Abstract
Saturated fatty acids (SFAs) have been shown to induce endoplasmic reticulum (ER) stress and chronic inflammatory responses, as well as alter sphingolipid metabolism. Disruptions in ER stress and sphingolipid metabolism have also been implicated in intestinal inflammation. Therefore, to elucidate the roles of SFAs in ER stress and inflammation in intestinal epithelial cells, we examined myristate (C14:0) and palmitate (C16:0). Myristate, but not palmitate, induced ER stress signaling, including activation of inositol-requiring enzyme 1 (IRE1) and X-box binding protein 1 (XBP1) signaling. Myristate significantly increased C14-ceramide levels, whereas palmitate increased several long-chain ceramides. To define the role of ceramide synthases (CerSs) in myristate-induced ER stress, we used the pharmacologic inhibitor, fumonisin B1 (FB1), and small interfering RNA (siRNA) for CerS5 and 6, the primary isoforms that are involved in C14-ceramide generation. FB1 and siRNA for CerS5 or 6 suppressed myristate-induced C14-ceramide generation and XBP1 splicing (XBP1s). Moreover, increased XBP1s induced the downstream expression of IL-6 in a CerS5/6-dependent manner. In addition, a myristate-enriched milk fat–based diet, but not a lard-based diet, increased C14-ceramide, XBP1s, and IL-6 expression in vivo. Taken together, our data suggest that myristate modulates ER stress and cytokine production in the intestinal epithelium via CerS5/6 and C14-ceramide generation.—Choi, S., Snider, J. M., Olakkengil, N., Lambert, J. M., Anderson, A. K., Ross-Evans, J. S., Cowart, L. A., Snider, A. J. Myristate-induced endoplasmic reticulum stress requires ceramide synthases 5/6 and generation of C14-ceramide in intestinal epithelial cells.
Keywords: ER stress, IL-6, XBP1
Endoplasmic reticulum (ER) stress and the unfolded protein response are evolutionarily conserved signaling mechanisms by which to overcome stress in the ER, which results in a pause for new protein synthesis and the induction of chaperone proteins to restore normal ER function. The unfolded protein response has 3 major signaling branches: activating transcription factor 6 (ATF6), pancreatic ER kinase-like ER kinase (PERK), and inositol requiring enzyme 1 (IRE1). ATF6 is activated by cleavage at the Golgi apparatus. PERK autophosphorylation results in the phosphorylation of its substrate, eukaryotic translation initiation factor 2α (eIF2α), and increased expression of ATF4 (1–3). Phosphorylation of IRE1 triggers its endonuclease activity, which results in alternative splicing of X-box binding protein 1 (XBP1) that then acts as a potent transcription factor [XBP1 splicing (XBP1s)] (4). Initially, ER stress acts as an adaptation to cellular stress; however, when stress exceeds the adaptive capacity of cells, the induction of C/EBP homologous protein (CHOP) expression and caspase-3 activity lead to apoptosis (5, 6). ER stress can be initiated by several triggers, including disruption of cellular redox regulation, and, recently, high-fat diet (HFD) and saturated fatty acids (SFAs), specifically C16:0 palmitate, have emerged as potent inducers of ER stress (7–10).
Sphingolipids, long thought to be only structural components of cell membranes, have emerged over the last 2 decades as bioactive lipids with distinct and important biologic functions (11). Ceramide, the central lipid in sphingolipid metabolism, can be generated either de novo via serine palmitoyltransferase (SPT) followed by the action of ceramide synthases (CerSs), or in the salvage/recycling pathway that also involves the action of CerS, but not SPT. SPT condenses serine (glycine/alanine) and palmitoyl-CoA (myristoyl-CoA/stearoyl-CoA) to generate the sphingoid backbone of sphingolipids. SPT actively functions as a complex with 3 subunits, SPTLC1-SPTLC2, SPTLC3-SPTSSA, or SPTSSB. SPTLC1 anchors the enzyme to the membrane, SPTSSA or SPTSSB stimulate the activity of SPT, and SPTLC2 or SPTLC3 determine substrate preference (12, 13). Whereas isozyme with SPTLC2 preferentially uses C16:0 palmitoyl-CoA, which generates sphingolipids with d18:0 and d18:1 sphingoid backbones (canonical dihydrosphingosine and sphingosine, respectively), it has recently been demonstrated that SPTLC3 can use fatty acids (FAs) other than palmitate, such as myristate (C14:0), which results in the generation of sphingolipids with d16:0/d16:1 backbones (14). Furthermore, it has been reported that sphingolipids with the d16:0/d16:1 backbone, such as d16:0-dihydrosphingosine (dhSph), elicit potent effects, including the induction of cell death in cardiomyocytes (15). In addition to differences in the sphingoid backbone, ceramides can also vary in their acyl-chain length. CerS, of which there are 6 isoforms, preferentially use specific FAs to generate ceramide. For example, CerS5 and CerS6 preferentially use C14- or C16-SFAs as their substrates (16). Both SPT and CerS use fatty acyl-CoAs as their substrates; thus, oversupply of dietary SFA has been shown to promote the elevation of endogenous ceramide levels [reviewed in Choi and Snider (17)].
As a bioactive lipid, ceramide mediates several cell-stress responses, which suggests that dysregulated ceramide levels disrupt normal cellular functions. In addition, direct exposure to short-chain ceramides, such as C2- or C6-ceramide, was shown to elicit significant ER stress with increased alternative splicing of XBP1 and phosphorylation of eIF2α (18, 19). Chemotherapy-induced long-chain ceramide (e.g., C14- and C16-ceramide) has also been suggested as a possible modulator of PERK signaling (20); however, the role of elevated long-chain ceramide in SFA-induced ER stress remains unclear.
Our laboratory has previously demonstrated that sphingolipids and their metabolizing enzymes play roles in the intestinal inflammation. These studies suggested roles for sphingosine kinase 1 (21) and 3 ceramidases—neutral (22), alkaline (23), and acid ceramidase (24)—which suggests a critical role for sphingolipids in the regulation of inflammatory response in the intestinal epithelium. Moreover, ER stress is a critical mechanism in mediating inflammation and has been implicated in animal models of inflammatory bowel disease (IBD) (25) and clinical studies (26). In addition, it has been established that XBP1 directly binds to the promoter of several proinflammatory cytokine genes, such as TNF-α and IL-6 (27), which suggests that ER stress–induced XBP1 may contribute to intestinal inflammation via regulation of cytokine expression. Together, these studies led us to question whether HFD or SFA alter ER stress, sphingolipid metabolism, and inflammation in the intestine.
In the current study, we demonstrate the effects of SFAs on ER stress and the subsequent inflammatory responses in intestinal epithelial cells (IECs). Of interest, palmitate, which is known to induce ER stress in metabolic cells and tissues, such as liver, pancreas, and skeletal muscle, did not cause ER stress in IECs. Instead, C14:0 myristate significantly induced ER stress and IL-6 expression. We further provide evidence that the myristate-induced generation of C14-ceramide was indeed required for XBP splicing and IL-6 generation. Moreover, mice that were fed a milk fat–based diet (MFBD), which is high in myristate, exhibited increased ER stress and expression of XBP1s target genes in the small intestine. Together, this study suggests that myristate modulates ER stress responses and subsequent proinflammatory cytokine expression via CerS5/6 and C14-ceramide generation in IECs. We discuss the implications of these results in diet and the intestinal inflammation.
MATERIALS AND METHODS
Reagents and materials
DMEM, penicillin-streptomycin, Lipofectamine, RNAiMax, PureLink RNA Mini Kit, Pierce ECL Substrate, and Pierce BCA Protein Assay Kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Fetal bovine serum was from GE Healthcare Life Sciences (Logan, UT, USA). Anti–phospho-eIF2α and anti–glyceraldehyde 3-phosphate dehydrogenase were from Cell Signaling Technology (Danvers, MA, USA). Anti–phospho-IRE1α was purchased from Novus Biologicals (Littleton, CO, USA). Anti-IRE1α and horseradish peroxidase–labeled secondary Abs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). qScript cDNA SuperMix was from Quantabio (Beverly, MA, USA). iTAQ master mix was purchased from Bio-Rad (Hercules, CA, USA). FA-free, low-endotoxin bovine serum albumin (BSA), myristate, palmitate, and myriocin were from MilliporeSigma (Burlington, MA, USA). Fumonisin B1 (FB1) was from Enzo Life Sciences (Farmingdale, NY, USA). Tunicamycin was purchased from Tocris Bioscience (Bristol, United Kingdom). d,l-Threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) was from Cayman Chemical (Ann Arbor, MI, USA).
Animals and diets
In all studies, C57BL/6 male and female mice (The Jackson Laboratory, Bar Harbor, ME, USA) were given water and chow ad libitum. Lard-based diet (LBD; 60% of calories from fat, TD. 06414), MFBD (60% of calories from fat, TD.09766), and an isocaloric low-fat diet (16.8% of calories from fat, TD.08810) were purchased from Envigo (Indianapolis, IN, USA). For HFD studies, six 8-wk-old male mice were placed in each group and maintained on diets for a total of 12 wk. At 12 wk, small intestines were isolated, snap frozen, and homogenized for analysis. All animal procedures were approved by the Ralph H. Johnson VA Medical Center and Medical University of South Carolina Institutional Animal Care and Use Committees, and all studies conducted were based on U.S. National Institutes of Health (NIH; Bethesda, MD, USA) and the American Veterinary Medical Association guidelines.
Cell culture
IEC6 rat intestinal epithelial cells were originally purchased from American Type Culture Collection (Manassas, VA, USA). DMEM was supplemented with 0.1 U/ml bovine insulin (MilliporeSigma) and 10% (v/v) fetal bovine serum. Cells were kept in a humidified incubator at 37°C with 5% CO2. Cells were seeded and treated as needed 24 h after plating. Cells were grown to a final confluence of ∼80%. Before SFA treatment, cells were serum starved for 8 h. Pretreatments with pharmacologic inhibitors were carried out 1 h before the addition of SFAs unless otherwise indicated.
SFA treatment
SFAs were prepared as described by Ross et al. (28). In brief, SFAs were dissolved in 100% ethanol to a concentration of 100 mM, portioned into aliquots, and dried under nitrogen. Before cell treatment, SFAs were reconstituted to designated concentrations using DMEM that was supplemented with 2% FA-free, low-endotoxin BSA. SFAs were conjugated to BSA via brief sonication, with incubation at 55°C for 15 min, and were cooled to 37°C. Before SFA treatment, cells were serum starved for 8 h, then the media were changed to media containing SFAs conjugated to BSA for the indicated times.
Small interfering RNA
Twenty-four hours after plating, cells were transfected with small interfering RNA (siRNA) using Lipofectamine RNAiMax according to the manufacturer’s protocol. Forty-eight hours post-transfection, media were changed and cells were treated as indicated. Silencer Select siRNAs (Thermo Fisher Scientific) used in this study were as follows: siIRE1α (s176364), siCerS2 (s160555), siCerS5 (s173034), and siCerS6 (s172504). AllStar Negative Control siRNA (Qiagen, Hilden, Germany) was used as a negative control.
Immunoblot analysis
Cells were washed with ice-cold PBS, then directly lysed in ice-cold RIPA buffer that contained protease inhibitor cocktail and phosphatase inhibitor cocktail (MilliporeSigma). Equal amounts of protein (20 μg) were boiled in NuPAGE LDS sample buffer (Thermo Fisher Scientific) and separated by SDS-PAGE (4–15%, Tris-HCl) using the Bio-Rad Criterion system. Separated proteins were then transferred onto nitrocellulose membranes (Bio-Rad) and blocked with 5% nonfat milk in PBS-0.05% Tween-20 for at 1 h at room temperature. Primary Abs (diluted 1:1000 or 1:5000) for glyceraldehyde 3-phosphate dehydrogenase were then added to membranes and incubated at 4°C overnight. Membranes were washed 3 times with PBS-0.05% Tween-20, then incubated with horseradish peroxidase–conjugated secondary Abs (diluted 1:5000) for 1 h at room temperature. Membranes were washed 3 times with PBS-0.05% Tween-20, then incubated with Pierce ECL Substrate and exposed to X-ray films (Thermo Fisher Scientific) that were then processed and scanned.
RNA extraction and real-time quantitative RT-PCR
RNA extraction was performed using the PureLink RNA Mini Kit according to the manufacturer’s protocol. One microgram of RNA was used for cDNA synthesis using the qScript cDNA SuperMix according to the manufacturer’s protocol.
Real-time RT-PCR was carried out using the Applied Biosystems 7500 Real-Time PCR System (Foster City, CA, USA). The following TaqMan probes (Thermo Fisher Scientific) were used: rat CHOP (Rn00492098_g1), rat ERdj4 (Rn01473728_m1), rat IL-6 (Rn01410330_m1), rat CerS5 (Rn01532865_m1), rat CerS6 (Rn01747117_m1), rat CerS2 (Rn01762797_g1), mouse ERdj4 (Mm01622956_s1), mouse IL-6 (Mm00446190_m1), and rat β-actin (Rn00667869_m1) and mouse β-actin (ID: Mm02619580_g1) as housekeeping genes. Cycle threshold (Ct) values were obtained for each gene of interest and β-actin. ΔCt values were calculated, and we calculated the relative gene expression normalized to control samples from ΔΔCt values.
XBP1 RT-PCR splicing analysis
ER stress–induced processing of XBP1 mRNA was evaluated by RT-PCR and restriction site analysis (29). The 601-bp RT-PCR product—encompassing the IRE1 cleavage site of XBP1—was amplified using the following XBP1 primers: forward, 5′-AAA CAG AGT AGC GCA GAC TGC-3′ and reverse, 5′-GGA TCT CTA AAA CTA GAG GCT TGG TG-3′. The thermal cycling profile consisted of 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. As only unspliced XBP1 has a restriction site for PstI, RT-PCR products were incubated with PstI at 37°C for 5 h, followed by separation on 2% agarose gels. Optical density (OD) of bands representing XBP1s and XBP1u were measured by ImageJ program (NIH).
Mass spectrometry to measure sphingolipid levels
For lipid extraction, cells were washed with ice-cold PBS, then directly lysed with 2 ml of cell extraction mixture (2:3 70% isopropanol/ethyl acetate) followed by gentle scraping of the cell from the culture plate. Small intestine tissues were directly homogenized in a cell extraction mixture using FastPrep-24 from MP Biomedicals (Santa Ana, CA, USA). Lysate and homogenate were then transferred to 15-ml Falcon tubes. Lipid extracts were analyzed by the Lipidomics Core Facility at Stony Brook University Medical Center. Data were normalized by total inorganic phosphate present in the sample (30) after a Bligh and Dyer extraction (31). Sphingolipid levels were expressed as picomoles per nanomole of inorganic phosphate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Data are presented as means ± sem and analyzed by 2-tailed, unpaired Student’s t test or 2-way ANOVA with a Bonferroni posttest to correct for multiple comparisons, as appropriate. Values of P < 0.05 were considered statistically significant.
RESULTS
Myristate induces ER stress in intestinal epithelial cells
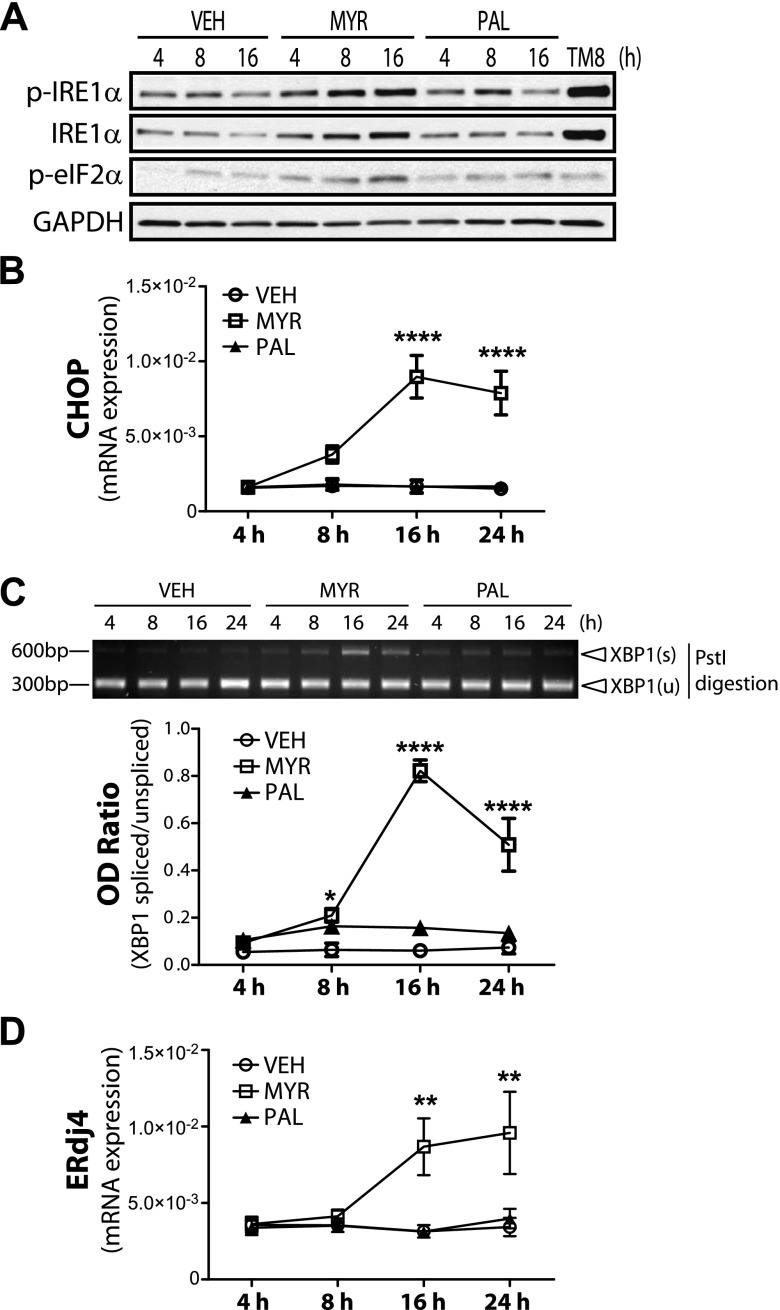
Diets that are high in SFAs have been implicated in many detrimental effects, including ER stress, in several metabolic tissues, such as liver, skeletal muscle, and adipose tissue (7, 9, 32, 33). In the current study, we set out to demonstrate the effect of different SFAs on ER stress in IECs. To this end, we examined the effects of 2 common dietary FAs, myristate (C14:0) and palmitate (C16:0), in IEC6 cells. Cells were stimulated with either vehicle (2% FA-free, low-endotoxin BSA), 0.6 mM myristate, or palmitate for up to 24 h. Of interest, only myristate elevated ER stress markers, including the expression and phosphorylation of IRE1α, as well as phosphorylation of eIF2α, with increased expression of its downstream target gene, CHOP (Fig. 1A, B). To assess signals downstream of IRE1α, we examined XBP1s upon SFA treatment (Fig. 1C). Levels of XBP1s were determined as described in Materials and Methods (29). XBP1s increased after 8 h treatment with myristate, with maximal response at 16 h (Fig. 1C). Expression of ERdj4, a known target gene for XBP1s, was also significantly elevated after 16 h of treatment with myristate (Fig. 1D). To ensure that the FA-free BSA did not trigger ER stress in IECs, we compared vehicle-treated cells with nontreated cells. FA-free BSA did not alter the expression of CHOP or ERdj4 expression (Supplemental Fig. 1) compared with nontreated cells. These results suggest that myristate, but not palmitate, is a potent inducer of ER stress in IECs.
Figure 1.
Myristate induces ER stress in intestinal epithelial cells. IEC6 cells were treated with 0.6 mM myristate (Myr), palmitate (Pal), vehicle (Veh; 2% BSA), or 5 μg/ml tunicamycin (TM) for the indicated times. A) Western blot analysis was performed to assess relative phosphorylation of IRE1α and eIF2α. B, D) mRNA levels of ER stress marker CHOP (B) and chaperone protein ERdj4 (D) were analyzed by quantitative RT-PCR and normalized to β-actin. C) XBP1s was analyzed using RT-PCR and PstI digestion (upper panel) and quantified as OD ratio using ImageJ; lower panel). Data represent means ± sem, n = 3. *P < 0.05, **P < 0.01, ****P < 0.0001 compared with Veh.
Myristate significantly increases C14-ceramide generation
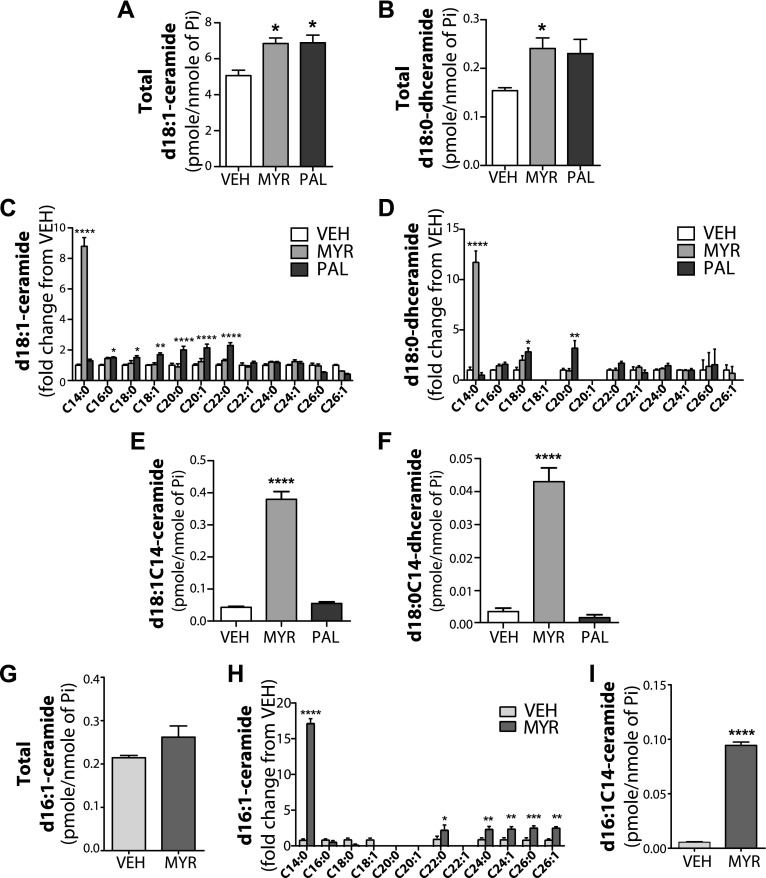
Previous studies have shown that HFD administration and/or SFA treatment altered sphingolipid metabolism, which resulted in the accumulation of ceramide in various tissues and cell types [reviewed in Choi and Snider (17)]. Ceramide has been also implicated in lipotoxicity, including ER stress (18, 19). Moreover, FA incorporation into either the sphingoid backbone vs. the N-acyl-chain of ceramide may have different capacities to induce ER stress (15). To assess the effect of SFAs on sphingolipid metabolism in IECs, we measured d18:1- and d16:1-ceramide and d18:0-dihydroceramide levels upon SFA treatment. Both myristate and palmitate increased levels of total d18:1-ceramide and d18:0-dihydroceramide to a similar extent, which suggests that these SFAs are similarly incorporated into sphingolipids in these cells (Fig. 2A, B). Palmitate treatment increased several species of d18:1-ceramides, including d18:1C16-ceramide and longer-chain d18:1 ceramides, up to a fatty acyl length of C22 (Fig. 2C). Palmitate also increased several species of d18:0-dihyrdoceramides (Fig. 2D); however, upon myristate treatment, only d18:1C14-ceramide and d18:0C14-dihydroceramide were significantly elevated (Fig. 2C, D). Figure 2E, F shows the mass levels of these ceramides. Investigating the effects of FAs on the formation of ceramides that contained shorter sphingoid bases (d16:0 and d16:1), results demonstrate that myristate treatment modestly increased total d16:1-ceramide, but dramatically increased d16:1C14-ceramide in IEC6 cells (Fig. 2G, H). Figure 2I shows the mass levels of d16:1C14-ceramide after myristate. These data demonstrate the selective effects of myristate on d16:1C14-, d18:0C14-, and d18:1C14-ceramides and therefore suggest that the generation of C14-ceramide/dihydroceramide, but not C16, may contribute to myristate-induced ER stress.
Figure 2.
Myristate induces the generation of C-14 ceramide in intestinal epithelial cells. IEC6 cells were treated with 0.6 mM myristate (Myr), palmitate (Pal), vehicle (Veh) for 16 h. Sphingolipids were analyzed by electrospray ionization tandem mass spectrometry in the Stony Brook University Lipidomics Shared Resource Core and normalized to total lipid phosphate (Pi). A–I) Total d18:1-ceramide (A), total d18:0-dihydroceramide (B), individual species of d18:1-ceramide (C), individual species of d18:0-dihydroceramide (D), d18:1C14-ceramide (E), d18:0C14-dihydroceramide (F), total d16:1-ceramide (G), individual species of d16:1-ceramide (H), and d16:1C14-ceramide (I). Data represent means or fold change ± sem, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with Veh.
CerS inhibition suppresses myristate-induced d18:1C14-ceramide and XBP1s
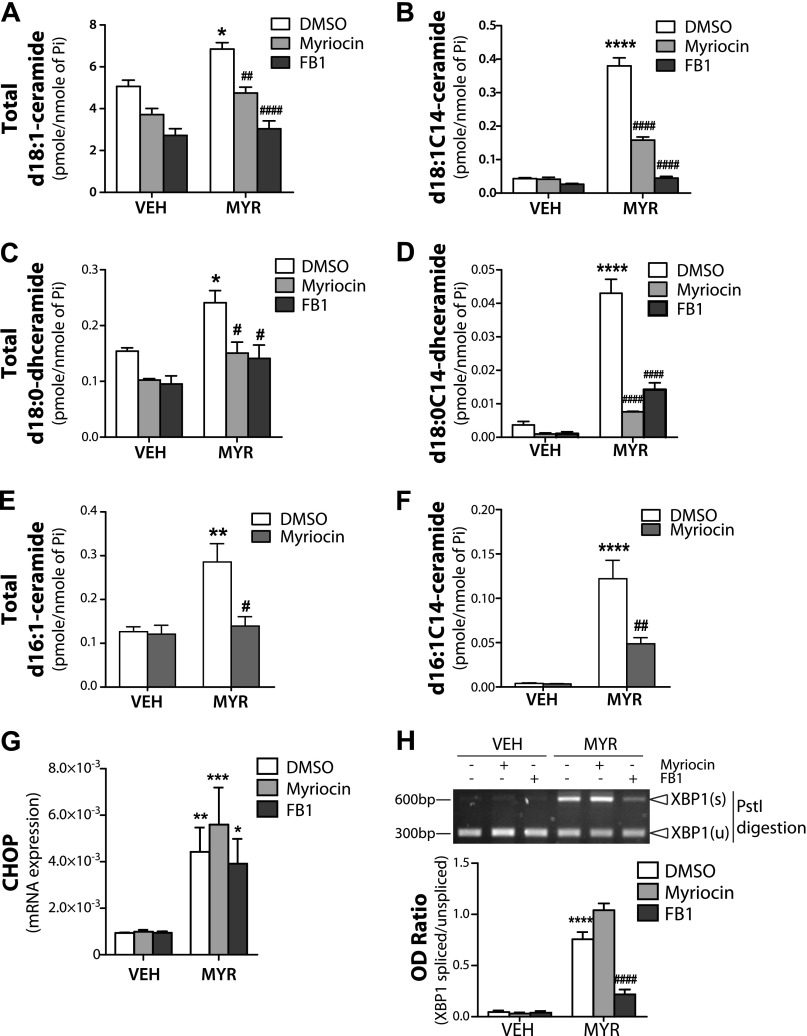
Myristate specifically augmented C14-ceramide/dihydroceramide generation; therefore, we next set out to determine whether de novo ceramide or CerS-generated ceramide were required for myristate-induced ER stress. To this end, we used pharmacologic inhibitors for SPT and CerS (myriocin and FB1, respectively). Both myriocin and FB1 significantly reduced total d18:1-ceramide levels, even in vehicle-treated cells, with FB1 exerting a more pronounced effect than myriocin (Fig. 3A). Upon analysis of d18:1-ceramide species, myriocin significantly decreased myristate-induced d18:1C14-ceramide, whereas FB1 almost completely abolished myristate-induced d18:1C14-ceramide generation (Fig. 3B). In addition to d18:1-ceramide, both inhibitors suppressed myristate-induced total d18:0-dihydroceramide and d18:0C14-dihydroceramide (Fig. 3C, D). SPT synthesis of d16:1/d16:0-sphingoid backbone requires myristate; thus, myristate-induced total d16:1-ceramide and d16:1C14-ceramide were notably reduced by myriocin treatment (Fig. 3E, F).
Figure 3.
CerS inhibition suppresses myristate-induced C14:0-ceramide generation and XBP1s. IEC6 cells were pretreated with 100 nM myriocin (Myr; SPT inhibitor) or 50 mM FB1 (CerS inhibitor) for 1 h, then treated with 0.6 mM MYR or vehicle (Veh) for 16 h. A–F) Total d18:1-ceramide (A) and d18:1C14-ceramide (B), total d18:0-dihydroceramide (C), d18:0C14-dihydroceramide (D), total d16:1-ceramide (E), and d16:1C14-ceramide (F) levels were determined by electrospray ionization tandem mass spectrometry in the Stony Brook University Lipidomics Shared Resource Core and normalized to total lipid phosphate (Pi). G) mRNA levels of CHOP were analyzed by quantitative RT-PCR and normalized to β-actin. H) XBP1s was analyzed using RT-PCR and PstI digestion (upper panel) and quantified as OD ratio using ImageJ (lower panel). Data represent means ± sem, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with Veh; #P < 0.05, ##P < 0.01, ####P < 0.0001 compared with DMSO Myr treatment.
To determine a role for the sphingoid backbone in myristate-induced ER stress, we examined the ER stress markers, CHOP and XBPs, in response to FB1 and myriocin. Neither FB1, nor myriocin suppressed myristate-induced CHOP expression (Fig. 3G); however, FB1 significantly inhibited XBP1s upon myristate treatment (Fig. 3H). These data demonstrate the selective effects of FB1 vs. myriocin on the ER stress response to myristate, which suggests the preferential involvement of the recycling pathway (inhibited by FB1, but not myriocin) vs. the de novo pathway (inhibited by both). Coupled with the ceramide analysis, these results also suggest that d18:1C14-ceramide or d18:0C14-dihydroceramide, generated by CerS, may play key roles in myristate-induced XBP1 regulation.
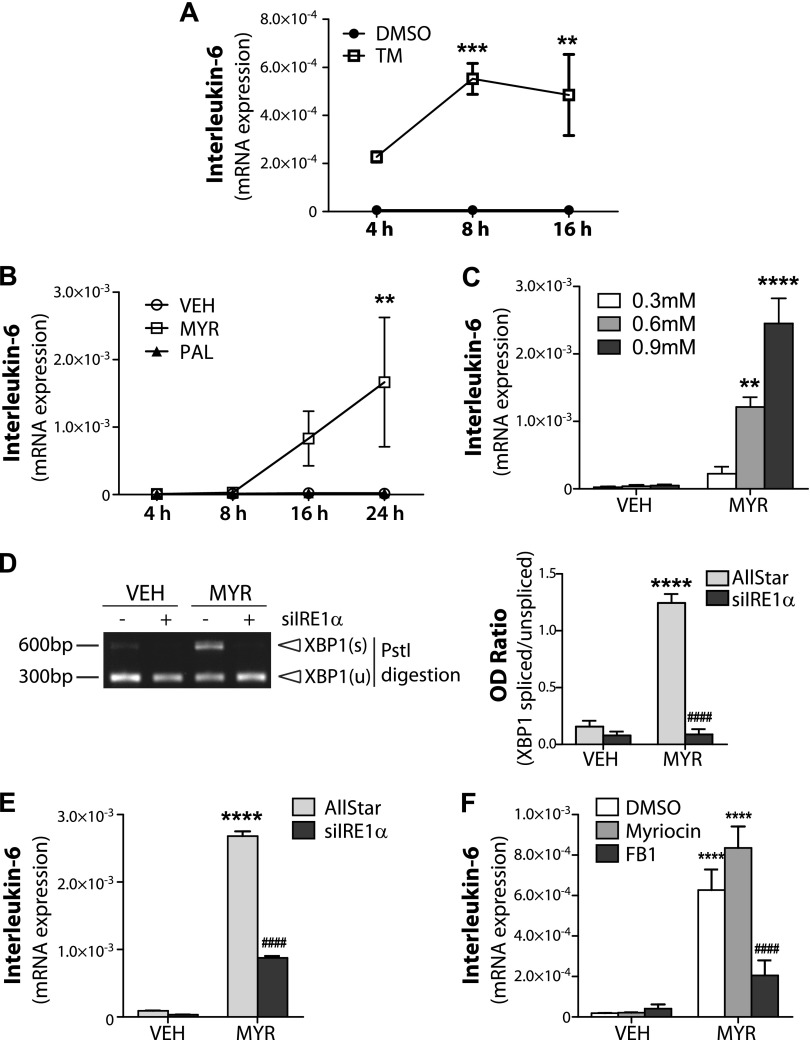
XBP1 induces IL-6 expression in response to myristate
As XBP1s functions as a transcription factor and has been demonstrated to promote transcription of IL-6 in macrophages (27), we hypothesized that XBP1 may regulate IL-6 in a similar manner in IECs. We first examined the effects of ER stress in IL-6 induction in IECs using the known ER stress inducer, tunicamycin. Indeed, tunicamycin robustly increased IL-6 mRNA expression as early as 4 h post-treatment, and this became significant between 8 and 16 h (Fig. 4A). Next, myristate induction of IL-6 was determined to be both time (Fig. 4B) and dose dependent (Fig. 4C). To determine that XBP1 is required for the induction of IL-6 in response of myristate, we used siRNA for IRE1α, the upstream regulator of XBP1. IRE1α knockdown abolished XBP1s (Fig. 4D) and significantly suppressed myristate-induced IL-6 expression (Fig. 4E). As FB1 decreased myristate-induced XBP1s, we examined the effect of FB1 on myristate-induced IL-6 expression. Pretreatment with FB1, but not myriocin, inhibited myristate-induced IL-6 expression (Fig. 4F). Together, these data demonstrate that myristate-induced ER stress increases IL-6 expression and requires CerS activity and XBP1s.
Figure 4.
Myristate (Myr) -induced ER stress induces IL-6 expression in IEC6. IEC6 cells were treated with 5 μg/ml tunicamycin (TM) at indicated times (A); treated with 0.6 mM Myr or vehicle (Veh) for indicated times (B); treated with indicated concentrations of MYR for 16 h (C); transfected with 10 nM AllStar negative control siRNA (D) or IRE1α siRNA (E), or pretreated 100 nM myriocin or 50 mM FB1 for 1 h (F), then treated with 0.6 mM Myr or Veh for 16 h. mRNA expression levels of IL-6 were analyzed by quantitative RT-PCR and normalized to β-actin (A–C, E, F). D) XBP1s was analyzed using RT-PCR and PstI digestion (left panel) and quantified as OD ratio using ImageJ (right panel). Data represent means ± sem, n ≥ 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with Veh; ####P < 0.0001 compared with AllStar/DMSO Myr treatment.
CerS5/6 are required for myristate-induced XBP1s and IL-6 expression
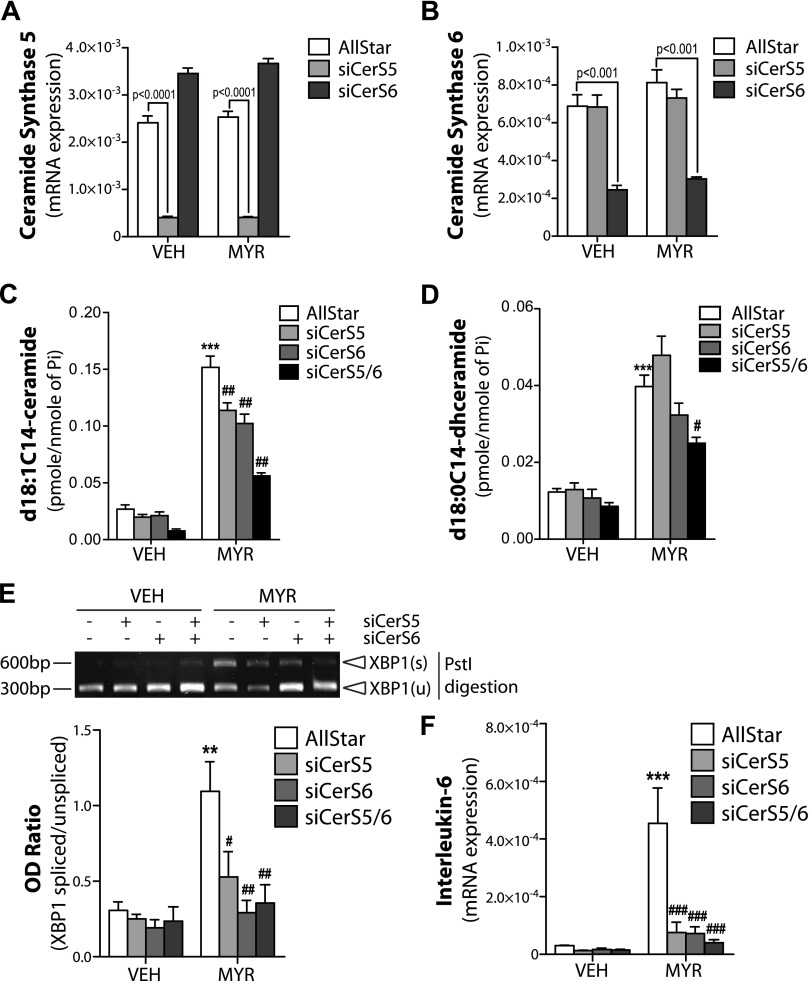
Among the 6 isoforms of CerS, CerS5 and 6 are known to generate C14-ceramide. To specify which isoform is involved in myristate-induced ER stress, we used siRNA against CerS5 or CerS6. We first validated that each siRNA knocked down their respective target without altering the expression of the other CerS isoform (Fig. 5A, B). We next determined C14-ceramide/dihydroceramide level in cells with CerS 5 or 6 siRNA. Both CerS5 and CerS6 siRNA significantly, but only partly, decreased d18:1C14-ceramide levels; however, d18:0C14-dihydroceramide levels were not significantly altered (Fig. 5C, D). When both siRNAs were used in combination, a significant decrease in the levels of both d18:1C14-ceramide and d18:0C14-dihydroceramide was detected (Fig. 5C, D).
Figure 5.
Myristate (Myr) induces ER stress and IL-6 expression in a CerS5/6-dependent manner. IEC6 cells were transfected with 10 nM AllStar negative control siRNA or siRNA targeted against CerS5 and/or CerS6 and treated with 0.6 mM MYR or vehicle (Veh) for 16 h. A, B) mRNA of CerS 5 (A) or 6 (B) were normalized to β-actin. C, D) d18:1C14-ceramide (C) and d18:0C14-dihydroceramide (D) levels were determined by electrospray ionization tandem mass spectrometry in the Stony Brook University Lipidomics Shared Resource Core and normalized to total lipid phosphate (Pi). E) XBP1s was analyzed using RT-PCR and PstI digestion (upper panel) and quantified as OD ratio using ImageJ (lower panel). F) mRNA expression of IL-6 were analyzed by quantitative RT-PCR and normalized to β-actin. Data represent means ± sem, n = 3. **P < 0.01, ***P < 0.001 compared with Veh; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with AllStar Myr treatment.
To define the role for CerS5 and 6 in myristate-induced XBP1s, we analyzed XBP1s after knock down of CerS5 and CerS6 with their respective siRNAs. Indeed, both CerS5 siRNA and CerS6 siRNA reduced myristate-induced XBP1s, with the double knockdown completely ablating XBPs (Fig. 5E). In addition, myristate-induced IL-6 levels were also significantly suppressed in cells with CerS5 or CerS6 siRNA (Fig. 5F).
It has been demonstrated that knockdown of CerS2, which generates very long-chain ceramides, induces the generation of long-chain ceramides; therefore, we examined the effects of CerS2 siRNA on d18:1C14-ceramide levels and the induction of ER stress. Myristate-induced ERdj4 and IL-6 expression was augmented in CerS2 siRNA cells (Supplemental Fig. 2A, B). Total d18:1 ceramide levels and d18:1C24:1-ceramide, the known product of CerS2, were significantly reduced by CerS2 siRNA (Supplemental Fig. 2C, D). Of interest, CerS2 siRNA altered the basal level of d18:1C16-ceramide, but not d18:1C14-ceramide; however, CerS2 siRNA augmented myristate-induced increases in d18:1C14-ceramide levels, but not d18:1C16-ceramide (Supplemental Fig. 2E, F). This supported our hypothesis that d18:1C14-ceramide is critical to myristate-induced pathobiology. Together, these data establish that CerS5 and CerS6 and the generation of d18:C14-ceramide are necessary for myristate-induced ER stress, XBP1s, and IL-6 expression.
Myristate-induced ER stress predominantly involves d18:C14-ceramide
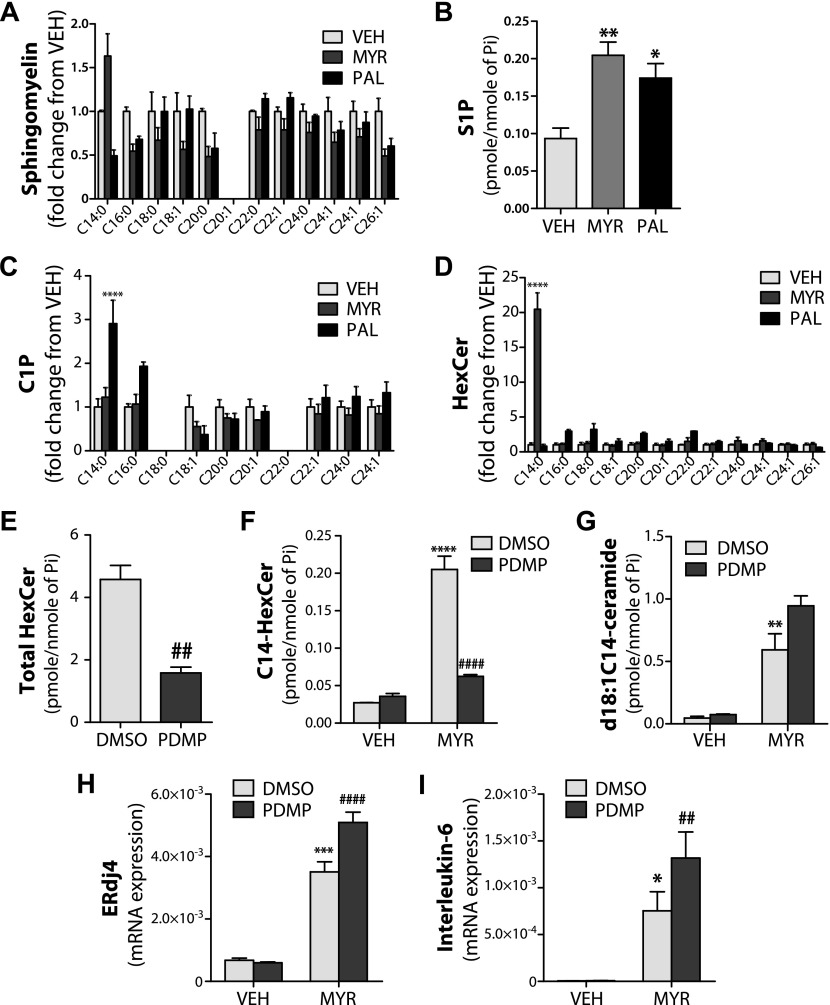
Ceramide can be used to generate numerous other sphingolipid species, including sphingomyelin (SM), hexosylceramide (HexCer), ceramide 1-phosphate (C1P), and sphingosine 1-phosphate (S1P); therefore, in addition to ceramide, we measured other sphingolipid species that could be generated from ceramide and potentially induce ER stress. C14 SM was elevated by myristate treatment; however, this did not reach statistical significance (Fig. 6A). S1P levels were elevated by both myristate and palmitate (Fig. 6B), which suggests that S1P is unlikely to be the lipid involved in myristate-induced ER stress. C1P, specifically C14 and C16-C1P, increased only with palmitate treatment (Fig. 6C). Of interest, myristate selectively increased C14-HexCer, which suggests the potential involvement of glucosylceramide species (Fig. 6D). To determine the role of HexCer in myristate-induced ER stress, we used the pharmacologic inhibitor of glucosylceramide synthase, PDMP. PDMP suppressed the basal generation of HexCer (Fig. 6E). Moreover, PDMP decreased myristate-induced C14-HexCer (Fig. 6F) and increased d18:1C14-ceramide, although this did not reach statistical significance (Fig. 6G). In addition, PDMP augmented the myristate-induced expression of XBP1s target genes, ERdj4 and IL-6 (Fig. 6H, I). These data suggest that d18:1C14-ceramide, and not SM, S1P, C1P, or HexCer, is the predominant lipid involved in myristate-induced ER stress.
Figure 6.
SFAs alter sphingolipid metabolism in intestinal epithelial cells. IEC6 cells were treated with 0.6 mM myristate (Myr), palmitate (PAL), vehicle (Veh) for 16 h. A–D) Individual species of sphingomyelin (A), S1P (B), C1P (C), and HexCer (D) were analyzed by electrospray ionization tandem mass spectrometry in the Stony Brook University Lipidomics Shared Resource Core and normalized to total lipid phosphate (Pi). E–I) IEC6 cells were pretreated 25 µM PDMP for 1 h, then treated with 0.6 mM Myr or Veh for 16 h. Total HexCer (E), C14-HexCer (F), and d18:1C14-ceramide (G) levels were analyzed and normalized to total lipid Pi. mRNA of ERdj4 (H) or IL-6 (I) were analyzed by quantitative RT-PCR and normalized to β-actin. Data represent means ± sem, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with Veh; ##P < 0.01, ####P < 0.0001 compared with DMSO/DMSO Myr treatment.
d18:1C14-ceramide and expression of XBP1 target gene were increased in intestines from MBFD mice
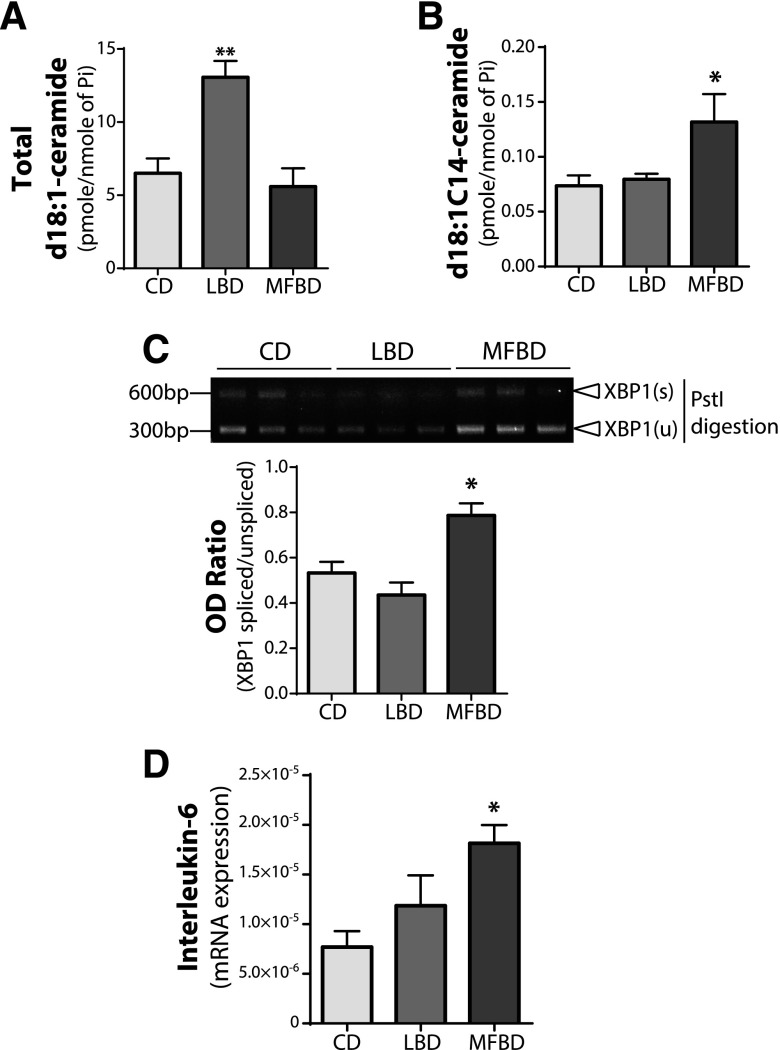
To investigate the effect of myristate on the ER stress response in intestinal tissues, we maintained mice on an LBD, MFBD, or isocaloric low-fat control diet for 12 wk. Both the LBD and MFBD contained similar levels of palmitate (∼30%), yet the C14:0 myristate content in MFBD was ∼5-fold higher than that of LBD (34, 35). We first examined the small intestine for changes in ceramide levels. Total d18:1-ceramide levels were elevated by LBD, but not by MFBD (Fig. 7A), and only d18:1C14-ceramide was significantly augmented in MFBD mice (Fig. 7B). XBP1s was significantly increased in small intestine tissues from MFBD mice (Fig. 7C), but not those fed the LBD or control diet. Moreover, IL-6 was significantly increased in the small intestine of only MFBD mice (Fig. 7D). Together, these data demonstrate that a diet with high myristate content, such as the MFBD, increases d18:1C14-ceramide and the induction of ER stress, which potentially results in subsequent pathobiology in the intestine.
Figure 7.
MFBD increases C14-ceramide, ER stress, and IL-6 expression in intestinal tissues. C57BL6 mice were fed LBD, MFBD, or an isocaloric control diet (CD) for 12 wk. A, B) Total d18:1-ceramide (A) and d18:1C14-ceramide (B) levels in the small intestine were analyzed by electrospray ionization tandem mass spectrometry in the Stony Brook University Lipidomics Shared Resource Core and normalized to total lipid Pi. C) XBP1s in the small intestine was analyzed using RT-PCR and PstI digestion, then a representative gel image was taken (upper panel) and quantified as OD ratio using ImageJ (lower panel). D) mRNA expression levels of IL-6 in the small intestine were analyzed by quantitative RT-PCR and normalized to β-actin. Data represent means ± sem, n ≥ 4. *P < 0.05, **P < 0.01 compared with CD.
DISCUSSION
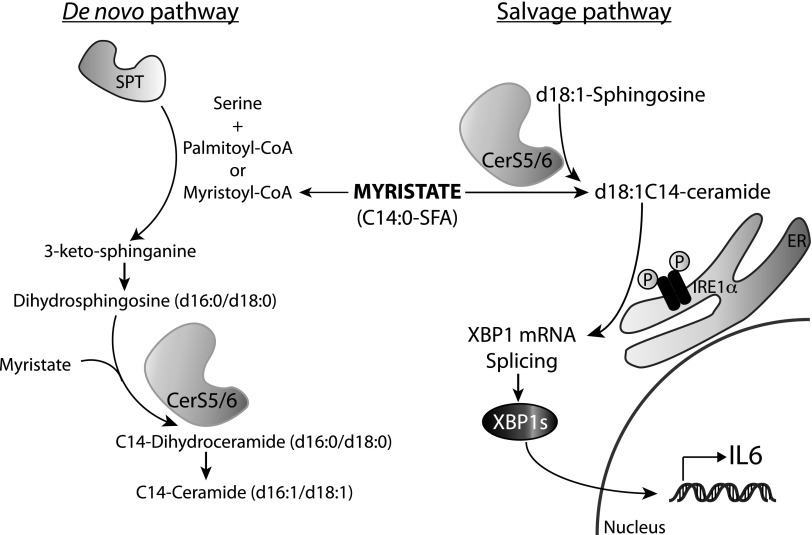
It has been previously shown that SFAs, specifically palmitate, induce such detrimental effects as ER stress and chronic inflammatory signaling in metabolic tissues and cells (7–10, 33). ER stress has been demonstrated to be associated with inflammation, specifically in IBD (25, 26); therefore, we set out to determine whether SFAs induced ER stress and/or inflammatory cytokines in intestinal epithelial cells, as the effects of specific FAs in these cells remain largely unknown. In the current study, we show that myristate treatment of IECs resulted in a potent induction of ER stress, specifically XBP1s and the expression of downstream target genes, such as ERdj4 and IL-6. In an effort to elucidate the mechanism of myristate-specific responses in ER stress in IECs, we determined a novel role for ceramide and CerS in myristate-induced ER stress. Specifically, we have demonstrated that CerS5 and 6 and their product, d18:0C14-ceramide, generated via the recycling pathway regulates IRE1-XBP1 signaling and subsequent IL-6 expression (summarized in Fig. 8). In addition, our in vivo study with myristate-enriched MFBD suggests that CerS5/6-derived long-chain ceramides may be potential factors that regulate ER stress–induced inflammatory response in intestinal tissues.
Figure 8.
Proposed model for myristate-induced ER stress and inflammatory cytokine expression via CerS5/6. Myristate treatment promotes ceramide generation via 2 distinct pathways, the de novo pathway and the salvage pathway. CerSs, which are involved in both pathways, produce (dihydro)ceramide by catalyzing the N-acylation of (dihydro)sphingosine. CerS-derived ceramide from the salvage pathway, specifically CerS5/6 and its product, C14-ceramide, activates IRE1 and induces XBP1s which results in increased expression of IL-6.
Ceramide levels are regulated by de novo synthesis of sphingolipids and the salvage pathway. HFD/palmitate-induced generation of ceramide has been demonstrated to be primarily a result of increased de novo synthesis via SPT [reviewed in Choi and Snider (17)]. However, Hu et al. (36) demonstrated that palmitate can be incorporated into d18:1C16-ceramide via in the N-acyl chain in the presence of myriocin, implicating CerS activity upon the inhibition of SPT. Our data demonstrate that myristate robustly increased d18:1C14-ceramide (Fig. 2), but did not alter d18:0-dhSph upon CerS inhibition (data not shown), which suggests that myristate augmented d18:1C14-ceramide generation via CerS rather than de novo synthesis. In contrast, d16:1-ceramide synthesis is regulated by de novo synthesis, as SPTLC3 is essential for incorporating myristate into the sphingoid backbone (14). Indeed, our data demonstrate that inhibition of SPT by myriocin abolished myristate-induced total d16:1-ceramide generation (Fig. 3E) and significantly suppressed d16:1C14-ceramide (Fig. 3F), which demonstrates the regulation of d16-sphingolipids by de novo synthesis. A recent study by Russo et al. (15) reported that d18:0- and d16:0-dhSph played distinct roles in myristate-induced cellular biology in that d16:0-dhSph induced cell death, whereas d18:0-dhSph stimulated autophagy in cardiomyocytes. In the current study, we determined that d16-ceramides were generated in response to myristate, but were not involved in myristate-induced ER stress, which demonstrates that specific sphingolipid species are required for myristate-induced ER stress in IECs.
Recently, the different roles of ceramides from specific pathways have been implicated in the regulation of apoptosis (37, 38). UV-C irradiation and TNF-α treatment significantly increased ceramides, particularly C16-ceramide, which resulted in increased apoptosis. FB1 treatment or CerS5/6 double-knockdown prevented UV-C–induced plasma membrane permeability in late-stage apoptosis and significantly reduced C14- and C16-ceramide (37). Similarly, TNF-α–induced caspase-3/7 activation, plasma membrane permeability, and C16-ceramide generation were suppressed by CerS inhibition with either FB1 or siRNA against CerS6 (38). However, SPT inhibition with myriocin did not prevent these irradiation- or TNF-α–induced apoptotic processes, although myriocin inhibited elevation in C16-ceramide (37, 38). These data suggest different roles for specific ceramides that are dependent on the enzymes involved in their generation, implicating different pathways or cellular compartments. We show here that myristate-induced C14-ceramide was decreased by both FB1 and myriocin (Fig. 3B); however, only FB1 suppressed myristate-induced XBP1s and IL-6 expression (Figs. 3H and 4F). Furthermore, knockdown of CerS5 or CerS6 prevented myristate-induced regulation of XBP1 and IL-6. These data suggest that CerS-derived ceramide via the salvage pathway, not the de novo pathway, is required for myristate-induced ER stress.
Our data are consistent with published reports that show the possible involvement of intracellular C14- and C16-ceramide levels in ER stress. Both of these ceramides have been implicated in the activation of both the PERK and IRE1α pathways in cells that were transfected with CerS2 siRNA (39). Similarly, these ceramides have been implicated in PERK activation in response to chemotherapeutics (20). In contrast, Wei et al. (8) have reported that CerS inhibition with FB1 did not inhibit palmitate-induced CHOP expression in H4IIE liver cells. It is possible that FB1 may not be effective in the suppression of CHOP expression, as CHOP can be regulated by all 3 major ER stress signaling branches. In addition, our data demonstrate that FB1 did not prevent myristate-induced CHOP expression (Fig. 3G), whereas it inhibited myristate-induced XBP1s specifically (Fig. 3H). These results suggest that specific ceramide species are critical for the modulation of cellular responses, including ER stress.
As a hub in sphingolipid metabolism, ceramide can be converted into multiple species of sphingolipid, such as SM, C1P, glucosylceramide, and S1P. These sphingolipid species have been known to be involved in various cellular functions, including inflammation [reviewed in Hannun and Obeid (40)]. Our study demonstrates that S1P levels were significantly increased by both palmitate and myristate, which implies that S1P is unlikely to be involved in myristate-induced ER stress in IECs (Fig. 6B). C14-HexCer was selectively increased by myristate; however, inhibition of glucosylceramide synthase and decreased generation of C14-HexCer did not suppress myristate-induced ER stress or IL-6 expression. Indeed, when combined with myristate treatment, PDMP augmented the levels of d18:1C14-ceramide and significantly increased myristate-induced XBP1 target genes (Fig. 6). These results suggest that myristate-induced ER stress is more likely a result of the generation of d18:1C14-ceramide than of other sphingolipid species.
ER stress has recently emerged as modulator of the regulation of proinflammatory signaling. Although all 3 branches of ER stress signaling can act as regulators in inflammatory signaling via NF-κB and AP-1 activation (41), XBP1 has recently been shown to control the expression of proinflammatory cytokines, such as IL-6 (27, 42, 43). XBP1 has also been shown to directly bind the IL-6 promoter to induce its expression in macrophages (27) and melanoma cells (43), which supports our conclusion that myristate-induced IL-6 expression is downstream of XBP1s in IECs. In addition, the spliced form of XBP1 has been found to be highly expressed in inflamed lesions in patients with IBD (26, 44). Furthermore, IL-6 treatment in Caco-2 monolayers has been shown to increase permeability via claudin-2 (45), which suggests that XBP1 could regulate intestinal permeability via IL-6. These studies, coupled with our data, suggest that ER stress, XBP1, and IL-6 may play key roles in intestinal permeability and inflammation, specifically in the setting of IBD.
High-fat dairy products, such as butter and cheese, are one of the main sources of dietary FAs, especially myristate. In epidemiologic studies, high consumption of milk or butter fat has shown significant correlation with insulin resistance and metabolic disease in humans (46, 47). Along these lines, MFBD, which had higher myristate content compared with LBD and was used in our study, induced cardiac hypertrophy and dysfunction, concomitant with increased d18:1C14-ceramide in C57BL/6 mice (15, 34). In the current study, we extended our investigation of MFBD effects on the proinflammatory response in the intestine. Our data indicate that maturation of XBP1 and expression of IL-6 were significantly augmented in small intestine tissues from MFBD mice, as well as d18:1C14-ceramide levels (Fig. 7), which implicates increased ER stress in response to the consumption of a diet high in dairy fat. These results suggest that C14-ceramide generated via CerS5 and CerS6 may be a critical regulator of ER stress in the intestine, with additional studies on the involvement of CerS5 and CerS6 in vivo needed.
In summary, the current study demonstrates that myristate, and not palmitate, induced XBP1s and subsequent IL-6 expression in IECs. This ER stress response required CerS5 or CerS6 generation of d18:1C14-ceramide, which suggests that specific FAs may preferentially be incorporated into sphingolipids and elicit specific responses in intestinal function and pathobiology.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Lina M. Obeid for expertise and suggestions for examining CerS, and Yusuf A. Hannun for expert advice in sphingolipid biology (both from the Stony Brook University School of Medicine). The authors also thank the Stony Brook University Lipidomics Shared Resource Core for lipid analysis. This work was supported by the Veteran’s Administration Career Development Award (to A.J.S.); U.S. National Institutes of Health (NIH), National Cancer Institute Grant P01-CA097132 (to A.J.S.); NIH, National Heart, Lung, and Blood Institute Grant R01-HL117233; and the Department of Veteran’s Affairs Merit Grant I01BX000200 (to L.A.C.). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government. The authors declare no conflicts of interest.
Glossary
- ATF
activating transcription factor
- BSA
bovine serum albumin
- C1P
ceramide 1-phosphate
- CerS
ceramide synthase
- CHOP
C/EBP homologous protein
- dhSph
dihydrosphingosine
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- FA
fatty acid
- FB1
fumonisin B1
- HexCer
hexosylceramide
- HFD
high-fat diet
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- IRE1
inositol requiring enzyme 1
- LBD
lard-based diet
- MFBD
milk fat–based diet
- OD
optical density
- PDMP
d,l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- PERK
pancreatic ER kinase-like ER kinase
- S1P
sphingosine 1-phosphate
- SFA
saturated fatty acid
- siRNA
small interfering RNA
- SM
sphingomyelin
- SPT
serine palmitoyltransferase
- UPR
unfolded protein response
- XBP1
X-box binding protein 1
- XBP1s
X-box binding protein 1 splicing
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Choi wrote the manuscript and performed conceptual and experimental design, as well as the generation of data and analyses for cell culture and in vivo experiments; J. M. Snider performed lipid analyses from cell culture experiments; N. Olakkengil performed RNA analyses from cell culture experiments; J. M. Lambert, A. K. Anderson, and J. S. Ross-Evans performed in vivo HFD experiments and collected tissues for analysis; L. A. Cowart contributed to experimental design, data analysis, and manuscript editing; A. J. Snider conceived the original hypothesis, provided the necessary funding for materials and methods, and supervised the entire project.
REFERENCES
- 1.Ye J., Rawson R. B., Komuro R., Chen X., Davé U. P., Prywes R., Brown M. S., Goldstein J. L. (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 [DOI] [PubMed] [Google Scholar]
- 2.Harding H. P., Zhang Y., Ron D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 [DOI] [PubMed] [Google Scholar]
- 3.Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., Bell J. C., Hettmann T., Leiden J. M., Ron D. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 [DOI] [PubMed] [Google Scholar]
- 5.Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., Ron D. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi H., Wang H.-G. (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 279, 45495–45502 [DOI] [PubMed] [Google Scholar]
- 7.Özcan U., Cao Q., Yilmaz E., Lee A.-H., Iwakoshi N. N., Özdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 8.Wei Y., Wang D., Topczewski F., Pagliassotti M. J. (2006) Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291, E275–E281 [DOI] [PubMed] [Google Scholar]
- 9.Guo W., Wong S., Xie W., Lei T., Luo Z. (2007) Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am. J. Physiol. Endocrinol. Metab. 293, E576–E586 [DOI] [PubMed] [Google Scholar]
- 10.Lai E., Bikopoulos G., Wheeler M. B., Rozakis-Adcock M., Volchuk A. (2008) Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 294, E540–E550 [DOI] [PubMed] [Google Scholar]
- 11.Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 12.Yasuda S., Nishijima M., Hanada K. (2003) Localization, topology, and function of the LCB1 subunit of serine palmitoyltransferase in mammalian cells. J. Biol. Chem. 278, 4176–4183 [DOI] [PubMed] [Google Scholar]
- 13.Han G., Gupta S. D., Gable K., Niranjanakumari S., Moitra P., Eichler F., Brown R. H., Jr., Harmon J. M., Dunn T. M. (2009) Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. USA 106, 8186–8191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornemann T., Penno A., Rütti M. F., Ernst D., Kivrak-Pfiffner F., Rohrer L., von Eckardstein A. (2009) The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 284, 26322–26330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo S. B., Tidhar R., Futerman A. H., Cowart L. A. (2013) Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J. Biol. Chem. 288, 13397–13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizutani Y., Kihara A., Igarashi Y. (2005) Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem. J. 390, 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi S., Snider A. J. (2015) Sphingolipids in high fat diet and obesity-related diseases. Mediators Inflamm. 2015, 520618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contreras C., González-García I., Martínez-Sánchez N., Seoane-Collazo P., Jacas J., Morgan D. A., Serra D., Gallego R., Gonzalez F., Casals N., Nogueiras R., Rahmouni K., Diéguez C., López M. (2014) Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 9, 366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z., Xia Y., Li B., Xu H., Wang C., Liu Y., Li Y., Li C., Gao N., Li L. (2014) Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca2+ homeostasis in human adenoid cystic carcinoma cells. Cell Biosci. 4, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park M. A., Zhang G., Martin A. P., Hamed H., Mitchell C., Hylemon P. B., Graf M., Rahmani M., Ryan K., Liu X., Spiegel S., Norris J., Fisher P. B., Grant S., Dent P. (2008) Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol. Ther. 7, 1648–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snider A. J., Kawamori T., Bradshaw S. G., Orr K. A., Gilkeson G. S., Hannun Y. A., Obeid L. M. (2009) A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 23, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snider A. J., Wu B. X., Jenkins R. W., Sticca J. A., Kawamori T., Hannun Y. A., Obeid L. M. (2012) Loss of neutral ceramidase increases inflammation in a mouse model of inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 99, 124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K., Xu R., Snider A. J., Schrandt J., Li Y., Bialkowska A. B., Li M., Zhou J., Hannun Y. A., Obeid L. M., Yang V. W., Mao C. (2016) Alkaline ceramidase 3 deficiency aggravates colitis and colitis-associated tumorigenesis in mice by hyperactivating the innate immune system. Cell Death Dis. 7, e2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espaillat M. P., Snider A. J., Qiu Z., Channer B., Coant N., Schuchman E. H., Kew R. R., Sheridan B. S., Hannun Y. A., Obeid L. M. (2018) Loss of acid ceramidase in myeloid cells suppresses intestinal neutrophil recruitment. FASEB J. 32, 2339–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., Blumberg R. S. (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negroni A., Prete E., Vitali R., Cesi V., Aloi M., Civitelli F., Cucchiara S., Stronati L. (2014) Endoplasmic reticulum stress and unfolded protein response are involved in paediatric inflammatory bowel disease. Dig. Liver Dis. 46, 788–794 [DOI] [PubMed] [Google Scholar]
- 27.Martinon F., Chen X., Lee A. H., Glimcher L. H. (2010) TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross J. S., Hu W., Rosen B., Snider A. J., Obeid L. M., Cowart L. A. (2013) Sphingosine kinase 1 is regulated by peroxisome proliferator-activated receptor α in response to free fatty acids and is essential for skeletal muscle interleukin-6 production and signaling in diet-induced obesity. J. Biol. Chem. 288, 22193–22206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams B. L., Lipkin W. I. (2006) Endoplasmic reticulum stress and neurodegeneration in rats neonatally infected with borna disease virus. J. Virol. 80, 8613–8626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Veldhoven P. P., Bell R. M. (1988) Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim. Biophys. Acta 959, 185–196 [DOI] [PubMed] [Google Scholar]
- 31.Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 32.Deldicque L., Cani P. D., Philp A., Raymackers J.-M., Meakin P. J., Ashford M. L. J., Delzenne N. M., Francaux M., Baar K. (2010) The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299, E695–E705 [DOI] [PubMed] [Google Scholar]
- 33.Wang D., Wei Y., Pagliassotti M. J. (2006) Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147, 943–951 [DOI] [PubMed] [Google Scholar]
- 34.Russo S. B., Baicu C. F., Van Laer A., Geng T., Kasiganesan H., Zile M. R., Cowart L. A. (2012) Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 122, 3919–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geng T., Hu W., Broadwater M. H., Snider J. M., Bielawski J., Russo S. B., Schwacke J. H., Ross J., Cowart L. A. (2013) Fatty acids differentially regulate insulin resistance through endoplasm reticulum stress-mediated induction of tribbles homologue 3: a potential link between dietary fat composition and the pathophysiological outcomes of obesity. Diabetologia 56, 2078–2087 [DOI] [PubMed] [Google Scholar]
- 36.Hu W., Bielawski J., Samad F., Merrill A. H., Jr., Cowart L. A. (2009) Palmitate increases sphingosine-1-phosphate in C2C12 myotubes via upregulation of sphingosine kinase message and activity. J. Lipid Res. 50, 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullen T. D., Jenkins R. W., Clarke C. J., Bielawski J., Hannun Y. A., Obeid L. M. (2011) Ceramide synthase-dependent ceramide generation and programmed cell death: involvement of salvage pathway in regulating postmitochondrial events. J. Biol. Chem. 286, 15929–15942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernández-Corbacho M. J., Canals D., Adada M. M., Liu M., Senkal C. E., Yi J. K., Mao C., Luberto C., Hannun Y. A., Obeid L. M. (2015) Tumor necrosis factor-α (TNFα)-induced ceramide generation via ceramide synthases regulates loss of focal adhesion kinase (FAK) and programmed cell death. J. Biol. Chem. 290, 25356–25373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spassieva S. D., Mullen T. D., Townsend D. M., Obeid L. M. (2009) Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem. J. 424, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannun Y. A., Obeid L. M. (2018) Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19, 175–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg A. D., Kaczmarek A., Krysko O., Vandenabeele P., Krysko D. V., Agostinis P. (2012) ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol. Med. 18, 589–598 [DOI] [PubMed] [Google Scholar]
- 42.Kim S., Joe Y., Kim H. J., Kim Y. S., Jeong S. O., Pae H. O., Ryter S. W., Surh Y. J., Chung H. T. (2015) Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J. Immunol. 194, 4498–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C., Zhang X. (2017) IRE1α-XBP1 pathway promotes melanoma progression by regulating IL-6/STAT3 signaling. J. Transl. Med. 15, 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogaert S., De Vos M., Olievier K., Peeters H., Elewaut D., Lambrecht B., Pouliot P., Laukens D. (2011) Involvement of endoplasmic reticulum stress in inflammatory bowel disease: a different implication for colonic and ileal disease? PLoS One 6, e25589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki T., Yoshinaga N., Tanabe S. (2011) Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 286, 31263–31271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lovejoy J. C., Champagne C. M., Smith S. R., DeLany J. P., Bray G. A., Lefevre M., Denkins Y. M., Rood J. C. (2001) Relationship of dietary fat and serum cholesterol ester and phospholipid fatty acids to markers of insulin resistance in men and women with a range of glucose tolerance. Metabolism 50, 86–92 [DOI] [PubMed] [Google Scholar]
- 47.Nestel P. J. (2008) Effects of dairy fats within different foods on plasma lipids. J. Am. Coll. Nutr. 27, 735S–740S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.