Abstract
Cobalamin [Cbl (or B12)] deficiency causes megaloblastic anemia and a variety of neuropathies. However, homeostatic mechanisms of cyanocobalamin (CNCbl) and other Cbls by vascular endothelial cells are poorly understood. Herein, we describe our investigation into whether cultured bovine aortic endothelial cells (BAECs) perform transcytosis of B12, namely, the complex formed between serum transcobalamin and B12, designated as holo-transcobalamin (holo-TC). We show that cultured BAECs endocytose [57Co]-CNCbl-TC (source material) via the CD320 receptor. The bound Cbl is transported across the cell both via exocytosis in its free form, [57Co]-CNCbl, and via transcytosis as [57Co]-CNCbl-TC. Transcellular mobilization of Cbl occurred in a bidirectional manner. A portion of the endocytosed [57Co]-CNCbl was enzymatically processed by methylmalonic aciduria combined with homocystinuria type C (cblC) with subsequent formation of hydroxocobalamin, methylcobalamin, and adenosylcobalamin, which were also transported across the cell in a bidirectional manner. This demonstrates that transport mechanisms for Cbl in vascular endothelial cells do not discriminate between various β-axial ligands of the vitamin. Competition studies with apoprotein- and holo-TC and holo-intrinsic factor showed that only holo-TC was effective at inhibiting transcellular transport of Cbl. Incubation of BAECs with a blocking antibody against the extracellular domain of the CD320 receptor inhibited uptake and transcytosis by ∼40%. This study reveals that endothelial cells recycle uncommitted intracellular Cbl for downstream usage by other cell types and suggests that the endothelium is self-sufficient for the specific acquisition and subsequent distribution of circulating B12 via the CD320 receptor. We posit that the endothelial lining of the vasculature is an essential component for the maintenance of serum-tissue homeostasis of B12.—Hannibal, L., Bolisetty, K., Axhemi, A., DiBello, P. M., Quadros, E. V., Fedosov, S., Jacobsen, D. W. Transcellular transport of cobalamin in aortic endothelial cells.
Keywords: vitamin B12, transcobalamin, vascular endothelium, MMACHC processing, exocytosis
Cobalamin (Cbl/B12) is a water-soluble vitamin whose deficiency is a leading cause of megaloblastic anemia (1). Nutritional and genetic deficiencies of vitamin B12 are a public health issue worldwide (2). Because Cbl is exclusively synthesized by a small group of bacteria and archaea (3), humans rely on a dietary daily intake of 2.4 µg of vitamin B12 (4). Cbl is essential for the synthesis of methionine from homocysteine by methylcobalamin (MeCbl)-dependent methionine synthase (MS) in the cytosol, and for the 5′-deoxyadenosylcobalamin (AdoCbl)-dependent conversion of methylmalonyl-coenzyme A to succinyl-coenzyme A by methylmalonyl-coenzyme A mutase (MCM) in mitochondria (5). Humans possess a complex network of extracellular and intracellular proteins for the transport, processing, and use of Cbl (6–8). Free, dietary Cbl binds first to human haptocorrin (HC) present in the upper gastrointestinal tract. The HC-Cbl complex undergoes proteolysis in the small intestine, and the released Cbl binds to intrinsic factor (IF), a B12-binder secreted by gastric parietal cells (7). IF-Cbl is absorbed by ileal enterocytes in a receptor-mediated process involving cubilin and amnionless (9). After proteolysis of the IF-Cbl complex in enterocytes, Cbl binds to apoprotein-transcobalamin (apo-TC) to form holo-TC and then enters portal circulation (10). Systemic circulation distributes holo-TC to all cells in the body (7). Examination of total serum B12 shows that 75–80% is bound to the slow-exchanging glycoprotein HC. Approximately 20–25% of the total serum B12 is bound to the fast-exchanging protein TC. HC binds all Cbls as well as most B12 analogs that originate from the gradual decay of Cbls. The exact role of serum HC has not been elucidated but it may serve as a vehicle to store or remove B12 analogs by the liver (11). Most (if not all) cells in the body express receptors for holo-TC, the latter being considered the bioactive form of the circulating B12 pool (12).
Cells take up holo-TC via endocytosis, mediated by the TC receptor (TCblR/CD320) (13–21).The holo-TC complex is then degraded in lysosomes, and free Cbl is transported into the cytosol by lysosomal proteins Cbl complementation group F (cblF) (22–24) and cblJ (25, 26), which render the micronutrient available for processing and trafficking by the methylmalonic aciduria and homocystinuria type C (MMACHC/cblC) (27, 28) and methylmalonic aciduria type D and homocystinuria (MMADHC/cblD) (29–31) proteins, respectively. An additional group of proteins deliver the micronutrient to its final intracellular destinations, cytosolic MS and mitochondrial MCM (27, 32, 33). Cbl can also be mobilized across cellular compartments by transcellular transport. Transcytosis of protein-bound Cbl (IF-Cbl or TC-Cbl) has been documented in kidney cells, enterocytes, and adenocarcinoma cells (34–41). Endocytosis and transcytosis of holo-TC have also been proposed as important mechanisms for the mobilization of maternal Cbl in the placenta (42–45). Investigation of maternofetal Cbl transfer in a CD320 knockout mouse model showed that, in the absence of a functional CD320 receptor, Cbl could be supplied to the fetus by uptake of holo-TC by megalin, a multiligand receptor (46).
An elusive aspect of vitamin B12 metabolism concerns the lack of a distinct correlation between serum and tissue levels of the micronutrient (47–50) and an unclarified role of the vascular endothelium in that homeostasis. We have previously shown that bovine aortic endothelial cells (BAECs) can take up and process Cbl to generate the 2 cofactors MeCbl and AdoCbl (27, 51). Although all cell types require Cbl, the endothelium may be critical for maintaining tissue-serum homeostasis of the micronutrient. In this study, we employed BAECs, in an in vitro model of the vascular endothelium, to investigate transcytosis of the bioactive protein complex holo-TC. This is the first study, to our knowledge, to demonstrate: 1) transcytosis of holo-TC in endothelial cells; 2) the existence of 2 operative transport pathways, one direct, characterized by transcellular transport of unmodified [57Co]-CNCbl either by transcytosis (as [57Co]-CNCbl-TC) or by exocytosis (as free [57Co]-CNCbl), and a second route involving the processing of [57Co]-CNCbl for coenzyme formation and export of newly made [57Co]-Cbl forms; and 3) the dependence of the Cbl transcellular transport routes for CD320, the cellular receptor for holo-TC.
MATERIALS AND METHODS
Cell culture
Primary isolates of BAECs at passages between 4 and 10 were grown in Cbl-free, folate-free Ham’s F12/DMEM, supplemented with 5% fetal bovine serum (FBS; 33 pM total Cbl) and 200 nM N5-methyltetrahydrofolate. BAECs were seeded in transwells [polyethylene-terephthalate (PET), 0.45 μm, 354442; BD Biosciences, Franklin Lakes, CA, USA] coated with rat-tail tendon collagen I, at an initial confluency of 90%.
Cell culture conditions of transcellular transport experiments
The conditioned culture medium of cells grown in transwells was replaced every 48 h. Confluent monolayers suitable for transcellular transport experiments were obtained after 1 wk in culture. Human aortic endothelial cells (HAECs, primary cultures isolated in-house from discarded aorta segments), were grown under the same conditions. Because establishing tight monolayers of HAECs proved more difficult (intercellular gaps were sometimes observed), most of the experiments were performed with the bovine counterpart. The experimental conditions chosen for transcytosis were selected to eliminate or substantially reduce the contribution of Cbl efflux that could interfere with the assessment of transcellular transport. Cbl efflux occurs in the presence of cellular Cbl overload (supraphysiologic doses), the rapid turnover of Cbl metabolism (leukemia cells) (52), in which cells harboring inborn errors of Cbl metabolism (53) and cellular vitamin B12 cannot be used. Cells were grown in Cbl-deficient culture medium for ≥4 passages before transfer to transwell inserts. Only physiologic concentrations of holo-TC (110 pM) were used to test for transcytosis (Supplemental Fig. S1). Those cell culture conditions were shown to support active Cbl processing by the cblC pathway, as shown in Hannibal et al. (51).
Integrity of the endothelial monolayer
The integrity of the endothelial monolayer selected for the experiments was assessed by 3 methods: 1) visual examination by phase-contrast microscopy (Supplemental Fig. S2), 2) measuring leakage across transwells, and 3) electrical conductance measurements for transcellular resistance (Supplemental Fig. S3). Evaluation of para-cellular leakage was performed by filling up the transwell with culture medium and monitoring liquid transfer into the empty bottom chamber overnight. A transcellular resistance of ≥155 mΩ was considered acceptable for further experimentation, in line with previously suggested guidelines (54). Under our experimental conditions, the transcellular resistance of BAECs in PET coated with collagen I was 170 ± 10 mΩ (n = 30). Transcellular resistance measurements were performed with an Ag/AgCl electrode (Millicell-Electrical Resistance System; MilliporeSigma, Billerica, MA, USA).
Preparation of holo-TC and holo-IF
Holo-TC and -IF proteins containing bound Cbl were prepared by incubating [57Co]-CNCbl with 4-fold excess of apo-TC [bovine recombinant (54)] or apo-IF (Beta Innovations, Belmont, MA, USA) overnight, at 37°C, in fresh culture medium to be used in the transcytosis experiments. The final concentration of holo-TC or -IF was 110 pM in all experiments. The quality of the preparation (ideally 100% of the [57Co]-CNCbl would be protein bound) was determined by spin-filtration of an aliquot of the preparation in an Amicon filter system (cutoff ∼5000 Da; MilliporeSigma). This method consistently produced adequate quantities of both holo-proteins (>99% of the Cbl bound; Supplemental Fig. S1).
Transcytosis of [57Co]-holo-TC in BAECs
Transcytosis experiments were performed on transwell inserts (PET, coated with collagen I; BD Biosciences) previously reconstituted with culture medium as directed by the manufacturer. Six-well plates were seeded with BAECs (initial cell density ≥90%), and the cells were allowed to grow for 48 h. The conditioned cultured medium (2 ml/chamber) was then replaced with fresh medium, and the cells were allowed to grow for 5–7 d. The transcytosis experiments were initiated by replacement of the conditioned culture medium with fresh medium containing radioactive holo-TC (prepared as described above) to either the upper or the bottom chambers. Transport across the endothelial monolayer was monitored by counting the radioactivity of a 50-µl aliquot from each chamber (source and receiving chamber) at the following times: 0.5, 1, 2, 4, 6, 9, 12, 24 and 98 h. Cell cultures were protected from light at all times for further analysis of intracellular and extracellular Cbl.
Production of apo-TC by BAECs
The production of apo-TC by cultured BAECs was assessed by measuring the unsaturated Cbl binding capacity (UCBC) assay, according to a method published by Gottlieb et al. (55). That assay measures the ability of biologic fluids to bind vitamin B12. Briefly, 25 µl of a stock solution of [57Co]-CNCbl (10 ng/ml, 200,000 dpm/ml) was added to 0.2 ml of conditioned culture medium or to controls and calibrators with known UCBC values. Unbound [57Co]-CNCbl was removed by addition of hemoglobin-coated charcoal. The charcoal was pelleted by centrifugation, and the radioactivity of the supernatants and pellets were measured in a γ-spectrometer (Gamma 4000; Beckman-Coulter, Fullerton, CA, USA). Control experiments, in which [57Co]-CNCbl was incubated with B12-free conditioned culture medium, followed by purification by CM-Sephadex C-50 (GE17-0220-01; GE Healthcare Life Sciences, Little Chalfont St. Giles, United Kingdom) and comparison with purified bovine recombinant apo- and holo-TC showed that the measured UCBC represented mostly (if not only) apo-TC as the major B12 binder secreted into the culture medium of BAECs.
Expression of TC and CD320 in BAECs
The expression of TC in BAECs was examined by Western blot with an antibody raised against human TC (rabbit anti-human TC; 189871; Abcam, Cambridge, United Kingdom). The primary antibody was used at a dilution of 1:500 and the secondary antibody at 1:1000 (polyclonal goat anti-rabbit). Expression of TC was examined in conditioned culture medium (undiluted) and in whole cell extracts of BAECs (Supplemental Fig. S4A). Whole cell extracts under near-native conditions were prepared with the Whole Cell Extraction Kit (ab113475; Abcam). One confluent T25-culture flask of BAECs was used to prepare a total cell extract with a final volume of 100 µl. From that extract, 15–20 µl (∼30 µg total protein) was loaded on an SDS-PAGE for Western blotting. The expression of CD320 in cultured BAECs was examined with the CD320 ELISA (8D6A) Kit (ab213759; Abcam) based on a human antibody, according to the manufacturer’s instruction. The sensitivity of that ELISA assay was <10 pg/ml, and the dynamic range for quantification is 156–10,000 pg/ml (instructions booklet; Abcam). Conditioned culture medium (100 µl) without BAECs (time, 0; which determines basal levels of contaminant CD320 derived from supplemental 10% FBS), and after 3, 7, and 11 d of growth was used for the analysis. Whole-cell extracts were prepared as previously described. From that extract, 50 µl (∼60–70 µg total protein) were used in the ELISA CD320 measurement. Results of the CD320 quantification are provided in Supplemental Fig. S4B.
Purification of [57Co]-CNCbl-TC from conditioned culture medium
An aliquot (5 ml) of conditioned culture medium was taken, and the [57Co]-CNCbl-TC fraction was purified by CM-Sephadex C-50, using a protocol published by Quadros et al. (57). Briefly, 2 g of dry resin were preswollen in equilibration buffer [0.02 M Na2HPO4 (pH 5.4); 0.1 M NaCl). The conditioned culture medium was added to the equilibrated resin and allowed to rock overnight at room temperature. The pH of the conditioned culture medium was adjusted to 5.4 before incubation with CM-Sephadex C-50. Unbound proteins were removed by sequential washes with 50 ml equilibration buffer, followed by 25 ml of wash buffer [0.02 M Na2HPO4 (pH 5.4); 0.1 M NaCl). Fractions of 5 ml were collected. Bound [57Co]-CNCbl holo-TC was eluted with 25 ml 0.02 M Na2HPO4 (pH 5.8) and 1.0 M NaCl. Fractions of 2.5 ml were collected, and an aliquot of 0.1 ml was analyzed for radioactivity in a γ-spectrometer and at an absorbance of 280 nm. Under our experimental conditions, [57Co]-CNCbl holo-TC eluted in the first 10 ml. Because the concentration of holo-TC in the conditioned culture medium was too low for direct assessment by absorbance at 280 nm, the presence of a radioactive peak eluting at the exact retention time of authentic, pure holo-TC permitted confirmation of results.
Fate of [125I]-holo-TC upon uptake and transcellular transport by BAECs
Bovine recombinant apo-TC was iodinated ([125I]) as described by Dan and Cutler (37), using the IODO Beads iodination reagent (Thermo Fisher Scientific, Waltham, MA, USA), which permits real-time monitoring of transport and turnover. [125I]-holo-TC was produced by incubation of [125I]-apo-TC with 1.2 M excess of CNCbl at 37°C, 3 h before the experiment. Transcytosis of [125I]-holo-TC, [57Co]-holo-TC, and free [57Co]-CNCbl in parallel experiments was determined after 24 h. An aliquot of the conditioned culture medium in the bottom compartment (receiving chamber) was analyzed by spin filtration using Amicon spin filters (MW cutoff of 5000 Da), followed by counting of the filtrate and filter-retained (protein-bound) radioactivity in a γ-spectrometer. The protein-bound fraction containing the [125I] tracer was concentrated and run on a native PAGE (Supplemental Fig. S5) and subjected to phosphorimager analysis to determine the MW of the [125I]-protein species (Supplemental Fig. S6).
Identification of intracellular and transcytosed Cbl forms
Cells were grown for a total of 96 h in the presence of [57Co]-CNCbl-TC, protected from light to prevent photodecomposition of newly made [57Co]-MeCbl and [57Co]-AdoCbl. A change of medium was performed at 48 h, using fresh medium containing [57Co]-CNCbl-TC, prepared as previously described. Cbls were extracted from cells or from the conditioned culture medium and identified using our method previously published in Hannibal et al. (51).
Characterization of transcytosed Cbl forms (protein bound vs. free)
To determine whether Cbl exits the cells in its free form or bound to protein, cells were grown in transwells as previously described, and the [57Co]-CNCbl-TC (source material) was added to the upper compartment. The bottom compartment consisted of culture medium with or without 1 μM CNCbl (unlabeled) incorporated to trap any apo-TC secreted by BAECs in culture. An aliquot (0.25 ml) of the conditioned culture medium in the receiving compartment was removed after 24 h and passed through a spin filter with a MW cutoff of 5000 Da. The filtrate represented the fraction containing “free Cbl” (or Cbl bound to low MW proteins/peptides), whereas the filter retains “protein-bound Cbl,” including holo-TC (46 kDa).
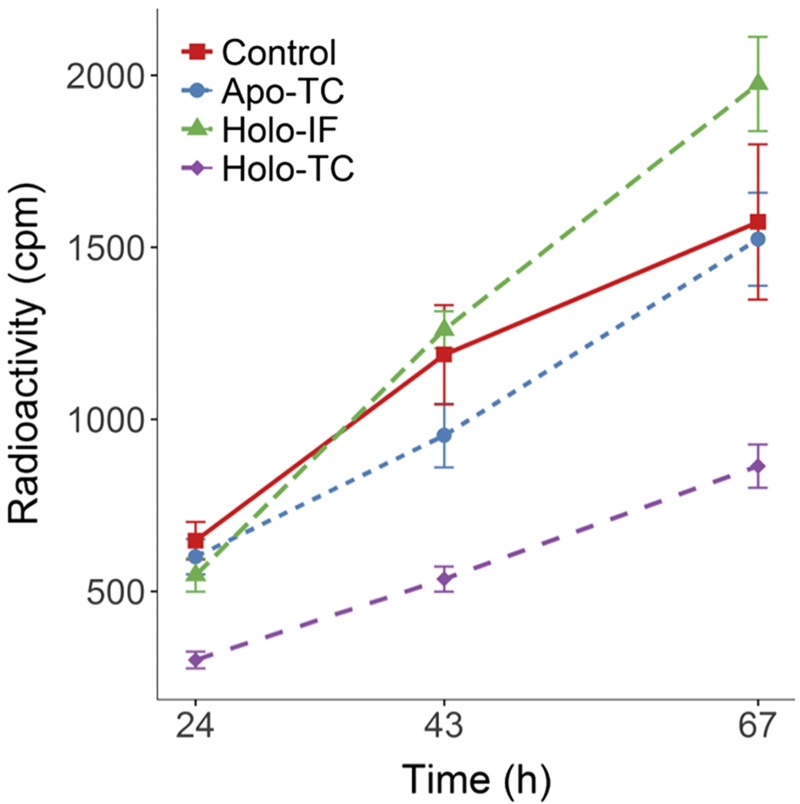
Competition experiments with apo-TC, holo-TC, and holo-IF
The specificity of the transcytosis pathway in endothelial cells was assessed through competition experiments with the Cbl transport proteins. The competitors used were apo-TC, CNCbl-TC (unlabeled), and CNCbl-IF (unlabeled). All competing proteins were added simultaneously in 48-fold excess, with respect to [57Co]-CNCbl-TC. Transcytosis of [57Co]-CNCbl-TC in the absence (control) or in the presence of the competitors was determined at 24, 43, and 67 h.
Blocking of holo-TC uptake and internalization in BAECs by a specific antibody against the human TCblR
Anti-TCblR mouse mAb [raised against the extracellular domain of the human TC receptor (15)] was used in these experiments. BAECs were grown in 6-well plates until ∼60% confluent. The conditioned culture medium was then replaced with fresh medium with or without 10 μg/ml of anti-TCblR antibodies. Cells were preincubated with the anti-TCblR antibody at 4°C for 1 h before the addition of [57Co]-CNCbl-TC. Cell cultures were then transferred to regular culture conditions (37°C) and allowed to grow for 10 h.
Involvement of the proteolytic pathway in transcellular transport of holo-TC
A typical experiment was performed with [57Co]-CNCbl-TC (110 pM). Cells were grown for 24 h in the presence of 200 μg/ml leupeptin (in both the transwell and the receiving chamber) or without the inhibitor (control). Exogenous [57Co]-CNCbl-TC was eliminated by washing the cells 3 times with PBS. Transcellular transport of [57Co]-CNCbl-TC was chased for 5 h in the presence or absence of leupeptin at 200 μg/ml, as previously described. The radioactivity measured in the receiving chamber represented the total Cbl that underwent transcellular transport, both by transcytosis and by exocytosis.
Lysosomal processing and fate of [125I]-holo-TC after cellular uptake
To determine the fate of holo-TC taken up by the cells in culture, bovine recombinant apo-TC was iodinated ([125I]), as described in Dan and Cutler (37) with IODO Beads iodination reagent (Thermo Fisher Scientific). Further details are provided in the Supplemental Data.
Expression and purification of monomeric red fluorescent protein fusion with full-length TC
To ensure that the TCblR-TC recognition axis was operative in cultured endothelial cells under our experimental conditions, uptake of monomeric red fluorescent protein fusion with full-length TC (Ds-RED-TC) by BAECs was monitored by spectral laser scanning confocal fluorescence microscopy (Leica TCS-SP-AOBS; Leica Microsystems GmbH, Germany). A ×63 oil immersion objective lens was used. Recombinant human TC was cloned into the pDsRed-N1 vector to yield a fusion protein with a fluorescent tag at the C terminus (15). Apo–Ds-Red-TC was purified by CM-Sephadex ion-exchange chromatography, as described in Quadros et al. (57).
Data analysis
Graphs were produced with R Studio software (v.1.0.143; RStudio, Boston, MA, USA). All results are shown as means ± sd. Pooling of samples was kept to a minimum and performed only when necessary to achieve detection of the radioactive species.
RESULTS
Secretion of apo-TC, uptake of holo-TC, and expression of CD320 by BAECs
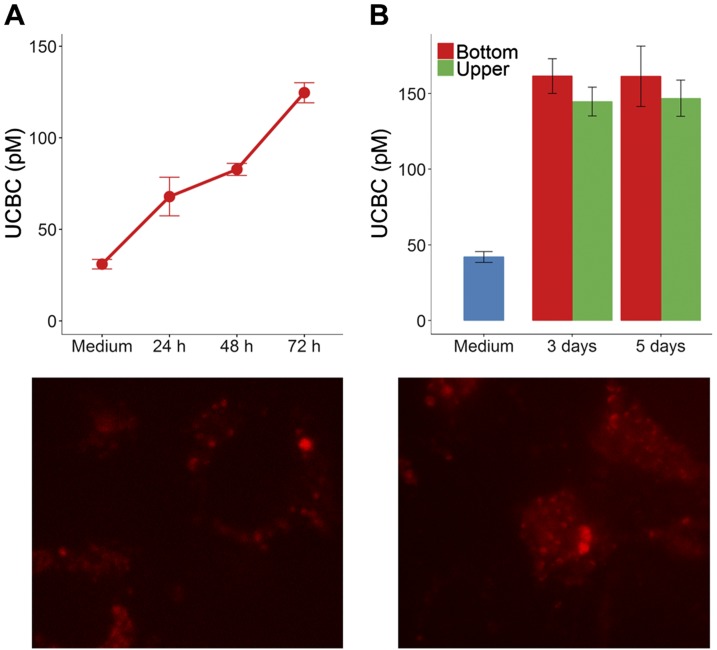
The production of apo-TC in the upper and bottom chambers of the transwell culture dishes was examined over time to evaluate the functional properties of the endothelial monolayer under our experimental conditions. Confluent BAECs steadily produced apo-TC over time (Fig. 1A), reaching a maximum UCBC value of 140–150 pM after 3–5 d (Fig. 1B). Western blotting with an antibody raised against human TC demonstrated the presence of TC in whole-cell extracts of BAECs (Supplemental Fig. S4A). Ds-RED–holo-TC uptake by BAECs was examined by confocal microscopy. Under our experimental conditions, Ds-RED–holo-TC segregated to the surface of the cell and underwent internalization within 2 h (Fig. 1C), as previously documented in other cell types (15, 46). Immunodetection of CD320 receptor indicated low levels of soluble CD320 in the conditioned culture medium and substantial amounts of the receptor in whole-cell extracts of BAECs (Supplemental Fig. S4B).
Figure 1.
Secretion of apo-TC by BAECs cultured in transwell inserts and internalization of Ds-RED-holo-TC. A) Confluent BAECs were cultured in Ham’s/F12 supplemented with 5% FBS and 200 nM N5-MeTHF, for 72 h without medium exchange. An aliquot of the conditioned culture medium was collected at specific times and examined for UCBCs. An aliquot taken at t = 0 h represents the UCBCs derived from FBS in the culture medium (herein labeled as “medium”). B). Comparison of maximum UCBC values in the bottom and upper compartments of the transwell after 3 and 5 d. C) Confocal microscopy visualization of membrane assembly (left) and internalization (right) of Ds-RED-holo-TC on cultured BAECs. Cells were incubated with Ds-RED-holo-TC for 3 h at 37°C, before washing and imaging. Original magnification, ×63.
Transcytosis of [57Co]-CNCbl-TC in BAECs
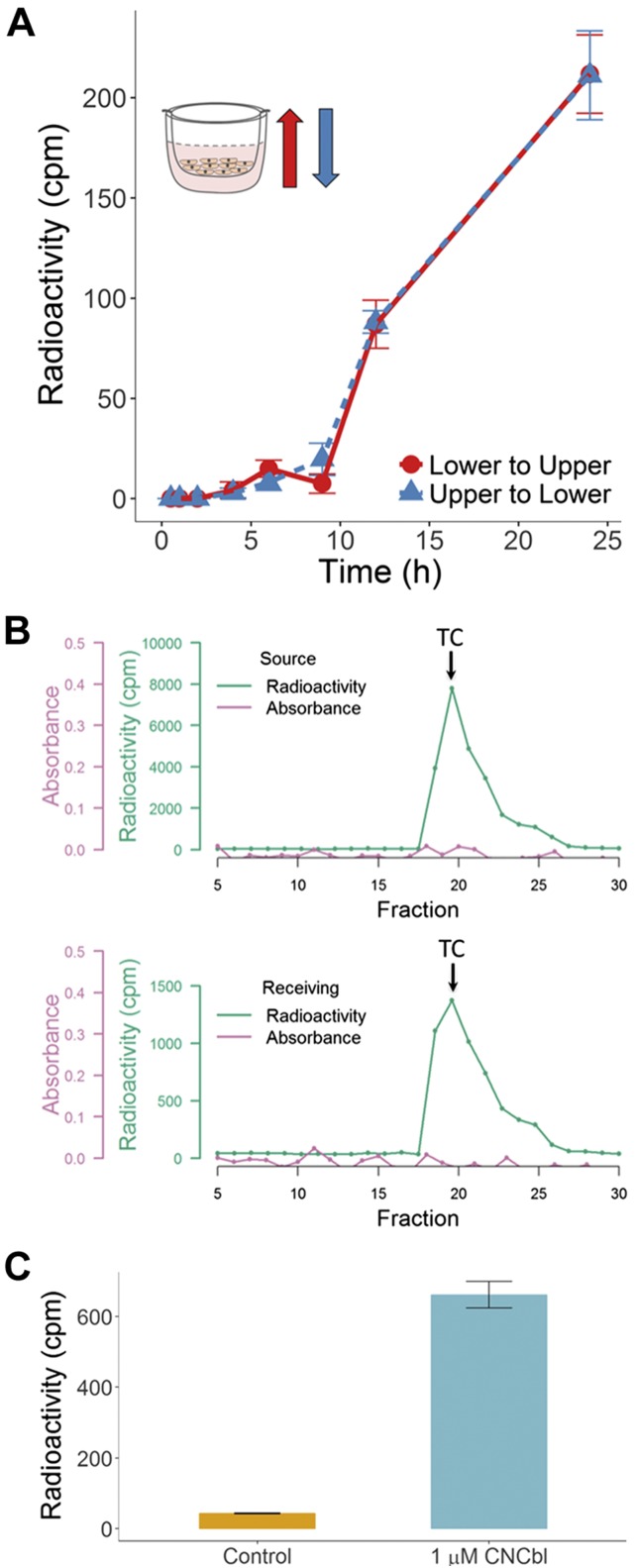
Transcytosis of [57Co]-CNCbl-TC in endothelial cells was investigated for the first time, to our knowledge, using transwell culture dishes. Fig. 2A shows a time course for transcytosis of [57Co]-CNCbl-TC in BAECs. The kinetic records of transport from the upper to the bottom chamber and vice versa were comparable. This suggests that transport of [57Co]-CNCbl-TC across BAECs occurs in both directions; hence, endothelial cells do not display any distinct functional poles, in contrast to what has been reported for intestinal epithelial cells (37). The total recovery of [57Co]-Cbl in the receiving chambers was approximately the same, regardless of the location of the source (Supplemental Table S1).
Figure 2.
Transcellular transport of [57Co]-holo-TC in BAECs is bidirectional and releases holo-TC as well as free Cbl. A) An aliquot of conditioned culture medium (50 µl) was taken at the indicated times from the receiving compartments, and the radioactivity was determined. A control for cell-independent permeability, consisting of PET/collagen I–coated inserts without cells, was subtracted at each time point. No transcytosis was observed during the first 6 h of incubation with holo-TC. Inset) Expected direction of transport when [57Co]-CNCbl-TC was added to the upper chamber (blue arrow) and when [57Co]-CNCbl-TC was added to the lower chamber (red). B) BAECs were cultured in the presence of [57Co]-CNCbl-TC for 24 h. An aliquot of the conditioned culture medium in the source and receiving chambers was subjected to CM-Sephadex chromatography. Presence of a radioactive signal at the expected elution fraction of holo-TC (arrows) demonstrated that in both fractions, [57Co]-CNCbl is bound to TC. C) BAECs were incubated with [57Co]-holo-TC for 24 h, with the receiving chamber supplemented with 1 μM unlabeled CNCbl. An aliquot of the conditioned culture medium was spin-filtered through Amicon mini-filters (MW cutoff 5000 Da), and the radioactivity present in the filter (holo-TC) and filtrate (free Cbl) were determined. The figure shows [57Co]-Cbl recovered in the respective filtrates, which corresponds to free Cbl. In the presence of excess CNCbl, most of the [57Co]-Cbl appears to be in its free form, whereas the opposite was observed in the absence of CNCbl. Thus, [57Co]-holo-TC detected in A corresponds to transcytosed [57Co]-holo-TC as well as exocytosed [57Co]-CNCbl that bound to newly secreted apo-TC produced by cells in the receiving chamber.
Endocytosis of [57Co]-CNCbl-TC leads to exocytosis of protein-free [57Co]-Cbl and transcytosis of [57Co]-CNCbl-TC
Under our experimental conditions, all of the transcytosed [57Co]-Cbl appeared to be bound to TC (Fig. 2B and Supplemental Table S1). That finding, however, did not preclude the possibility that [57Co]-Cbl exited the cell in free form and readily bound to endogenously made apo-TC, secreted by the BAECs into the culture medium. A second experiment was performed in which transcytosis of [57Co]-CNCbl-TC was monitored either in the presence or absence of saturating amounts (1 μM) of unlabeled CNCbl, added to the receiving compartment to trap all the newly secreted apo-TCs. The results of that experiment are summarized in Fig. 2C. Very little [57Co]-Cbl was present in free form under control culture conditions (no addition of unlabeled CNCbl). Trapping of newly made apo-TC with CNCbl resulted in ∼5–10% [57Co]-CNCbl-TC (transcytosis), and most of the endocytosed source [57Co]-holo-TC was exported out of the cell as free [57Co]-CNCbl (exocytosis).
Identification of intracellular and secreted Cbl forms
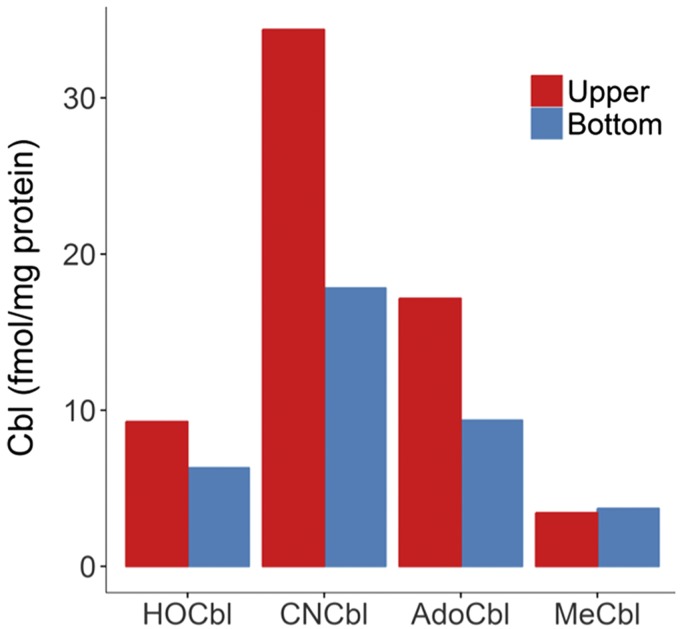
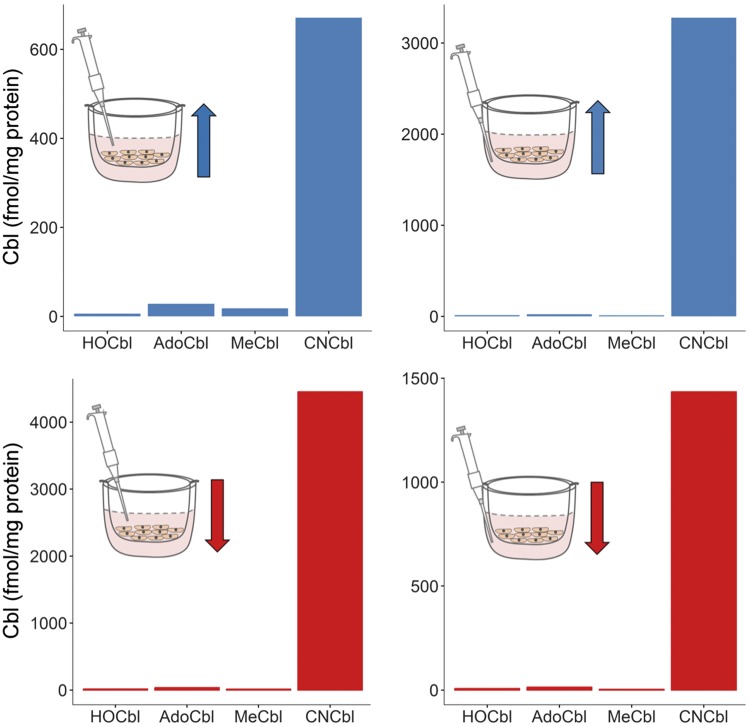
Intracellular and extracellular [57Co]-Cbl forms were determined in cell lysates and in conditioned culture medium of BAECs grown in the presence of [57Co]-CNCbl-TC. Figure 3 shows the intracellular Cbl profile for 12 pooled samples. In addition to the source ligand [57Co]-CNCbl, [57Co]-AdoCbl appeared to be the major intracellular form present in the BAECs, in agreement with the literature (51). The profile of intracellular Cbl was similar regardless of the placement of the source [57Co]-CNCbl-TC (upper or lower chamber). Figure 4 shows the [57Co]-Cbl profile of extracellular Cbls. The major extracellular form was the source [57Co]-CNCbl. [57Co]-HOCbl, [57Co]-AdoCbl, and [57Co]-MeCbl were also transported out of the cell, although to a lesser extent. In addition to [57Co]-CNCbl, [57Co]-AdoCbl was the second most-abundant Cbl form exported by the BAECs, a pattern that mimics the intracellular Cbl pool.
Figure 3.
Intracellular Cbl pool under experimental conditions of transcellular transport. Cells were incubated with [57Co]-CNCbl-TC for 96 h, washed with PBS, and the Cbls were extracted and identified as previously published in Hannibal et al. (51). Each bar represents 12 pooled samples of the same experimental condition (samples were pooled to achieve a reproducible and reliable readout of radioactivity). Blue bars: The radioactive source was added to the upper compartment. Red bars: The radioactive source was added to the bottom compartment.
Figure 4.
Extracellular Cbl pool under experimental conditions of transcellular transport. Cells were incubated with [57Co]-CNCbl-TC for 96 h. Cbls from an aliquot of the conditioned culture medium (0.8 ml) from the upper and lower compartments were extracted and identified, as previously described (26, 50). Arrows indicate direction of transcellular transport. The position of the pipette indicates which compartment of the transcytosis culture system the conditioned cultured medium taken from for analysis. Each bar represents a measurement of 12 pooled samples.
Evidence that endocytosis of [57Co]-CNCbl-TC is mediated by CD320
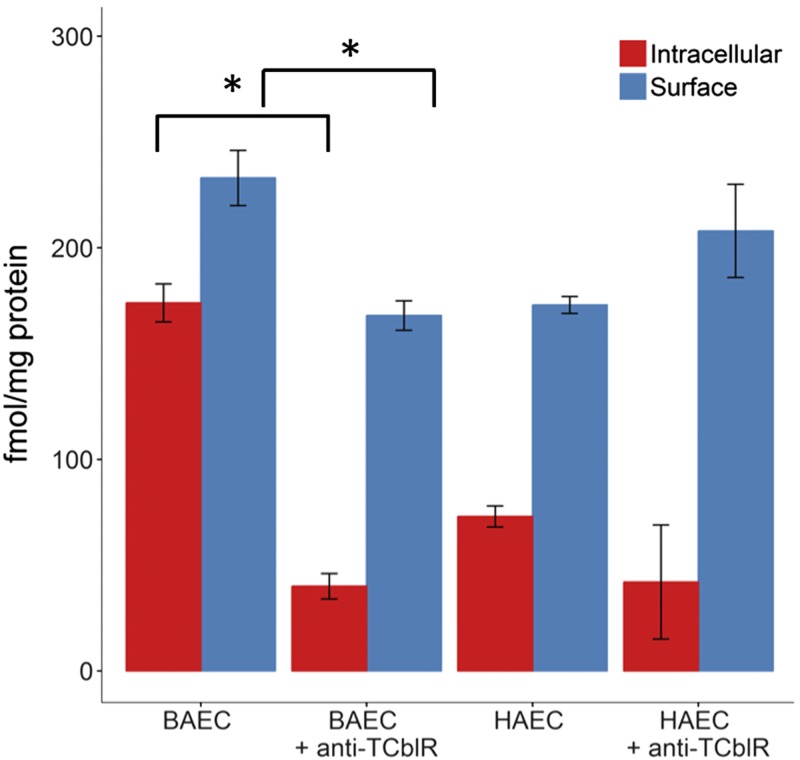
A competition experiment was performed to investigate whether endocytosis of [57Co]-CNCbl-TC and its downstream transport events (transcytosis and exocytosis) are mediated by the TC receptor CD320 (also called TCblR) in endothelial cells. The results are shown in Fig. 5. No inhibition of the transcellular transport of [57Co]-CNCbl was observed in the presence of excess apo-TC or holo-IF, as the levels of [57Co]-CNCbl resembled those found in the control experiment for all time points. Addition of holo-TC reduced the transcellular mobilization of [57Co]-CNCbl-TC by ∼40% with respect to the control. Those results suggested that, under our experimental conditions, the uptake and transcellular transport of [57Co]-CNCbl-TC were primarily mediated by the CD320 receptor. To further investigate the role of the CD320 receptor in the cellular transport of [57Co]-Cbl, an uptake experiment was performed in the presence or absence of a blocking antibody that was raised against the TC binding domain of human CD320. Because of the high similarity of the human and bovine TC and CD320 genes, the experiment was performed with BAECs as well as HAECs. Figure 6 shows intracellular and surface- associated [57Co]-CNCbl after an uptake study in the presence or absence of anti-CD320. In both BAECs and HAECs, a reduction in the intracellular recovery of [57Co]-CNCbl was observed in the presence of the anti-CD320 antibody.
Figure 5.
Competition of the transcellular transport of [57Co]-holo-TC with nonradioactive holo-TC, apo-TC, and holo-IF. Cells were incubated in the presence or absence of potential effectors for 24, 43, and 67 h. Apo-TC and holo-IF were supplied in 48-fold excess with respect to the concentration of [57Co]-CNCbl-TC. Only holo-TC (nonradioactive) inhibited transcellular transport, indicating that this route is specific for the TC receptor CD320 in endothelial cells. Under our experimental conditions, no other cellular receptors in endothelial cells could take up [57Co]-CNCbl-TC to enable detectable transcellular transport.
Figure 6.
Endocytosis of [57Co]-holo-TC in the presence or absence of anti-TCblR (CD320). Confluent cells were grown in 6-well plates and incubated with an antibody raised against the TC binding domain of the CD320 receptor, for 10 h. The antibody was added at a final concentration of 10 μg/ml. Overall, HAECs took up less [57Co]-CNCbl-TC compared with BAECs, which could be related to species-specific differences in affinity of their respective CD320 receptors for the bovine holo-TC used in this study. A reduction in the intracellular recovery of [57Co]-CNCbl-TC was observed in the presence of the anti-CD320 antibody for aortic endothelial cells of both species. Statistically significant differences were found only for the experiments performed with BAECs (Student t test). *P < 0.001.
Involvement of the lysosomal degradation pathway in the transcellular transport of Cbl
The presence of 200 μg/ml leupeptin (36) did not affect the transcellular mobilization of [57Co]-CNCbl (data not shown), suggesting little or no direct involvement of the lysosomal degradation pathway. This finding is in contrast with the reported observations on the transcytosis of holo-IF in caco-2 cells, where addition of leupeptin appeared to inhibit transcytosis of holo-IF, albeit modestly. In general, involvement of lysosomal degradation in transcellular transport appears to be of limited importance, and specific for the cell type and the Cbl binding protein (Table 1) (37).
TABLE 1.
Transcellular transport of vitamin B12 and its binding proteins in cultured cells and tissues
| Cell type | Transport protein | Characteristic | Reference |
|---|---|---|---|
| BAECs | Holo-TC | Bidirectional, insensitive to leupeptin, Cbl enters the cell as holo-TC and exits both in free form (>90%) and also bound to TC (5–10%). | Present study |
| Transcytosis and exocytosis inhibited by CD320 antibody raised against TC binding domain | |||
| Transcellular transport does not discriminate between different β-axial ligands of Cbl | |||
| Transcytosis and exocytosis occur for nonprocessed, as well as processed, Cbl | |||
| Polarized opossum kidney epithelial cells | IF-Cbl | Transcytosis of holo-IF inhibited by cerulenin and tunicamycin. | 36, 40 |
| Cerulenin caused accumulation of Cbl in the lysosome | |||
| Putative IF-Cbl receptor expression modulated by N-glycosylation | |||
| Uptake of IF-Cbl by receptor-mediated endocytosis | |||
| Human intestinal epithelial cells, Caco-2 cells | Holo-TC | Transcytosis of holo-TC has directionality (apical to basolateral different from basolateral to apical sides) | 35, 39 |
| Apical to basolateral transcytosis of holo-TC insensitive to chloroquine or leupeptin, and the opposite was observed for basolateral to apical transcytosis of holo-TC | |||
| Existence of lysosomal and nonlysosomal pathways for transcytosis of holo-TC | |||
| No transcytosis of HC-Cbl | |||
| Caco-2 cells | Putative TC receptor | Transcytosis from basolateral to apical membranes of Caco-2 cells | 85 |
| Inhibited by Brefeldin A and tunicamycin but insensitive to wortmannin and leupeptin | |||
| Transcytosis of putative TC receptor mediated by megalin | |||
| Caco-2 cells | IF-Cbl | Transcytosis of IF-Cbl and holo-TC, inhibited by chloroquine | 36 |
| Holo-TC | Transcytosis of holo-TC inhibited by TC antibodies. | ||
| 80% of the source Cbl underwent transcytosis; the rest underwent intracellular processing to MeCbl and AdoCbl | |||
| Caco-2 cells | IF-Cbl | Transcytosis of IF-Cbl inhibited by leupeptin | 37 |
| Transcytosis involves an intracellular pool of free Cbl | |||
| Guinea pig ileum | IF-Cbl | Internalization of IF-Cbl by enterocytes and transfer of IF to blood circulation by transcytosis | 41 |
| TC-Cbl | Secretion of TC; synthesis, secretion, and binding of newly absorbed Cbl. |
Previous studies did not investigate whether transcellular transport led to mobilization of protein-bound (transcytosis) or free Cbl (exocytosis). The term “transcytosis” in such studies may thus represent a combination of both means of Cbl transport in different cell types.
A transcellular transport experiment with [125I]-holo-TC to track the fate of holo-TC
Approximately 40% of the source [125I]-holo-TC added to the culture medium underwent turnover into low-MW peptides (<5000 Da) after 24 h. However, a significant fraction of the intact source [125I]-holo-TC reached the receiving chamber via transcytosis, as can be seen in the phosphorimager record of conditioned culture medium after 24 h (Supplemental Fig. S5). That finding, along with the insensitivity of transcytosis to the lysosomal inhibitor leupeptin, further confirmed that 2 distinct routes exist for the transcellular transport of Cbl in endothelial cells, namely, transcytosis of holo-TC and exocytosis of protein-free Cbl.
DISCUSSION
Background
All cells in the body obtain vitamin B12 through the internalization of holo-TC by receptor-mediated endocytosis (13–21). Plasma holo-TC is an early marker of changes in vitamin B12 homeostasis (58–60), and a reliable indicator of vitamin B12 absorption and assimilation (61). The relationship between plasma (circulation) and tissue levels of Cbl (47–50) is unknown, and results from this study suggest that the vascular endothelium may have an important role in that homeostasis. Early in vitro studies showed that most cell types could contribute to TC present in circulation (62). Detailed examination of human umbilical vein endothelial cells in culture and within a naked umbilical vein segment demonstrated that this cell type secretes copious amounts of TC and at rates that could account for the total TC pool in blood (63). The role of the vascular endothelium in B12 homeostasis is also relevant to inborn errors of metabolism that lead to functional Cbl deficiency, whereby serum levels of B12 and holo-TC are often within the normal range, but cells are metabolically (functionally) deficient (64).
The endothelium as a source of apo-TC
Expression of TC has been demonstrated in a variety of human cells (62, 65–70), including endothelial cells (63, 71–73). mRNA expression data from the human protein atlas shows that both TC and CD320 are expressed in essentially all cell types, including 3 types of endothelial cells (74–77).
Here, we examined endothelial transcytosis of bioactive vitamin B12 [i.e., [57Co]-CNCbl-TC (holo-TC)], and exocytosis of protein-free [57Co]-CNCbl under controlled experimental conditions that mimic normal physiology in terms of: 1) concentration of supplied holo-TC, 2) cell culture medium composition, and 3) competency of the endothelial cell monolayer. Results from this study demonstrated that cultured BAECs actively express and secrete apo-TC and that a maximum UCBC of ∼140 pM is reached in the conditioned medium after 3 d. That level of apo-TC remained constant under our experimental conditions after 5 d in culture. Those findings are consistent with the expression of TC in other endothelial cell types (63, 71–73). The UCBC values found in the conditioned medium of BAECs were within the range of holo-TC concentrations found in human plasma (12) but were somewhat less than the total TC found in humans not supplemented with vitamin B12 (≤1 nM). This suggests that the endothelium could indeed function as an important source of apo-TC. Our finding that cultured BAECs expressed substantial amounts of CD320 (500–800 pg/mg total protein) suggests that a fully functional TC-CD320 system exists for the cellular import of Cbl.
Endothelial transcytosis of holo-TC
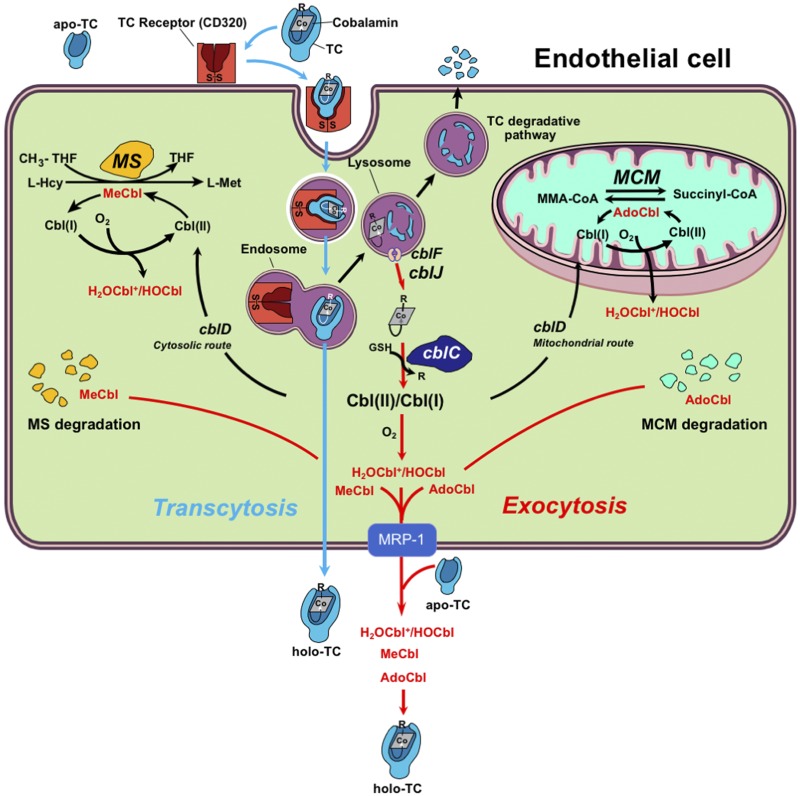
We show that holo-TC, the bioactive fraction of circulating B12, is first, endocytosed, and then, transcytosed, in endothelial cells in a bidirectional manner. Under our experimental conditions, transcytosis of holo-TC represented 8% of the total source [57Co]-CNCbl-TC supplied to cultured cells at the beginning of the experiment. To our knowledge, this is the first study demonstrating endothelial transcytosis of holo-TC. Most of the endocytosed [57Co]-CNCbl-TC partitioned into 3 possible fates (Fig. 7): 1) exocytosis yielding protein-free [57Co]-CNCbl, 2) intracellular retention of [57Co]-CNCbl without chemical modification, and 3) intracellular processing of [57Co]-CNCbl by the enzyme MMACHC/cblC (26, 32) with subsequent conversion to [57Co]-MeCbl and [57Co]-AdoCbl by MS and MCM. That is consistent with previous observations that the major reservoirs of Cbl in cells are the enzymes MS and MCM. Our data uncovered that the transcellular transport of Cbl did not discriminate between the chemical structures of its β-axial ligand (methyl, adenosyl, hydroxo, cyano). Saturation of endogenously produced apo-TC with excess, unlabeled CNCbl in the receiving transcytosis chamber showed that, although transcytosis begins with Cbl bound to its carrier protein TC in the source chamber, Cbl exits the cell on the other side both bound to TC (5–10% of the total radioactivity, transcytosis) as well as in its free form (90–95% of the total radioactivity, exocytosis). Those 2 characteristics indicate that, in addition to transcytosis of holo-TC, endothelial cells may also employ the MRP1/ABCC1 transport system described in intestinal epithelial cells (8) to relay the micronutrient to other cells. In fact, expression of MRP1/ABCC1 has been demonstrated in BAECs (78) and in brain endothelial cells (79). Under physiologic conditions, in which no excess CNCbl is present in the extracellular milieu, exocytosed Cbl would rebind to apo-TC to form holo-TC (Fig. 7), the latter becoming available to supply underlying tissues with the micronutrient.
Figure 7.
Transcellular and intracellular metabolism of Cbl. Endothelial cells lining blood vessels and arteries secrete apo-TC. Apo-TC is the main carrier of dietary Cbl to all cells in the body. Cells take up holo-TC by absorptive endocytosis via the CD320 receptor. The holo-TC-CD320 complex is internalized and processed through the lysosomal pathway. Free Cbl is shuttled out of the lysosome via transporter proteins CblF and CblJ. Once in the cytosol, the cblC enzyme performs processing (reductive removal of the β-axial ligand) of the incoming Cbl, and the newly processed vitamin is directed to acceptor enzyme MS in the cytosol and MCM. That trafficking event is guided by direct interactions of catalytically active cblC with the adaptor protein cblD in the cytosol. Here, we demonstrated that Cbls can also be mobilized across the cell by either bypassing the lysosomal and processing route (transcytosis) or going through it (exocytosis), the latter permitting the recycling of uncommitted pools of MeCbl and AdoCbl originating from the degradation of MS and MCM. For clarity, the major pathway where RCbl bypasses CblC-processing and is exocytosed without change, is not shown. Because of the large surface of the endothelium, the finding of active Cbl import, processing, and transcellular transport suggests that this cellular type could have an essential role in maintaining blood–solid tissue homeostasis of vitamin B12. CoA, coenzyme A.
Cells recycle uncommitted B12 pools
We identified exocytosis of not only the source material, [57Co]-CNCbl, but also the corresponding processed forms [57Co]-HOCbl, [57Co]-MeCbl, and [57Co]-AdoCbl. Thus, endothelial cells have mechanisms to recycle uncommitted pools of those Cbl forms for further use by recipient underlying cells. This could be an important route to salvage the scarce micronutrient. Normal proteolytic turnover of MS and MCM, the only sources of MeCbl and AdoCbl in the cell, would lead to the release of their bound cofactors, which could either be internally cannibalized by the cell via the MMACHC/cblC processing protein to reenter the Cbl catalytic cycle or exocytosed for further use by other cell acceptors. The finding that endothelial cells exocytose hydroxocobalamin [the oxidation product of cob(I)alamin and cob(II)alamin] suggests that this transcellular transport pathway can also intercept reduced Cbl [cob(II)alamin and cob(I)alamin] produced by the processing enzyme MMACHC/cblC, as well as Cbl that exits the catalytic cycles of MS and MCM (Fig. 7).
Routes of endothelial transcellular transport and lysosomal involvement
Under our experimental conditions, transcellular transport of MMACHC/cblC-processed Cbl forms seemed to occur in parallel with the cblC-independent route, and a summary of those findings is provided in Fig. 7. The degradative pathway requires lysosomal destruction of holo-TC, to release the Cbl molecule for further biochemical processing and delivery to MS and MCM. The nondegradative route provides a direct mechanism whereby TC carries Cbl to adjacent cells and tissues, via transcellular transport. That pathway might include pH-dependent dissociation of holo-TC in lysosomes. followed by separate excretion of both molecules into the extracellular milieu; at which point, they might recombine again. Although in our experimental model of the endothelium, ∼40% of the source [125I]-holo-TC underwent degradation, the relative contribution of each pathway under physiologic conditions remains to be determined.
Endothelial transcytosis and exocytosis are mediated by the CD320 receptor
Effective blocking of Cbl uptake by an antibody against the TC-binding domain of soluble CD320 (sCD320) leads to inhibition of transcellular transport. Of interest is the presence of soluble variants of the TCblR CD320 receptor (soluble CD320) (13, 80–84) in human fluids, which could transiently impair endothelial import and, therefore, transcellular transport of B12. The sCD320 was found in concentrations 2–3-fold higher than holo-TC is found in human plasma, and in vitro studies showed that sCD320 coprecipitates with holo-TC after a 1:1 stoichiometry (84). The interaction between soluble and cellular CD320, as well as the proportion of their active surface concentrations in blood, presents an interesting problem for a future investigation. To date, transcytosis of Cbl has been documented for its binders IF and TC in renal and intestinal cells (Table 1), but no reports exist, to our knowledge, for transcytosis of HC in any cell type. Transcytosis of Cbl in polarized intestinal cells shows a preference toward the type and expression of the protein binder in apical and basolateral sides of the cell (34, 35, 37, 39, 85), which is in contrast to our results with vascular endothelial cells.
Role of the vascular endothelium in B12 homeostasis
Altogether, these findings show that in addition to secreting TC, the vascular endothelium has an active role in the uptake, intracellular MMACHC/cblC-mediated processing, trafficking, and transcellular transport of cobalamins, much in contrast to its generally assumed quiescent state (mostly confined to secretion of apo-TC). Our results with vascular endothelial cells demonstrate active transcytosis of bioactive vitamin B12 and warrant further investigation in other physiologically relevant endothelial compartments, such as the blood–brain barrier. Brain microvascular endothelial cells are the major component of the blood–brain barrier (86). Selective transcytosis through brain endothelial cells has been shown to affect the function of its associated proteins, such as astrocytes, by enabling transduction by adeno-associated virus (AAV9), a promising tool in gene therapy (87, 88). Studies performed with rats showed that administration of CNCbl leads to predominant accumulation of this Cbl form in the brain, whereas administration of HOCbl leads to substantial localization of the vitamin in the liver (89). This suggests that some selection mechanisms affect the distribution of Cbl across tissues, and it is thus plausible that the endothelial lining of the general vasculature, as a large surface and an important source of the transport protein TC, may have a key role in orchestrating micronutrient uptake in different organs, including Cbl access to the brain. The rapid clearance of holo-TC in human plasma of about 60–120 min (90) suggests that endothelial cells could quickly respond to varying cellular demands for apo-TC and cobalamins. From a metabolic standpoint, endothelial cells lack an active transsulfuration pathway, as demonstrated by negligible or no activity of the enzyme cystathionine-β-synthase (91), and therefore, the presence of active cellular processing of B12 and transcytosis could represent an essential mechanism for the homeostasis of homocysteine via its remethylation to form methionine. In sum, the vascular endothelium exhibits active routes for the handling of B12, a micronutrient that is crucial in 1-carbon metabolism, which challenges the paradigm of its quiescent nature under nonproliferating conditions.
Our findings suggest that the vascular endothelium may be a central component for serum-tissue homeostasis of vitamin B12. The work was performed on an in vitro model of the vascular aortic endothelium, which itself represents a shortcoming. It remains to be investigated whether a similar mechanism operates for the transcellular transport of Cbl in endothelial cells of other body compartments, such as vascular capillaries and the blood–brain barrier. Our culture conditions do not incorporate pulsatile blood flow, but rather, static cell growth conditions that are not physiologic. Further studies employing isolated aortas or veins maintained under continuous pulsatile flow of culture medium are required to elucidate whether sheer-stress has a role in endothelial homeostasis of vitamin B12. Our selected conditions of concentration of holo-TC of 100–140 pM mimic physiology but do not include excess apo-TC, which is typically found in humans who do not receive vitamin B12 supplements. In addition, humans are exposed to small daily doses of dietary B12, which could predictably modify the time course of biosynthesis of apo-TC and the relative ratios of apo-TC to holo-TC, thus affecting the availability of holo-TC for transcytosis. Individual variation in the levels of holo-TC has been documented (92), but such effects have not been accounted for in experimental models of the endothelial vasculature.
In vivo studies concerning Cbl transport across tissues are lacking. The recent development of relevant animal models presents a great opportunity for further research. For example, 2 studies generated and characterized Mrp1 (Abcc1) knockout mice and showed that genetic deletion of Mrp1 has a protective role against endothelial damage (93, 94). It would be interesting to examine the transport of Cbl in mouse endothelial cells that lack Mrp1. One of the researchers (E.V.Q.) has generated and characterized Cbl transport in a knockout mouse model of the TC receptor CD320 (95–97). Surprisingly, the CD320 knockout mice did not exhibit systemic Cbl deficiency, which suggested marked differences in Cbl transport between rodents and humans. A specific and severe depletion of Cbl was, however, detected in the central nervous system of the CD320 knockout mice, which manifested with elevated homocysteine and methylmalonic acid and an abnormal methylation status (97). Noteworthy, differences between rodents and humans have also been documented for plasmatic Cbl binders. Work by Hygum et al. (98) demonstrated that mice do not carry a gene for haptocorrin and that the function of haptocorrin seen in humans is performed by the sole Cbl plasma transporter in rodents, TC. In the context of our findings, we predict that in vivo studies with animal models carrying defective Cbl transporters and/or receptors will help us to understand the organ specificity of Cbl transcellular transport and whether such mechanisms are conserved among species.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was funded by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grant HL71907 (to D.W.J.). L.H. acknowledges intramural funding support from the Department of Pediatrics, University of Freiburg Medical Center. The authors declare no conflicts of interest.
Glossary
- AdoCbl
5′-deoxyadenosylcobalamin
- apo
apoprotein
- BAEC
bovine aortic endothelial cell
- Cbl
cobalamin
- cblA–J
cobalamin complementation group A–J
- CD320
TC receptor (TCblR)
- CNCbl
cyanocobalamin (vitamin B12)
- Ds-RED-TC
monomeric red fluorescent protein fusion with full-length TC
- FBS
fetal bovine serum
- HAEC
human aortic endothelial cell
- HC
haptocorrin
- HOCbl
hydroxocobalamin
- IF
intrinsic factor
- MCM
methylmalonyl-coenzyme A mutase
- MeCbl
methylcobalamin
- MMACHC
methylmalonic aciduria and homocystinuria type C (cblC)
- MMADHC
methylmalonic aciduria type D and homocystinuria (cblD)
- MRP1
multidrug resistance associated protein 1
- MS
methionine synthase
- PET
polyethylene-terephthalate
- TC
transcobalamin
- TCblR
transcobalamin receptor (CD320)
- UCBC
unsaturated cobalamin binding capacity
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. Hannibal designed and executed experiments and wrote the manuscript; K. Bolisetty executed the experiments; A. Axhemi and P. M. DiBello provided technical assistance and executed experiments; E. V. Quadros and S. Fedosov designed the experiments and provided methods; D. W. Jacobsen designed the experiments and wrote the manuscript; and all authors contributed significantly to the conception of the research and the preparation of the final version of the manuscript.
REFERENCES
- 1.Green R. (2017) Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 129, 2603–2611 [DOI] [PubMed] [Google Scholar]
- 2.Hannibal L., Lysne V., Bjørke-Monsen A. L., Behringer S., Grünert S. C., Spiekerkoetter U., Jacobsen D. W., Blom H. J. (2016) Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front. Mol. Biosci. 3, 27; erratum: 4, 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helliwell K. E., Wheeler G. L., Leptos K. C., Goldstein R. E., Smith A. G. (2011) Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol. Biol. Evol. 28, 2921–2933 [DOI] [PubMed] [Google Scholar]
- 4.U.S. Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline (1998) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline, National Academies Press, Washington D.C. [PubMed] [Google Scholar]
- 5.Banerjee R. (2006) B12 trafficking in mammals: a for coenzyme escort service. ACS Chem. Biol. 1, 149–159 [DOI] [PubMed] [Google Scholar]
- 6.Fedosov S. N. (2012) Physiological and molecular aspects of cobalamin transport. Subcell. Biochem. 56, 347–367 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen M. J., Rasmussen M. R., Andersen C. B., Nexø E., Moestrup S. K. (2012) Vitamin B12 transport from food to the body’s cells—a sophisticated, multistep pathway. Nat. Rev. Gastroenterol. Hepatol. 9, 345–354 [DOI] [PubMed] [Google Scholar]
- 8.Beedholm-Ebsen R., van de Wetering K., Hardlei T., Nexø E., Borst P., Moestrup S. K. (2010) Identification of multidrug resistance protein 1 (MRP1/ABCC1) as a molecular gate for cellular export of cobalamin. Blood 115, 1632–1639 [DOI] [PubMed] [Google Scholar]
- 9.Fyfe J. C., Madsen M., Højrup P., Christensen E. I., Tanner S. M., de la Chapelle A., He Q., Moestrup S. K. (2004) The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 103, 1573–1579 [DOI] [PubMed] [Google Scholar]
- 10.Quadros E. V., Regec A. L., Khan K. M., Quadros E., Rothenberg S. P. (1999) Transcobalamin II synthesized in the intestinal villi facilitates transfer of cobalamin to the portal blood. Am. J. Physiol. 277, G161–G166 [DOI] [PubMed] [Google Scholar]
- 11.Morkbak A. L., Poulsen S. S., Nexo E. (2007) Haptocorrin in humans. Clin. Chem. Lab. Med. 45, 1751–1759 [DOI] [PubMed] [Google Scholar]
- 12.Nexo E., Hoffmann-Lücke E. (2011) Holotranscobalamin, a marker of vitamin B12 status: analytical aspects and clinical utility. Am. J. Clin. Nutr. 94, 359S–365S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendt J. F., Quadros E. V., Nexo E. (2011) Soluble transcobalamin receptor, sCD320, is present in human serum and relates to serum cobalamin—establishment and validation of an ELISA. Clin. Chem. Lab. Med. 50, 515–519 [DOI] [PubMed] [Google Scholar]
- 14.Lai S. C., Nakayama Y., Sequeira J. M., Quadros E. V. (2011) Down-regulation of transcobalamin receptor TCblR/CD320 by siRNA inhibits cobalamin uptake and proliferation of cells in culture. Exp. Cell Res. 317, 1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W., Nakayama Y., Sequeira J. M., Quadros E. V. (2011) Characterizing monoclonal antibodies to antigenic domains of TCblR/CD320, the receptor for cellular uptake of transcobalamin-bound cobalamin. Drug Deliv. 18, 74–78 [DOI] [PubMed] [Google Scholar]
- 16.Quadros E. V., Lai S. C., Nakayama Y., Sequeira J. M., Hannibal L., Wang S., Jacobsen D. W., Fedosov S., Wright E., Gallagher R. C., Anastasio N., Watkins D., Rosenblatt D. S. (2010) Positive newborn screen for methylmalonic aciduria identifies the first mutation in TCblR/CD320, the gene for cellular uptake of transcobalamin-bound vitamin B12. Hum. Mutat. 31, 924–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pangilinan F., Mitchell A., VanderMeer J., Molloy A. M., Troendle J., Conley M., Kirke P. N., Sutton M., Sequeira J. M., Quadros E. V., Scott J. M., Mills J. L., Brody L. C. (2010) Transcobalamin II receptor polymorphisms are associated with increased risk for neural tube defects. J. Med. Genet. 47, 677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W., Sequeira J. M., Nakayama Y., Lai S. C., Quadros E. V. (2010) Characterization of the promoter region of TCblR/CD320 gene, the receptor for cellular uptake of transcobalamin-bound cobalamin. Gene 466, 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quadros E. V., Nakayama Y., Sequeira J. M. (2009) The protein and the gene encoding the receptor for the cellular uptake of transcobalamin-bound cobalamin. Blood 113, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quadros E. V., Nakayama Y., Sequeira J. M. (2005) The binding properties of the human receptor for the cellular uptake of vitamin B12. Biochem. Biophys. Res. Commun. 327, 1006–1010 [DOI] [PubMed] [Google Scholar]
- 21.Alam A., Woo J. S., Schmitz J., Prinz B., Root K., Chen F., Bloch J. S., Zenobi R., Locher K. P. (2016) Structural basis of transcobalamin recognition by human CD320 receptor. Nat. Commun. 7, 12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gailus S., Suormala T., Malerczyk-Aktas A. G., Toliat M. R., Wittkampf T., Stucki M., Nürnberg P., Fowler B., Hennermann J. B., Rutsch F. (2010) A novel mutation in LMBRD1 causes the cblF defect of vitamin B12 metabolism in a Turkish patient. J. Inherit. Metab. Dis. 33, 17–24 [DOI] [PubMed] [Google Scholar]
- 23.Rutsch F., Gailus S., Miousse I. R., Suormala T., Sagné C., Toliat M. R., Nürnberg G., Wittkampf T., Buers I., Sharifi A., Stucki M., Becker C., Baumgartner M., Robenek H., Marquardt T., Höhne W., Gasnier B., Rosenblatt D. S., Fowler B., Nürnberg P. (2009) Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism. Nat. Genet. 41, 234–239 [DOI] [PubMed] [Google Scholar]
- 24.Rutsch F., Gailus S., Suormala T., Fowler B. (2011) LMBRD1: the gene for the cblF defect of vitamin B12 metabolism. J. Inherit. Metab. Dis. 34, 121–126 [DOI] [PubMed] [Google Scholar]
- 25.Kim J. C., Lee N. C., Hwu P. W., Chien Y. H., Fahiminiya S., Majewski J., Watkins D., Rosenblatt D. S. (2012) Late onset of symptoms in an atypical patient with the cblJ inborn error of vitamin B12 metabolism: diagnosis and novel mutation revealed by exome sequencing. Mol. Genet. Metab. 107, 664–668 [DOI] [PubMed] [Google Scholar]
- 26.Coelho D., Kim J. C., Miousse I. R., Fung S., du Moulin M., Buers I., Suormala T., Burda P., Frapolli M., Stucki M., Nürnberg P., Thiele H., Robenek H., Höhne W., Longo N., Pasquali M., Mengel E., Watkins D., Shoubridge E. A., Majewski J., Rosenblatt D. S., Fowler B., Rutsch F., Baumgartner M. R. (2012) Mutations in ABCD4 cause a new inborn error of vitamin B12 metabolism. Nat. Genet. 44, 1152–1155 [DOI] [PubMed] [Google Scholar]
- 27.Hannibal L., Kim J., Brasch N. E., Wang S., Rosenblatt D. S., Banerjee R., Jacobsen D. W. (2009) Processing of alkylcobalamins in mammalian cells: a role for the MMACHC (cblC) gene product. Mol. Genet. Metab. 97, 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerner-Ellis J. P., Tirone J. C., Pawelek P. D., Doré C., Atkinson J. L., Watkins D., Morel C. F., Fujiwara T. M., Moras E., Hosack A. R., Dunbar G. V., Antonicka H., Forgetta V., Dobson C. M., Leclerc D., Gravel R. A., Shoubridge E. A., Coulton J. W., Lepage P., Rommens J. M., Morgan K., Rosenblatt D. S. (2006) Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat. Genet. 38, 93–100; erratum: 957 [DOI] [PubMed] [Google Scholar]
- 29.Gherasim C., Hannibal L., Rajagopalan D., Jacobsen D. W., Banerjee R. (2013) The C-terminal domain of CblD interacts with cblC and influences intracellular cobalamin partitioning. Biochimie 95, 1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coelho D., Suormala T., Stucki M., Lerner-Ellis J. P., Rosenblatt D. S., Newbold R. F., Baumgartner M. R., Fowler B. (2008) Gene identification for the cblD defect of vitamin B12 metabolism. N. Engl. J. Med. 358, 1454–1464 [DOI] [PubMed] [Google Scholar]
- 31.Plesa M., Kim J., Paquette S. G., Gagnon H., Ng-Thow-Hing C., Gibbs B. F., Hancock M. A., Rosenblatt D. S., Coulton J. W. (2011) Interaction between MMACHC and MMADHC, two human proteins participating in intracellular vitamin B12 metabolism. Mol. Genet. Metab. 102, 139–148 [DOI] [PubMed] [Google Scholar]
- 32.Hannibal L., DiBello P. M., Jacobsen D. W. (2013) Proteomics of vitamin B12 processing. Clin. Chem. Lab. Med. 51, 477–488 [DOI] [PubMed] [Google Scholar]
- 33.Kim J., Hannibal L., Gherasim C., Jacobsen D. W., Banerjee R. (2009) A human vitamin B12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins. J. Biol. Chem. 284, 33418–33424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pons L., Guy M., Lambert D., Hatier R., Guéant J. (2000) Transcytosis and coenzymatic conversion of [57Co]cobalamin bound to either endogenous transcobalamin II or exogenous intrinsic factor in Caco-2 cells. Cell. Physiol. Biochem. 10, 135–148 [DOI] [PubMed] [Google Scholar]
- 35.Bose S., Seetharam S., Dahms N. M., Seetharam B. (1997) Bipolar functional expression of transcobalamin II receptor in human intestinal epithelial Caco-2 cells. J. Biol. Chem. 272, 3538–3543 [DOI] [PubMed] [Google Scholar]
- 36.Ramanujam K. S., Seetharam S., Dahms N. M., Seetharam B. (1994) Effect of processing inhibitors on cobalamin (vitamin B12) transcytosis in polarized opossum kidney cells. Arch. Biochem. Biophys. 315, 8–15 [DOI] [PubMed] [Google Scholar]
- 37.Dan N., Cutler D. F. (1994) Transcytosis and processing of intrinsic factor-cobalamin in Caco-2 cells. J. Biol. Chem. 269, 18849–18855 [PubMed] [Google Scholar]
- 38.Ramanujam K. S., Seetharam S., Seetharam B. (1992) Leupeptin and ammonium chloride inhibit intrinsic factor mediated transcytosis of [57Co]cobalamin across polarized renal epithelial cells. Biochem. Biophys. Res. Commun. 182, 439–446 [DOI] [PubMed] [Google Scholar]
- 39.Ramanujam K. S., Seetharam S., Ramasamy M., Seetharam B. (1991) Expression of cobalamin transport proteins and cobalamin transcytosis by colon adenocarcinoma cells. Am. J. Physiol. 260, G416–G422 [DOI] [PubMed] [Google Scholar]
- 40.Ramanujam K. S., Seetharam S., Dahms N. M., Seetharam B. (1991) Functional expression of intrinsic factor-cobalamin receptor by renal proximal tubular epithelial cells. J. Biol. Chem. 266, 13135–13140 [PubMed] [Google Scholar]
- 41.Guéant J. L., Gérard A., Monin B., Champigneulle B., Gérard H., Nicolas J. P. (1988) Radioautographic localisation of iodinated human intrinsic factor in the guinea pig ileum using electron microscopy. Gut 29, 1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider H., Miller R. K. (2010) Receptor-mediated uptake and transport of macromolecules in the human placenta. Int. J. Dev. Biol. 54, 367–375 [DOI] [PubMed] [Google Scholar]
- 43.Perez-D’Gregorio R. E., Miller R. K. (1998) Transport and endogenous release of vitamin B12 in the dually perfused human placenta. J. Pediatr. 132, S35–S42 [DOI] [PubMed] [Google Scholar]
- 44.Fernandes-Costa F., Metz J. (1979) Transplacental transport in the rabbit of vitamin B12 bound to human transcobalamin I, II and III. Br. J. Haematol. 43, 625–630 [DOI] [PubMed] [Google Scholar]
- 45.Quadros E. V., Sai P., Rothenberg S. P. (1994) Characterization of the human placental membrane receptor for transcobalamin II–cobalamin. Arch. Biochem. Biophys. 308, 192–199 [DOI] [PubMed] [Google Scholar]
- 46.Arora K., Sequeira J. M., Quadros E. V. (2017) Maternofetal transport of vitamin B12: role of TCblR/CD320 and megalin. FASEB J. 31, 3098–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lysne V., Strand E., Svingen G. F., Bjørndal B., Pedersen E. R., Midttun Ø., Olsen T., Ueland P. M., Berge R. K., Nygård O. (2016) Peroxisome proliferator-activated receptor activation is associated with altered plasma one-carbon metabolites and B-vitamin status in rats. Nutrients 8, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devalia V., Hamilton M. S., Molloy A. M.; British Committee for Standards in Haematology (2014) Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 166, 496–513 [DOI] [PubMed] [Google Scholar]
- 49.Carmel R. (2000) Current concepts in cobalamin deficiency. Annu. Rev. Med. 51, 357–375 [DOI] [PubMed] [Google Scholar]
- 50.Solomon L. R. (2005) Cobalamin-responsive disorders in the ambulatory care setting: unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood 105, 978–985, author reply 1137 [DOI] [PubMed] [Google Scholar]
- 51.Hannibal L., Axhemi A., Glushchenko A. V., Moreira E. S., Brasch N. E., Jacobsen D. W. (2008) Accurate assessment and identification of naturally occurring cellular cobalamins. Clin. Chem. Lab. Med. 46, 1739–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quadros E. V., Jacobsen D. W. (1995) The dynamics of cobalamin utilization in L-1210 mouse leukemia cells: a model of cellular cobalamin metabolism. Biochim. Biophys. Acta 1244, 395–403 [DOI] [PubMed] [Google Scholar]
- 53.Hannibal L., DiBello P. M., Yu M., Miller A., Wang S., Willard B., Rosenblatt D. S., Jacobsen D. W. (2011) The MMACHC proteome: hallmarks of functional cobalamin deficiency in humans. Mol. Genet. Metab. 103, 226–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuma P., Hubbard A. L. (2003) Transcytosis: crossing cellular barriers. Physiol. Rev. 83, 871–932 [DOI] [PubMed] [Google Scholar]
- 55.Fedosov S. N., Berglund L., Nexo E., Petersen T. E. (1999) Sequence, S-S bridges, and spectra of bovine transcobalamin expressed in Pichia pastoris. J. Biol. Chem. 274, 26015–26020 [DOI] [PubMed] [Google Scholar]
- 56.Gottlieb C., Lau K. S., Wasserman L. R., Herbert V. (1965) Rapid charcoal assay for intrinsic factor (If), gastric juice unsaturated B12 binding capacity, antibody to if, and serum unsaturated B12 binding capacity. Blood 25, 875–884 [PubMed] [Google Scholar]
- 57.Quadros E. V., Rothenberg S. P., Pan Y. C., Stein S. (1986) Purification and molecular characterization of human transcobalamin II. J. Biol. Chem. 261, 15455–15460 [PubMed] [Google Scholar]
- 58.Bor M. V., Nexø E., Hvas A. M. (2004) Holo-transcobalamin concentration and transcobalamin saturation reflect recent vitamin B12 absorption better than does serum vitamin B12. Clin. Chem. 50, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 59.Nexo E., Hvas A. M., Bleie Ø., Refsum H., Fedosov S. N., Vollset S. E., Schneede J., Nordrehaug J. E., Ueland P. M., Nygard O. K. (2002) Holo-transcobalamin is an early marker of changes in cobalamin homeostasis: a randomized placebo-controlled study. Clin. Chem. 48, 1768–1771 [PubMed] [Google Scholar]
- 60.Herbert V. (1994) Staging vitamin B12 (cobalamin) status in vegetarians. Am. J. Clin. Nutr. 59(Suppl 5), 1213S–1222S [DOI] [PubMed] [Google Scholar]
- 61.Von Castel-Roberts K. M., Morkbak A. L., Nexo E., Edgemon C. A., Maneval D. R., Shuster J. J., Valentine J. F., Kauwell G. P., Bailey L. B. (2007) Holo-transcobalamin is an indicator of vitamin B12 absorption in healthy adults with adequate vitamin B12 status. Am. J. Clin. Nutr. 85, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 62.Fràter-Schröder M., Porck H. J., Erten J., Müller M. R., Steinmann B., Kierat L., Arwert F. (1985) Synthesis and secretion of the human vitamin B12-binding protein, transcobalamin II, by cultured skin fibroblasts and by bone marrow cells. Biochim. Biophys. Acta 845, 421–427 [DOI] [PubMed] [Google Scholar]
- 63.Quadros E. V., Rothenberg S. P., Jaffe E. A. (1989) Endothelial cells from human umbilical vein secrete functional transcobalamin II. Am. J. Physiol. 256, C296–C303 [DOI] [PubMed] [Google Scholar]
- 64.Watkins D., Rosenblatt D. S. (2013) Lessons in biology from patients with inborn errors of vitamin B12 metabolism. Biochimie 95, 1019–1022 [DOI] [PubMed] [Google Scholar]
- 65.Zhao H., Ruberu K., Li H., Garner B. (2016) Cell type-specific modulation of cobalamin uptake by bovine serum. PLoS One 11, e0167044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Begley J. A., Colligan P. D., Chu R. C. (1994) Synthesis and secretion of transcobalamin II by cultured astrocytes derived from human brain tissue. J. Neurol. Sci. 122, 57–60 [DOI] [PubMed] [Google Scholar]
- 67.Ramanujam K. S., Seetharam S., Seetharam B. (1991) Synthesis and secretion of cobalamin binding proteins by opossum kidney cells. Biochem. Biophys. Res. Commun. 179, 543–550 [DOI] [PubMed] [Google Scholar]
- 68.Schohn H., Guéant J. L., Girr M., Nexø E., Baricault L., Zweibaum A., Nicolas J. P. (1991) Synthesis and secretion of a cobalamin-binding protein by HT 29 cell line. Biochem. J. 280, 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamani L., Gibbs B. F., Gilfix B. M., Watkins D., Hosack A., Rosenblatt D. S. (2008) Transcobalamin in cultured fibroblasts from patients with inborn errors of vitamin B12 metabolism. Mol. Genet. Metab. 95, 104–106 [DOI] [PubMed] [Google Scholar]
- 70.Li N., Seetharam S., Rosenblatt D. S., Seetharam B. (1994) Expression of transcobalamin II mRNA in human tissues and cultured fibroblasts from normal and transcobalamin II-deficient patients. Biochem. J. 301, 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soda R., Tavassoli M., Jacobsen D. W. (1985) Receptor distribution and the endothelial uptake of transcobalamin II in liver cell suspensions. Blood 65, 795–802 [PubMed] [Google Scholar]
- 72.Carmel R., Neely S. M., Francis R. B., Jr (1990) Human umbilical vein endothelial cells secrete transcobalamin II. Blood 75, 251–254 [PubMed] [Google Scholar]
- 73.Regec A. L., Quadros E. V., Rothenberg S. P. (2002) Transcobalamin II expression is regulated by transcription factor(s) binding to a hexameric sequence (TGGTCC) in the promoter region of the gene. Arch. Biochem. Biophys. 407, 202–208 [DOI] [PubMed] [Google Scholar]
- 74.Thul P. J., Åkesson L., Wiking M., Mahdessian D., Geladaki A., Ait Blal H., Alm T., Asplund A., Björk L., Breckels L. M., Bäckström A., Danielsson F., Fagerberg L., Fall J., Gatto L., Gnann C., Hober S., Hjelmare M., Johansson F., Lee S., Lindskog C., Mulder J., Mulvey C. M., Nilsson P., Oksvold P., Rockberg J., Schutten R., Schwenk J. M., Sivertsson Å., Sjöstedt E., Skogs M., Stadler C., Sullivan D. P., Tegel H., Winsnes C., Zhang C., Zwahlen M., Mardinoglu A., Pontén F., von Feilitzen K., Lilley K. S., Uhlén M., Lundberg E. (2017) A subcellular map of the human proteome. Science 356, 816–819 [DOI] [PubMed] [Google Scholar]
- 75.Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C. A., Odeberg J., Djureinovic D., Takanen J. O., Hober S., Alm T., Edqvist P. H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J. M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. (2015) Proteomics: tissue-based map of the human proteome. Science 347, 1260419 [DOI] [PubMed] [Google Scholar]
- 76.Uhlen M., Oksvold P., Fagerberg L., Lundberg E., Jonasson K., Forsberg M., Zwahlen M., Kampf C., Wester K., Hober S., Wernerus H., Björling L., Ponten F. (2010) Towards a knowledge-based human protein atlas. Nat. Biotechnol. 28, 1248–1250 [DOI] [PubMed] [Google Scholar]
- 77.Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F., Sanli K., von Feilitzen K., Oksvold P., Lundberg E., Hober S., Nilsson P., Mattsson J., Schwenk J. M., Brunnström H., Glimelius B., Sjöblom T., Edqvist P. H., Djureinovic D., Micke P., Lindskog C., Mardinoglu A., Ponten F. (2017) A pathology atlas of the human cancer transcriptome. Science 357, eaan2507. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi K., Tatsunami R., Sato K., Tampo Y. (2012) Multidrug resistance associated protein 1 together with glutathione plays a protective role against 4-hydroxy-2-nonenal-induced oxidative stress in bovine aortic endothelial cells. Biol. Pharm. Bull. 35, 1269–1274 [DOI] [PubMed] [Google Scholar]
- 79.Zhang W., Mojsilovic-Petrovic J., Andrade M. F., Zhang H., Ball M., Stanimirovic D. B. (2003) The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 17, 2085–2087 [DOI] [PubMed] [Google Scholar]
- 80.Abuyaman O., Torring N., Obeid R., Nexo E. (2016) First trimester serum levels of the soluble transcobalamin receptor, holo-transcobalamin, and total transcobalamin in relation to preeclampsia risk. Scand. J. Clin. Lab. Invest. 76, 641–644 [DOI] [PubMed] [Google Scholar]
- 81.Abuyaman O., Nexo E. (2015) The soluble transcobalamin receptor (sCD320) is present in cerebrospinal fluid and correlates to dementia-related biomarkers tau proteins and amyloid-beta. Scand. J. Clin. Lab. Invest. 75, 514–518 [DOI] [PubMed] [Google Scholar]
- 82.Hoffmann-Lücke E., Arendt J. F., Nissen P. H., Mikkelsen G., Aasly J. O., Nexo E. (2013) Three family members with elevated plasma cobalamin, transcobalamin and soluble transcobalamin receptor (sCD320). Clin. Chem. Lab. Med. 51, 677–682 [DOI] [PubMed] [Google Scholar]
- 83.Arendt J. F., Nexo E. (2012) Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoS One 7, e45979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abuyaman O., Andreasen B. H., Kronborg C., Vittinghus E., Nexo E. (2013) The soluble receptor for vitamin B12 uptake (sCD320) increases during pregnancy and occurs in higher concentration in urine than in serum. PLoS One 8, e73110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bose S., Kalra S., Yammani R. R., Ahuja R., Seetharam B. (2007) Plasma membrane delivery, endocytosis and turnover of transcobalamin receptor in polarized human intestinal epithelial cells. J. Physiol. 581, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malinovskaya N. A., Komleva Y. K., Salmin V. V., Morgun A. V., Shuvaev A. N., Panina Y. A., Boitsova E. B., Salmina A. B. (2016) Endothelial progenitor cells physiology and metabolic plasticity in brain angiogenesis and blood–brain barrier modeling. Front. Physiol. 7, 599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Merkel S. F., Andrews A. M., Lutton E. M., Mu D., Hudry E., Hyman B. T., Maguire C. A., Ramirez S. H. (2017) Trafficking of adeno-associated virus vectors across a model of the blood–brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. J. Neurochem. 140, 216–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber-Adrian D., Heinen S., Silburt J., Noroozian Z., Aubert I. (2017) The human brain endothelial barrier: transcytosis of AAV9, transduction by AAV2: an editorial highlight for ‘trafficking of adeno-associated virus vectors across a model of the blood–brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells.’ J. Neurochem. 140, 192–194 [DOI] [PubMed] [Google Scholar]
- 89.Kornerup L. S., Fedosov S. N., Juul C. B., Greibe E., Heegaard C. W., Nexo E. (2017) Tissue distribution of oral vitamin B12 is influenced by B12 status and B12 form: an experimental study in rats. Eur. J. Nutr. 57, 1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hom B. L., Olesen H. A. (1969) Plasma clearance of 57cobalt-labelled vitamin B12 bound in vitro and in vivo to transcobalamin I and II. Scand. J. Clin. Lab. Invest. 23, 201–211 [DOI] [PubMed] [Google Scholar]
- 91.Chen P., Poddar R., Tipa E. V., Dibello P. M., Moravec C. D., Robinson K., Green R., Kruger W. D., Garrow T. A., Jacobsen D. W. (1999) Homocysteine metabolism in cardiovascular cells and tissues: implications for hyperhomocysteinemia and cardiovascular disease. Adv. Enzyme Regul. 39, 93–109 [DOI] [PubMed] [Google Scholar]
- 92.Brokner M., Hager H. B., Lindberg M. (2017) Biological variation of holotranscobalamin and cobalamin in healthy individuals. Scand. J. Clin. Lab. Invest. 77, 433–436 [DOI] [PubMed] [Google Scholar]
- 93.Jehle J., Müller C. F. H., Aksoy A., Zimmer S., Nickenig G., Tiyerili V. (2017) Genetic disruption of multidrug resistance-associated protein 1 improves endothelial function and attenuates atherosclerosis in MRP1−/− LDLr−/− double knockout mice. Arch. Med. Sci. 13, 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neuser J., Fraccarollo D., Wick M., Bauersachs J., Widder J. D. (2016) Multidrug resistance associated protein-1 (MRP1) deficiency attenuates endothelial dysfunction in diabetes. J. Diabetes Complications 30, 623–627 [DOI] [PubMed] [Google Scholar]
- 95.Arora K., Sequeira J. M., Hernández A. I., Alarcon J. M., Quadros E. V. (2017) Behavioral alterations are associated with vitamin B12 deficiency in the transcobalamin receptor/CD320 KO mouse. PLoS One 12, e0177156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernàndez-Roig S., Lai S. C., Murphy M. M., Fernandez-Ballart J., Quadros E. V. (2012) Vitamin B12 deficiency in the brain leads to DNA hypomethylation in the TCblR/CD320 knockout mouse. Nutr. Metab. (Lond.) 9, 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lai S. C., Nakayama Y., Sequeira J. M., Wlodarczyk B. J., Cabrera R. M., Finnell R. H., Bottiglieri T., Quadros E. V. (2013) The transcobalamin receptor knockout mouse: a model for vitamin B12 deficiency in the central nervous system. FASEB J. 27, 2468–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hygum K., Lildballe D. L., Greibe E. H., Morkbak A. L., Poulsen S. S., Sorensen B. S., Petersen T. E., Nexo E. (2011) Mouse transcobalamin has features resembling both human transcobalamin and haptocorrin. PLoS One 6, e20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.