Abstract
Dysregulation of the tightly controlled protein phosphorylation networks that govern cellular behavior causes cancer. The membrane-associated, intracellular protein tyrosine phosphatase PTP4A3 is overexpressed in human colorectal cancer and contributes to cell migration and invasion. To interrogate further the role of PTP4A3 in colorectal cancer cell migration and invasion, we deleted the Ptp4a3 gene from murine colorectal tumor cells. The resulting PTP4A3−/− cells exhibited impaired colony formation, spheroid formation, migration, and adherence compared with the paired PTP4A3fl/fl cells. We replicated these phenotypic changes using the new small-molecule, allosteric PTP4A3 inhibitor JMS-053. A related structure, JMS-038, which lacked phosphatase inhibition, displayed no cellular activity. Reduction in cell viability and colony formation by JMS-053 occurred in both mouse and human colorectal cell lines and required PTP4A3 expression. Ptp4a3 deletion increased the expression of extracellular matrix (ECM) and adhesion genes, including the tumor suppressor Emilin 1. JMS-053 also increased Emilin 1 gene expression. Moreover, The Cancer Genome Atlas genomic database revealed human colorectal tumors with high Ptp4a3 expression had low Emilin 1 expression. These chemical and biologic reagents reveal a previously unknown communication between the intracellular PTP4A3 phosphatase and the ECM and support efforts to pharmacologically target PTP4A3.—McQueeney, K. E., Salamoun, J. M., Ahn J. G., Pekic, P., Blanco, I. K., Struckman, H. L., Sharlow, E. R., Wipf, P., Lazo, J. S. A chemical genetics approach identifies PTP4A3 as a regulator of colon cancer cell adhesion.
Keywords: phosphatase, inhibitor, migration, extracellular matrix, Emilin
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the United States (1). The 5-yr survival rate for localized CRC stages is 90%, but the rate drops to 14% in patients with advanced disseminated CRC, which is not amenable to surgery (2). Recently, there has been a sharp increase in the number of young adults diagnosed with a more advanced disease stage (3). Thus, there is an urgent need for new molecular targets and therapies for CRC.
Cancer is a disease of aberrant cellular signaling often caused by altered intracellular protein phosphorylation. Although a focus on kinases as potential targets for correcting this dysregulation is common, far less effort has been dedicated to manipulating the activity of phosphatases. Members of the protein tyrosine phosphatase (PTP) superfamily have a central role in controlling the phosphorylation status and function of many eukaryotic proteins. Thus, it is unsurprising that PTPs would be key participants in cancer (4). PTP4A3 is a nonclassic intracellular PTP reported to be overexpressed in many types of cancers (5–15). Elevated PTP4A3 levels promote cellular invasion, motility, and angiogenesis (7, 16, 17), which are characteristics associated with highly malignant cancers. No definitive PTP4A3 substrates have been established, but alterations in PTP4A3 levels affect several signaling pathways, notably Rho GTPases, SRC, TGF-β, and PI3K-AKT, supporting a pivotal role in oncogenesis (17–20).
Interactions between malignant cells and the extracellular matrix (ECM) are paramount for tumor progression (21–23). Cell adhesion to the ECM not only prevents programmed cell death (22) but also controls a host of other cell processes, including proliferation, migration, and cell polarity (24–27). Although PTP4A3 has been linked to changes in expression levels of Ezrin, integrins, and matrix metalloproteinases, the impact of this collective network alteration on the adherence and interaction with the ECM has yet to be explored in colorectal cancer (11, 20, 28–31).
We previously developed and characterized a Ptp4a3 gene deletion mouse model, enabling a more comprehensive analysis of the pathologic role of PTP4A3 in CRC (32). Ptp4a3-null mice were viable with no observable gross histologic abnormalities. When exposed to a carcinogen in a widely used model of colitis-induced CRC (33), Ptp4a3-null mice developed fewer tumors than wild-type mice. When CRC cells were isolated from the tumors of Ptp4a3 wild-type and Ptp4a3-null mice and cultured on a lethally irradiated rat cell feeder layer, Ptp4a3-null cells produced fewer in vitro colonies by limited dilution analysis and subcutaneous tumors than cells isolated from Ptp4a3 wild-type mice (34). Conclusions obtained with these 2 cell populations should be viewed with caution, however, because the isolated CRC tumor cells were exposed to a mutagen and may harbor other independent genetic differences. Therefore, in the current study, we generated a feeder layer–independent tumor cell pair to probe further the role of PTP4A3 in CRC.
Although genetic approaches have enormous power, pharmacological tools often provide a compelling orthogonal method for discerning the biochemical functions of enzymes. We recently described the synthesis of a highly potent, small-molecule PTP4A3 inhibitor, 7-imino-2-phenylthieno[3,2-c]pyridine-4,6(5H,7H)-dione or JMS-053 (35). We have now used this compound, along with an inactive analog, to demonstrate the role of PTP4A3 phosphatase activity in the oncogenic phenotypes provoked by PTPA3 overexpression. The combined use of the newly developed cell pair and a small molecule inhibitor revealed a function for PTP4A3 in controlling cellular interactions with the ECM, which could be relevant to PTP4A3’s involvement in the malignant cell phenotype and in tumor progression.
MATERIALS AND METHODS
Cells and reagents
Mouse CRC cells were derived from our previously described primary colon tumor epithelial cells, which were obtained from Ptp4a3fl/fl mice treated with azoxymethane and dextran sodium sulfate (34). Mouse CRC cells were initially plated on a confluent feeder layer of lethally irradiated (80 Gy) LA7 rat mammary tumor cells (CRL-2283; American Type Culture Collection, Manassas, VA, USA) at ∼80,000 cells/cm2 and in DMEM/F12 medium with 0.5% fetal bovine serum (FBS), 25 mg/ml gentamycin (MilliporeSigma, St. Louis, MO, USA), and 1% insulin-transferrin/selenium (Mediatech, Manassas, VA, USA). When cells reached ∼70% confluence, Ptp4a3fl/fl cells were detached from the feeder layer by incubation with Earle’s balanced salt solution/1 mM EGTA/1% HEPES followed by 0.25% trypsin/0.1% EDTA as previously described (34). A mouse CRC feeder layer–independent cell population was generated by serially decreasing the feeder layer at the time of passaging (approximately once per week with a 20% reduction in number of cells in feeder layer) and increasing the FBS concentration (15% increase in concentration at time of passage). After 7 passages, cells were successfully maintained in DMEM/F12 medium with 7.5% FBS, 25 mg/ml gentamycin, and 1% insulin-transferrin/selenium. We confirmed the C57BL/6J origin of the resulting cell population and the lack of contamination by any residual rat feeder layer cells with short tandem repeat profiling (CellCheck; Idexx BioResearch, Columbia, MO, USA). The feeder layer–independent cell population was divided into 2 pools and infected with an adenovirus expressing either Cre recombinase accompanied by a green fluorescent protein (GFP) marker or GFP alone. Four days after infection, GFP-expressing cells were isolated by fluorescence-activated cell sorting (FACS). The resulting cell populations were expanded and either used immediately or frozen in liquid nitrogen for future use. The sorted GFP-infected population is referred to herein as PTP4A3fl/fl, and the sorted Cre/GFP-infected population is referred to as PTP4A3−/−. Cells were discarded after 20 passages. PTP4A3 mRNA levels were determined by real-time quantitative RT-PCR (qRT-PCR), and protein levels were determined by Western blotting as described below. HCT116 and DLD-1 cells were purchased from the American Type Culture Collection and cultured in McCoy’s 5A or RPMI medium, respectively, with 10% FBS, penicillin (100 IU/ml), streptomycin (100 µg/ml), and glutamine (10 mM). Cells were passaged <22 times and then discarded.
All reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA) unless otherwise noted. The synthesis and chemical characterization of the PTP4A3 inhibitor, JMS-053, and the inactive congener, JMS-038, were previously published by our group (35).
Fluorescent cell imaging
Cells were plated in CellCarrier-96 Ultra microplates (PerkinElmer, Waltham, MA, USA) at 30,000 cells/well and grown at 37°C in 5% CO2. At 48 h, cells were fixed in 4% paraformaldehyde for 10 min, permeabilized in 0.5% Triton X-100 for 5 min, and stained with 100 nM Acti-stain 488 phalloidin (Cytoskeleton, Denver, CO, USA). To visualize nuclei, cells were stained for 5 min with Hoechst 33342. The Operetta CLS (PerkinElmer) was used to obtain confocal images using a ×20 water objective.
Western blot analysis
Cell lysates were generated by removing growth medium, washing cells 3 times in ice-cold PBS, adding lysis buffer, detaching cells from the plate with a scraper, and then placing cells in a microcentrifuge tube on ice. The cell lysate was passed through a 26-gauge needle (3 times), followed by sonication using three 10-s pulses, and clarified by centrifugation for 10 min at 4°C and 7000 g. Tissue lysates were generated by placing samples in ice-cold lysis buffer, using a Potter-Elvehjem homogenizer, and passing homogenate through a 25-gauge needle (3 times), followed by sonication using three 10-s pulses, and clarified by centrifugation for 10 min at 4°C and 7000 g. Cells and tissues were lysed in 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate containing 1 time Complete Mini EDTA-free Protease Inhibitor (Roche, Basel, Switzerland), and phosphatase inhibitor cocktail (MilliporeSigma). Total protein in the lysate was quantified by a bicinchoninic acid assay (Thermo Fisher Scientific). A total of 30 µg of protein was separated using Novex SDS-PAGE reagents and transferred to a PVDF membrane. Membranes were blocked in 5% dry milk in Tris-buffered saline with 0.1% Tween-20 and incubated with primary antibodies overnight followed by secondary fluorescent antibodies according to the manufacturer’s instructions. We used the following commercially available primary antibodies: PTP4A3 (6484), GAPDH (2118), Ezrin (3145), and phospho-Ezrin (Thr567)/Radixin (Thr564)/Moesin (Thr558) Antibody (3141) (Cell Signaling Technology, Danvers, MA, USA). Membranes were imaged using the Odyssey imaging system (Li-Cor Biotechnology, Lincoln, NE, USA).
Cell proliferation assay
Cell proliferation rates were determined with a CyQuant Direct Cell Proliferation Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells in midlogarithmic growth phase were detached as described previously, centrifuged, resuspended in complete medium with 10% FBS, and counted. Cells were resuspended, and 1.5 × 103 cells per well were added to black, clear-bottom, 96-well plates. CyQuant reagent was added at 24, 30, 45, 55, 71, and 77 h and incubated at 37°C for 30 min. The fluorescence was measured using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA, USA) at 480 nm excitation and 535 nm emission.
Colony formation assay
Soft agar assays were performed in 12-well plates by adding 1.25 ml complete medium containing 0.6% agar and allowing it to solidify and generate a base layer. Cells were detached with 0.25% trypsin/ 0.1% EDTA, centrifuged at 100 g for 3 min, counted, and then resuspended in complete medium containing 0.4% agar at a final concentration of 1.5 × 104 cells/1.25 ml per well. Complete medium (300 μl) was added on top of soft agar in each well every 4 d. After 14 d, cells were fixed using 10% ethanol and 10% acetic acid and then stained using 0.01% Crystal Violet. Mouse CRC cell colonies were manually counted with a microscope (IX70; Olympus, Tokyo, Japan).
Spheroid growth assays
For the scaffold-free model, PTP4A3fl/fl and PTP4A3−/− cells were harvested and plated (500 cells/100 μl) into individual wells of a 96-well, round-bottom plate coated in polyHEMA to prevent cell attachment. Cells were grown in an incubator at 37°C in 5% CO2. After 48 h, images of spheroids were taken using an Olympus IX70 with a ×10 objective.
For human CRC viability assays, test and control compounds (15 μl) were added to each well of a 384-well, ultralow-attachment spheroid microplate (Corning, Corning, NY, USA), with vehicle and positive controls being 0.5% and 10% DMSO, respectively. DLD-1 and HCT116 cells were seeded (100 cells/10 μl) into each well of the microtiter plate. The microtiter plates were incubated for 48 h (37°C, 5% CO2), and 25 μl of CellTiterGlo 3D (Promega, Fitchburg, WI, USA) reagent was added. Plates were incubated while shaking for 30 min at room temperature. Luminescence data were captured on a SpectraMax M5 multimodal plate reader. EC50 values were calculated using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
Migration assays
Differences in cell migration were quantified by our previously described in vitro scratch wound healing assay (36). Cells were grown to confluence in 24-well tissue culture plates (BD Biosciences, San Jose, CA, USA). Each well (n = 3/genotype or treatment) was scratched longitudinally with a pipette tip and incubated for 16 h to allow gap closure. Cell migration images were captured, and the distance was determined by measuring the gap distance between cell fronts following inward migration using ImageJ Fiji software (National Institutes of Health, Bethesda, MD, USA).
For transwell migration assays, chambers were assembled using 8 μm polyethylene terephthalate membrane transwell inserts (Costar) as upper chambers and 24-well plates as lower chambers. Cells were detached from the monolayer with 0.25% trypsin/0.1% EDTA as previously described, centrifuged, and resuspended in medium containing 0.5% FBS and placed (2.5 × 105 cells/well) in the upper chamber. The lower chamber contained cell medium supplemented with 10% FBS to act as a chemo-attractant to stimulate migration. After 20 h, cells were fixed in 100% methanol and stained with 0.2% Crystal Violet for 30 min to visualize cells. The cells that migrated to the bottom of the membrane were counted manually with an Olympus IX70 microscope.
Impedance-based adhesion assay
Impedance-based adhesion assays were performed using the xCelligence Real-Time Cell Analyzer Dual Plate in combination with the E-Plate 16 (Acea Biosciences, San Diego, CA, USA). The Real-Time Cell Analyzer Dual Plate instrument was placed in an incubator (37°C in 5% CO2) for all experiments. The background measurements for each experiment were determined by filling each well with 100 μl of medium, allowing the plate to equilibrate for 30 min, and calibrating the instrument immediately prior to adding cells. Cells at >60% confluence were detached and seeded in an E-plate in a total volume of 100 μl. Impedance readings were taken every 10 min over the course of 25 h. To determine the effect of small molecules, we pretreated cells with the appropriate concentration of compounds for 3 h in serum-free medium, harvested the cells as previously described, and plated the cells with compound or vehicle.
RhoA GTPase activity assay
RhoA activation was detected by the luminescence-formatted RhoA G-LISA Activation Assay (Cytoskeleton) according to the manufacturer’s instructions. After a 24-h serum starvation in DMEM/F12 supplemented with l-glutamine, murine tumor cells at ∼50% confluence were pretreated in serum-free medium for 1 h with compounds in a final DMSO concentration of 0.5%, as was the vehicle control. Cells were then stimulated for 30 min with DMEM/F12 containing 10 ng/ml platelet-derived growth factor (PDGF)-β with treatment in a final DMSO concentration of 0.5%. Cells were harvested on ice and snap-frozen in liquid nitrogen. Total protein was determined using Precision Red Advanced protein assay reagent (Cytoskeleton). Twenty-five micrograms of protein per Rho-GTP affinity well were incubated at 4°C for 30 min on an orbital shaker to facilitate binding. As a positive control, 1 ng of purified recombinant RhoA was bound to the affinity well. The provided primary and secondary antibodies were used at a 1:250 and 1:500 dilution, respectively. Luminescence data were captured on a SpectraMax M5 multimode plate reader set to 25-ms integration time after 2 min incubation with luminescence detection reagent. Data were normalized to total RhoA as determined by the total RhoA ELISA (Cytoskeleton).
qRT-PCR and Next-Gen sequencing
For all RNA analyses, total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. For qRT-PCR, a total of 1 μg of mRNA was treated to eliminate genomic DNA and converted to cDNA using the RT2 First Strand Kit (Qiagen). The validated primer pairs for amplification of target genes were purchased from Qiagen and used with SYBR Green/Rox qPCR mastermix. Quantitative PCR was performed by incubating at 95°C for 10 min followed by 39 cycles of 95°C for 15 s and 60°C for 1 min while monitoring reactions on a BioRad CFX Connect Real-Time PCR Detection System. A melt curve analysis was included to ensure the absence of nonspecific primer binding.
For Next-Gen sequencing, RNA samples were quantified with a Nanodrop spectrophotometer and underwent quality check with Agilent BioAnalyzer. Only samples with an RNA Integrity Number of 7.5 or above were used for RNA-Seq. Sequencing libraries for the RNA-Seq were constructed following the instructions of the Illumina TrueSeq RNA sample preparation by the manufacturer. Individually barcoded sequencing libraries were pooled, denatured, and analyzed with an llumina NextSeq 500 for a paired-end sequencing of 75 sequencing cycles according to the manufacturer’s recommended protocol. Paired-end sequencing data were quality checked and filtered using the apps provided in BaseSpace, an analysis portal provided by the manufacturer. Alignment was performed using Bowtie 2 in conjunction with TopHat.
Gene expression array
The expression profile of 84 key genes affecting cellular adhesion was determined using a 96-well format murine ECM and adhesion molecule RT2 Profiler PCR array (Qiagen) according to the manufacturer’s instructions. For normalization, the array contains 6 housekeeping genes. Plates for quantitative PCR were run on a BioRad CFX Connect Real-Time PCR detection system using RT2 SYBR Green/Rox qPCR Mastermix (Qiagen). Data analysis was performed by the ΔΔCt method on the web-based software available through the manufacturer (http://pcrdataanalysis.sabiosciences.com). Three PCR microarray replicates were used per cell population.
Clinical gene expression
PTP4A3 mRNA expression data were obtained using cBioPortal (http://www.cbioportal.org/) and the Cancer Cell Line Encyclopedia (Agilent microarray), which contained 967 cell lines, or the The Cancer Genome Atlas (TCGA) Provisional colorectal adenocarcinoma database (RNA Seq v.2), which contained 379 tumor samples. Data analyses were conducted in December 2017. In the Cancer Cell Line Encyclopedia, 70 (7%) of the tumor cell lines had elevated PTP4A3 levels (z-score threshold of 2.0). In The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov/) colorectal database 52 patient tumor samples (14%) with highly elevated PTP4A3 mRNA (z-score threshold of 2.0). Gene expression pattern visualization by heatmap used the SVG data file downloaded from cBioPortal imported into Graphpad Prism 7.0. The data were sorted based on degree of altered expression in each gene, and 30% of the total samples were depicted. Overall survival was calculated using a Kaplan-Meier program in cBioPortal from patients with tumors that overexpressed Ptp4a3 or matrix metallopeptidase (Mmp) 1 or underexpressed Emilin 1. Fbln 1, Vcan, integrin subunit β2 (Itgb2) and Itgb3, or thrombospondin 3.
Statistics
All statistical analyses were performed with GraphPad Prism 7.0. Data are presented as means ± sd or sem. We calculated significance values with Student’s t test for comparisons involving 2 groups or with 1- or 2-way ANOVA for comparisons involving >2 groups. A value of P < 0.05 was considered statistically significant. Each experiment represents at least 3 biological replicates and 3 technical replicates (per independent experiment).
RESULTS
Generation of paired PTP4A3fl/fl and PTP4A3−/− murine CRC cell lines
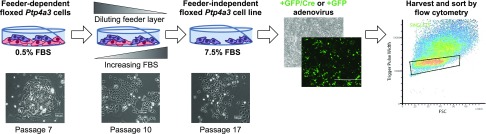
We previously described Ptp4a3 wild-type and null cells derived from primary colon tumor epithelial cells of Ptp4a3fl/fl and Ptp4a3−/− mice treated with azoxymethane and dextran sodium sulfate (32, 34). These cells, however, require the presence of a monolayer of lethally irradiated rat mammary carcinoma cells to survive, which complicates analyses and limits the overall usefulness of the cells. Furthermore, the Ptp4a3 wild-type and the companion Ptp4a3 null cells were derived from mice treated with a carcinogen, which likely produces additional mutations that could alter the genotype and phenotype of the resulting cells. For these reasons, we developed feeder layer–independent cells that could be used to specifically remove the Ptp4a3 gene and yield a unique paired model. Our overall experimental scheme is illustrated in Fig. 1. Over the course of 17 passages, the Ptp4a3 wild-type cells were liberated from the feeder layer and proliferated in the presence of medium with 7.5% FBS. The resulting cells were infected with an adenovirus expressing either Cre recombinase accompanied by a GFP marker or GFP alone. Four days after infection, GFP-positive cells were isolated by FACS, and the sorted populations were plated on plastic and cultured. There was no obvious difference in the nuclear/cytoplasmic ratio between the 2 cell populations (Fig. 2A). Relative to Ptp4a3 gene expression in the normal colon of a Ptp4a3fl/fl mouse, whole tumor lysate showed an ∼8-fold increase in Ptp4a3 mRNA, whereas the original Ptp4a3 wild-type cells and the GFP-expressing Ptp4a3fl/fl cells (hereafter referred to as PTP4A3fl/fl cells) demonstrated an ∼12-fold increase in expression (Fig. 2B). Infection with the adenovirus expressing GFP did not alter Ptp4a3 mRNA levels compared with the uninfected cells. The Ptp4a3 null cells showed no detectable mRNA, and the Cre/GFP-infected cells (hereafter referred to as PTP4A3−/− cells) had almost a complete loss of Ptp4a3 mRNA (Fig. 2B). In agreement with these findings, the PTP4A3−/− cells lacked PTP4A3 protein by Western blotting (Fig. 2C). In addition, the loss of PTP4A3 protein did not result in a significant compensatory change in the mRNA levels of the 2 other members of the PTP4A family, Ptp4a1 and Ptp4a2 (Fig. 2D). There were no differences in the levels of total Ezrin or phosphorylated Ezrin as measured by Western blotting in the base levels of PTP4A3fl/fl and PTP4A3−/− cells and upon treatment with 2.5 µM JMS-053 (Supplemental Fig. 1).
Figure 1.
Experimental workflow of the development of a uniquely paired PTP4A3fl/fl and PTP4A3−/− murine CRC cell lines. Cells isolated from the chemically induced colorectal tumors of Ptp4a3 wild-type mice were made feeder layer independent by serially decreasing the irradiated LA7 rat mammary feeder layer cells while increasing exposure to FBS to a final concentration of 7.5% by passage 17. The resulting cells were infected with either a GFP/Cre or a GFP adenovirus. Four days after infection, GFP-positive cells were isolated by FACS and used to generate the PTP4A3fl/fl and PTP4A3−/− cells.
Figure 2.
Validation of PTP4A3fl/fl and PTP4A3−/− murine CRC tumor cell lines. A) Fluorescence confocal images of PTP4A3fl/fl and PTP4A3−/− cells using Acti-stain 488 phalloidin to visualize the cytoskeleton (green) and Hoechst to visualize the nuclei (blue). Original magnification, ×20. Scale bars, 100 µm. B) qRT-PCR analysis of Ptp4a3 gene expression in normal mouse colon, murine tumor, uninfected Ptp4a3fl/fl and Ptp4a3−/− cells, and Cre/GFP- and GFP-infected Ptp4a3fl/fl cells. Relative to normal colon tissue. Error bars indicate sem. N.D., not detected. ***P < 0.0001 (determined by 1-way ANOVA; n = 4). C) PTP4A3 protein as determined by Western blotting. GAPDH was used as the loading control (n = 4). D) Quantitative PCR analysis of Ptp4a1, Ptp4a2, and Ptp4a3 gene expression in PTP4A3fl/fl and PTP4A3−/− cells (n = 4). Error bars indicate sem.
PTP4A3 deletion or inhibition reduces CRC colony formation, spheroid formation, migration, and adhesion
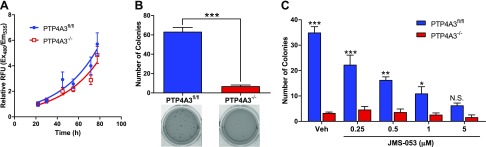
When PTP4A3fl/fl and PTP4A3−/− cells were grown on a 2-dimensional (2D) plastic monolayer, we detected no significant difference in the growth rate (Fig. 3A), consistent with previously published observations of an unchanged proliferation rate of Ptp4a3 wild-type cancer cells after small interfering RNA (siRNA) suppression of PTP4A3 (37). Despite the similar growth rate on a 2D monolayer, a striking difference in the growth of the 2 cell populations was observed when they were suspended in soft agar. Anchorage-independent colony formation and cell growth on a semisolid medium, such as soft agar, has been a fundamental method to define in vitro transformation because it is associated with tumor cell aggressiveness in vivo, tumorigenesis, and metastatic potential (38). It is notable, therefore, that the soft agar colony-forming efficiency of PTP4A3fl/fl cells was 15-fold greater than their PTP4A3−/− counterpart (Fig. 3B). This is consistent with, but more striking than, the decreases previously reported with siRNA (37). The small molecule thienopyridone was previously described to inhibit anchorage-independent cell growth of RKO and HT-29 CRC cells grown in soft agar with an EC50 ≈7.5 µM (37, 39). JMS-053 was 10 times more potent than thienopyridone and inhibited anchorage-independent growth in PTP4A3fl/fl cells in a concentration-dependent manner with an EC50 ≈0.35 µM (Fig. 3C). Treatment of PTP4A3−/− cells with JMS-053 had no significant effect on colony formation. Moreover, there was no statistically significant difference in soft agar colonies between the PTP4A3fl/fl and PTP4A3−/− cells upon treatment with 5 µM JMS-053 (Fig. 3C).
Figure 3.
Inhibition or loss of PTP4A3 did not alter 2D growth but decreased colony formation. A) Growth rate of PTP4A3fl/fl and PTP4A3−/− cells grown as monolayer on plastic (n = 6). Error bars indicate sem. B) PTP4A3−/− cells displayed a marked decrease in colony formation when grown in soft agar compared with PTP4A3fl/fl cells. Representative images of cells stained with crystal violet seen below graph (n = 3). Error bars indicate sem. ***P < 0.001. C) Concentration-dependent reduction in anchorage-independent colony formation of PTP4A3fl/fl cells, but not PTP4A3−/− cells, after exposure to JMS-053 (n = 3). Error bars indicate sem. N.S., not significant. *P < 0.02, **P < 0.0005, ***P < 0.0001 (determined by 2-way ANOVA).
Three-dimensional culture conditions are generally regarded as superior to 2D tumor cell models because 3-dimensional cell cultures possess several features of tumors, such as cell-cell interaction, hypoxia, drug penetration, response and resistance, and production/deposition of ECM (40). These factors can shift growth dependence away from the phenotype of unrestrained proliferation, which is dominant in standard 2D cultures. Therefore, we examined the growth of our paired lines in scaffold-free conditions. Homotypic cell interactions in a scaffold-free environment were assessed by plating between 500 and 5000 cells in polyHEMA-coated round-bottom plates to examine the morphology of the spheroid 48 h later. PTP4A3fl/fl cells formed well-defined spheroids, whereas PTP4A3−/− cells failed to form organized spheroids at all cell concentrations (Fig. 4).
Figure 4.
Loss of the PTP4A3 impaired tumor cell spheroid formation. PTP4A3−/− cells were unable to form spheroids when cultured in cell repellent plates. The indicated numbers of PTP4A3fl/fl and PTP4A3−/− cells were plated, and images of spheroids were obtained 48 h later using a ×10 objective. Scale bars, 50 µm.
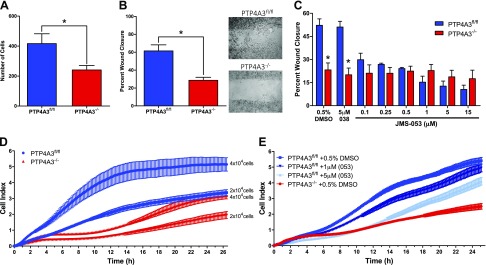
Overexpression of PTP4A3 has been shown to increase cell migration (17, 20), whereas reduction by siRNA (41) or complete gene deletion (36) decreases cell migration. Consistent with these observations, PTP4A3−/− cells displayed a ∼40% reduction in transwell migration (Fig. 5A) and a >50% decrease in migration as measured by a scratch wound assay (Fig. 5B). Furthermore, this reduction in migration appeared to be dependent on PTP4A3 phosphatase activity because there was a JMS-053 concentration-dependent decrease in PTP4A3fl/fl cell migration in the wound healing assay (Fig. 5C). The EC50 for cell migration in the PTP4A3fl/fl was ∼340 nM. The inactive congener JMS-038 did not inhibit cell migration at concentrations as high as 5 μM (Fig. 5C). Treatment with ≤15 µM JMS-053 had no significant effect on PTP4A3−/− cell wound closure.
Figure 5.
Inhibition or loss of PTP4A3 reduced cell migration and adhesion. A) Loss of Ptp4a3 decreased cell migration as measured by a transwell assay (n = 4). Error bars indicate sem. *P < 0.05. B) Loss of PTP4A3 decreased cell migration as measured by a scratch wound healing assay (n = 3). Error bars indicate sem. *P < 0.001. C) Concentration response of PTP4A3fl/fl cells upon JMS-053 treatment in scratch wound healing assay (n = 3). Error bars indicate sem. *P > 0.001 (determined by 2-way ANOVA). D, E) Real-time monitoring of cell impedance using xCelligence biosensor, which detects cell attachment, spreading, and number based on changes in impedance, reported as cell index (n = 4). Error bars indicate sem.
The ability of circulating tumor cells to attach to a secondary site is a fundamental step during invasion and metastasis (42). Therefore, we examined differences in cellular adhesion using an impairment of the impedance of electrical current across the cell culture surface, reported as cell index. PTP4A3−/− cells exhibited reduced cell index compared with PTP4A3fl/fl cells (Fig. 5D). Likewise, treatment of PTP4A3fl/fl cells with 1 or 5 µM JMS-053 replicated the reduction in cellular impedance seen in PTP4A3−/− cells (Fig. 5E).
A 48-h exposure to JMS-053 inhibited human HCT116 and DLD-1 CRC spheroid cell growth with EC50 values of 2.6 ± 0.4 and 2.9 ± 0.7 μM, respectively (Fig. 6A, B), which demonstrated that growth inhibition was not unique to the mouse CRC cells. No growth inhibition or cytotoxicity was seen with the control compound JMS-038 (Fig. 6A, B). HCT116 colony formation in soft agar was inhibited with an EC50 of 1.1 ± 0.1 µM (Fig. 6C, D). No inhibition of HCT116 colony formation was observed with concentrations of JMS-038 as high as 5 µM.
Figure 6.
Pharmacological inhibition of PTP4A3 with JMS-053 in human CRC cell lines reduced spheroid viability and colony formation. A, B) Open square, JMS-053; closed circle, JMS-038. JMS-053 inhibited DLD-1 (A) and HCT116 (B) colon cancer cell viability when cells were cultured as spheroids with EC50 values of 2.6 ± 0.4 and 2.9 ± 0.7 µM, respectively. C, D) JMS-053 inhibited HCT116 colony formation with an EC50 of 1.1 ± 0.1 µM. C) Total number of colonies counted in each treatment group (n = 3). Error bars indicate sem. *P < 0.05, **P < 0.0001. D) EC50 curve plotting the data shown in C (n = 3). Error bars indicate sem.
PTP4A3 deletion or inhibition reduces RhoA activation
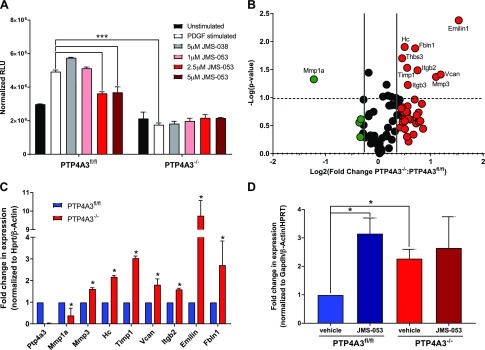
RhoA GTPase has well-defined roles in the regulation cytoskeletal dynamics, which are essential for cancer cell migration, invasion, and metastasis (43). We previously demonstrated that JMS-053 caused a significant inhibition of FBS-stimulated RhoA activation in ovarian cancer cells (44). PDGF-β is potent inducer of cell migration, which is mediated by RhoA activation and the resultant cytoskeletal rearrangement (45). A 30-min exposure of PTP4A3fl/fl cells to PDGF-β (10 ng/ml) caused a 1.7-fold activation of RhoA but no activation in PTP4A3−/− cells (Fig. 7A), confirming the central role of PTP4A3 in mediating RhoA activation by PDGF-β. JMS-053 (2.5–5 μM) markedly inhibited the PDGF-β–mediated RhoA activation in PTP4A3fl/fl cells. In contrast, the inactive congener JMS-038 at 5 μM failed to inhibit PDGF-β–mediated RhoA activation in PTP4A3fl/fl cells. We observed no effect on RhoA activation with JMS-053 or JMS-038 in PTP4A3−/− cells (Fig. 7A).
Figure 7.
Gene expression of ECM and adhesion genes in PTP4A3fl/fl and PTP4A3−/− cells. A) PDGF-β (10 ng/μl) activated RhoA in PTP4A3fl/fl but not PTP4A3−/− cells, and JMS-053 inhibited RhoA activation (n = 3). Error bars indicate sem. ***P < 0.0001. B) Volcano plot of differences in mRNA gene expression. Changes in relative gene expression from Qiagen Extracellular Matrix and Adhesion PCR Array of PTP4A3−/− vs. PTP4A3fl/fl cells are shown. Genes with ≥1.5-fold overexpression are noted in red, and genes with ≥1.5-fold underexpression are noted in green (n = 3). The dashed line indicates P < 0.05. C) Confirmation by qRT-PCR of gene expression changes in PTP4A3−/− cells identified in RT-PCR array. mRNA levels relative to Hprt and Gapdh (n = 4). Error bars indicate sem. D) Emilin 1 mRNA expression after 48 h of treatment of murine tumor cells with JMS-053 mimics the effect of genetic loss of PTP4A3. mRNA levels relative to β-actin, Hprt, and Gapdh (n = 4). Error bars indicate sem. *P > 0.05.
PTP4A3 regulates the expression of key ECM and adhesion genes
Cell migration, colony formation, spheroid formation, and adhesion require highly coordinated interactions with the ECM (46). Therefore, we next examined the changes in the expression of genes encoding key adhesion and ECM proteins caused by the absence of PTP4A3. Of the 84 genes examined by a commercially available PCR array, 8 were significantly overexpressed in PTP4A3−/− cells compared with PTP4A3fl/fl cells: Emilin 1, fibulin 1, tissue inhibitor of metalloproteinase 1, Vcan, thrombospondin 1, Itgb2, Itgb3, hemolytic complement, and Mmp3 (Fig. 7B). Mmp1 was significantly suppressed. All of these changes were confirmed by qRT-PCR, with Emilin 1 being the most highly overexpressed (Fig. 7C). Furthermore, upon treatment of PTP4A3fl/fl cells with JMS-053 for 48 h, the increase in Emilin 1 mRNA expression seen in PTP4A3−/− cells was replicated (Fig. 7D). There was no significant impact of the JMS-053 treatment on PTP4A3−/− cells (Fig. 7D). We have also begun an unbiased RNA sequencing study of PTP4A3fl/fl and PTP4A3−/− cells and have found 1379 up-regulated and 1021 down-regulated genes (Supplemental Fig. 2). Notably, Vcan, collagen 5α1, collagen 6α1 and 6α3, and Fbln 7 were significantly up-regulated in PTP4A3fl/fl cells compared with PTP4A3−/− cells. We are currently examining changes in the expression of these and other genes using qRT-PCR and Western blotting.
We next examined the expression of these adhesion and ECM genes in the 967 human cell lines in the Cancer Cell Encyclopedia and the TCGA colorectal adenocarcinoma database (cBioPortal). In both the Cancer Cell Encyclopedia (Fig. 8A) and the TCGA datasets (Fig. 8B), high expression of PTP4A3 mRNA is generally accompanied by low expression of the adhesion and ECM interacting genes identified as up-regulated in the PTP4A3−/− cells, as illustrated in the heatmap by the isolation of the PTP4A3-high (red) subset in the top row. In the TGCA patient samples (Fig. 8B), not only were PTP4A3-high expressers isolated, but also high expression of the ECM and adhesion genes of interest appeared to co-occur, as illustrated by the clustering of high expressers (red) in individual samples. Of the 379 cases with RNA-Seq data, 52 (14%) had increased expression of Ptp4a3, whereas 97 (26%) had altered expression of Ptp4a3 and/or one of the other genes identified in our ECM PCR array panel. Moreover, a significant reduction in the overall survival was observed in patients with tumor samples having altered expression of Ptp4a3 or any of the identified ECM and adhesion genes (Fig. 8C).
Figure 8.
Changes in Ptp4a3 and ECM and adhesion genes in human cancer cells and samples from patients with CRC. A, B) Cancer Cell Line Encyclopedia (A) and TCGA Provisional CRC sample (B) heat map of the expression levels of Ptp4a3 and genes previously identified with the PCR array. Red indicates overexpression, and green indicates underexpression. Each bar represents an individual patient sample. C) Kaplan-Meier plot of overall survival of patients who had altered gene expression of Ptp4a3 or the identified adhesion and ECM genes. P = 0.032.
DISCUSSION
CRC remains one of the deadliest cancers worldwide, and the incidence of the disease continues to increase (2). PTP4A3 mRNA and protein overexpression in metastatic CRC has been noted by others (5, 7), and we found an increase in PTP4A3 mRNA expression in 14% of the CRC tumors sequenced in the TCGA database. The ramifications of PTP4A3 overexpression in CRC are unclear because definitive PTP4A3 substrates have yet to be identified. Studies using transient PTP4A3 overexpression systems implicate a number of signaling molecules and cellular pathways; however, we do not have a solid understanding of the mechanism by which PTP4A3 influences these signaling mechanisms and subsequent cellular responses. It appears that, within these transient expression systems, PTP4A3 has a central role in regulating the phosphorylation status of important intracellular signaling proteins, yet, paradoxically, hyper- rather than hypophosphorylation of tyrosines, serines, and threonines has been observed in cells with elevated levels of PTP4A3 (47). Such results imply that the observed changes in the phosphorylation status of the signaling proteins are an indirect effect of PTP4A3 activity (28). Moreover, these transient overexpression systems can suffer from experimental artifacts associated with the expression of superphysiological levels of PTP4A3. Because our PTP4A3fl/fl cells endogenously express detectable PTP4A3 protein, PTP4A3 function and physiologic substrates may be more directly identified. Coupling this cell line with its paired PTP4A3−/− cell line partner provides a controlled experimental system to further delineate the functionality of PTP4A3 phosphatase and its role in cellular signaling.
To confirm the cellular effects observed with PTP4A3 genetic knockdown, we also used the pan-PTP4A small molecule inhibitor JMS-053 to inhibit PTP4A3 activity. JMS-053 is the most potent known PTP4A family–specific inhibitor (35, 44) and is a considerable improvement over thienopyridone, a previously described small-molecule pan-PTP4A inhibitor (37). As a pan-PTP4A inhibitor, JMS-053 is a useful chemical probe for the PTP4A subfamily and can be used to determine functional roles of the individual PTP4A family members (35, 37). For example, our results suggest PTP4A3, and not PTP4A1 or PTP4A2, is the major family member determinant of migration and invasion in PTP4A3fl/fl CRC cells, based on the observation that treatment of PTP4A3−/− cells with JMS-053 produced no further changes in these processes. Thus, JMS-053, coupled with our loss-of-function paired CRC cell lines, provides us with complementary orthogonal methods to interrogate and confirm PTP4A3 phosphatase functionality.
One of the more well-characterized PTP4A3 cellular roles is as a regulator of cancer cell proliferation. Specifically, studies using PTP4A3 overexpression or transient suppression systems (29, 48, 49) led to the general conclusion that PTP4A3 overexpression increased cell proliferation, whereas reduction of PTP4A3 either had no effect (17, 41) or reduced cell proliferation (50). Similarly, using Ptp4a3 wild-type CRC cells grown on the lethally irradiated rat LA7 mammary epithelial cells, with their attendant heterotypic interactions, we observed a faster proliferation rate compared with the Ptp4a3 null cells (34). However, once the feeder layer was eliminated and the cells were grown independently on plastic, the resulting PTP4A3fl/fl and PTP4A3−/− cell lines shared similar growth rates. These results suggest the ECM and hetero- or homotypic interactions influence aspects of cell’s phenotype with respect to PTP4A3 expression. Our observation that PTP4A3−/− cells display a marked inhibition in their ability to form spheroids also suggests a functional role for PTP4A3 in mediating homotypic cellular interactions.
Homotypic and heterotypic tumor cell interactions with the ECM are pivotal components of the epithelial-mesenchymal transition and the subsequent metastatic process. As tumor cells alleviate their necessity to adhere to the ECM, they are able to evade programmed cell death induced by loss of anchorage and subsequently migrate to and invade distant sites (51). PTP4A3 is a regulator of the epithelial-mesenchymal transition (52), so it is perhaps unsurprising that PTP4A3 is a regulatory element of the cell’s interaction with the ECM. However, the scope of PTP4A3’s involvement in the regulation of the ECM was previously unknown. Our PCR microarray screen suggests that at least 10 ECM-related genes are regulated by PTP4A3. The most provocative is Emilin 1, a member of the Emilin family of proteins, which binds specifically to elastin and is a key component of the ECM in tumor tissue (53, 54). Previous studies demonstrated that when Emilin 1 is absent, it alters cell-ECM interactions and enriches the opportunity for migration and invasion, suggesting that Emilin 1 functions as a tumor suppressor (55). We demonstrate that, upon genetic ablation and pharmacological inhibition of PTP4A3, we enhance Emilin 1 gene expression. Thus, overexpression of PTP4A3 may override cellular control mechanisms, leading to tumor migration and metastasis. Similarly, Fbln 1 is reported to function as a tumor suppressor (56). Fbln 1 is involved in matrix reorganization, and high expression correlates with improved patient prognosis. PTP4A3 may be acting to reduce the amount of Fbln 1 present, thereby disrupting the matrix organization and leading to an altered interaction of the cells with the ECM. Our initial RNA sequencing studies further indicate that PTP4A3 can regulate the ECM in a complex manner, with significant elevation in the mRNA coding for Vcan, collagen 5α1, collagen 6α1 and 6α3, and Fbln 7 being seen in PTP4A3−/− cells. A more detailed investigation into these changes should provide valuable insight into the actions of PTP4A3 in CRC.
The mechanism by which PTP4A3 alters ECM mRNA levels is unclear, and addressing this question is complicated by PTP4A3’s complex role in cancer biology, the anonymity of its direct substrates, and the limited available tools for PTP4A3 as well as target genes. Consequently, new cellular models and cell-active, well-credentialed, chemical probes, such as JMS-053, provide valuable reagents to help clarify its functionality. In the current study, we exploited these tools to demonstrate that not only does PTP4A3 have a profound effect on the cell’s ability to migrate but also that PTP4A3 is driving this phenotype through the cells’ interactions with the ECM. This is exemplified in the changes in integrin (Itgb2) and matrix metalloproteinase expression (Mmp1a). In this same vein, the changes in the cell’s ability to adhere can be traced back to changes in expression of these key genes. The integrins function to both bind and respond to the ECM surrounding the cell, and changing the expression of any of these genes alters the cellular response to differing matrix types. Matrix metalloproteinases are essential components in the breakdown and turnover of specific matrix proteins. Given that the expression of molecules within these families was altered, it is unsurprising that the genetic and pharmacologic inhibition of PTP4A3 resulted in differences in the strength of the adhesion of the cells to plastic. This may be because of the way these cells interact with the self-produced matrix due to a decrease in the level of Mmp1a and an increase in Itgb2. We cannot exclude a role for RhoA GTPase, which is activated by growth factors and integrins and has a central role in controlling the transmembrane ECM-cytoskeleton interactions and focal adhesion (57). Our study and the study by Fiordalisi et al. (20) demonstrate that RhoA activity can be controlled by PTP4A3.
PTP4A3 is an enigmatic phosphatase. Some investigators have even suggested that PTP4A3 is not a phosphatase but rather acts as a pseudophosphatase, which functions to regulate magnesium homeostasis through a nonezymatic interaction with CNNM3 (58). It has also been suggested that PTP4A3 does have phosphatase activity, but toward lipids, specifically phosphoinositides, rather than proteins (59). Regardless, alterations in this small phosphatase affect a wide array of signaling networks. The implications of alterations in the expression levels of phosphatases reach far beyond any individual direct substrate, and understanding the impact of changes in these networks is essential to understanding how these molecules drive oncogenic phenotypes. PTP4A3 expression is increased in CRC cells, and this increase leads to enhanced migration, spheroid formation, and adhesion. These phenotypes are directly linked to PTP4A3 phosphatase activity and are driven by changes in mRNA expression of genes controlling the cells’ interaction with the ECM. The changes provide a foundation for the negative impact elevated PTP4A3 mRNA has on disease-free survival with CRC. We have developed valuable cell lines and a potent and specific PTP4A3 small molecule inhibitor. Our cellular and pharmacological reagents should assist in the process of understanding how this intracellular protein communicates with the ECM.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Alex Cheung (University of Virginia) for assistance in spheroid assay development, Dr. Mark Zimmerman (University of Pittsburgh) for providing tumor tissue from the colorectal tumors of Ptp4a3fl/fl and Ptp4a3−/− mice, Sophie Lewandoski (Oberlin College, Oberlin, OH, USA) for help in characterizing the JMS-053 and JMS-038 compounds, and Yongde Bao (University of Virginia Genomic Analysis and Technology Core) for assistance with RNA sequencing. This research was supported by National Institutes of Health (NIH) National Cancer Institute Grants R21CA191944 (to J.S.L.), F31CA196062 (to K.E.M.), and P30 CA44579, NIH National Institute of General Medical Sciences Grant T32GM007055, and NIH Office of the Director Grant S10OD021723 (to J.S.L.); the Farrow Fellowship (to K.E.M.); the Fiske Drug Discovery Fund (to J.S.L.); the Owens Foundation (to J.S.L.); the Ivy Foundation (to J.S.L.); and by discretionary funding from Boehringer-Ingelheim Pharmaceuticals (Ridgefield, CT, USA) (to P.W.). The authors declare no conflicts of interest.
Glossary
- 2D
2-dimensional
- CRC
colorectal cancer
- ECM
extracellular matrix
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- Itgb
integrin subunit β
- Mmp
matrix metalloproteinase
- PDGF
platelet-derived growth factor
- PTP
protein tyrosine phosphatase
- qRT-PCR
quantitative RT-PCR
- siRNA
small interfering RNA
- TCGA
The Cancer Genome Atlas
- Vcan
versican
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. E. McQueeney, J. M. Salamoun, P. Pekic, J. G. Ahn, I. K. Blanco, and H. L. Struckman designed and conducted the research; E. R. Sharlow, P. Wipf, and J. S. Lazo participated in research design; K. E. McQueeney, J. M. Salamoun, E. R. Sharlow, P. Wipf, and J. S. Lazo contributed new reagents and tools; K. E. McQueeney, E. R. Sharlow, P. Wipf, and J. S. Lazo performed data analysis; and K. E. McQueeney, E. R. Sharlow, P. Wipf, and J. S. Lazo wrote the manuscript.
REFERENCES
- 1. National Cancer Institute. (2016) SEER Cancer Stat Facts: Colon and Rectum Cancer, Vol. 2017, National Cancer Institute, Bethesda, MD, USA. [Google Scholar]
- 2.Siegel R. L., Miller K. D., Fedewa S. A., Ahnen D. J., Meester R. G. S., Barzi A., Jemal A. (2017) Colorectal cancer statistics, 2017. CA Cancer J. Clin. 67, 177–193 [DOI] [PubMed] [Google Scholar]
- 3.Connell L. C., Mota J. M., Braghiroli M. I., Hoff P. M. (2017) The rising incidence of younger patients with colorectal cancer: questions about screening, biology, and treatment. Curr. Treat. Options Oncol. 18, 23 [DOI] [PubMed] [Google Scholar]
- 4.Julien S. G., Dubé N., Hardy S., Tremblay M. L. (2011) Inside the human cancer tyrosine phosphatome. Nat. Rev. Cancer 11, 35–49 [DOI] [PubMed] [Google Scholar]
- 5.Saha S., Bardelli A., Buckhaults P., Velculescu V. E., Rago C., St Croix B., Romans K. E., Choti M. A., Lengauer C., Kinzler K. W., Vogelstein B. (2001) A phosphatase associated with metastasis of colorectal cancer. Science 294, 1343–1346 [DOI] [PubMed] [Google Scholar]
- 6.Kato H., Semba S., Miskad U. A., Seo Y., Kasuga M., Yokozaki H. (2004) High expression of PRL-3 promotes cancer cell motility and liver metastasis in human colorectal cancer: a predictive molecular marker of metachronous liver and lung metastases. Clin. Cancer Res. 10, 7318–7328 [DOI] [PubMed] [Google Scholar]
- 7.Bardelli A., Saha S., Sager J. A., Romans K. E., Xin B., Markowitz S. D., Lengauer C., Velculescu V. E., Kinzler K. W., Vogelstein B. (2003) PRL-3 expression in metastatic cancers. Clin. Cancer Res. 9, 5607–5615 [PubMed] [Google Scholar]
- 8.Xing X., Peng L., Qu L., Ren T., Dong B., Su X., Shou C. (2009) Prognostic value of PRL-3 overexpression in early stages of colonic cancer. Histopathology 54, 309–318 [DOI] [PubMed] [Google Scholar]
- 9.Polato F., Codegoni A., Fruscio R., Perego P., Mangioni C., Saha S., Bardelli A., Broggini M. (2005) PRL-3 phosphatase is implicated in ovarian cancer growth. Clin. Cancer Res. 11, 6835–6839 [DOI] [PubMed] [Google Scholar]
- 10.Hao R. T., Zhang X. H., Pan Y. F., Liu H. G., Xiang Y. Q., Wan L., Wu X. L. (2010) Prognostic and metastatic value of phosphatase of regenerating liver-3 in invasive breast cancer. J. Cancer Res. Clin. Oncol. 136, 1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gari H. H., DeGala G. D., Ray R., Lucia M. S., Lambert J. R. (2016) PRL-3 engages the focal adhesion pathway in triple-negative breast cancer cells to alter actin structure and substrate adhesion properties critical for cell migration and invasion. Cancer Lett. 380, 505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Den Hollander P., Rawls K., Tsimelzon A., Shepherd J., Mazumdar A., Hill J., Fuqua S. A., Chang J. C., Osborne C. K., Hilsenbeck S. G., Mills G. B., Brown P. H. (2016) Phosphatase PTP4A3 promotes triple-negative breast cancer growth and predicts poor patient survival. Cancer Res. 76, 1942–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ooki A., Yamashita K., Kikuchi S., Sakuramoto S., Katada N., Waraya M., Kawamata H., Nishimiya H., Nakamura K., Watanabe M. (2011) Therapeutic potential of PRL-3 targeting and clinical significance of PRL-3 genomic amplification in gastric cancer. BMC Cancer 11, 122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J., Chong P. S., Lu X., Cheong L. L., Bi C., Liu S. C., Zhou Y., Tan T. Z., Yang H., Chung T. H., Zeng Q., Chng W. J. (2014) Phosphatase of regenerating liver-3 is regulated by signal transducer and activator of transcription 3 in acute myeloid leukemia. Exp. Hematol. 42, 1041–1052.e1-2 [DOI] [PubMed] [Google Scholar]
- 15.Laurent C., Valet F., Planque N., Silveri L., Maacha S., Anezo O., Hupe P., Plancher C., Reyes C., Albaud B., Rapinat A., Gentien D., Couturier J., Sastre-Garau X., Desjardins L., Thiery J. P., Roman-Roman S., Asselain B., Barillot E., Piperno-Neumann S., Saule S. (2011) High PTP4A3 phosphatase expression correlates with metastatic risk in uveal melanoma patients. Cancer Res. 71, 666–674 [DOI] [PubMed] [Google Scholar]
- 16.Al-Aidaroos A. Q., Yuen H. F., Guo K., Zhang S. D., Chung T. H., Chng W. J., Zeng Q. (2013) Metastasis-associated PRL-3 induces EGFR activation and addiction in cancer cells [Erratum]. J. Clin. Invest. 123, 3459–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang F., Liang J., Wang W. Q., Sun J. P., Udho E., Zhang Z. Y. (2007) PRL3 promotes cell invasion and proliferation by down-regulation of Csk leading to Src activation. J. Biol. Chem. 282, 5413–5419 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Li Z. F., He J., Li Y. L., Zhu G. B., Zhang L. H., Li Y. L. (2007) Expression of the human phosphatases of regenerating liver (PRLs) in colonic adenocarcinoma and its correlation with lymph node metastasis. Int. J. Colorectal Dis. 22, 1179–1184 [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y., Liu X. Q., Rajput A., Geng L., Ongchin M., Zeng Q., Taylor G. S., Wang J. (2011) Phosphatase PRL-3 is a direct regulatory target of TGFbeta in colon cancer metastasis. Cancer Res. 71, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiordalisi J. J., Keller P. J., Cox A. D. (2006) PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res. 66, 3153–3161 [DOI] [PubMed] [Google Scholar]
- 21.Buchheit C. L., Weigel K. J., Schafer Z. T. (2014) Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat. Rev. Cancer 14, 632–641 [DOI] [PubMed] [Google Scholar]
- 22.Frisch S. M., Francis H. (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddig P. J., Juliano R. L. (2005) Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 24, 425–439 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M. A., Assoian R. K. (2001) Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553–2560 [DOI] [PubMed] [Google Scholar]
- 25.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration: integrating signals from front to back. Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 26.Ojakian G. K., Schwimmer R. (1988) The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J. Cell Biol. 107, 2377–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park C. C., Zhang H., Pallavicini M., Gray J. W., Baehner F., Park C. J., Bissell M. J. (2006) Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 66, 1526–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walls C. D., Iliuk A., Bai Y., Wang M., Tao W. A., Zhang Z. Y. (2013) Phosphatase of regenerating liver 3 (PRL3) provokes a tyrosine phosphoproteome to drive prometastatic signal transduction. Mol. Cell. Proteomics 12, 3759–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., Quah S. Y., Dong J. M., Manser E., Tang J. P., Zeng Q. (2007) PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 67, 2922–2926 [DOI] [PubMed] [Google Scholar]
- 30.Foy M., Anézo O., Saule S., Planque N. (2017) PRL-3/PTP4A3 phosphatase regulates integrin β1 in adhesion structures during migration of human ocular melanoma cells. Exp. Cell Res. 353, 88–99 [DOI] [PubMed] [Google Scholar]
- 31.Forte E., Orsatti L., Talamo F., Barbato G., De Francesco R., Tomei L. (2008) Ezrin is a specific and direct target of protein tyrosine phosphatase PRL-3. Biochim. Biophys. Acta 1783, 334–344 [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman M. W., Homanics G. E., Lazo J. S. (2013) Targeted deletion of the metastasis-associated phosphatase Ptp4a3 (PRL-3) suppresses murine colon cancer. PLoS One 8, e58300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maltzman T., Whittington J., Driggers L., Stephens J., Ahnen D. (1997) AOM-induced mouse colon tumors do not express full-length APC protein. Carcinogenesis 18, 2435–2439 [DOI] [PubMed] [Google Scholar]
- 34.Cramer J. M., Zimmerman M. W., Thompson T., Homanics G. E., Lazo J. S., Lagasse E. (2014) Deletion of Ptp4a3 reduces clonogenicity and tumor-initiation ability of colitis-associated cancer cells in mice. Stem Cell Res. (Amst.) 13, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salamoun J. M., McQueeney K. E., Patil K., Geib S. J., Sharlow E. R., Lazo J. S., Wipf P. (2016) Photooxygenation of an amino-thienopyridone yields a more potent PTP4A3 inhibitor. Org. Biomol. Chem. 14, 6398–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerman M. W., McQueeney K. E., Isenberg J. S., Pitt B. R., Wasserloos K. A., Homanics G. E., Lazo J. S. (2014) Protein-tyrosine phosphatase 4A3 (PTP4A3) phosphatase promotes vascular endothelial growth factor signaling and enables endothelial cell motility. J. Biol. Chem. 289, 5904–5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daouti S., Li W. H., Qian H., Huang K. S., Holmgren J., Levin W., Reik L., McGady D. L., Gillespie P., Perrotta A., Bian H., Reidhaar-Olson J. F., Bliss S. A., Olivier A. R., Sergi J. A., Fry D., Danho W., Ritland S., Fotouhi N., Heimbrook D., Niu H. (2008) A selective phosphatase of regenerating liver phosphatase inhibitor suppresses tumor cell anchorage-independent growth by a novel mechanism involving p130Cas cleavage. Cancer Res. 68, 1162–1169 [DOI] [PubMed] [Google Scholar]
- 38.Saiga T., Ohbayashi T., Tabuchi K., Midorikawa O. (1987) A model for tumorigenicity and metastatic potential: growth in 1.0% agar cultures. In Vitro Cell. Dev. Biol. 23, 850–854 [DOI] [PubMed] [Google Scholar]
- 39.Hoeger B., Diether M., Ballester P. J., Köhn M. (2014) Biochemical evaluation of virtual screening methods reveals a cell-active inhibitor of the cancer-promoting phosphatases of regenerating liver. Eur. J. Med. Chem. 88, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanoni M., Piccinini F., Arienti C., Zamagni A., Santi S., Polico R., Bevilacqua A., Tesei A. (2016) 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 6, 19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian F., Li Y. P., Sheng X., Zhang Z. C., Song R., Dong W., Cao S. X., Hua Z. C., Xu Q. (2007) PRL-3 siRNA inhibits the metastasis of B16-BL6 mouse melanoma cells in vitro and in vivo [Erratum]. Mol. Med. 13, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers A. F., Groom A. C., MacDonald I. C. (2002) Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 [DOI] [PubMed] [Google Scholar]
- 43.Orgaz J. L., Herraiz C., Sanz-Moreno V. (2014) Rho GTPases modulate malignant transformation of tumor cells. Small GTPases 5, e29019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McQueeney K. E., Salamoun J. M., Burnett J. C., Barabutis N., Pekic P., Lewandowski S. L., Llaneza D. C., Cornelison R., Bai Y., Zhang Z. Y., Catravas J. D., Landen C. N., Wipf P., Lazo J. S., Sharlow E. R. (2017) Targeting ovarian cancer and endothelium with an allosteric PTP4A3 phosphatase inhibitor. Oncotarget 9, 8223–8240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Donatis A., Comito G., Buricchi F., Vinci M. C., Parenti A., Caselli A., Camici G., Manao G., Ramponi G., Cirri P. (2008) Proliferaition versus migration in platelet-derived growth factor signaling: the key role of endocytosis. J. Biol. Chem. 283, 19948–19956 [DOI] [PubMed] [Google Scholar]
- 46.Hynes R. O. (2009) The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y., Lian S., Meng L., Qu L., Shou C. (2017) Antibody array revealed PRL-3 affects protein phosphorylation and cytokine secretion. PLoS One 12, e0169665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slørdahl T. S., Abdollahi P., Vandsemb E. N., Rampa C., Misund K., Baranowska K. A., Westhrin M., Waage A., Rø T. B., Børset M. (2016) The phosphatase of regenerating liver-3 (PRL-3) is important for IL-6-mediated survival of myeloma cells. Oncotarget 7, 27295–27306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing C., Lu X. X., Guo P. D., Shen T., Zhang S., He X. S., Gan W. J., Li X. M., Wang J. R., Zhao Y. Y., Wu H., Li J. M. (2016) Ubiquitin-specific protease 4-mediated deubiquitination and stabilization of PRL-3 is required for potentiating colorectal oncogenesis. Cancer Res. 76, 83–95 [DOI] [PubMed] [Google Scholar]
- 50.Gari H. H., DeGala G. D., Lucia M. S., Lambert J. R. (2016) Loss of the oncogenic phosphatase PRL-3 promotes a TNF-R1 feedback loop that mediates triple-negative breast cancer growth. Oncogenesis 5, e255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taddei M. L., Giannoni E., Fiaschi T., Chiarugi P. (2012) Anoikis: an emerging hallmark in health and diseases. J. Pathol. 226, 380–393 [DOI] [PubMed] [Google Scholar]
- 52.Lai W., Liu L., Zeng Y., Wu H., Xu H., Chen S., Chu Z. (2013) KCNN4 channels participate in the EMT induced by PRL-3 in colorectal cancer. Med. Oncol. 30, 566 [DOI] [PubMed] [Google Scholar]
- 53.Zanetti M., Braghetta P., Sabatelli P., Mura I., Doliana R., Colombatti A., Volpin D., Bonaldo P., Bressan G. M. (2004) EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell. Biol. 24, 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rabajdova M., Urban P., Spakova I., Saksun L., Dudic R., Ostro A., Caprnda M., Kruzliak P., Adamek M., Marekova M. (2016) The crucial role of emilin 1 gene expression during progression of tumor growth. J. Cancer Res. Clin. Oncol. 142, 2397–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danussi C., Petrucco A., Wassermann B., Modica T. M., Pivetta E., Del Bel Belluz L., Colombatti A., Spessotto P. (2012) An EMILIN1-negative microenvironment promotes tumor cell proliferation and lymph node invasion. Cancer Prev. Res. (Phila.) 5, 1131–1143 [DOI] [PubMed] [Google Scholar]
- 56.Xu Z., Chen H., Liu D., Huo J. (2015) Fibulin-1 is downregulated through promoter hypermethylation in colorectal cancer: a CONSORT study. Medicine (Baltimore) 94, e663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geiger B., Bershadsky A., Pankov R., Yamada K. M. (2001) Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2, 793–805 [DOI] [PubMed] [Google Scholar]
- 58.Kostantin E., Hardy S., Valinsky W. C., Kompatscher A., de Baaij J. H., Zolotarov Y., Landry M., Uetani N., Martínez-Cruz L. A., Hoenderop J. G., Shrier A., Tremblay M. L. (2016) Inhibition of PRL-2 CNNM3 protein complex formation decreases breast cancer proliferation and tumor growth. J. Biol. Chem. 291, 10716–10725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McParland V., Varsano G., Li X., Thornton J., Baby J., Aravind A., Meyer C., Pavic K., Rios P., Köhn M. (2011) The metastasis-promoting phosphatase PRL-3 shows activity toward phosphoinositides. Biochemistry 50, 7579–7590 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.