy between microscopic and macroscopic invasion. Microscopic invasion was more aggressive than macroscopic invasion. As aforementioned, positive surgical margins were located not only in the dura mater near the cavernous sinus or clivus, where additional resection was difficult, but also in the nasal mucosa or masseter, which appeared normal when directly visualized during surgery. However, intraoperative evaluation using frozen sections is inherently challenging because of occasionally significant frozen artifacts that obscure diagnostic histology. Therefore, accurate intraoperative or postoperative margin surveillance was difficult in certain cases.
Nevertheless, we decided the treatment strategy based on this limited information. In this study, we classified pathological surgical margin status as MN, MMP, or WMP. Local control was poor for patients with WMP and favorable for those with MN or MMP. Of the six patients with MMP, five had chemosensitive sarcomas and one had a chemoresistant sarcoma. MMP tumors maybe controlled by postoperative treatment, and radical skull base surgery is indicated for these patients. To avoid WMP in patients undergoing skull base surgery for head and neck sarcoma, detailed preoperative plans, including imaging studies and pathological analysis, are necessary.
The appropriate timing of radiotherapy for sarcoma invading the skull base is controversial. According to the Intergroup Rhabdomyosarcoma Study-IV (IRS-IV) and D9803studies, early radiation therapy may improve the local control for patients with intracranial extension rhabdomyosarcoma 4 12 ; however, according to the subgroup analysis of the IRS-VI and D9803 studies, delaying radiotherapy for patients with cranial nerve palsy or cranial base bony erosion may not increase the rate of local failure (local failure rate for radiotherapy expected week: week 12 versus week 0, 28% versus 15%, p = 0.22). 13 Moreover, these treatments rely on the initial surgery. In the present analysis, patients with MN or MMP resection reported a good local control. We recommend performing skull base surgery prior to radiotherapy, to avoid the risk of postoperative complications, such as liquorrhea and poor wound healing. Omitting a postoperative radiotherapy, antecedent surgery was acceptable.
Conclusions
Conservative resection with close surgical margins may be acceptable for chemosensitive sarcomas with a good response to induction chemotherapy. Resection margin status is an important predictive factor for local recurrence after radical skull base surgery of chemosensitive sarcomas with a poor response to induction chemotherapy and chemoresistant sarcomas. Microscopic invasion is more aggressive than gross invasion; however, microscopic microresidual tumor may be controlled by postoperative treatment.
This was a nonrandomized retrospective study with a small sample size. Further studies are needed to clarify the role of skull base surgery for sarcoma.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 16K20232.
Footnotes
Conflicts of Interest The authors declare no conflict of interest.
References
- 1.Montogomery P Q, Evans P HR, Gullane P J. London: Informa; 2009. Principles and Practice of Head and Neck Surgery and Oncology. 2nd ed. [Google Scholar]
- 2.Fletcher C D. Distinctive soft tissue tumors of the head and neck. Mod Pathol. 2002;15(03):324–330. doi: 10.1038/modpathol.3880526. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan B, Bell R S, Bramwell V HC. Oxford: Oxford University Press; 2002. Sarcomas of the soft tissue; pp. 2495–2523. [Google Scholar]
- 4.Arndt C A, Stoner J A, Hawkins D S et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children's oncology group study D9803. J Clin Oncol. 2009;27(31):5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grier H E, Krailo M D, Tarbell N J et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(08):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 6.Malempati S, Hawkins D S. Rhabdomyosarcoma: review of the Children's Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59(01):5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodeberg D A, Wharam M D, Lyden E R et al. Delayed primary excision with subsequent modification of radiotherapy dose for intermediate-risk rhabdomyosarcoma: a report from the Children's Oncology Group Soft Tissue Sarcoma Committee. Int J Cancer. 2015;137(01):204–211. doi: 10.1002/ijc.29351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi K, Matsumoto F, Kodaira M et al. Significance of delayed primary excision in localized nonmetastatic adult head and neck rhabdomyosarcoma. Cancer Med. 2016;5(10):2708–2714. doi: 10.1002/cam4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohno K, Tsunoda A, Shirakura S, Takahashi N, Kishimoto S. The approaches and outcomes of skull base surgery for pediatric sarcoma after initial therapy. Auris Nasus Larynx. 2011;38(02):208–214. doi: 10.1016/j.anl.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Bentz B G, Bilsky M H, Shah J P, Kraus D. Anterior skull base surgery for malignant tumors: a multivariate analysis of 27 years of experience. Head Neck. 2003;25(07):515–520. doi: 10.1002/hed.10250. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara N, Sasaki T, Asakage T et al. Long-term outcome following radical temporal bone resection for lateral skull base malignancies: a neurosurgical perspective. J Neurosurg. 2008;108(03):501–510. doi: 10.3171/JNS/2008/108/3/0501. [DOI] [PubMed] [Google Scholar]
- 12.Crist W M, Anderson J R, Meza J L et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 13.Spalding A C, Hawkins D S, Donaldson S S et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: an analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys. 2013;87(03):512–516. doi: 10.1016/j.ijrobp.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


