Abstract
Introduction Brainstem gliomas (BsG) account for 10 to 15% of pediatric brain tumors. Surgery is the preferred treatment for focal and exophytic lesions. Sodium fluorescein has been proven safe and effective in resection of malignant brain tumors.
Objective The objective was to o analyze the safety and effectiveness of this approach, to evaluate intraoperative fluorescein imaging, and to measure the safety of chosen dose for pediatric patients.
Methods Twelve cases were enrolled between March 2014 and September 2016 in Beijing Tiantan Hospital. All of the patients received 2.5 mg/kg of sodium fluorescein before opening the dura; the intraoperative fluorescence enhancement was observed, and the degree of satisfaction and consistency with the neuronavigation were evaluated.
Results With a mean age of 7.5 years, there were eight cases located within the pontine, three in the medullary oblongata, and one in the tectal plate. Histological results were astrocytoma, glioblastoma, oligodendroglioma, and pilocytic astrocytoma. Under the fluorescein module of the microscope, the tumors were recognizable enough to help surgeons to discriminate the lesion from non-fluorescent tissue, with a consistency of 83% with the neuronavigation. Total removal was accomplished in nine cases, while the mean percentage of resection of the other cases was 93.7%. The Karnofsky performance score (KPS) showed no significant differences between pre-operation and discharge, but there was a difference between pre-operation and 6-month follow-up.
Conclusion The fluorescein-guided surgery is useful for demarcating the tumor margin and works well with other navigation and monitoring devices. A safe dose of sodium fluorescein (2.5 mg/kg) was proven effective for children.
Keywords: sodium fluorescein, pediatric brainstem gliomas, yellow 560 nm, surgery, safe dose
Introduction
Brainstem gliomas (BsG) are a heterogeneous group of tumors that account for 10 to 15% of pediatric brain tumors. 1 Most of these neoplasms are biologically malignant tumors that extensively infiltrate the surrounding tissue, while others are relatively benign and focal. The classification of BsG is mainly based on magnetic resonance imaging (MRI) appearances. In general, they can be grouped into diffuse or focal tumors, which include dorsal exophytic, cervicomedullary, and tectal subtypes.
While radiation is considered as the primary or even the only therapy for diffuse intrinsic brainstem gliomas (DIPG), the therapeutic goal of focal BsG is safe and total surgical removal. 2 In most cases, a well-defined interface between the tumor and neural tissue indicates a gross total resection (GTR) or subtotal resection (STR) resulting in a favorable prognosis. 3 However, the deep location and complicated anatomical structures of the brainstem areas make the surgical maneuverability in those areas really challenging, even with experienced neurosurgeons or with the widespread application of surgical adjuvant techniques, such as neuronavigation system, electrophysiological monitoring, or even intraoperative MRI.
Fluorescence-guided surgery utilizing sodium fluorescein has been proven to be a safe and effective adjunct for the removal of malignant brain tumors. 4 5 6 7 The primary advantage of fluorescence-guided surgery is to facilitate the resection by demarcating the tumor margin from the normal brain tissue in real time, with relatively easy preparation and low cost. 8 9
In this retrospective study, we report the first experiences using sodium fluorescein in pediatric patients with BsG. The major goal of the study is to analyze the safety and effectiveness of this approach. We also evaluate intraoperative fluorescein imaging and measure the safety of the chosen dose for pediatric patients.
Methods
Patient Selection
Twelve children with BsG treated between March 2014 and September 2016 in the Department of Neurosurgery of Beijing Tiantan Hospital were enrolled in this study. Inclusion criteria were as follows: (1) age < 18 years, (2) newly diagnosed or suspicious, untreated BsG, and (3) the lesion identified as a focal exophytic tumor and enhanced remarkably on MRI. Exclusion criteria included the followings: (1) histological diagnosis other than glioma, (2) previously treated, recurrent tumors, (3) diffuse intrinsic or unresectable BsG, (4) no or mild enhancement on MRI after contrast, and (5) a Karnofsky performance score (KPS) < 70, and (6) medical conditions that prevent the surgery or use of sodium fluorescein, such as coagulopathy, renal, and hepatic dysfunction. There were eight males and four females with ages ranging from 2 to 18 years. Information about the patients and tumors has been summarized in Table 1 .
Table 1. Clinical characteristics of the patients.
| Patient no. | Sex | Age | Location | Approaches | Histology | Percentage of resection | complication | KPS at | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Admission | Discharge | Follow-up | ||||||||
| 1 | M | 5 | R pons | Subtemporal | GBM | 95% | 70 | 70 | 80 | |
| 2 | F | 7 | R pons | Subtemporal | GBM | 100% | Hypoproteinemia | 90 | 90 | 90 |
| 3 | M | 10 | L pons | Subtemporal | GBM | 100% | 70 | 80 | 80 | |
| 4 | M | 18 | RL medulla | Posterior median | Astrocytoma | 100% | Tracheotomy | 80 | 70 | 80 |
| 5 | M | 2 | R medulla | Far lateral | Astrocytoma | 88% | Pulmonary infection | 90 | 70 | 80 |
| 6 | M | 4 | L pons | Retrosigmoid | GBM | 100% | 80 | 80 | 90 | |
| 7 | M | 4 | L tectal | Poppen | Astrocytoma | 98% | 80 | 80 | 80 | |
| 8 | M | 5 | L pons | Retrosigmoid | Astrocytoma | 100% | 100 | 100 | 100 | |
| 9 | M | 7 | R pons | Retrosigmoid | Oligodendroglioma | 100% | Dystaxia | 90 | 80 | 80 |
| 10 | F | 8 | L pons | Retrosigmoid | Pilocytic astrocytoma | 100% | 90 | 90 | 90 | |
| 11 | F | 12 | R medulla | Posterior median | Astrocytoma | 100% | Dystaxia | 100 | 90 | 100 |
| 12 | F | 9 | R pons | Subtemporal | Oligodendroglioma | 100% | 80 | 80 | 90 | |
Abbreviations: GBM, glioblastoma multiforme; KPS, Karnofsky performance score; L, left; R, right.
Clinical and Radiological Assessment
All patients' medical histories were recorded, including laboratory tests. The KPS was used to evaluate general physical performances. Pre- and post-contrast MRI was submitted no earlier than 5 days before the surgery and no later than 3 days after the surgery. Diffusion tensor image (DTI) was prepared for the use of neuronavigation during the operations. Surgical approaches were confirmed by the same chief operator (Z.L.). The KPS was evaluated at admission, discharge, and 6-month follow-up. Percentage of resection was calculated by comparing of the measured volume of the residual tumor on the post-operation MRI with the volume measurement on the pre-operation MRI. All complications and adverse effects of sodium fluorescein were thoroughly documented.
Operative Protocol
After anesthesia, 0.05 mL of 10% sodium fluorescein was used to perform a sensitivity test. The intraoperative use of sodium fluorescein was allowed if the test proved negative. A predefined dose of 2.5 mg/kg was injected intravenously through the central line before opening the dura. Microsurgery was performed under the PENTERO 900 (Carl Zeiss, Oberkochen, Germany) equipped with a YELLOW 560 nm filter which allowed for switching the illumination between fluorescence and white light. A neuronavigation system (iPlan Cranial 3.0, Brainlab, Munich, Germany) was routinely used for surgical planning, tumor localization, and orientation. Electrophysiological monitoring is mandatory due to the sensitive nature of the brain area and the high possibility of displaced or involved motor and sensor tracts. The surgical procedures were performed by the same neurosurgeon (Z.L.) who also judged the effectiveness of sodium fluorescein as “helpful” or “unhelpful.” Helpful was defined as the tumor being enhanced and visualized by sodium fluorescein with a demarcated margin from normal tissue, while unhelpful was considered as mild enhancement, vague visualization or blurred tumor–brain interface. The tumor was removed with standard microsurgical techniques. At the end stage of resection, the consistency between the fluorescein guide and the neuronavigation guide was carefully compared. After the operations, all patients were followed up in the neurological intensive care unit (NICU) after surgery and were transferred to the regular ward as soon as their condition became stable.
Results
Clinical, Radiological, and Histopathological Characteristics
Among the patients, eight (67%) were male, and four (33%) were female. The mean age was 7.6 years old. The common clinical presentation was hemiparesis, headache, eye movement dysfunction, and cranial nerve deficit. All lesions were enhanced on the pre-operative MRI. There were eight cases (67%) located within the pontine, three (25%) in the medullary oblongata, and one (8%) in the tectal plate. Histological analysis found the most common type was low grade (WHO II) astrocytoma (five cases, 42%); others were glioblastoma multiforme (GBM) (WHO IV, four cases, 33%), oligodendroglioma (WHO II, two cases, 17%), and pilocytic astrocytoma (WHO I, one case, 8%).
Intraoperative Fluorescence Enhancement
During surgery, the fluorescence was not visible under the white light of the microscope until switching to the fluorescein module. All tumors were enhanced vividly by sodium fluorescein and stained yellowish green, while normal tissue remained in its natural color and unstained. It was convenient to switch the illumination between white light and fluorescence with one button on the grip. Sodium fluorescein was able to make the tumors recognizable enough to help the surgeons to discriminate the lesion from non-fluorescent tissue. Tumors were resected as much as possible unless the electrophysiological monitoring showed abnormalities, in which case the resection had to be stopped. Nevertheless, the operator considered the efficacy of fluorescein-guided operation as 100% helpful. At the end of surgery, the correspondence between the fluorescein guidance and neuronavigation was compared by placing the probe at the border of surgical cavity in each case. The consistency was 83% (10/12).
Percentage of Resection and Outcome
No adverse or allergic reactions were observed related to the use of sodium fluorescein both during and after the operations. For nine cases, total removal of the tumor was accomplished, while others had a mean percentage of resection of 93.7% (from 88 to 98%). The main complications were tracheotomy, pulmonary infection, hypoproteinemia, and dystaxia, and with subsequent therapy and rehabilitation, there were no permanent systemic or neurological deficits at the 6-months follow-up. There were no significant differences between the KPS of pre-operation and discharge ( p = 0.166), while the KPS of pre-operation and 6-months follow-up showed statistically significant differences ( p = 0.031).
Illustrative Case
A 5-year-old boy presented with fall attack for 2 weeks and developed headache for 2 days before seeing a doctor. On physical examination, significant hemiparesis of left limbs was noted. An MRI showed a heterogeneous lesion located within the pontine ( Fig. 1 ). The tumor enhanced vividly and heterogeneously. DTI revealed most of the tracts were displaced posteriorly and contralaterally, with a little posteriorly and ipsilaterally by the tumor ( Fig. 2 ). The pre-operative surgical plan was made, and a safe entry zone was chosen according to the DTI imaging. He underwent a subtemporal craniotomy under general anesthesia ( Fig. 3a ). As his weight was 23 kg, 57.5 mg (2.5 mg/kg) of sodium fluorescein had been injected intravenously before the dura was opened. Under the microscope with the fluorescence module, the tumor became greenish, and the normal brain remained its natural color ( Fig. 3b ). The tumor was resected in the white light of the microscope with the assistance of neuronavigation and electrophysiological monitoring. After the resection, the fluorescence module was switched on again to check the surgery field, showing a very small amount of residual tumor, which could not be safety removed because of its adherence to vital nuclei ( Fig. 3c and d ). Postoperative MRI showed a subtotal removal of the tumor, which was a glioblastoma confirmed by pathological examination ( Fig. 4 ). The patient was discharged at 10 days postoperatively without any new neurological deficits. At the follow-up 6 months later, the patients had received radiotherapy and chemotherapy. The patient died 1 year after the operation because of tumor recurrence, which had led to coma and hypoxemia.
Fig. 1.
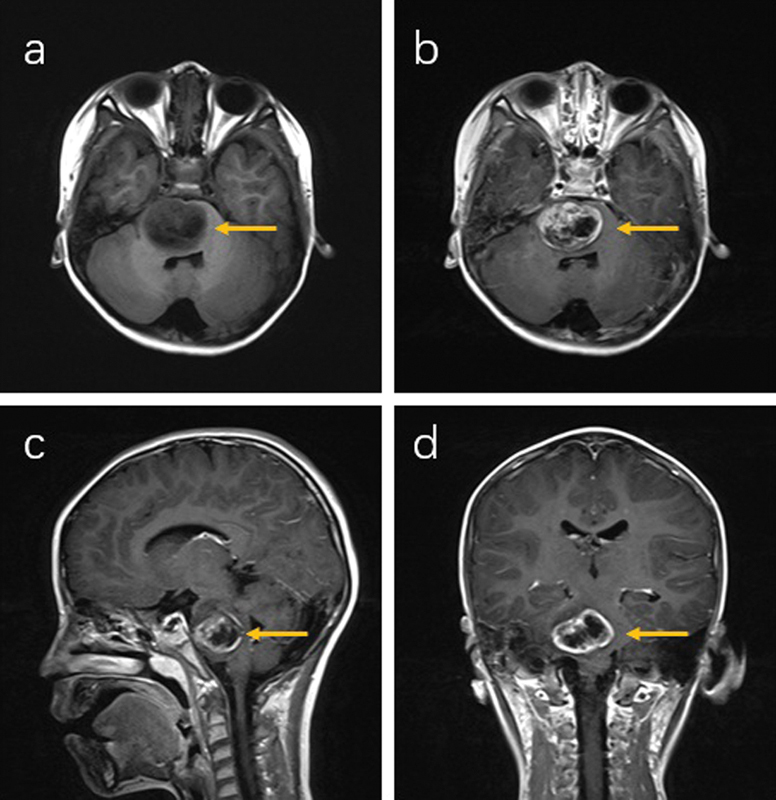
Preoperative MRI of patient 1 (right pons). Tumor (yellow arrow) was isointensity in non-contrast T1 image ( a ) and gadolinium-enhanced in post-contrast T1 images ( b – d ) with central necrosis, which suggested a high-grade glioma. MRI, magnetic resonance imaging.
Fig. 2.
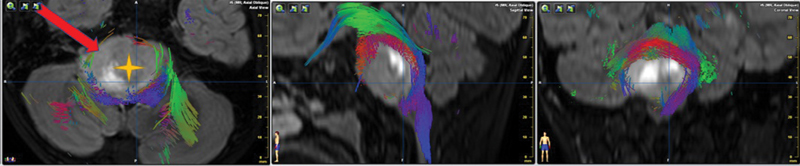
Neuronavigation strategy. By merging the images of MRI and DTI, the tumor (yellow cross) was shown in the right pons with the brainstem tract displaced posteriorly and contralaterally, with a little posteriorly and ipsilaterally. A subtemporal approach (red arrow, indicated the direction of the approach) was therefore chosen to avoid most of the tract. DTI, diffusion tensor image; MRI, magnetic resonance imaging.
Fig. 3.
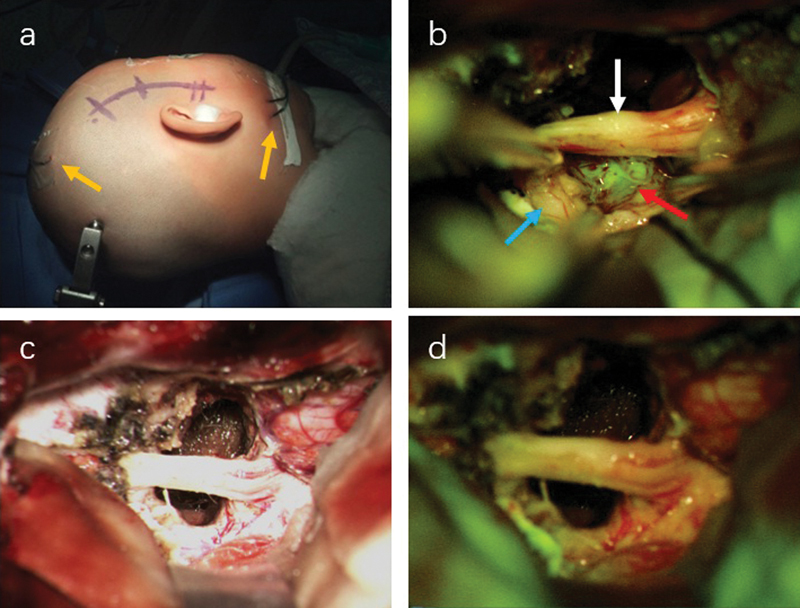
Surgical approach and intraoperative fluorescence enhancement of patient 1. Craniotomy is taken by subtemporal approach ( a ) with the electrophysiological monitoring (yellow arrow). The sodium fluorescein is injected before opening the dura. ( b ) Under the microscope with fluorescence module, the tumor was greenish red arrow), and the trigeminal nerve (white arrow) and normal brain (blue arrow) remained in their natural color. After the resection, the surgical field was compared between white light ( c ) and fluorescence module ( d ), which showed no greenish display obviously.
Fig. 4.
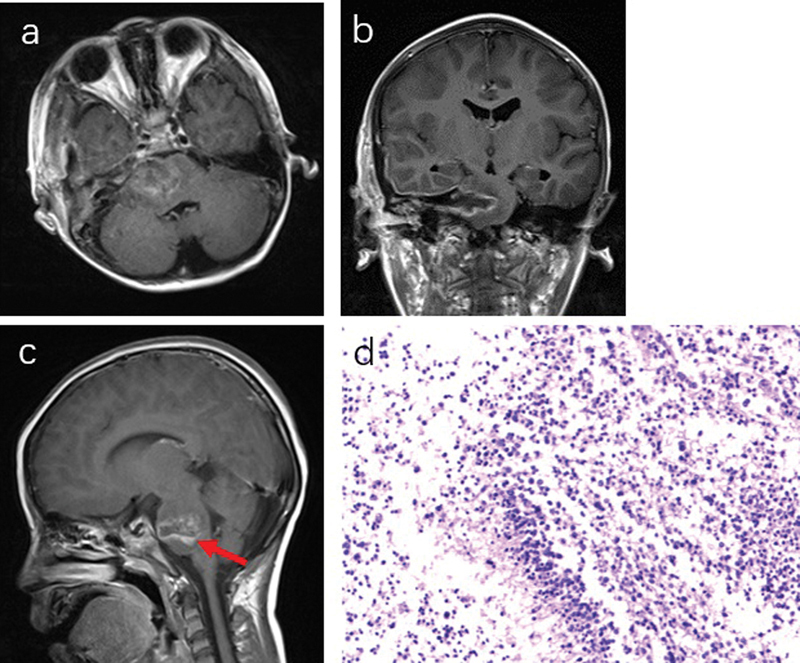
( a and b ) Postoperative MRI (3 days after operation) of patient 1. The tumor was extensive, but not completely removed ( c , red arrow), with the histological examination of glioblastoma ( d ) (WHO IV, GFAP + , Olig2 + , and K i -67, 60%). GFAP, glial fibrillary acidic protein; MRI, magnetic resonance imaging; WHO IV, World Health Organization grade IV.
Discussion
Pediatric brainstem glioma accounts for 10 to 20% of central nervous system tumors in children and 58 to 80% of all brainstem tumors. 1 2 10 It could occur at any location within the brainstem and can be classified into diffuse and focal types based on MRI features. The treatment modality includes surgery, radiation therapy, and chemotherapy. It is almost not possible to safely remove a diffuse tumor in the brainstem due to the long tracts and nuclei involved. Thus, surgery is reserved for focal, exophytic tumors, which are more amenable to resection. 11 Despite the application of intraoperative adjuvant techniques, such as neuronavigation, neurophysiological monitoring, and the popularization of intraoperative ultrasound, the surgical procedure is still associated with high morbidity and mortality. The application of fluorescence-guided surgery has shown significant benefit in surgery for high-grade gliomas, 4 5 cerebral metastases, 6 7 primary central nervous system lymphomas, 12 and even benign lesions, such as meningiomas and abscess. 13 14 The goal of surgery is to reduce the tumor as much as possible and to seek a balance point between the GTR and postoperative neurological function.
Sodium fluorescein has been widely and safely used in ophthalmology for retinal angiography. 15 16 It can pass through a damaged blood–brain barrier and accumulates in tumor tissue, thus producing fluorescence that could be detected by microscope with or without a filter, even with the naked eye. 17 Most studies have demonstrated that the enhancement pattern of fluorescein was similar to the contrast enhancement on MRI. 18 19 20 The typical appearance of fluorescein is to turn the tumor yellowish-green, while the peripheral brain tissue remains in its natural color.
In 1982, Murray reported usage of sodium fluorescein for resection of malignant brain tumors. 21 The illumination system was a conventional surgical headlight. He found that the sensitivity and specificity of fluorescein guidance was 84.7 and 94.7%, respectively. With the popularization of the microscope, Shinoda et al and Koc et al reported separate studies utilizing sodium fluorescein as an adjunct in glioblastoma surgery. 17 22 The authors found that the use of this substances was “simple and safe” in facilitating the surgery, and they achieved a higher resection rate. In 2013, Acerbi et al published a report of fluorescein-guided surgery for grade IV gliomas under a specific microscope equipped with a filter (YELLOW 560 nm). 23 They concluded that the advancement in the microscope could help better visualize the tumor while reducing the dose. Recently, the First National European Pharmacologic Agency has approved sodium fluorescein for neurosurgical use reflecting its safety and efficacy.
The dose of sodium fluorescein used in older series was primarily 20 mg/kg to visualize the tumor under white light. 7 17 22 With advances in surgical microscopes and the dedicated filter (YELLOW 560 nm), a relatively low dose could be used, which in most series was less than 10 mg/kg. Some authors, even reported the dose of 2 to 4 mg/kg. 4 24 There was no significant difference in the ability to enhance the tumor. Until now, we have not found any guidance on using sodium fluorescein in pediatric patients of brainstem tumors. In our experience, we have used a 5 mg/kg in adults with no apparent adverse effect or poor visualization. As a consequence, we used half dose (2.5 mg/kg) in children and found that it could be well tolerated. Given it was the first use of sodium fluorescein in pediatric patients, we carefully observed and documented any side effects that were reported in the literature. Someone reported a severe anaphylactic shock brought on by 20 mg/kg of sodium fluorescein, which might be due to the high dose used. 25 Several authors suggested that the rate of allergy was very low while using a low dose of sodium fluorescein, ranging from 2∼5 mg/kg. 9 In our series, we did not observe any apparent adverse effects associated with sodium fluorescein, although greenish urine was noted. No permanent or serious side effects occurred.
After the administration of sodium fluorescein, the tumors were visualized under the fluorescein module 20 to 30 minutes later. Therefore, we usually had finished the administration procedures before the dura was opened. In our 12 cases, no tumor failed to be visualized. It has been noted that the enhancing pattern of fluorescein is similar to contrast enhancement due to the mechanism of penetrating the disrupted blood–brain barrier. 19 20 According to our inclusion criteria, every tumor was enhanced on pre-operative MRI. Thus, it is certain that all tumors were stained and illuminated under the microscope. However, several reports have demonstrated that in rare cases the tumor is not enhanced as expected indicating a potential pattern different from the MRI contrast, which may require further experimental study. 26 27
At the end of resection, we investigated the consistency between the fluorescein guide and neuronavigation guide. In most cases, we found strong correspondence between the fluorescein-demonstrated border and the contrast-enhancing tumor margin on intraoperative navigation, 28 29 except for two cases in which the navigation system lost its reliability and accuracy due to brain shifting or other reasons.
In all cases, the fluorescence provided a clear-cut margin that could be distinguished between the tumor and brain tissue. We did not find partial enhancement or no enhancement after administration of sodium fluorescein as some authors have reported. 4 5 It may be due to our limited patient number which could create a false negative result.
Unlike supratentorial malignant tumors, for which the extent of resection (EOR) has a predictive value in the outcome, 30 31 the goal for BsG should always be maximally safe resection. In one case, the neurophysiological monitoring showed that amplitudes were significantly reduced during tumor resection, so the manipulation had to stop, and the fluorescein-enhanced tumor remnants were left for radiation.
The KPS at discharge was not higher than that at admission, but it seems that the performance status had improved to the level of presentation at 6-month follow-up. The reason may be the vulnerability of the brainstem and the compactness of important nuclei, which may be temporarily impaired shortly after the operation.
Conclusions
In this study, we investigated the benefit of using sodium fluorescein in guiding brainstem surgery in pediatric patients. We achieved promising results and proved that fluorescein-guided surgery is useful in demarcating the tumor margin and works well with other navigation and monitoring devices. A safe dose of sodium fluorescein (2.5 mg/kg) is proven to be effective for children in our cohort. Although the precision of fluorescein is satisfying, it should be emphasized that each of the adjuvant techniques (fluorescein-guide surgery, neuronavigation, intraoperative ultrasound, and neurophysiological monitor) has advantages and disadvantages and plays a complementary role when combined. Only utilizing these together can achieve better surgical outcomes.
Conflicts of Interest The authors declare no conflicts of interest.
Financial Support
This work was supported by grants from Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support [ZYLX201608].
These authors have contributed equally to this work .
References
- 1.Kaplan A M, Albright A L, Zimmerman R A et al. Brainstem gliomas in children. A children's cancer group review of 119 cases. Pediatr Neurosurg. 1996;24(04):185–192. doi: 10.1159/000121036. [DOI] [PubMed] [Google Scholar]
- 2.Farmer J P, McNeely P D, Freeman C R . New York: Thieme; 2008. Brainstem Gliomas; pp. 640–654. [Google Scholar]
- 3.Halperin E C, Constine L S, Tarbell N J . London: Lippincott Williams & Wilkins; 2005. Tumors of the posterior fossa and spinal canal. Pediatric Radiation Oncology. [Google Scholar]
- 4.Acerbi F, Cavallo C, Broggi M et al. Fluorescein-guided surgery for malignant gliomas: a review. Neurosurg Rev. 2014;37(04):547–557. doi: 10.1007/s10143-014-0546-6. [DOI] [PubMed] [Google Scholar]
- 5.Schebesch K M, Proescholdt M, Höhne J et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery--a feasibility study. Acta Neurochir (Wien) 2013;155(04):693–699. doi: 10.1007/s00701-013-1643-y. [DOI] [PubMed] [Google Scholar]
- 6.Schebesch K M, Hoehne J, Hohenberger C et al. Fluorescein sodium-guided resection of cerebral metastases—experience with the first 30 patients. Acta Neurochir (Wien) 2015;157(06):899–904. doi: 10.1007/s00701-015-2395-7. [DOI] [PubMed] [Google Scholar]
- 7.Okuda T, Kataoka K, Yabuuchi T, Yugami H, Kato A. Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium. J Clin Neurosci. 2010;17(01):118–121. doi: 10.1016/j.jocn.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Berger M S. The fluorescein-guided technique. Neurosurg Focus. 2014;36(02):E6. doi: 10.3171/2013.11.FOCUS13535. [DOI] [PubMed] [Google Scholar]
- 9.Schebesch K M, Brawanski A, Hohenberger C, Hohne J. Fluorescein sodium-guided surgery of malignant brain tumors: history, current concepts, and future projects. Turk Neurosurg. 2016;26(02):185–194. doi: 10.5137/1019-5149.JTN.16952-16.0. [DOI] [PubMed] [Google Scholar]
- 10.Freeman C R, Farmer J P. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(02):265–271. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 11.Teo C, Siu T L. Radical resection of focal brainstem gliomas: is it worth doing? Childs Nerv Syst. 2008;24(11):1307–1314. doi: 10.1007/s00381-008-0647-z. [DOI] [PubMed] [Google Scholar]
- 12.Schebesch K M, Hoehne J, Hohenberger C et al. Fluorescein sodium-guided surgery in cerebral lymphoma. Clin Neurol Neurosurg. 2015;139:125–128. doi: 10.1016/j.clineuro.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 13.da Silva C E, da Silva V D, da Silva J L. Sodium fluorescein in skull base meningiomas: a technical note. Clin Neurol Neurosurg. 2014;120:32–35. doi: 10.1016/j.clineuro.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Höhne J, Brawanski A, Schebesch K M. Fluorescence-guided surgery of brain abscesses. Clin Neurol Neurosurg. 2017;155:36–39. doi: 10.1016/j.clineuro.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Rabb M F, Burton T C, Schatz H, Yannuzzi L A. Fluorescein angiography of the fundus: a schematic approach to interpretation. Surv Ophthalmol. 1978;22(06):387–403. doi: 10.1016/0039-6257(78)90134-0. [DOI] [PubMed] [Google Scholar]
- 16.Yannuzzi L A, Rohrer K T, Tindel L J et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93(05):611–617. doi: 10.1016/s0161-6420(86)33697-2. [DOI] [PubMed] [Google Scholar]
- 17.Shinoda J, Yano H, Yoshimura S et al. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg. 2003;99(03):597–603. doi: 10.3171/jns.2003.99.3.0597. [DOI] [PubMed] [Google Scholar]
- 18.Hesselink J R, Press G A. MR contrast enhancement of intracranial lesions with Gd-DTPA. Radiol Clin North Am. 1988;26(04):873–887. [PubMed] [Google Scholar]
- 19.Diaz R J, Dios R R, Hattab E M et al. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J Neurosurg. 2015;122(06):1360–1369. doi: 10.3171/2015.2.JNS132507. [DOI] [PubMed] [Google Scholar]
- 20.Neira J A, Ung T H, Sims J S et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J Neurosurg. 2017;127(01):111–122. doi: 10.3171/2016.7.JNS16232. [DOI] [PubMed] [Google Scholar]
- 21.Murray K J. Improved surgical resection of human brain tumors: Part I. A preliminary study. Surg Neurol. 1982;17(05):316–319. doi: 10.1016/0090-3019(82)90298-1. [DOI] [PubMed] [Google Scholar]
- 22.Koc K, Anik I, Cabuk B, Ceylan S. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg. 2008;22(01):99–103. doi: 10.1080/02688690701765524. [DOI] [PubMed] [Google Scholar]
- 23.Acerbi F, Broggi M, Eoli M et al. Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: preliminary results in 12 cases. Acta Neurochir (Wien) 2013;155(07):1277–1286. doi: 10.1007/s00701-013-1734-9. [DOI] [PubMed] [Google Scholar]
- 24.Rey-Dios R, Cohen-Gadol A A. Technical principles and neurosurgical applications of fluorescein fluorescence using a microscope-integrated fluorescence module. Acta Neurochir (Wien) 2013;155(04):701–706. doi: 10.1007/s00701-013-1635-y. [DOI] [PubMed] [Google Scholar]
- 25.Dilek O, Ihsan A, Tulay H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J Clin Neurosci. 2011;18(03):430–431. doi: 10.1016/j.jocn.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Neira J A, Ung T H, Sims J S et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J Neurosurg. 2017;127(01):111–122. doi: 10.3171/2016.7.JNS16232. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Rey-Dios R, Roberts D W, Valdés P A, Cohen-Gadol A A.Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies World Neurosurg 201482(1-2):175–185. [DOI] [PubMed] [Google Scholar]
- 28.Catapano G, Sgulò F G, Seneca V, Lepore G, Columbano L, di Nuzzo G. Fluorescein-guided surgery for high-grade glioma resection: an intraoperative “contrast-enhancer”. World Neurosurg. 2017;104:239–247. doi: 10.1016/j.wneu.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Ung T H, Kellner C, Neira J A et al. The use of fluorescein sodium in the biopsy and gross-total resection of a tectal plate glioma. J Neurosurg Pediatr. 2015;16(06):732–735. doi: 10.3171/2015.5.PEDS15142. [DOI] [PubMed] [Google Scholar]
- 30.De Bonis P, Anile C, Pompucci A et al. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013;115(01):37–43. doi: 10.1016/j.clineuro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Orringer D, Lau D, Khatri S et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117(05):851–859. doi: 10.3171/2012.8.JNS12234. [DOI] [PubMed] [Google Scholar]


