Abstract
Macrophages are immune-sensing “big eater” phagocytic cells responsible for an innate, adaptive, and regenerative response. After myocardial infarction, macrophages predominantly clear the deceased cardiomyocyte apoptotic or necrotic neutrophils to develop a regenerative and reparative program with the activation of the lipoxygenase-mediated maresin (MaR) metabolome at the site of ischemic injury. The specialized proresolving molecule and macrophage mediator in resolving inflammation, MaR-1, produced by human macrophages, has potent defining effects that limit polymorphonuclear neutrophil infiltration, enhance uptake of apoptotic PMNs, regulate inflammation resolution and tissue regeneration, and reduce pain. In addition to proresolving and anti-inflammatory actions, MaR-1 displays potent tissue regenerative effects in stroke and is an antinociceptive. Macrophages actively participate in the biosynthesis of bioactive MaR-2, which exhibits anti-inflammatory, proresolving, and atherosclerotic effects. A new class of macrophage-derived molecules, MaR conjugates in tissue regeneration, is identified that regulates phagocytosis and the repair and regeneration of damaged tissue. The presented review provides a current summary of the effect of MaR in resolution pathophysiology, with relevance to a cardiac repair program.—Jadapalli, J. K., Halade, G. V. Unified nexus of macrophages and maresins in cardiac reparative mechanisms.
Keywords: myocardial infarction, myocardium repair, myocardium regeneration, inflammation, resolution of inflammation
Prolonged ischemia in cardiac tissue, triggering heart attack or myocardial infarction (MI), is one of the major causes of myocardium injury and thereby morbidity and mortality worldwide. In the United States, more than 7 million people have cardiac infarctions each year (1, 2). With the advancement of medical technology in the past few decades, the survival after MI has significantly improved because of early percutaneous revascularization therapy and drug treatment; however, more than 45% reperfused or nonreperfused patients develop heart failure related to collateral injury. Therefore, the current focus is the discovery of new therapeutic strategies to treat the conditions that can delay advanced heart failure (3–5). This paradigm provokes immunologists to focus on ischemic heart disease, as cardiologists are more interested in the intricacies of cardiomyocyte interaction with the immune system. Recent reports indicate that atherogenesis, atheroprogression, and atherosclerosis are not merely a lipid storage disorder, but is also driven by immune system dysfunction that initiates the convergence of cardiology and immunology (6).
Overactivation of the stress-related neurohormonal axis or narrowing of a coronary vessel results in an acutely diminished oxygen supply to the myocardium, which leads to necrosis, micro or macro MI, and acute inflammation, widely known as a heart attack or MI (3). As the human heart has limited regeneration capacity, restoration of the oxygen supply, in addition to infarct healing, is much dependent on the inflammatory and resolving responses, which are sterile (7). After MI, the innate inflammatory response induced by MI instigates cardiac repair processes toward either a resolving or nonresolving pathway (8). This inflammatory response results in predominant infiltration of peripheral neutrophils and monocytes that eventually differentiate into macrophages. These are involved at different stages of infarct healing, ultimately leading to scar formation, and harmonize remodeling to preserve cardiac function (9–11). On-time leukocyte entry failure (delayed “get-in” signal) at the site of injury or prolonged residual time at the site of infarction (delayed “get-out” signal) causes development of nonresolving, uncontrolled chronic inflammation in the cardiac repair program (12–15).
MACROPHAGES AND COMPLEX TERMINOLOGY
Ilya Ilyich Mechnikov, a 1908 Nobel Laureate, first discovered the phagocytic role of macrophages (“big eaters”) that are termed phagocytes (16). Macrophages are large myeloid cells that display stellate morphology and are characterized by the presence of pseudopodia, nonspecific esterase, and phagocytic granules, which give them a foamy appearance. Resident and monocyte-derived macrophages have different roles in almost every aspect of an organism’s pathobiology (Fig. 1, cardiac pathophysiology), from embryonic development, homeostasis, electrical conduction, cardiac repair, and regeneration to immune responses against pathogens (17). In adult mammals, macrophages are found in all tissues, with the highest presence in the microenvironment of solid tumors, where they display a comprehensive anatomic and functional diversity of surface markers, depending on the microenvironment (18). Macrophages play a vibrant and multitasking role in the innate immune response to injury, and, with the integral role of phagocytosis, macrophages also regulate several wound-healing pathways, including dismantling and removing cell debris, development of mature infarct scar, and angiogenesis (9, 19). In vitro, macrophage phenotypes are the classically activated M1 macrophages (proinflammatory type) and the alternatively activated M2 macrophages (anti-inflammatory type or reparative) (20–24). In a petri dish, macrophage exposure to IFN-γ and LPS leads to M1 (inflammatory) polarization, with cytotoxic and antitumoral properties, whereas M2 (reparative or resolving) macrophages display immunoregulatory effects. The M2 phenotype is subdivided into M2a, M2b, and M2c subtypes. These M2 macrophages polarize and acquire a different functional role in response to environment-derived signals. In particular, M2a is formed on exposure to IL-4 and -13 and M2b on combined exposure to TLR or IL-1R agonists and immune complexes and promotes type II responses and immunoregulatory functions, whereas M2c macrophages (induced by IL-10) are more related to suppression of immune responses and tissue remodeling (20, 25–27). In vivo, this heterogeneous classification is challenging because of the temporal dynamics of resident vs. infiltrating macrophages in wound healing. The tension between the host defense (proinflammatory) and reparative (proresolving) properties of the mononuclear phagocytes in infarcted myocardium requires caution against indiscriminate targeting of macrophages (28). In cardiac healing, the reparative macrophages are classified based on cluster of differentiation (CD)206 (mannose receptor), but the degree to which CD206 is relevant to human healing in heart failure pathology remains unclear. The infiltrating macrophage shows biphasic activation after MI, with the first-responder M1 macrophages showing an exclusively proinflammatory character based on gene expression profiling. However, the late-responder M2 macrophages show a mixed character, expressing high levels of both reparative and proinflammatory genes (29). First, when macrophages expand from their native environments and ultimately lose their tissue-specific gene expression, it becomes difficult for them to monitor changes and secondary activation, with diverse stimuli relevant to mediators present in the local milieu that regulate deviation in the M1/M2 spectrum. For instance, like macrophage colony-stimulating factor or granulocyte macrophage colony-stimulating factor, dependent macrophage activation network revealed considerable deviation from the M1/M2 axis making it even more complicated to differentiate the overlapping phenotypes and macrophage functional capacity. In the peritoneal domain, the macrophages comprise small and large cells. Large peritoneal macrophages disappear after infection or injury and are replaced by blood monocyte–derived small macrophages that express lower levels of CD11b (surface marker for monocytes, neutrophils, and macrophages) and F4/80 (macrophage marker) but express high levels of major histocompatibility complex (MHC)-II (derived from extracellular proteins) (30, 31). Thus, macrophages are multitasking, diversified cell types with a precise residential phenotype and diversified postinjury or -infection markers.
Figure 1.
Unified roles of macrophage chemistry in cardiac pathology. Macrophages facilitate the conduction, plaque progression, and regenerative mechanisms in cardiovascular disease. Likewise, macrophages and allied cells biosynthesize maresin (MaR) and tissue-regenerating factors in cardiac pathobiology to facilitate the resolution program.
Defining the clear margin between the M1/M2 macrophages by measuring their transcripts is also not always reliable because of the diversified local milieu of each organ—for instance, during augmentation or attenuation certain cell markers consider M1 or M2 as equivalent. In some cases, markers may break from the rule entirely. Macrophages of the same tissue have differential origins—that is, from the yolk sac or hematopoietic stem cells (HSCs) or both. For instance, microglia (brain, yolk sac), Kupffer cells (liver, yolk sac+HSCs), alveolar macrophages (lungs, HSCs), red pulp macrophages (spleen, HSCs+marginal), Langerhans cells (skin, yolk sac+HSCs), dermal macrophages (skin, HSCs), C-C chemokine receptor (CCR)2− macrophages (heart, yolk sac+HSCs), CCR2+ macrophages (heart, HSCs), kidney macrophages (HSCs), peritoneal macrophages (HSCs), and LyC6+, Ly6C− monocytes (blood, HSCs). They do not rely on specific marking, and that it makes them hard to categorize; also, macrophages have a tendency to switch from M1 to M2, making them even more difficult to distinguish (32–38). Another recent classification based on macrophage origins (monocyte vs. locally derived tissue residents), environmental stimuli (different organs and different stimuli during steady state and inflammation), and time (development, stages of inflammation, microenvironment, and aging). Murray et al. (39) suggested stimuli dependent terminology, although such methodology goes beyond the simple dichotomy suggested using M1/M2 model. Still, this futuristic approach has its inherent limitations.
ROLE OF MACROPHAGES IN MYOCARDIUM HEALING
Macrophages can be of embryonic origin or can be resident in many organ tissues in the steady state condition. Resident macrophages contribute to homeostasis and development, whereas in the setting of injury or infection, they coordinate tissue repair and regeneration processes, making them prominent candidates for therapeutic intervention (26). The disruption of homeostasis associated with ageing and the absence of inflammation or injury, within the myocardium and other organs, results not only in the recruitment of blood monocyte–derived macrophages but also in the permanent replacement of embryonically established resident macrophage populations (32, 40). This replacement is reflected by dynamic changes in the resident cardiac macrophage markers and decreasing cardiac macrophage self-renewal over time (39). However, during post-MI inflammation or after macrophage depletion, Ly6Chi monocytes contribute to all 4 macrophage populations, whereas resident macrophages numerically expand through proliferation (32).
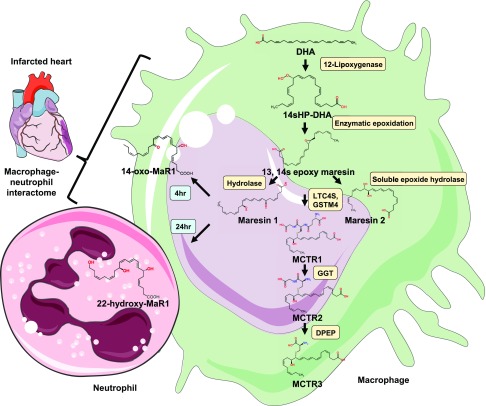
In infarcted hearts, the chemokine expression profile is modulated over time; thus, change in the edematous chemical gradient sequentially and actively recruits Ly6Chi and Ly6Clo monocytes via CCR2 and CX3C chemokine receptor (CX3CR)-1, respectively. Ly6Chi monocytes control early (phase I) and exhibit phagocytic, proteolytic, and inflammatory functions. Ly6Clo monocytes govern later (phase II), and have attenuated inflammatory properties, and express VEGF. Therefore Ly6Chi monocytes digest damaged tissue, whereas Ly6Clo monocytes promote healing via myofibroblast accumulation, angiogenesis, and deposition of collagen (28). In the heart, tissue-resident cardiac macrophages are marked as CX3CR1 cells that can be found throughout the myocardium (26). Transcriptional analysis of residential cardiac macrophages revealed that monocyte-derived macrophages coordinate cardiac inflammation while playing outmoded but minor roles in antigen sampling and efferocytosis (32). Recently, it was reported that CX3CR1 residential macrophages couple electrically to cardiomyocytes in the distal AV node via connexin-43 containing gap junctions. This process aids in depolarizing resting cardiomyocytes via connexin-43 by reducing their action potential upstroke velocity and overshoot, and aids early repolarization, altered conditions showed the irregular heartbeat condition may be one of the considerable factors leading to heart failure (17). The relative balance between pathologic inflammatory pathways and on time tissue reparative processes (physiologic inflammation) defines the trajectory of heart failure development (41). The questions remain unanswered of how macrophages respond to the organ-specific milieu and what the secretome of macrophages is that contribute to the feed-forward resolving and nonresolving inflammation in heart failure pathology. The age-related immunosenescence leads to alterations in their composition, gene expression, and function precede increased cardiac fibrosis and impairments in the inflammatory response typical of the aging heart (42). From a translational perspective, whether other metabolic disease pathologies alter macrophage function, secretome, and phenotype in aging, needs to be investigated (14, 43). In a recent study, Halade et al. (44) quantitated leukocyte populations in the splenic reservoir and infarcted LV and further determined the concentrations of specialized proresolving mediators that limit cardiac inflammation post-MI. This study clearly suggests that leukocytes mobilize from the spleen to the infarcted heart to produce resolvins, lipoxins, maresins, and protectins in the infarcted heart during the acute inflammatory response, suggesting that acute inflammatory response coincides with resolving response in cardiac healing. Furthermore, the depletion of macrophages correlated with the imperceptible levels of proresolving mediators. Thus, macrophages and the macrophage interactome with innate leukocyte players are essential for the biosynthesis of bioactive mediators and cardiac healing (Fig. 2).
Figure 2.
Biosynthesis of MaR and its metabolites coordinated by the macrophage–neutrophil alliance. Shown is the overall representative sequence of biosynthesis of both MaR-1 and -2, the MCTRs produced in macrophages, and the macrophage–neutrophil interactome formed from MaR-1. DPEP, dipeptidase; GGT, γ-glutamyltransferase; GSTM4, GST-μ4; LTC4S, leukotriene C4 synthase.
ORCHESTRATION OF MONOCYTES AND MACROPHAGES INTO THE ISCHEMIC HEART
In the normal steady state, most cardiac macrophages are embryonically derived, and a smaller contribution is derived from HSCs. At the time of cardiac ischemic injury or inflammation, Ly6Chi monocytes migrate into tissue and undergo proliferation, and compete with resident cardiac macrophages proliferation to re-establish the steady state in the macrophage pool. The pool of resident cardiac macrophages has undergone ‘‘ontological reprogramming’’ as a result and now is primarily composed of adult-derived macrophages. In conditions such as the absence of circulating blood monocytes (as in CCR2−mice), resident cardiac macrophages are fully capable of rebuilding steady-state levels solely through in-situ proliferation (45–48). At baseline, the adult heart contained 2 immunosurveillance resident macrophages (MHC-IIloCCR2− and MHC−IIhiCCR2−) subsets, 2 monocyte-derived macrophages (MHC-IIhiCCR2+), and (MHC-IIloCR2+) subsets. The neonatal heart included one resident macrophage (MHC-IIloCR2−) and 1 monocyte-derived (MHC-IIloCCR2+) subset. In response to injury, the neonatal heart selectively expanded the number of MHC-IIloCCR2− macrophages and did not recruit additional CCR2+ monocytes. In contrast, the injured adult heart selectively recruited monocytes and MHC-IIhiCCR2+ monocyte-derived macrophages. An additional surface marker such as tyrosine-protein kinase Mer and cluster of differentiation-64 confirms that MHC-IIloCR2−, MHC-IIhiCCR2−, and MHC-IIhiCCR2+ cells represent macrophages, whereas MHC-IIloCCR2+ cells are monocytes in both the neonatal and adult heart (49). A flow cytometry–based quantitative approach or qualitative immunostaining revealed the rapid expansion of macrophages in the neonatal heart that resolved within 5 d after injury. In contrast, monocytes infiltrated the adult heart after injury and transitioned to macrophages progressively (50, 51). Together, these data indicate that, in response to injury, the population of resident CCR2− reparative cardiac macrophages expands in the neonatal heart, whereas the adult heart recruits CCR2+ monocyte-derived macrophages (51). The consequences of this difference are regeneration vs. repair. The neonatal heart has a unique and transient ability for myocardial regeneration after mild-to-moderate cardiac injury by surgical apical resection, cryoablation, or MI (50–57). The amplified regenerative response can be supported by a resolution of inflammation with the biosynthesis of resolving lipids and reactive cardiomyocyte dedifferentiation and redifferentiation (56). The regenerative response in newborn mice is marked with angiogenesis, which is scar formation in adults. Our knowledge is still limited, but initial research in this model has led to the understanding that myocardial regeneration in the neonatal heart is dependent on diversified macrophage phenotypes and transcription profiles (32, 54, 57). When a myocardial resection was performed, fibrin clots sealed the resection site and created a matrix for recruiting myeloid cells, and macrophages secreted growth factors and paracrine factors that promote regeneration (58–61). Inhibition of myocardial regeneration by anti-inflammatory glucocorticoids was seen in zebrafish (60). Thus, the mechanism of immune system control in myocardial regeneration and repair is an important area of research that has therapeutic implications in human cardiac healing.
MACROPHAGES AND REPARATIVE MECHANISMS
Aurora and colleagues (54) were the first to show with the quantitative approach of flow cytometry that macrophages are essential for neonatal heart regeneration. In the intact heart, without MI, macrophages are significantly more abundant at d 1 after birth than on d 14. Seven days after birth, post-MI d 1 macrophages appear more abundantly overall in the heart and are distributed throughout the myocardium, both at the infarct and in remote areas, whereas on d 14 after birth, there were fewer post-MI d 1 macrophages, and they were almost exclusively localized to the infarct zone (54). The regenerative capacity of the neonatal heart was eliminated by macrophage depletion (62). Macrophage depletion did not reduce cardiomyocyte proliferation but was associated with impaired myocardial vascularization and regeneration (54). These findings indicate the importance of macrophages in the regeneration of the neonatal heart and suggest that newborn (d 1) mice are rich in M2 macrophages, with high regenerative angiogenic potential, distinctive from the profibrotic and scar-forming activities of adult monocytes/macrophages (54). These fundamental insights have been gained from cardiac regeneration in fish, salamander, and neonatal mouse; are translatable; and have paved the way to study cardiac repair in humans (54, 63). As in newborn mice, newborn humans with signs of ischemic injury or heart attack heal faster than adult humans; the mechanism that facilitates this healing is unclear (64).
The neonatal heart is responsive to cardiac inflammation with the progression of MHC-IIloCCR2− embryo-derived macrophages (51). These macrophages have superior phagocytic, angiogenic, and reparative properties that enhance the regenerative capacity of the neonatal heart (65). In contrast, adult mice respond with the recruitment of inflammatory MHC-IIhiCCR2+ monocytes that replace the reparative resident CCR2− macrophages and lead to adverse cardiac remodeling and dysfunction. Inhibiting the accumulation of CCR2+ monocytes in the injured myocardium preserves reparative cardiac MHC-IIlo macrophages in the adult heart and improves coronary angiogenesis and reduced inflammation (51).
Han et al. (52) recently showed that cardiomyocyte proliferation induced by acute inflammation after apical resection results in infiltration of Ly6G inflammatory leukocytes. When the regenerative response in neonatal mouse heart was blocked after immunosuppression with dexamethasone, it showed that IL-6 is essential for cardiomyocyte proliferation. The same results were observed when deletion of the cardiomyocyte-specific signal transducer and activation of transcription-3 caused the downregulation of IL-6 signaling and decreased reactive cardiomyocyte proliferation after apical resection (52). These results are in harmony with recent profiling studies that indicated that cardiomyocyte signal transducer and activation of transcription-3 mediate injury-induced myocyte proliferation and heart regeneration in the adult zebrafish (60, 66) and the neonatal mouse heart (67). This study suggests that the immune system stimulates the regenerative response in the mouse heart.
Aging is also a primary factor in MI-induced heart failure pathology superimposed on additional risk factors, such as obesity, insulin resistance, and hypertension (8, 43). A recent study revealed that aging impairs the phagocytotic capacity of brain macrophages (microglia) (68). In addition to aging, macrophages exhibit diminished ability to phagocytose apoptotic cells, indicating systemic metabolic dysfunction, which is often seen in old obese mice (69). This effect shows that diminished macrophage phagocytotic activity with aging results in failed resolution and accumulation of damage-associated molecular patterns in aged animals (70) and confirms that aging-related immunosenescence leads to a decreased immunosurveillance capacity of macrophages and thereby ultimately affects immunoregulatory and tolerogenic mechanisms of the immune system in aging (71, 72).
MARESINS
Macrophages also participate in the biosynthesis of specialized proresolving mediators (SPMs), which are bioactive autacoids with both anti-inflammatory and proresolving properties derived from fatty acid immunometabolism. SPMs are enzymatically biosynthesized from essential fatty acids, with different stereochemistries. Recently, a new family of macrophage-derived mediators was identified and named maresins (MaRs) for macrophage mediator resolving inflammation (73). These newly identified bioactive lipid mediators display potent anti-inflammatory and proresolving actions inhibiting neutrophil infiltration in vivo and stereoselectively stimulating macrophage phagocytosis and efferocytosis to enhance the clearance of inflammation without affecting the innate response (74, 75). MaRs are a family of bioactive lipid mediators biosynthesized by activated macrophages from docosahexaenoic acid (DHA) via human 12-lipoxygenase (LOX) (75). Chemically, MaR-1 is 7R,14S, dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid, and is the first member of the MaR family to be identified. The complete stereochemistry of MaR-1 has been established in several in vitro and in vivo models. MaR-1 also possesses potent tissue regenerative and antinociceptive effects (74). Recently a new bioactive MaR metabolome was identified as 13R,14S dihydroxy-4Z,7Z,9E,11E,16Z,19Z-hexaenoic acid (Mar-2), which displays anti-inflammatory, proresolving, and antiatherosclerotic effects (Table 1) (76, 77).
TABLE 1.
Various roles of MaRs
| Bioactive resolution | Action | Model system/cell type | Reference |
|---|---|---|---|
| MaR-1 (7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid) | ↓ PMN infiltration, ↑ phagocyte clearance | Murine peritonitis/lung | 110 |
| Antinociceptive | Mouse/pain | 111 | |
| ↓ PMN infiltration and cytokine expression as well as NF-κB activation | Mouse/colitis | 112 | |
| ↑ Tissue regeneration in Planaria | Planaria/regeneration | 90 | |
| Phenotype switch | Human macrophages | 75 | |
| ↑ Apoptotic PMNs | Human macrophages | 90 | |
| ↑ Phagocytosis and efferocytosis | Human macrophages | 73, 113 | |
| ↓ Cytokine release with organic dust | Human bronchial epithelial cells | 114 | |
| Identified in synovial fluid extracts | Human/rheumatoid arthritis | 115 | |
| MaR-2 (13R,14S-dihydroxy-4Z,7Z,9E,11Z,16Z,19Z-DHA) | ↓ PMN infiltration | Human macrophages | 76 |
| ↑ Phagocytosis and efferocytosis | Human macrophages | 76, 116 |
In macrophages, MaR-1 biosynthesis (Fig. 2) is initiated by 12-LOX from DHA, producing 14S-hydroperoxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid, the hydroperoxy intermediate, which undergoes further conversion via enzymatic 13(14)-epoxidation. This epoxide intermediate is hydrolyzed enzymatically via an acid-catalyzed nucleophilic attack by water at carbon 7, resulting in the introduction of a hydroxyl group at that position and a double bond rearrangement to form the stereochemistry of bioactive MaR1 (7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid) with potent proresolution properties (74, 75). The 13S,14S-epoxy-MaR the intermediate in MaR1 synthesis is also the precursor of MaR2. This is the product of DHA biosynthesize by 12-LOX to produce the 14S-hydroperoxide that is converted to the 13S,14S-epoxy-MaR and finally converted by a soluble epoxide hydrolase into MaR2 (78).
MARESINS ROLE IN THE CLEARANCE OF ATHEROSCLEROTIC INFLAMMATION
Atherogenesis and atheroprogression have long been regarded as signs of chronic low-grade inflammation. However, modern concepts also view atherosclerosis as a low-grade chronic consequence of failed resolution (79, 80). Resolution is crucially orchestrated by a switch from inflammatory to resolving by specialized lipid mediators in the affected tissue (81), an event that may be impaired during atheroprogression (82, 83). In contrast to acute inflammatory situations, atherogenesis and atheroprogression are characterized by the persistence of the initiating stimulus, including hypertension, hyperlipidemia, and oxidative stress.
Macrophages in atherosclerotic lesions actively participate in lipoprotein ingestion, and their accumulation gives rise to the formation of foam cells filled with lipid droplets (Table 1) (84). Accumulation of foam cells in the lesions results in lipid storage and growth of atherosclerotic plaque. This effect was determined by the increase in several M1 macrophages in progressing plaques, and a greater number of M2 macrophages present in regressing plaques, where they were involved in tissue repair and remodeling (85). Macrophages are highly heterogeneous, plastic cells that can rapidly adapt their phenotype/function and change the chemical milieu to facilitate resolution. During the inflammatory phase, they phagocytose necrotic cells and intensify inflammation by releasing proteases, proinflammatory cytokines/chemokines, and reactive oxygen species. Later these macrophages, in the reparative phase, undergo transformation and exert profibrotic functions by activating collagen production and neovascularization. A timely resolution of each phase is crucial because spontaneous, prolonged inflammation and fibrosis may be detrimental and determine whether progression to heart failure or repair of cardiac tissue occurs (86).
The milieu of progressive atherosclerotic lesions increases the synthesis of leukotriene (LT)B4 driven by 5-LOX (79, 80). Also, the local cytokine environment favors the accumulation of inflammatory over reparative macrophages (87). LTB4 and prostaglandin (PG)E2 are produced in abundance at sites of inflammation (88–90), suggesting that their production by classic inflammatory macrophages favors the synthesis of inflammatory lipid mediators, in comparison to their reparative macrophage counterparts (91).
Systematically, LTB4 potentiates atherosclerosis by increasing the expression of fatty acid translocase/CD36 and C-C motif chemokine ligand-2, thereby inducing a positive feedback loop to recruit leukocytes (92, 93). PGE2 and PGD2 have been reported to have a dual, paradoxical role, functioning as a proinflammatory and proresolving mediators, depending on their location (94). In the context of atherosclerosis, PGE2 is associated with disease progression, mediated by activation of platelets (89) and, consequently, monocyte recruitment (95). In general, lipid mediators are mostly produced by a myeloid cells, where the same cell or combination of cells can often be a source of different classes of lipid mediators, depending on the substrate and temporal injury setting. Under inflammatory conditions, neutrophils (96, 97) and macrophages (91, 98) preferentially synthesize LTB4 and PGE2 over MaR-1 and resolvin (Rv)-D-2. Also, endothelial (99) and epithelial cells (100) add to the pool of LTB4 and PGE2 production. Upon a switch toward resolution, macrophages (reparative) and neutrophils (possibly second wave neutrophils) also synthesize MaR-1 (75) and RvD-2 (91, 101). However, during chronic inflammation, such as atherosclerosis it is likely that, overall, the inflammatory environment prevails, favoring the synthesis of inflammatory lipid mediators. MaR-1 and RvD-2 act on lesional macrophages, inducing phenotype changes toward a reparative phenotype. These reparative macrophages reduce the inflammatory environment by enhancing secretion of TGF-β and decreasing the concentrations of secreted IL-6 and TNF-α. The resolving milieu aids in reducing macrophage accumulation and smaller necrotic core sizes and instructs smooth muscle cells to produce collagen, resulting in stable thickening of the fibrous cap and increased plaque stability, eventually resulting in a halt of atheroprogression.
MACROPHAGES AND MaRs NEXUS
Recently, two new macrophage–neutrophil interactomes that are formed from MaR-1 when metabolized in macrophages and neutrophils—14-oxo-MaR-1 and 22-OH-MaR-1—were identified, respectively. Incubation of human macrophages with MaR-1 biosynthesize 14-oxo-MaR-1, reaching a maximum level at 4 h, whereas the primary product with human neutrophils was 22-OH-MaR-1, reaching a maximum at 24 h (102) (Fig. 2). Each of these newly identified molecules retained at low concentrations the bioactions of their parent SPMs—that is, MaR-1, with human macrophages. 22-OH-MaR-1 gave a biphasic dose response and up-regulated E. coli phagocytosis by human macrophages. This response is suggestive of the engagement of a high- and a low-affinity receptor by 22-OH-MaR-1 as observed for other proresolving mediators including RvD-1 (103). Also, both MaR-1 and its metabolite 22-OH-MaR-1 act at the human leukotriene-1 receptor and affect LTB4 signaling. These results recognize heretofore unidentified molecules and establish unique functions for the macrophage-derived MaR-1 bioactive metabolome (102, 104).
Macrophages also produce a family of bioactive peptide-conjugated mediators called MaR conjugates in tissue regeneration (MCTRs). These recently identified innovative MCTRs regulate the mechanisms in inflammation resolution, as well as tissue regeneration establishing links between local inflammatory exudates and tissue regeneration via newly identified chemical signals (105). MCTR compounds are produced from the 13(S),14S-epoxide MaR intermediate during the MaR biosynthesis. This epoxide intermediate is then enzymatically converted to MCTRs. MCTR-1 (13R-glutathionyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-DHA) is biosynthesized in the presence of leukotriene C4 synthase and γ-glutamyltransferase-μ4 in human macrophages. γ-Glutamyl transferase aids in the conversion of MCTR-1 to MCTR2 (13R-cysteinylglycinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-DHA), which is then further converted to MCTR-3 (13R-cysteinyl,14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-DHA) by a dipeptidase. The direct emphasis on the MCTR biosynthetic pathway and the enzymes that catalyze these reactions, along with their relation with MaRs in human macrophages are unclear (106). The simplified and overall nexus between the MaR and MCTR metabolome and macrophages is demonstrated in Fig. 2.
MaRs AS THERAPEUTIC TARGETS
The resolution of inflammation is an active process orchestrated by lipid SPMs. Uncontrolled and chronic inflammation facilitates tissue destruction and impedes healing. With the significant roles of MaR-1 in regulating phagocytic functions, tissue regeneration, and wound healing properties, it can be used as a therapeutic agent, as it impedes the rate of inflammation during atherosclerosis by limiting the preferential synthesis of LTB4 by inhibiting the production of PGE2. MaR-1 can also be employed as a therapeutic target as MCTR-1, -2 and -3 (Table 2) conjugates derived from MaR-1 counter both vascular and negative inotropic actions that are stimulated by LTD4, by blocking the MK571 receptor in tissue regeneration (107).
TABLE 2.
Different actions of MCTRs in inflammation-resolution as well as tissue regeneration
| Name of the action | Order of potency between MCTRs |
|---|---|
| Stimulating tissue regeneration | MCTR3 ≈ MCTR2 > MCTR1 |
| Macrophage phagocytic responses | MCTR3 > MCTR1 > MCTR2 |
| Resolution of live bacterial infections | MCTR3 ≥ MCTR2 > MCTR1 |
| Controlling exudate eicosanoids during infections | MCTR1 > > MCTR3 > MCTR2 |
Data from ref. 106.
MaR-1 is effective in controlling inflammation in periodontal infections with unresolved inflammation and leukocyte-mediated tissue destruction (108), and restoring the reparative function of nonhealing diabetic wounds impaired the macrophages (109). MaR-1 has also been tested in the treatment of diabetic nephropathy by inhibiting nucleotide binding and oligomerization domain-like receptor family pyrin domain–containing-3 and controls the inflammation rate in renal failure (110).
FUTURE PROSPECTIVE
What is first in cardiac healing and repair after ischemic injury? Is it inflammation, or is it a resolution of inflammation, or do both overlap in a time-dependent manner to set the stage for healing program of myocardium after MI (44)? In response to injury, infection, or stress, an acute inflammatory response primarily involves early tissue edema within seconds to minutes followed by infiltration of neutrophils within minutes to hours and later mononuclear cells, some of which transition at maturity to macrophages. During host-mediated acute inflammation that resolves and returns the infarcted cardiac tissue to homeostasis by formation of SPMs, such as lipoxins, Rvs, protectins, and MaRs (44). The main hallmark of chronic inflammation (particularly in aging marked with decreased biosynthesis of SPMs) is when acute inflammation fails to resolve, or excessive inflammation leads to bystander tissue injury. However, the defective or delayed resolution also may result in chronic inflammation, which relates to other human diseases. Resolution defects may arise because of one of the following reasons: decreased SPM formation, ineffective LOX, inhibition of SPM sites of action, and perhaps genetic variation or different pharmacologic treatment that interferes with resolution physiology. Identification of the mediators and mechanisms underlying the resolution and regulation of inflammation will provide new insights into the pathobiology of human disease and lead to the development of novel therapeutic approaches to such diseases as atheroprogression characterized by excess or chronic inflammation.
SUMMARY
Several lines of evidence point out that inflammation and resolution of inflammation are primordial and inherent components of the wound-healing mechanism, including that for cardiac ischemic injury. Now, it is time to decode the therapeutic potential of MaR biomolecules involved in the resolution process with the inbuilt endogenously enzymatic process, to amplify the resolution program, identify the factors that contribute to resolution deficit, impair MaR formation in response to injuries such as aging, and test whether restoration of these molecules improves myocardial healing in a translational prospective. Therefore, a detailed study of resolution biology with major emphasis on comprehensive molecular and cellular mechanisms is necessary in acute and chronic heart failure pathology. Furthermore, a precise focus of human macrophage plasticity and function in the steady-state condition or in a setting of injury could guide the development of macrophage-derived molecules in patients with ischemic cardiac diseases. Finally, we should test in the clinical setting whether a single MaR molecule or a combination of specialized proresolving molecules is necessary to accelerate a resolution program without affecting the innate response to control major ischemic disease pathology and to delay heart failure.
ACKNOWLEDGMENTS
Funding was provided by U.S. National Institutes of Health (NIH) National Center for Complementary and Integrative Health Grant AT006704, and NIH National Heart, Lung, and Blood Institute Grant HL132989 (both to G.V.H.); and The University of Alabama at Birmingham Pittman Scholar Award (to G.V.H.). The authors declare no conflicts of interest.
Glossary
- CCR2
C-C chemokine receptor
- CD
cluster of differentiation
- CX3CR
CX3C chemokine receptor
- DHA
docosahexaenoic acid
- HSC
hemapoietic stem cell
- LOX
lipoxygenase
- LT
leukotriene
- MaR
maresin
- MCTR
maresin conjugates in tissue regeneration
- MHC
major histocompatibility complex
- MI
myocardial infarction
- PG
prostaglandin
- PMN
polymorphonuclear neutrophil
- Rv
resolvin
- SPM
specialized proresolving mediator
AUTHOR CONTRIBUTIONS
J. K. Jadapalli wrote the manuscript draft based on the outline and comments on a series of drafts; and G. V. Halade designed the review scheme and edited, revised, and approved the submission to the journal.
REFERENCES
- 1.Clarke S. A., Richardson W. J., Holmes J. W. (2016) Modifying the mechanics of healing infarcts: is better the enemy of good? J. Mol. Cell. Cardiol. 93, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kain V., Prabhu S. D., Halade G. V. (2014) Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res. Cardiol. 109, 444 [DOI] [PubMed] [Google Scholar]
- 3.Weinberger T., Schulz C. (2015) Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front. Physiol. 6, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhu S. D., Frangogiannis N. G. (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halade G. V., Kain V., Ingle K. A. (2018) Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology. Am. J. Physiol. Heart Circ. Physiol. 314, H255–H267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frantz S., Nahrendorf M. (2014) Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc. Res. 102, 240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leor J., Palevski D., Amit U., Konfino T. (2016) Macrophages and regeneration: lessons from the heart. Semin. Cell Dev. Biol. 58, 26–33 [DOI] [PubMed] [Google Scholar]
- 8.Tourki B., Halade G. (2017) Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J. 31, 4226–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horckmans M., Ring L., Duchene J., Santovito D., Schloss M. J., Drechsler M., Weber C., Soehnlein O., Steffens S. (2017) Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 38, 187–197 [DOI] [PubMed] [Google Scholar]
- 10.Lindsey M. L., Saucerman J. J., DeLeon-Pennell K. Y. (2016) Knowledge gaps to understanding cardiac macrophage polarization following myocardial infarction. Biochim. Biophys. Acta 1862, 2288–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Laan A. M., Ter Horst E. N., Delewi R., Begieneman M. P., Krijnen P. A., Hirsch A., Lavaei M., Nahrendorf M., Horrevoets A. J., Niessen H. W., Piek J. J. (2014) Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 35, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halade G. V., Kain V., Ingle K. A., Prabhu S. D. (2017) Interaction of 12/15-lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved postmyocardial infarction healing. Am. J. Physiol. Heart Circ. Physiol. 313, H89–H102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halade G. V., Kain V. (2017) Obesity and cardiometabolic defects in heart failure pathology. Compr. Physiol. 7, 1463–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halade G. V., Kain V., Black L. M., Prabhu S. D., Ingle K. A. (2016) Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany N.Y.) 8, 2611–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halade G. V., Kain V., Serhan C. N. (2018) Immune responsive resolvin D1 programs myocardial infarction-induced cardiorenal syndrome in heart failure. FASEB J. 32, 3717–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S. (2008) Elie Metchnikoff: father of natural immunity. Eur. J. Immunol. 38, 3257–3264 [DOI] [PubMed] [Google Scholar]
- 17.Hulsmans M., Clauss S., Xiao L., Aguirre A. D., King K. R., Hanley A., Hucker W. J., Wülfers E. M., Seemann G., Courties G., Iwamoto Y., Sun Y., Savol A. J., Sager H. B., Lavine K. J., Fishbein G. A., Capen D. E., Da Silva N., Miquerol L., Wakimoto H., Seidman C. E., Seidman J. G., Sadreyev R. I., Naxerova K., Mitchell R. N., Brown D., Libby P., Weissleder R., Swirski F. K., Kohl P., Vinegoni C., Milan D. J., Ellinor P. T., Nahrendorf M. (2017) Macrophages facilitate electrical conduction in the heart. Cell 169, 510–522.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen S. R., Schmid M. C. (2017) Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm. 2017, 9624760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kain V., Halade G. V. (2015) Big eater macrophages dominate inflammation resolution following myocardial infarction. J. Mol. Cell. Cardiol. 87, 225–227 [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 21.Ter Horst E. N., Hakimzadeh N., van der Laan A. M., Krijnen P. A., Niessen H. W., Piek J. J. (2015) Modulators of macrophage polarization influence healing of the infarcted myocardium. Int. J. Mol. Sci. 16, 29583–29591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. (1983) Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158, 670–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackaness G. B. (1962) Cellular resistance to infection. J. Exp. Med. 116, 381–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghassabeh G. H., De Baetselier P., Brys L., Noël W., Van Ginderachter J. A., Meerschaut S., Beschin A., Brombacher F., Raes G. (2006) Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood 108, 575–583 [DOI] [PubMed] [Google Scholar]
- 26.Pinto A. R., Paolicelli R., Salimova E., Gospocic J., Slonimsky E., Bilbao-Cortes D., Godwin J. W., Rosenthal N. A. (2012) An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7, e36814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gentek R., Hoeffel G. (2017) The innate immune response in myocardial infarction, repair, and regeneration. Adv. Exp. Med. Biol. 1003, 251–272 [DOI] [PubMed] [Google Scholar]
- 28.Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J. L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan X., Anzai A., Katsumata Y., Matsuhashi T., Ito K., Endo J., Yamamoto T., Takeshima A., Shinmura K., Shen W., Fukuda K., Sano M. (2013) Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 62, 24–35 [DOI] [PubMed] [Google Scholar]
- 30.Ghosn E. E., Cassado A. A., Govoni G. R., Fukuhara T., Yang Y., Monack D. M., Bortoluci K. R., Almeida S. R., Herzenberg L. A., Herzenberg L. A. (2010) Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl. Acad. Sci. USA 107, 2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadl A., Meher A. K., Sharma P. R., Lee M. Y., Doran A. C., Johnstone S. R., Elliott M. R., Gruber F., Han J., Chen W., Kensler T., Ravichandran K. S., Isakson B. E., Wamhoff B. R., Leitinger N. (2010) Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 107, 737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epelman S., Lavine K. J., Beaudin A. E., Sojka D. K., Carrero J. A., Calderon B., Brija T., Gautier E. L., Ivanov S., Satpathy A. T., Schilling J. D., Schwendener R., Sergin I., Razani B., Forsberg E. C., Yokoyama W. M., Unanue E. R., Colonna M., Randolph G. J., Mann D. L. (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M. B., Leboeuf M., Becker C. D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S. W., Forsberg E. C., Tanaka M., van Rooijen N., García-Sastre A., Stanley E. R., Ginhoux F., Frenette P. S., Merad M. (2013) Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orkin S. H., Zon L. I. (2008) Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samokhvalov I. M. (2014) Deconvoluting the ontogeny of hematopoietic stem cells. Cell. Mol. Life Sci. 71, 957–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S. E., Pollard J. W., Frampton J., Liu K. J., Geissmann F. (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 [DOI] [PubMed] [Google Scholar]
- 37.Yona S., Kim K. W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D. A., Perlman H., Malissen B., Zelzer E., Jung S. (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91; correction 1073–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahrendorf M., Swirski F. K. (2016) Abandoning M1/M2 for a network model of macrophage function. Circ. Res. 119, 414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., Natoli G., Saeij J. P., Schultze J. L., Shirey K. A., Sica A., Suttles J., Udalova I., van Ginderachter J. A., Vogel S. N., Wynn T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molawi K., Wolf Y., Kandalla P. K., Favret J., Hagemeyer N., Frenzel K., Pinto A. R., Klapproth K., Henri S., Malissen B., Rodewald H. R., Rosenthal N. A., Bajenoff M., Prinz M., Jung S., Sieweke M. H. (2014) Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dick S. A., Epelman S. (2016) Chronic heart failure and inflammation: what do we really know? Circ. Res. 119, 159–176 [DOI] [PubMed] [Google Scholar]
- 42.Pinto A. R., Godwin J. W., Chandran A., Hersey L., Ilinykh A., Debuque R., Wang L., Rosenthal N. A. (2014) Age-related changes in tissue macrophages precede cardiac functional impairment. Aging (Albany N.Y.) 6, 399–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halade G. V., Kain V., Ingle K. A., Prabhu S. D. (2017) Interaction of 12/15 lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved post-myocardial infarction healing. Am. J. Physiol. Heart Cir. Physiol. 313, H89–H102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halade G. V., Norris P. C., Kain V., Serhan C. N., Ingle K. A. (2018) Splenic leukocytes define the resolution of inflammation in heart failure. Sci. Signal. 11, eaao1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locati M., Deuschle U., Massardi M. L., Martinez F. O., Sironi M., Sozzani S., Bartfai T., Mantovani A. (2002) Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. J. Immunol. 168, 3557–3562 [DOI] [PubMed] [Google Scholar]
- 46.Perrier P., Martinez F. O., Locati M., Bianchi G., Nebuloni M., Vago G., Bazzoni F., Sozzani S., Allavena P., Mantovani A. (2004) Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J. Immunol. 172, 7031–7042 [DOI] [PubMed] [Google Scholar]
- 47.Scotton C. J., Martinez F. O., Smelt M. J., Sironi M., Locati M., Mantovani A., Sozzani S. (2005) Transcriptional profiling reveals complex regulation of the monocyte IL-1 β system by IL-13. J. Immunol. 174, 834–845 [DOI] [PubMed] [Google Scholar]
- 48.Epelman S., Lavine K. J., Randolph G. J. (2014) Origin and functions of tissue macrophages. Immunity 41, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautier E. L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K. G., Gordonov S., Mazloom A. R., Ma’ayan A., Chua W.-J., Hansen T. H., Turley S. J., Merad M., Randolph G. J.; Immunological Genome Consortium (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haubner B. J., Adamowicz-Brice M., Khadayate S., Tiefenthaler V., Metzler B., Aitman T., Penninger J. M. (2012) Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 4, 966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavine K. J., Epelman S., Uchida K., Weber K. J., Nichols C. G., Schilling J. D., Ornitz D. M., Randolph G. J., Mann D. L. (2014) Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart Proc. Natl. Acad. Sci. USA 111, 16029–16034; correction: 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han C., Nie Y., Lian H., Liu R., He F., Huang H., Hu S. (2015) Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res. 25, 1137–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darehzereshki A., Rubin N., Gamba L., Kim J., Fraser J., Huang Y., Billings J., Mohammadzadeh R., Wood J., Warburton D., Kaartinen V., Lien C. L. (2015) Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev. Biol. 399, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aurora A. B., Porrello E. R., Tan W., Mahmoud A. I., Hill J. A., Bassel-Duby R., Sadek H. A., Olson E. N. (2014) Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahmoud A. I., Porrello E. R., Kimura W., Olson E. N., Sadek H. A. (2014) Surgical models for cardiac regeneration in neonatal mice. Nat. Protoc. 9, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porrello E. R., Mahmoud A. I., Simpson E., Hill J. A., Richardson J. A., Olson E. N., Sadek H. A. (2011) Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konfino T., Landa N., Ben-Mordechai T., Leor J. (2015) The type of injury dictates the mode of repair in neonatal and adult heart. J. Am. Heart Assoc. 4, e001320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poss K. D., Wilson L. G., Keating M. T. (2002) Heart regeneration in zebrafish. Science 298, 2188–2190 [DOI] [PubMed] [Google Scholar]
- 59.Becker R. O., Chapin S., Sherry R. (1974) Regeneration of the ventricular myocardium in amphibians. Nature 248, 145–147 [DOI] [PubMed] [Google Scholar]
- 60.Huang W. C., Yang C. C., Chen I. H., Liu Y. M., Chang S. J., Chuang Y. J. (2013) Treatment of glucocorticoids inhibited early immune responses and impaired cardiac repair in adult zebrafish. PLoS One 8, e66613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jopling C., Sleep E., Raya M., Martí M., Raya A., Izpisúa Belmonte J. C. (2010) Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464, 606–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aurora A. B., Olson E. N. (2014) Immune modulation of stem cells and regeneration. Cell Stem Cell 15, 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Godwin J. W., Debuque R., Salimova E., Rosenthal N. A. (2017) Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen. Med. 2, 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haubner B. J., Schneider J., Schweigmann U., Schuetz T., Dichtl W., Velik-Salchner C., Stein J.-I., Penninger J. M. (2016) Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 118, 216–221 [DOI] [PubMed] [Google Scholar]
- 65.Calderone A. (2017) p38 MAPK and the compromised regenerative response of the infarcted adult heart. Cardiovasc. Regen. Med. 4, e1508 [Google Scholar]
- 66.Fang Y., Gupta V., Karra R., Holdway J. E., Kikuchi K., Poss K. D. (2013) Translational profiling of cardiomyocytes identifies an early Jak1/Stat3 injury response required for zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 110, 13416–13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Meara C. C., Wamstad J. A., Gladstone R. A., Fomovsky G. M., Butty V. L., Shrikumar A., Gannon J. B., Boyer L. A., Lee R. T. (2015) Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circ. Res. 116, 804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bliederhaeuser C., Grozdanov V., Speidel A., Zondler L., Ruf W. P., Bayer H., Kiechle M., Feiler M. S., Freischmidt A., Brenner D., Witting A., Hengerer B., Fändrich M., Ludolph A. C., Weishaupt J. H., Gillardon F., Danzer K. M. (2016) Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol. 131, 379–391 [DOI] [PubMed] [Google Scholar]
- 69.Li S., Sun Y., Liang C. P., Thorp E. B., Han S., Jehle A. W., Saraswathi V., Pridgen B., Kanter J. E., Li R., Welch C. L., Hasty A. H., Bornfeldt K. E., Breslow J. L., Tabas I., Tall A. R. (2009) Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ. Res. 105, 1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oishi Y., Manabe I. (2016) Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2, 16018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prelog M. (2006) Aging of the immune system: a risk factor for autoimmunity? Autoimmun. Rev. 5, 136–139 [DOI] [PubMed] [Google Scholar]
- 72.Linehan E., Fitzgerald D. C. (2015) Ageing and the immune system: focus on macrophages. Eur. J. Microbiol. Immunol. (Bp.) 5, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalli J., Serhan C. (2016) Macrophage proresolving mediators: the when and where. Microbiol. Spectr. 4, MCHD-0001-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serhan C. N., Dalli J., Karamnov S., Choi A., Park C.-K., Xu Z.-Z., Ji R.-R., Zhu M., Petasis N. A. (2012) Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26, 1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serhan C. N., Yang R., Martinod K., Kasuga K., Pillai P. S., Porter T. F., Oh S. F., Spite M. (2009) Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 206, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalli J., Zhu M., Vlasenko N. A., Deng B., Haeggström J. Z., Petasis N. A., Serhan C. N. (2013) The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 27, 2573–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deng B., Wang C. W., Arnardottir H. H., Li Y., Cheng C. Y., Dalli J., Serhan C. N. (2014) Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One 9, e102362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez A. R., Spur B. W. (2015) First total synthesis of the macrophage derived anti-inflammatory and pro-resolving lipid mediator Maresin 2. Tetrahedron Lett. 56, 256–259 [Google Scholar]
- 79.Viola J., Soehnlein O. (2015) Atherosclerosis: a matter of unresolved inflammation. Semin. Immunol. 27, 184–193 [DOI] [PubMed] [Google Scholar]
- 80.Libby P., Tabas I., Fredman G., Fisher E. A. (2014) Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 114, 1867–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merched A. J., Ko K., Gotlinger K. H., Serhan C. N., Chan L. (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fredman G., Hellmann J., Proto J. D., Kuriakose G., Colas R. A., Dorweiler B., Connolly E. S., Solomon R., Jones D. M., Heyer E. J., Spite M., Tabas I. (2016) An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 7, 12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bobryshev Y. V., Ivanova E. A., Chistiakov D. A., Nikiforov N. G., Orekhov A. N. (2016) Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis. BioMed Res. Int. 2016, 9582430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chistiakov D. A., Bobryshev Y. V., Nikiforov N. G., Elizova N. V., Sobenin I. A., Orekhov A. N. (2015) Macrophage phenotypic plasticity in atherosclerosis: the associated features and the peculiarities of the expression of inflammatory genes. Int. J. Cardiol. 184, 436–445 [DOI] [PubMed] [Google Scholar]
- 86.Sager H. B., Kessler T., Schunkert H. (2016) Monocytes and macrophages in cardiac injury and repair. J. Thorac. Dis. 9(Suppl 1), S30–S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leitinger N., Schulman I. G. (2013) Phenotypic polarization of macrophages in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 33, 1120–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cipollone F., Prontera C., Pini B., Marini M., Fazia M., De Cesare D., Iezzi A., Ucchino S., Boccoli G., Saba V., Chiarelli F., Cuccurullo F., Mezzetti A. (2001) Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation 104, 921–927 [DOI] [PubMed] [Google Scholar]
- 89.Gross S., Tilly P., Hentsch D., Vonesch J. L., Fabre J. E. (2007) Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J. Exp. Med. 204, 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. (1987) Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237, 1171–1176 [DOI] [PubMed] [Google Scholar]
- 91.Dalli J., Serhan C. N. (2012) Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsukawa A., Hogaboam C. M., Lukacs N. W., Lincoln P. M., Strieter R. M., Kunkel S. L. (1999) Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J. Immunol. 163, 6148–6154 [PubMed] [Google Scholar]
- 93.Subbarao K., Jala V. R., Mathis S., Suttles J., Zacharias W., Ahamed J., Ali H., Tseng M. T., Haribabu B. (2004) Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler. Thromb. Vasc. Biol. 24, 369–375 [DOI] [PubMed] [Google Scholar]
- 94.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 95.Von Hundelshausen P., Schmitt M. M. N. (2014) Platelets and their chemokines in atherosclerosis-clinical applications. Front. Physiol. 5, 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lämmermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmüller W., Parent C. A., Germain R. N. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He L. K., Liu L. H., Hahn E., Gamelli R. L. (2001) The expression of cyclooxygenase and the production of prostaglandin E2 in neutrophils after burn injury and infection. J. Burn Care Rehabil. 22, 58–64 [DOI] [PubMed] [Google Scholar]
- 98.Tian W., Jiang X., Tamosiuniene R., Sung Y. K., Qian J., Dhillon G., Gera L., Farkas L., Rabinovitch M., Zamanian R. T., Inayathullah M., Fridlib M., Rajadas J., Peters-Golden M., Voelkel N. F., Nicolls M. R. (2013) Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci. Transl. Med. 5, 200ra117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Viola J., Lemnitzer P., Jansen Y., Csaba G., Winter C., Neideck C., Silvestre-Roig C., Dittmar G., Döring Y., Drechsler M., Weber C., Zimmer R., Cenac N., Soehnlein O. (2016) Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Cir. Res. 119, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 100.Brogliato A. R., Antunes C. A., Carvalho R. S., Monteiro A. P., Tinoco R. F., Bozza M. T., Canetti C., Peters-Golden M., Kunkel S. L., Vianna-Jorge R., Benjamim C. F. (2012) Ketoprofen impairs immunosuppression induced by severe sepsis and reveals an important role for prostaglandin E2. Shock 38, 620–629 [DOI] [PubMed] [Google Scholar]
- 101.Sansbury B. E., Spite M. (2016) Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ. Res. 119, 113–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colas R. A., Dalli J., Chiang N., Vlasakov I., Sanger J. M., Riley I. R., Serhan C. N. (2016) Identification and actions of the maresin 1 metabolome in infectious inflammation. J. Immunol. 197, 4444–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Orr S. K., Colas R. A., Dalli J., Chiang N., Serhan C. N. (2015) Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L904–L911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., Serhan C. N. (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178, 3912–3917 [DOI] [PubMed] [Google Scholar]
- 105.Dalli J., Vlasakov I., Riley I. R., Rodriguez A. R., Spur B. W., Petasis N. A., Chiang N., Serhan C. N. (2016) Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA 113, 12232–12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dalli J., Sanger J. M., Rodriguez A. R., Chiang N., Spur B. W., Serhan C. N. (2016) Identification and actions of a novel third maresin conjugate in tissue regeneration: MCTR3. PLoS One 11, e0149319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiang N., Riley I. R., Dalli J., Rodriguez A. R., Spur B. W., Serhan C. N. (2018) New maresin conjugates in tissue regeneration pathway counters leukotriene D4-stimulated vascular responses. [E-pub ahead of print] FASEB J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang C.-W., Colas R. A., Dalli J., Arnardottir H. H., Nguyen D., Hasturk H., Chiang N., Van Dyke T. E., Serhan C. N. (2015) Maresin 1 biosynthesis and proresolving anti-infective functions with human-localized aggressive periodontitis leukocytes. Infect. Immun. 84, 658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong S., Lu Y., Tian H., Alapure B. V., Wang Q., Bunnell B. A., Laborde J. M. (2014) Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem. Biol. 21, 1318–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang S., Gao C., Long Y., Huang W., Chen J., Fan F., Jiang C., Xu Y. (2017) Maresin 1 mitigates high glucose-induced mouse glomerular mesangial cell injury by inhibiting inflammation and fibrosis. Mediators Inflamm. 2017, 2438247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abdulnour R.-E. E., Dalli J., Colby J. K., Krishnamoorthy N., Timmons J. Y., Tan S. H., Colas R. A., Petasis N. A., Serhan C. N., Levy B. D. (2014) Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA 111, 16526–16531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serhan C. N., Chiang N. (2013) Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol. 13, 632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marcon R., Bento A. F., Dutra R. C., Bicca M. A., Leite D. F. P., Calixto J. B. (2013) Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 191, 4288–4298 [DOI] [PubMed] [Google Scholar]
- 114.Dalli J., Consalvo A. P., Ray V., Di Filippo C., D’Amico M., Mehta N., Perretti M. (2013) Proresolving and tissue-protective actions of annexin A1-based cleavage-resistant peptides are mediated by formyl peptide receptor 2/lipoxin A4 receptor. J. Immunol. 190, 6478–6487 [DOI] [PubMed] [Google Scholar]
- 115.Chen L., Liu H., Wang Y., Xia H., Gong J., Li B., Yao S., Shang Y. (2016) Maresin 1 maintains the permeability of lung epithelial cells in vitro and in vivo. Inflammation 39, 1981–1989 [DOI] [PubMed] [Google Scholar]
- 116.Giera M., Ioan-Facsinay A., Toes R., Gao F., Dalli J., Deelder A. M., Serhan C. N., Mayboroda O. A. (2012) Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim. Biophys. Acta 1821, 1415–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buckley C. D., Gilroy D. W., Serhan C. N. (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]