Abstract
Objectives Boron neutron capture therapy (BNCT) is a nuclear reaction-based tumor cell-selective particle irradiation that occurs when nonradioactive Boron-10 is irradiated with low-energy neutrons to produce high-energy α particles (10B [ n , α] 7Li). Possible complications associated with extended surgical resection render high-grade meningioma (HGM) a challenging pathology and skull-base meningiomas (SBMs) even more challenging. Lately, we have been trying to control HGMs using BNCT. This study aims to elucidate whether the recurrence and outcome of HGMs and SBMs differ based on their location.
Design Retrospective review.
Setting Osaka Medical College Hospital and Kyoto University Research Reactor Institute.
Participants Between 2005 and 2014, 31 patients with recurrent HGM (7 SBMs) were treated with BNCT.
Main Outcome Measures Overall survival and the subgroup analysis by the anatomical tumor location.
Results Positron emission tomography revealed that HGMs exhibited 3.8 times higher boron accumulation than the normal brain. Although tumors displayed transient increases in size in several cases, all lesions were found to decrease during observation. Furthermore, the median survival time of patients with SBMs post-BNCT and after being diagnosed as high-grade were 24.6 and 67.5 months, respectively (vs non-SBMs: 40.4 and 47.5 months).
Conclusions BNCT could be a robust and beneficial therapeutic modality for patients with high-grade SBMs.
Keywords: boron neutron capture therapy, high-grade meningioma, skull base meningioma, recurrent, boronophenylalanine
Introduction
Both high-grade meningioma (HGM; World Health Organization [WHO] grades II and III) and high-grade glioma are pathologically challenging to control. Typically, the standard treatment options are gross total resection and radiotherapy following subtotal resection. Indeed, skull-base meningioma (SBM) is even more challenging because it often cannot be completely resected, and complications could follow extended surgical resection. The last few decades have witnessed an upsurge in the application of external beam radiotherapy (EBRT) and stereotactic radiosurgery (SRS). 1 2 3 However, to date, no data are available on long-term patients with high-grade SBMs. Recently, we have been trying to control high-grade SBMs by tumor cell-selective intensive particle radiation, boron neutron capture therapy (BNCT). 4 5 6 7
BNCT comprises nuclear capture and fission reactions that occur when nonradioactive Boron-10 ( 10 B) is irradiated with neutrons of appropriate energy to yield high-energy α particles ( 4 He) and recoiling lithium-7 ( 7 Li) nuclei. As these particles have path lengths of ∼5 to 9 μm, which is one cell diameter, their destructive effect is limited to cells containing 10 B; thus, contributing to a tumor-selective and significant tumoricidal activity with minimal damage to healthy tissues. 8
In 1950, Farr et al reported the first clinical trial of BNCT for patients with glioblastoma. 9 However, the poor selection of boron compounds and technical limitations led to a lack of neutron penetration, especially for deep-seated tumors, in the previously used version of BNCT. However, these issues have been resolved with the advancement and development of reactors used in this treatment. Furthermore, we modified several parts of the procedure and applied the modified BNCT to the treatment of high-grade gliomas, as well as the identification of HGMs. Previously, we reported the efficacy of BNCT for HGMs. 4 5 6
In our previous study, we focused on the early tumor shrinkage and adverse events and did not consider the long-term prognosis as a primary endpoint. Thus, this study includes an extended observation period (>2 years) and reports the results of re-evaluation from the perspective of the overall survival irrespective of whether it becomes a disadvantage for deep-seated tumors, which had been highlighted previously as a specific BNCT-related problem because of tissue penetration and decay of the neutron beam when only using the thermal neutron era. Moreover, this study compares SBMs and non-SBMs as a subclass about the tumor volume reduction, long-term control rate, including cumulative survival data, and treatment failure patterns post-BNCT.
Patients and Methods
Patients
Between June 2005 and May 2014, 33 patients with recurrent HGM were treated with BNCT at the Department of Neurosurgery, Osaka Medical College (Osaka, Japan). Of these, 9 patients were assigned with meningioma at the skull-base locations (SBMs group) and 24 patients were assigned to the non-SBMs group. Notably, all patients were referred to our institute for BNCT because of the uncontrolled tumor growth rate after repetitive surgeries and irradiations (EBRT or SRS). Table 1 summarizes the characteristics of all patients. This study was approved by the Ethical Committee of Osaka Medical College (No. 260 and 1218). Furthermore, each enrolled candidate was discussed and approved by the board of reviewers at Osaka Medical College and Kyoto University Research Reactor Institute.
Table 1. Patient and treatment characteristics of BNCT in HGMs.
| SBMs ( n = 9) |
Non-SBMs ( n = 24) | p | |
|---|---|---|---|
| Age at BNCT (years) | |||
| 57.1 | 58.7 | 0.77 | |
| Gender | |||
| Male | 2 | 9 | 0.68 |
| Female | 7 | 15 | |
| WHO grade | |||
| II | 3 | 9 | 0.69 |
| III | 6 | 15 | |
| T/N ratio of PET study | |||
| Mean | 4.0 | 3.7 | 0.47 |
| Number of prior surgeries (times) | |||
| Mean | 3.6 | 2.8 | 0.15 |
| Number of prior radiotherapies (times) | |||
| Mean | 1.8 | 2.1 | 0.56 |
| Minimum absorbed dose in tumor tissue (Gy-Eq) | |||
| Mean | 23.3 | 42.4 | a 0.001 |
| Maximum absorbed dose in tumor tissue (Gy-Eq) | |||
| Mean | 67.2 | 73.4 | 0.33 |
| Tumor depth (mm) | |||
| Mean | 76.1 | 49.9 | a < 0.001 |
| Number of BNCT(times) | |||
| Mean | 1.4 | 1.3 | 0.52 |
Abbreviations: BNCT, boron neutron capture therapy; tumor depth, distance from the scalp surface to the deepest part of the tumor; Gy-Eq, Gray Equivalent; HGMs, high-grade meningiomas; PET, positron emission tomography; SBMs, skull base meningiomas; T/N, the tumor-to-normal brain ratio; WHO, World Health Organization.
p < 0.05 was considered statistically significant.
Fluorine-18-Labeled BPA PET Analysis
All patients underwent fluorine-18 ( 18 F)labeled boronophenylalanine ( 18 F-BPA) positron emission tomography (PET) to assess the tracer uptake ability and distribution of BPA and to evaluate the boron concentrations in tumors before neutron irradiation. 4 5 6 10 11 12 In addition, the tumor-to-normal brain ratio of BPA uptake can be evaluated from 18 F-BPA PET, and the subsequent dose planning is based on this ratio. Table 1 represents the mean ratio of patients included in this study.
Clinical Regimen of BNCT for HGMs
We modified the clinical regimen of BNCT for HGMs slightly from that for malignant gliomas. 12 All patients were typically administered 500 mg/kg of BPA with or without 5 or 2.5 g of sodium borocaptate (BSH) per person. Both BPA and BSH were provided by the Stella Pharma Corporation (Osaka, Japan) in initial patients but were purchased primarily from Katchem Ltd. or Interpharma Praha (Czech Republic) later. However, as the study progressed, the procurement of BSH became difficult owing to its high cost. Thus, we omitted BSH administration in 26 patients.
BPA was administered only 2 hours before the neutron irradiation (200 mg/kg/h) and then reduced during the neutron irradiation (100 mg/kg/h) for maintaining the patients' blood BPA concentration throughout the neutron irradiation. When BSH was available, the compound solution was administered for 1 hour, starting at 12 hours before the neutron irradiation. We monitored the boron concentration in the blood by sampling every 1 to 2 hours after the initiation of the boron compound administration until the completion of the neutron irradiation. We assumed the boron concentrations from BSH in the tumor and brain tissue to be similar to the blood concentration from each patient. In tumors and normal brain tissues, we estimated the boron concentrations using the tumor-to-normal brain ratio of 18 F-BPA on PET imaging. Based on the contribution of each boron compound and the relative biological efficacy of neutron beams and compounds described previously, 6 13 14 we simulated the neutron fluence rate by the dose-planning system Simulation Environments for Radiotherapy Applications (SERA; Idaho National Engineering and Environmental Laboratory) and estimated the total irradiation doses to tumors and normal brain tissues. In addition, the duration of neutron irradiation was determined not to exceed 15 Gray Equivalent (Gy-Eq) to the normal brain. In this study, Gy-Eq implies an X-ray dose that can provide effects equivalent to the total BNCT radiation biologically. After the treatment, we re-estimated the doses administered precisely.
Assessment of Effectiveness
We assessed the efficacy of this treatment volumetrically in the serial radiographic analysis. The Gadolinium (Gd)-contrasted lesion on magnetic resonance imaging (MRI) was semiautomatically selected, and the area was calculated on each slice based on the contrast cut-off value (increased intensity on MRI by Gd) from the background. The area was calculated as the tumor volume by adding the areas of all slices using I-Response or SYNAPSE VINCENT software (Cedara Software Corp., Canada [unfortunately, this software is now no longer available] or Fujifilm Corp., Japan). Based on the diagnostic images pre-BNCT, we calculated the relative values of the tumor volume (percentage of control) from consecutive images obtained in each patient's follow-up evaluation. Then, we assessed changes in these values over time graphically and investigated for trends among our nine patients with SBMs undergoing BNCT.
Survival Analysis
We defined the patient survival in two ways: the number of months survived after diagnosis as HGM (SBMs or non-SBMs) and the number after the application of BNCT.
Statistical Analysis
In this study, statistical differences between means were calculated using the Student's unpaired t -test. In addition, we calculated the median survival time (MST) for each group using the Kaplan–Meier estimate.
Results
Absorbed Dose in the Tumor Tissue
In this study, the irradiation duration was planned such that it did not exceed 15 Gy-Eq in the normal brain tissue. The absorbed dose to the tumor tissue depended on both the boron concentration in the tumor tissue and the neutron irradiation time, which varied in each patient. Thus, the absorbed dose to the tumor tissue varied from patient to patient. The mean minimum absorbed dose in SBMs and non-SBMs was 23.3 Gy-Eq. (95% confidence interval [CI]: 15.1–31.6 Gy-Eq) and 42.4 Gy-Eq. (95% CI: 36.5–48.4 Gy-Eq), respectively ( Table 1 ). As SBMs were located deep into the brain, the mean tumor depth, evaluated as the direct distance from the scalp surface to the deepest part of the tumor, was significantly greater than that in non-SBMs, and the mean minimum absorbed dose in SBMs was significantly lower than that in non-SBMs. Furthermore, these absorbed doses were administered not in fractions but one-time irradiation in BNCT.
Volume Reduction in the Targeted Mass Lesions during Follow-Up
During observation, as in the initial cases of HGMs we reported previously, all patients with SBM exhibited a volume reduction in the mass lesions. 4 5 6 Although all tumors displayed a trend of gradual volume reduction, a transient increase was observed post-BNCT in some patients, usually within a month after irradiation, before decreasing again; this pattern was called pseudoprogression ( Fig. 1 ). 15 Fig. 2 represents an illustrative case of tumor shrinkage. The patient was a 49-year-old female with atypical meningioma developing at the anterior skull base. At 3 months post-BNCT, the patient exhibited a 30% volume reduction.
Fig. 1.
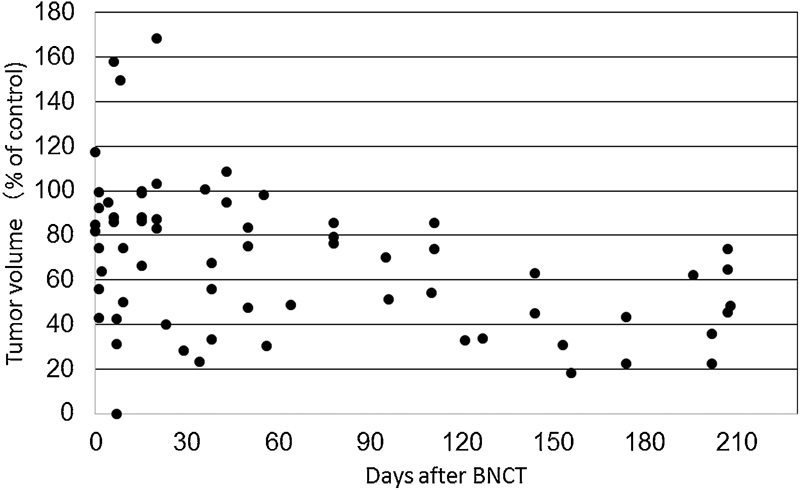
A scatterplot of the association between the tumor volume and the elapsed time (days) post-boron neutron capture therapy (BNCT). Day 0 means the date of the treatment, and the value (100%) plotted on day 0 was acquired 1 to 3 weeks before treatment. All values obtained from each patient from BNCT to relapse are plotted on the same graph.
Fig. 2.

Representative tumor shrinkage post-boron neutron capture therapy (BNCT) demonstrated on axial ( A , B , E , F , I , and J ) and sagittal ( C , D , G , H , K , and L ) Gd-enhanced magnetic resonance images. This case shows a marked tumor volume reduction post-BNCT. Images of an atypical meningioma before ( A – D ), 3 weeks after ( E – H ), and 3 months post-BNCT ( I – L ).
Treatment Failure
Table 2 represents the failure pattern after treatment. As reported previously, we experienced systemic metastasis, cerebrospinal fluid (CSF) dissemination, and distant intracranial recurrence outside the irradiation field. 5 6 Among nine patients who had their SBMs treated with BNCT, one patient demonstrated systemic metastasis, and only one patient presented the local tumor progression inside the irradiated field. No patient died because of the local tumor progression. Conversely, we observed the CSF leak in two of the nine patients with SBMs post-BNCT because of the rapid reduction in the tumor volume.
Table 2. Treatment failures.
| SBMs ( n = 9) | Non-SBMs ( n = 24) | |
|---|---|---|
| Recurrence | ||
| (In field) | 1 (11%) | 1 (4%) |
| (Out of field) | 3 (33%) | 1 (4%) |
| Systemic metastasis | 1 (11%) | 6 (25%) |
| CSF dissemination | 0 | 4 (17%) |
| Radiation necrosis | 0 | 1 (4%) |
Abbreviations: CSF, cerebrospinal fluid; SBMs, skull base meningiomas.
Survival after Diagnosis and Post-BNCT
Currently, four patients of this study are still alive, and the MSTs in SBMs post-BNCT and post-diagnosis were 24.6 and 67.5 months, respectively ( Fig. 3 ). In the MSTs post-BNCT or post-diagnosis, no significant differences were observed in SBMs and non-SBMs. Moreover, we established that the effect of BNCT could be anticipated for skull-base tumors that are located deep from the scalp surface. However, a comparison of our results to other reports is rather difficult because our patients were refractory to any existent treatments. Thus, our study cohort was biased because of multiple therapies with repetitive surgeries and radiotherapies ( Table 1 ). Furthermore, several patients in our study died because of systemic metastasis and recurrence outside of the radiation field, as noted earlier ( Table 2 ).
Fig. 3.

Graphs of the Kaplan–Meier survival curves post- boron neutron capture therapy (BNCT) ( A ) and post-diagnosis ( B ). The median survival times in skull-base meningiomas (SBMs) post-BNCT and diagnosis were 24.6 and 67.5 months, respectively.
Discussion
Meningiomas are the leading primary brain tumor in adults. According to the WHO 2000 and 2007 criteria, 90% of meningiomas are classified as benign (grade I) and 10% as high-grade (grade II and III). 16 17 The management of HGMs is difficult, and they remain controversial and under-investigated in prospective studies. Sun et al recommended strategies for HGMs: maximal safe resection of tumors and adjuvant radiotherapy after resection of tumors. 18 Although other treatments, including chemotherapy, for HGMs have been reported, no standard treatment has yet been established. 19
In particular, skull-base HGMs invade into the surrounding brain parenchyma and encase cranial nerves and major vessels. Thus, complete and safe resections of high-grade SBMs are not feasible in a substantial number of patients. In SBMs, HGMs occur far less frequently than non-SBMs. 20 21 22 Reportedly, patients with non-SBMs who experienced a recurrence were more likely to have a higher grade tumor at recurrence than patients with SBMs. 23 Notably, skull-base HGMs are extremely rare, and radiotherapy (EBRT or SRS) and proton beam therapy have been extensively applied over the last few decades. Nevertheless, to date, no data are available on the outcome of long-term patients with high-grade SBMs. We have tried to control high-grade SBMs by tumor-selective intensive particle radiation (BNCT).
Apparently, conventional EBRT at a total dose of 50 to 60 Gy was first used for HGMs. 1 24 In patients with complete gross total resection, postoperative adjuvant EBRT results in good local control. However, in patients with SBMs, EBRT does not offer satisfactory results because of difficulty in attaining safe, complete resections. Next, SRS with a mean marginal dose of 15 to 20 Gy has been applied to high-grade SBMs, as reported previously. 3 25 26 27 However, recurrent cases after repetitive surgeries and radiotherapies or large-sized tumor face a high risk of complications. In addition, SRS poses a high risk of radiation necrosis. More recently, particle radiotherapies using proton beams and carbon ion beams have used in cases of high-grade SBMs. 28 29 30 31 A comparison of our date to the data from these particle radiotherapies is rather difficult. Almost all reported series, including our cases, comprised a small number of patients and their clinical background, such as prior surgeries and radiotherapies, also varied. Notably, all patients with high-grade SBMs in this study were recurrent, complicated cases.
Our study demonstrates the efficacy of BNCT on prominent early tumor shrinkage, at least, for the treatment of high-grade SBMs. Moreover, it is an advantage of BNCT for uncontrollable high-grade SBMs in that the normal cranial nerve existing in the tumor and the vicinity of the tumor is preserved, and it is expected that the neurological function will be preserved as well. However, the long-term survival advantage of BNCT remains debatable owing to distant intracranial recurrence outside the radiation field, systemic metastasis, and CSF dissemination. Apparently, controlling deep-seated SBMs is difficult with single-dose irradiation. Based on our results, early treatment intervention and treatment by fractionated irradiation are imperative. Accelerator-based neutron sources will facilitate fractionated irradiation or multiple field irradiation, compared with the conventional reactor-based BNCT. In addition, the treatable depth limit remains unrecognized in BNCT. 32 As SBMs are deep-seated tumors, we need to develop a method to enhance the dose distribution and explore novel techniques for the administration of boron compounds. Furthermore, all patients in this study were introduced to our institute after radiotherapy using the maximum tolerable dose before BNCT; therefore, despite the tumor-selective nature of BNCT, radiation necrosis might have been inevitable. Hence, controlling radiation necrosis post-BNCT is a critical issue. Although CSF leakage that we experienced after irradiation was presumed to be because of rapid tumor shrinkage in SBMs, BNCT is relatively safe radiotherapy for treating recurrent tumors after multiple surgeries or radiotherapies with little injury to healthy tissue.
This study establishes that the efficacy of BNCT for SBMs is comparable to that for non-SBMs despite considerable attenuation of the neutron flux. The real value of the compound biological effectiveness (CBE) of BPA for HGMs might be higher than that for glioblastoma, and, perhaps, higher doses might be irradiated than the calculated numerical value. In contrast, it might be unnecessary to treat meningiomas with the physical dose comparable to the dose used for treating glioblastoma. Despite the possibility of controlling high-grade SBMs by other radiotherapies, adverse effects are unavoidable in the skull base. Thus, we consider that BNCT is effective and safe radiotherapy even for such situations. In our previous study, the CBE value for meningiomas was presented to be high 33 because of some reasons such as the structure of the vascular bed and the tumor to the blood barrier are different in meningiomas and gliomas. As high-grade SBMs are rare tumors, there is no large-scale clinical study compared with ours. Nonetheless, we intend to conduct an international multicenter registry in the future.
Conclusion
This preliminary study suggests that BNCT could be a promising therapeutic modality for patients with recurrent high-grade SBMs.
Acknowledgments
The work was partly supported by grant-in-aid for scientific research (B) (JSPS KAKENHI Grant No. 19390385) to Dr. Miyatake, and grant-in-aid for Young Scientists (B) (JSPS KAKENHI Grant No. 25861294 and No. 15K10345 to Y. Matsushita) from the Japan Society for the Promotion of Science (JSPS). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. All procedures were subjected to the Osaka Medical College Regulations for Clinical studies.
The authors would like to thank Enago ( www.enago.jp ) for the English language review.
Footnotes
Conflict of Interest The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Combs S E, Adeberg S, Dittmar J O et al. Skull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT) Radiother Oncol. 2013;106(02):186–191. doi: 10.1016/j.radonc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Kaul D, Budach V, Misch M, Wiener E, Exner S, Badakhshi H. Meningioma of the skull base: long-term outcome after image-guided stereotactic radiotherapy. Cancer Radiother. 2014;18(08):730–735. doi: 10.1016/j.canrad.2014.07.159. [DOI] [PubMed] [Google Scholar]
- 3.Pollock B E, Stafford S L, Link M J, Garces Y I, Foote R L. Single-fraction radiosurgery for presumed intracranial meningiomas: efficacy and complications from a 22-year experience. Int J Radiat Oncol Biol Phys. 2012;83(05):1414–1418. doi: 10.1016/j.ijrobp.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Tamura Y, Miyatake S, Nonoguchi N et al. Boron neutron capture therapy for recurrent malignant meningioma. Case report. J Neurosurg. 2006;105(06):898–903. doi: 10.3171/jns.2006.105.6.898. [DOI] [PubMed] [Google Scholar]
- 5.Miyatake S, Tamura Y, Kawabata S, Iida K, Kuroiwa T, Ono K.Boron neutron capture therapy for malignant tumors related to meningiomas Neurosurgery 2007610182–90., discussion 90–91 [DOI] [PubMed] [Google Scholar]
- 6.Kawabata S, Hiramatsu R, Kuroiwa T, Ono K, Miyatake S. Boron neutron capture therapy for recurrent high-grade meningiomas. J Neurosurg. 2013;119(04):837–844. doi: 10.3171/2013.5.JNS122204. [DOI] [PubMed] [Google Scholar]
- 7.Miyatake S, Kawabata S, Hiramatsu R et al. Boron neutron capture therapy for malignant brain tumors. Neurol Med Chir (Tokyo) 2016;56(07):361–371. doi: 10.2176/nmc.ra.2015-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coderre J A, Morris G M. The radiation biology of boron neutron capture therapy. Radiat Res. 1999;151(01):1–18. [PubMed] [Google Scholar]
- 9.Farr L E, Sweet W H, Robertson J S et al. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. Am J Roentgenol Radium Ther Nucl Med. 1954;71(02):279–293. [PubMed] [Google Scholar]
- 10.Imahori Y, Ueda S, Ohmori Y et al. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J Nucl Med. 1998;39(02):325–333. [PubMed] [Google Scholar]
- 11.Imahori Y, Ueda S, Ohmori Y et al. Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part I. Clin Cancer Res. 1998;4(08):1825–1832. [PubMed] [Google Scholar]
- 12.Miyatake S, Kawabata S, Kajimoto Y et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg. 2005;103(06):1000–1009. doi: 10.3171/jns.2005.103.6.1000. [DOI] [PubMed] [Google Scholar]
- 13.Morris G M, Coderre J A, Hopewell J W, Micca P L, Fisher C. Boron neutron capture irradiation of the rat spinal cord: effects of variable doses of borocaptate sodium. Radiother Oncol. 1996;39(03):253–259. doi: 10.1016/0167-8140(95)01693-7. [DOI] [PubMed] [Google Scholar]
- 14.Morris G M, Coderre J A, Micca P L, Fisher C D, Capala J, Hopewell J W. Central nervous system tolerance to boron neutron capture therapy with p-boronophenylalanine. Br J Cancer. 1997;76(12):1623–1629. doi: 10.1038/bjc.1997.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyatake S, Kawabata S, Nonoguchi N et al. Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro-oncol. 2009;11(04):430–436. doi: 10.1215/15228517-2008-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis D N, Schcithauer B W, Budka H, Kleihues P, Cavenee W Ket al. MeningiomasIn:, eds.World Health Organization Classification of Tumours. Pathology and genetics of tumours of the nervous system Lyon, France: IARC Press; 2000176–184. [Google Scholar]
- 17.Louis D N, Ohgaki H, Wiestler O D et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(02):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun S Q, Hawasli A H, Huang J, Chicoine M R, Kim A H. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus. 2015;38(03):E3. doi: 10.3171/2015.1.FOCUS14757. [DOI] [PubMed] [Google Scholar]
- 19.Kaley T, Barani I, Chamberlain M et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro-oncol. 2014;16(06):829–840. doi: 10.1093/neuonc/not330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelius J F, Slotty P J, Steiger H J, Hänggi D, Polivka M, George B. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta Neurochir (Wien) 2013;155(03):407–413. doi: 10.1007/s00701-012-1611-y. [DOI] [PubMed] [Google Scholar]
- 21.Kane A J, Sughrue M E, Rutkowski M J et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011;117(06):1272–1278. doi: 10.1002/cncr.25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sade B, Chahlavi A, Krishnaney A, Nagel S, Choi E, Lee J H.World Health Organization Grades II and III meningiomas are rare in the cranial base and spine Neurosurgery 200761061194–1198., discussion 1198 [DOI] [PubMed] [Google Scholar]
- 23.McGovern S L, Aldape K D, Munsell M F, Mahajan A, DeMonte F, Woo S Y. A comparison of World Health Organization tumor grades at recurrence in patients with non-skull base and skull base meningiomas. J Neurosurg. 2010;112(05):925–933. doi: 10.3171/2009.9.JNS09617. [DOI] [PubMed] [Google Scholar]
- 24.Adeberg S, Hartmann C, Welzel T et al. Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas--clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83(03):859–864. doi: 10.1016/j.ijrobp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Hakim R, Alexander E, III, Loeffler J Set al. Results of linear accelerator-based radiosurgery for intracranial meningiomas Neurosurgery 19984203446–453., discussion 453–454 [DOI] [PubMed] [Google Scholar]
- 26.Ojemann S G, Sneed P K, Larson D A et al. Radiosurgery for malignant meningioma: results in 22 patients. J Neurosurg. 2000;93 03:62–67. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 27.Stafford S L, Pollock B E, Foote R Let al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients Neurosurgery 200149051029–1037., discussion 1037–1038 [DOI] [PubMed] [Google Scholar]
- 28.Mizumoto M, Oshiro Y, Tsuboi K. Proton beam therapy for intracranial and skull base tumors. Transl Cancer Res. 2013;2(02):87–96. [Google Scholar]
- 29.Boskos C, Feuvret L, Noel G et al. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys. 2009;75(02):399–406. doi: 10.1016/j.ijrobp.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 30.Chan A W, Bernstein K D, Adams J A, Parambi R J, Loeffler J S. Dose escalation with proton radiation therapy for high-grade meningiomas. Technol Cancer Res Treat. 2012;11(06):607–614. doi: 10.7785/tcrt.2012.500267. [DOI] [PubMed] [Google Scholar]
- 31.Hug E B, Devries A, Thornton A F et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48(02):151–160. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai Y, Ono K. Improvement of dose distribution by central beam shielding in boron neutron capture therapy. Phys Med Biol. 2007;52(24):7409–7422. doi: 10.1088/0031-9155/52/24/014. [DOI] [PubMed] [Google Scholar]
- 33.Onishi K, Kawabata S, Miyata S et al. Evaluation of the biological effects of boron neutron capture therapy for the humanmalignant meningioma cell line IOMM-Lee. Bull Osaka Med Coll. 2009;55(01):9–19. [Google Scholar]


