Abstract
Previous evidence has suggested that dietary supplementation with a bioactive dietary polyphenol preparation (BDPP) rescues impairment of hippocampus-dependent memory in a mouse model of sleep deprivation (SD). In the current study, we extend our previous evidence and demonstrate that a mechanism by which dietary BDPP protects against SD-mediated cognitive impairment is via mechanisms that involve phosphorylation of the mammalian target of rapamycin complex 1 and its direct downstream targets, including the eukaryotic translation initiation factor 4E (eIF4E)–binding protein 1 (4E-BP1) and the ribosomal protein S6 kinase β-1 (p70S6K). In additional mechanistic studies in vitro, we identified the brain bioavailable phenolic metabolites derived from the metabolism of dietary BDPP that are responsible for the attenuation of SD-mediated memory impairments. On the basis of high-throughput bioavailability studies of brain bioavailable metabolites after dietary BDPP treatment, we found that select polyphenol metabolites [e.g., cyanidin-3′-O-glucoside and 3-(3′-hydroxyphenyl) propionic acid] were able to rescue mTOR and p70S6K phosphorylation in primary cortico-hippocampal neuronal cultures, as well as rescue 4E-BP1 phosphorylation in response to treatment with 4EGI-1, a specific inhibitor of eIF4E–eIF4G interaction. Our findings reveal a previously unknown role for dietary polyphenols in the rescue of SD-mediated memory impairments via mechanisms involving the promotion of protein translation.—Frolinger, T., Smith, C., Cobo, C. F., Sims, S., Brathwaite, J., de Boer, S., Huang, J., Pasinetti, G. M. Dietary polyphenols promote resilience against sleep deprivation–induced cognitive impairment by activating protein translation.
Keywords: memory consolidation, mTORC1, BDPP
Sleep deprivation (SD) is a public health epidemic of considerable health, social, and economic impact (1). Insufficient sleep is associated with deleterious effects on cognitive function (2, 3) is and highly comorbid with many neurodegenerative and neuropsychiatric disorders (4, 5); therefore, it is important to explore the molecular and cellular impacts of sleep loss in an effort to identify novel therapeutic approaches to counteract these effects.
SD compromises the function of the hippocampus that are crucial for the formation of spatial, contextual, and declarative memories (6, 7). In particular, SD disrupts the hippocampus-dependent consolidation of long-term memory (8–11) and synaptic plasticity in the hippocampal formation (12, 13). New gene expression, including the synthesis of new mRNAs and proteins, is a critical requirement for the conversion of the newly acquired memory to consolidated long-term memory (14, 15). At the molecular level, experience-dependent synaptic plasticity triggers the translation of mRNAs at remodeling synapses necessary for long-lasting changes in synaptic strength (16, 17). This is emerging as an important mechanism for persistent forms of synaptic plasticity both in vitro and in certain forms of memory consolidation (17).
Accumulating evidence has suggested that SD may affect protein translation, thus disrupting the mechanisms of memory consolidation in the hippocampus in experimental models. Hippocampus-dependent long-term memory is most strongly impacted by SD when animals are deprived of sleep during the first 5–6 h after learning (8, 18). This window of time coincides with the period of memory consolidation, which depends critically on protein synthesis (19–21). It has been reported that, of the various forms of synaptic plasticity, SD inhibits the induction of hippocampal long-term potentiation, a neural substrate of learning and memory (22). In particular, SD has been identified to impair late-phase long-term potentiation (23, 24), which correlates with long-term memory and requires the de novo synthesis of proteins (25–27).
Although most steps of protein translation—initiation, elongation, and mRNA sequestration—have been implicated in the regulation of plasticity-dependent translation (28, 29), the critical step for de novo protein production occurs at translation initiation, which is primarily controlled by the mammalian target of rapamycin complex 1 (mTORC1) pathway (30, 31). mTORC1, via its direct downstream target, eukaryotic initiation factor 4E (eIF4E) –binding protein 1 (4E-BP1), regulates the translation initiation of 5′ capped mRNA, which comprises most of the mRNA in the cell (31)·Phosphorylation of 4E-BP1 prevents its binding to eIF4E, which frees eIF4E to bind with eIF4G to stimulate translation. mTORC1 also promotes protein synthesis by activating the ribosomal protein S6 kinase β-1 (p70S6K) that phosphorylates the ribosomal protein S6 (RPS6) pathway to facilitate translation initiation (32, 33).
Recent studies of animal models have suggested that SD impairs the protein translation that is required for memory consolidation by impairing the mTORC1 signaling pathway. SD has been shown to decrease total mTORC1 protein abundance (34) after SD via mechanisms that result in impaired hippocampal protein synthesis (35).
We have previously reported that dietary supplementation with a bioactive dietary polyphenol preparation (BDPP) comprised of grape seed polyphenol extract (GSPE), concord grape juice, and resveratrol is effective in protecting against impaired performance in hippocampus-dependent memory tasks under conditions of SD, stress, and neurodegeneration (36–39). Evidence suggests that dietary polyphenols are effective in protecting against diverse mechanisms associated with cognitive function and general brain health, including oxidative stress and inflammation, as well as in promoting neuroplasticity and neurogenesis (40–44); however, the precise mechanism by which polyphenol metabolism confers benefits in cognitive and memory function is not yet clearly understood. Here, we demonstrate that the activation of mTORC1 pathway–dependent protein translation initiation may explain, in part, the mechanisms by which certain dietary polyphenols promote resilience to SD-mediated memory impairment.
MATERIALS AND METHODS
Chemicals
Polyphenol-free diet (AIN-93G) was purchased from Research Diets (New Brunswick, NJ, USA). Food-grade resveratrol was purchased from ChromaDex (Irvine, CA, USA). GSPE was purchased from Supplement Warehouse (Bolingbrook, IL, USA). One lot of resveratrol and 1 lot of GSPE were used for this particular study and were stored at 4°C in the dark. Concord purple grape juice (Welch Foods, Concord, MA, USA), malvidin-3-O-glucoside, cyanidin-3-O-glucoside (CYA), delphinidin-3-O-glucoside (DEL), quercetin-3′-O-glucuronide and, resveratrol-3′-O-glucuronide (Extrasynthesis, Genay Cedex, France), and 3-hydroxybenzoic acid (HBA), 3-(3′-hydroxyphenyl) propionic acid (HPP; MilliporeSigma, St. Louis, MO, USA) were obtained commercially. All tested compounds were analyzed by liquid chromatography–mass spectrometry and archived as previously reported (39, 45) in compliance with National Institutes of Health, National Center for Complementary and Integrative Health (Bethesda, MD, USA) product integrity guidelines.
Animals and treatment
C57BL/6J mice (n = 110) were purchased from The Jackson Laboratory (age 12 wk; Bar Harbor, ME, USA) and group housed (5 mice/cage) in the centralized animal care facility of the Center for Comparative Medicine and Surgery at the Icahn School of Medicine at Mount Sinai. All mice were allowed to adapt to the new environment for at least 2 wk and were tested at age 4–5 mo. To assess the effect of BDPP on memory consolidation in a model of acute SD, mice were randomly subdivided for 3 treatment groups, including the control (Ctrl) group of non-sleep-deprived (NSD) mice receiving regular drinking water (n = 40) and SD mice treated with either regular drinking water (n = 40) or with BDPP composed of GSPE, resveratrol, and concord grape juice (n = 30; SD + BDPP). BDPP treatment was delivered ad libitum via drinking water for 2 wk before and throughout behavioral testing. Calculated daily intake of GSPE was 200 mg/kg body weight (BW) (40, 46), resveratrol 400 mg/kg BW (45, 47), and total polyphenols from juice extract 183 mg/kg BW (48). These doses were chosen on the basis of the equivalent doses used in a previous study that showed efficacy in animal models for each component (38). BDPP was changed once every 2 d. To study the effect of BDPP on molecular mechanisms related to cognitive function in a model of SD, n = 15 mice per group were euthanized at the end of 5 h of SD, and brains were dissected for additional analyses. For all experiments, mice BW and food consumption were assessed once a week. Liquid consumption was assessed every 2 d. All animals were maintained on a 12-h light/dark cycle with lights on at 07:00 am in a temperature-controlled (20 ± 2°C) vivarium, and all procedures were approved by the Mount Sinai Institutional Animal Care and Use Committee.
SD
SD in mice was achieved using the Automated Sleep Deprivation System for Mice (Pinnacle Technology, Lawrence, KS, USA) (49, 50). The system used timed movements of the randomly rotating bar to prevent mice from sleeping. All mice were acclimated to the Automated Sleep Deprivation System for 30 min/d for 3 d before the SD procedure. To study the effect of SD on memory consolidation, mice were maintained in the Automated Sleep Deprivation System for 5 h, starting immediately after the 10-min training trial with ad libitum access to food and water/BDPP treatment. At the end of the 5 h of SD, mice were either euthanized for brain analyses or placed back in the home cage for the next day of behavioral testing.
Cognitive assessment of SD-induced memory impairment
To characterize the effect of polyphenol treatment in a model of SD-induced memory impairment, we conducted an object location (OL) test and a novel object recognition (NOR) test. For the OL test, mice were randomly selected for treatment groups, including NSD (n = 25), SD (n = 25), and SD + BDPP (n = 20). A separate group of mice were randomly selected for the NOR test and subdivided to NSD (n = 15), SD (n = 15), and SD + BDPP (n = 10) treatment groups. All animals were handled for 5 min/d 5 d before behavioral testing. All test trials were video recorded, tracked, and analyzed with ANY-Maze tracking software (v.5.1 beta; Stoelting, Wood Dale, IL, USA). Mice were habituated to the testing room for 30 min at the beginning of training and the test day. All tests were conducted by the same experimenter. General and locomotor activity were monitored for each test.
OL and NOR behavioral tasks
Training and test trials for OL and NOR tasks were conducted in square test boxes (40 × 40 × 35 cm) with even lighting conditions (30 ± 5 lux). Black and white external cues were placed on the walls of the test boxes. Towers of Lego (Lego Systems, Enfield, CT, USA) bricks (8-cm high and 3.2-cm wide, built in blue, yellow, red, and green bricks) and Falcon tissue culture flasks filled with sand were used as objects. Before experiments, both objects were tested with a separate cohort of mice to exclude the possibility that mice demonstrated a preference for either object. Two days before testing, we conducted habituation trials during which mice were allowed to explore the empty test box for 10 min. Twenty-four hours after the last habituation trial (d 3), we conducted a training phase. Training commenced 1 h after lights on (08:00 am). Training consisted of a 10-min exploration in the test box containing 2 identical objects (either Lego bricks or flasks). Directly after training, mice were either sleep deprived for 5 h or returned to their home cages. The next day, 24 h after the training session, mice were re-exposed to the context for 5 min, with 1 of the identical objects moved to a novel spatial location in the OL task or with 1 object changed to a novel object without changing its location in the NOR task. Placement of the moved/unmoved or changed/unchanged objects in OL and NOR tasks, respectively, followed a counterbalanced design between mice to control for location effects. Object interaction was defined as an event during which a mouse’s head was within 2 cm of the object and directed toward the object. The detection of object spatial novelty in the OL task was assessed by discrimination index (DI), calculated as follows: [(time exploring the moved object − time exploring the unmoved object)/(time exploring moved + unmoved) × 100] (51). Detection of the object’s novelty recognition in the NOR task was assessed by DI, calculated as follows: [(time exploring the changed object − time exploring the unchanged object)/(time exploring changed + unchanged) × 100] (51). After each trial, objects and boxes were cleaned with a Quatricide dilution to eliminate odor cues. Mice with <7 s of total object interaction in either trial were excluded from the analysis (52). In both tasks, mice with DI ± 20 at training were considered to have a significant location/object bias during training and were also excluded from analysis (51).
In vivo translation assay
Mice (n = 20) were fed with polyphenol-free diet for 2 wk before starting the experiment. Three weeks before SD experiments, mice were anesthetized with ketamine-xylazine and mounted onto a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) to allow for postoperative recovery. Intracerebroventricular cannulas (Plastics One, Roanoke, VA, USA) were implanted unilaterally at −0.5 mm anterioposterior, +1.0 mm mediolateral, and −2.4 dorsoventral from bregma. Implants were secured with a jewelry screw (Plastics One) and successive layers of dental cement. One week after cannula implantation, mice were randomly subdivided for 4 treatment groups, including NSD group injected with vehicle (n = 5), NSD group injected with puromycin (n = 5; NSD + puromycin), and SD groups treated with vehicle and injected with puromycin (n = 5; SD + puromycin) or treated with BDPP and injected with puromycin (n = 5; SD + BDPP + puromycin). BDPP or vehicle was delivered via drinking water for 14 d. Mice weekly BW, diet, and liquid consumption was monitored. On the day of the experiment, immediately after a 10-min training trail for OL tests, 25 μg in 1 μl puromycin (MilliporeSigma) or 1 μl vehicle was infused into the ventricle over 5 min. Mice in SD groups were then sleep deprived for 5 h, and mice in NSD groups were returned to home cage. At the end of 5 h of SD/NSD, contralateral hippocampus was dissected and flash frozen. Proteins were labeled using an adaptation of a modified nonradioactive surface sensing of translation (SUNSET) protocol (53, 54). Proteins labeled with puromycin were identified by Western blot analysis from hippocampal lysates.
Mouse primary embryonic cortico-hippocampal neuronal cultures
Primary cortico-hippocampal neurons were prepared from embryonic d 15 mouse embryos as previously described (55). Embryonic brain tissue was mechanically triturated and centrifuged. Neurons were seeded onto poly-d-lysine–coated 6-well plates and cultured in serum-free chemically defined Neurobasal medium that was supplemented with 2% B27, 0.5 mM l-glutamine, and 1% penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). Absence of astrocytes (<2%) was confirmed by the virtual absence of glial fibrillary acidic protein immunostaining (data not shown).
Dose response effect of polyphenols on gene expression
After 14 d of culture, neurons in the vehicle Ctrl group were treated with DMSO (MilliporeSigma), and neurons in treatment groups were treated with Malvidin-glucoside, CYA, DEL, quercetin-3′-O-glucuronideand, resveratrol, and resveratrol-3′-O-glucuronide at concentrations of 0.01, 0.1, and 1 nM, or with the phenolic acids HBA and HPP at concentrations of 0.01, 0.1, and 1 μM for 24 h. DMSO dilutions ranged from 105 to 107. Cells were stimulated with 15 ng/μl brain-derived neurotrophic factor (BDNF) (MilliporeSigma) for 1 h and then washed once with ice-cold PBS and subjected to RNA isolation. mRNA expression of Eif4e, Eif4g, Mtor, and P70s6k was assessed by RT-PCR. Dose response and EC50 were calculated using the sigmoidal dose-response (variable slope) curve equation (Prism 5; GraphPad Software, La Jolla, CA, USA). Potential cytotoxic effects of the individual polyphenols and their combination were tested using the quantitative colorimetric assay of lactate dehydrogenase (CytoTox 96; Promega, Madison, WI, USA).
In vitro SUNSET assay for effect of polyphenols on protein synthesis
After 14 d of culture, primary embryonic cortico-hippocampal neuronal cultures in the vehicle Ctrl group were treated with DMSO (MilliporeSigma), and primary embryonic cortico-hippocampal neuronal cultures in treatment groups were treated with a mix of CYA (0.1 nM) and HPP (0.15 μM) for 2 or 24 h and stimulated with 15 ng/μl BDNF for 1 h. Doses were determined on the basis of the calculated average EC50 of genes expression dose-response curves. SUNSET measurement of protein synthesis was performed as previously described (53). In brief, cells were treated with 25 μg/ml final concentration of puromycin for 10 min. Cells were then washed once with ice-cold PBS and lysed with Milliplex MAP Cell Signaling Universal Lysis Buffer containing phosphatase inhibitors, including 1 mM sodium orthovanadate and freshly prepared 1× protease inhibitor cocktail (MilliporeSigma, Billerica, MA, USA). Proteins were resolved by 4–20% PAGE, transferred to a nitrocellulose membrane, and blocked with 5% (w/v) milk. Puromycin was detected using Western blot analysis with an anti-puromycin Ab (1:1000; Kerafast, Boston, MA, USA) and normalized to glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology, Dallas, TX, USA) as loading Ctrl.
Specific polyphenols’ effect on translation pathway–related proteins
After 14 d of culture, neurons in the vehicle Ctrl group were treated with DMSO, and neurons in treatment groups were treated with CYA (0.1 nM); HPP (0.15 μM); a mix of CYA (0.1 nM) + HPP (0.15 μM), DEL (0.1 nM), and HBA (0.03 μM); or a mix of DEL (0.1 nM) + HBA (0.03 μM) for 24 h; and stimulated with 15 ng/μl BDNF for 1 h. To test the effect of the specific eFI4E–eIF4G inhibitor, 4EGI-1, we treated cell cultures untreated or treated with CYA (0.1 nM) + HPP (0.15 μM), with 25 μM 4EGI-1 (MilliporeSigma) for 45 min. All cell cultures were stimulated with 15 ng/μl BDNF for 1 h, then washed once with ice-cold PBS and lysed. The concentration of total and phosphorylated mTOR, p70S6K, RPS6, and 4E-BP1 in cell protein lysate was determined using commercially available ELISA kits (mTOR: PEL-mTOR-S2448-1; p70S6K: PEL-p70S6K-T421-1; S6: PEL-RPS6-S235-T-1; 4EBP: PEL-4EBP1-T36-T-1; RayBiotech, Norcross, GA, USA) according to the manufacturer’s recommended procedures.
Extraction of total protein and RNA from in vitro mouse primary embryonic cortico-hippocampal neuronal cultures or mouse hippocampal tissues
For the molecular investigation of the effect of BDPP in the SD model, a subset of mice was humanely killed by CO2 euthanasia immediately at the end of 5 h of SD after OL training. Hippocampi from each hemisphere were separately dissected, gently rinsed in ice-cold PBS, and snap frozen on dry ice for protein and RNA studies.
Total RNA from mouse hippocampal tissue or primary embryonic cortico-hippocampal neuronal cultures was isolated and purified using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Total RNA was eluted with nuclease-free water. The optical density ratio of 260/280 was measured using Nanodrop spectrophotometer (PeqLab Biotechnology, Erlangen, Germany) and ranged between 1.9 and 2.1. RNA samples were stored at −80°C before additional use.
Total protein was extracted from hippocampal tissue or cell cultures lysed with Milliplex MAP Cell Signaling Universal Lysis Buffer containing phosphatase inhibitors, including 1 mM sodium orthovanadate and freshly prepared 1× protease inhibitor cocktail (MilliporeSigma). Protein samples were stored at −80°C before additional use.
Pathway-focused gene expression analysis related to synaptic plasticity
Hippocampal RNA isolates (100 ng) were used to quantify the gene expression of mRNA transcripts related to synaptic plasticity (RT2 Profiler Mouse Synaptic Plasticity; Qiagen) containing primers for 84 targets and 5 housekeeping genes. Relative expression of each mRNA was normalized against the median of all 84 target genes using the equation 2−ΔΔCt where ΔΔCt = ΔCttreated − ΔCtCtrl and ΔCt = Cttarget-mRNA − Ctaverage of 5 housekeeping genes. Three biological replicates were used within each group. Gene expression changes were considered significant if they reached a value of P ≤ 0.05 and if they had a fold-change of ≥2 or ≤−2. The threshold of ±2 was based on the average experimental technical variance and biological variance. Three biological replicates were used.
Assessment of gene expression in total RNA from cells or mouse hippocampus tissues
RNA was isolated using RNeasy Mini Kit (Qiagen). One microgram of total hippocampal RNA and 400 ng of cells’ RNA were reverse transcribed with a SuperScript first-strand III kit (Thermo Fisher Scientific). Real-time RT-PCR was performed to confirm or identify genes of interest. Gene expression was measured in 4 replicates by quantitative RT-PCR using Maxima SYBR Green Master Mix (Fermentas, Waltham, MA, USA) in ABI Prism 7900HT. Hypoxanthine phosphoribosyltransferase (HPRT) expression level was used as an internal Ctrl. Data were normalized using the 2−ΔΔCt method (56). Levels of target gene mRNAs were expressed relative to those found in the hippocampal tissue of NSD mice for in vivo studies and to untreated cells + BNDF induction for cell cultures studies and plotted in GraphPad Prism. Primers are listed in Table 1.
TABLE 1.
Primers used in this study
| Primer, 5′–3′ | ||
|---|---|---|
| Gene | Forward | Reverse |
| Egr2 | TTGACCAGATGAACGGAGTG | TGCCCATGTAAGTGAAGGTC |
| c-fos | TCCGGTTCCTTCTATGCAGC | GTAAGTAGTGCAGCCCGGAG |
| Homer1 | CAATGGGACAGACGATGAGAG | GTTGCCTTTGAGGGTAGCTAG |
| Inhba | ATCACCTTTGCCGAGTCAG | ACTTTGGTCCTGGTTCTGTTAG |
| Arc | CTATACCGTTAGCCCCTATGC | CCTCGAAGATCTGTGTATCCAC |
| Cebpb | AGAACCCGCGGCCTTCTAC | GTCGTACATGGCAGGAGTCG |
| Eif4e | AGGACGGTGGCTGATCACA | TCTCTAGCCAGAAGCGATCGA |
| Eif4g | GGGAGCCGGAAATGGTTGTG | CTAGGGTAGAAGTGCTGGCG |
| 4e-bp1 | AGCCGTAGGACGCAATGATG | AGTCATTCCCCTGCAGTAGC |
| Mtor | CTGACCCCACGATGCTGAAA | TTCTGAGTCCCTGCTGCAAA |
| P70s6k | GCAAACTCTACCTCATCCTGG | GCTAGTGTGATCTCTGCCAG |
Western blot analysis and ELISA
For Western blot analysis, hippocampal protein samples were normalized on the basis of total protein content measured by BCA Protein Assay (23225; Thermo Fisher Scientific). Proteins were separated by 4–20% Tris-Glycine SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred to PVDF membranes. Membranes were blocked in 5% bovine serum albumin Tris-buffered saline/Tween 20 or 5% milk + 0.5% bovine serum albumin Tris-buffered saline/Tween 20 and incubated overnight at 4°C in primary Ab. Ab concentrations were at manufacturers’ recommendations; Egr2 (1:1000; Santa Cruz Biotechnology), c-Fos (1:1000; Cell Signaling Technology, Danvers, MA, USA), Homer1 (1:1000; Cell Signaling Technology), and puromycin 12D10 (1:5000; from 5 mg/ml stock; MilliporeSigma). Abs were then washed and incubated with appropriate horseradish peroxidase–conjugated goat anti-mouse or anti-rabbit IgG (1:5000; Santa Cruz Biotechnology) at room temperature for 1 h. Blots were washed and exposed via chemiluminescence using Kodak developer and quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA). Density of the signal was normalized to β-actin (1:1000; Cell Signaling Technology)
The concentration of total and phosphorylated mTOR, p70S6K, RPS6, and 4E-BP1 in hippocampal or cell protein lysate was determined using commercially available ELISA kits (mTOR: PEL-mTOR-S2448-1; p70S6K: PEL-p70S6K-T421-1; RPS6: PEL-RPS6-S235-T-1; 4E-BP1: PEL-4EBP1-T36-T-1; RayBiotech), according to the manufacturer’s recommended procedures.
Multipathway cell signaling assays
Luminex xMAP multiplexed immunoassay (MilliporeSigma) was used to evaluate levels of total and phosphorylated proteins in the hippocampal extracts from NSD vehicle-treated mice, SD vehicle-treated mice, and SD+ BDPP mice collected at the end of 5 h of SD that followed OL training sessions. Hippocampal tissue was lysed with Milliplex MAP Cell Signaling Universal Lysis Buffer containing phosphatase inhibitors, including 1 mM sodium orthovanadate and freshly prepared 1× protease inhibitor cocktail (MilliporeSigma). Phosphor-protein analyses used were as follows: cAMP-response element binding protein (CREB; Ser133), AKT (Ser473), Erk1/2 (Thr185/Tyr187), JNK (Thr183/Tyr185), p70S6K (Thr412), p38 (Thr189/Tyr182), STAT3 (Ser727), STAT5 (Tyr694/ 699), and NF-κB (Ser536). Twenty micrograms of protein lysate were used for the assays, according to the procedure provided by the manufacturer.
Statistical analysis
All values are expressed as means ± sem. For statistical analysis of behavioral tests, OL and NOR, as well as biochemical analyses comparing NSD group with testing groups, 1-way ANOVA followed by Bonferroni’s comparison was used. In all studies, outliers (2 sd from the mean) were excluded and the null hypothesis was rejected at the 0.05 level. All statistical analysis was performed using GraphPad Prism 5 software.
RESULTS
SD impairs memory consolidation and attenuates protein synthesis of synaptic plasticity genes in the hippocampus
To examine SD-mediated memory impairment, C57BL/6J mice were randomly grouped into 2 groups, NSD and SD mice, and fed on a polyphenol-free diet for 4 wk. Mice were then engaged in behavioral testing with OL. The OL task requires the hippocampus for memory encoding, consolidation, and retrieval (57, 58). For additional validation of our SD-induced memory impairment model, we performed behavioral testing for NOR memory in parallel groups of NSD and SD mice. NOR requires different critical brain regions, including the insular cortex (59), perirhinal cortex (60, 61), ventromedial prefrontal cortex (62), and hippocampus, with the necessity of the hippocampus varying on the basis of the exact experimental setup, found to be critical when conducted as described in this protocol (60, 63). In both the OL and NOR behavioral paradigms, after the training session on d 28, NSD mice were left undisturbed in their home cages and SD mice were kept awake for 5 h by an automated SD apparatus. The next day (d 29), mice were tested for memory performance (Fig. 1A). OL and NOR tests were used to assess cognition, specifically spatial memory and discrimination. These tasks involved exploiting rodents’ innate preference for novelty and were based on the spontaneous tendency of rodents to recognize when an object had been relocated or changed and spend more time exploring it than the familiar object (51, 64). The percentage of object interaction did not differ in SD mice compared with NSD mice and ranged from 38.36 to 33.09% in the OL task and from 24.25 to 26.80% in the NOR task (Supplemental Table 1); however, SD led to significantly impaired spatial memory of the location of an object (DI: 42.00 ± 6.61 NSD vs. −8.00 ± 13.94 SD; P = 0.01; Fig. 1B) and significantly impaired NOR memory (DI: 25.40 ± 5.02 NSD vs. 3.00 ± 5.58 SD; P = 0.02; Fig. 1C). We also found that SD mice demonstrated decreased phosphorylation of CREB compared with NSD mice. CREB signaling is essential for long-lasting changes in synaptic plasticity that mediate the conversion of short-term memory to long-term memory (65) (Supplemental Fig. 1). These results are supported by our previous findings (38) and suggest that 5 h of SD is sufficient to impair experience-based synaptic plasticity.
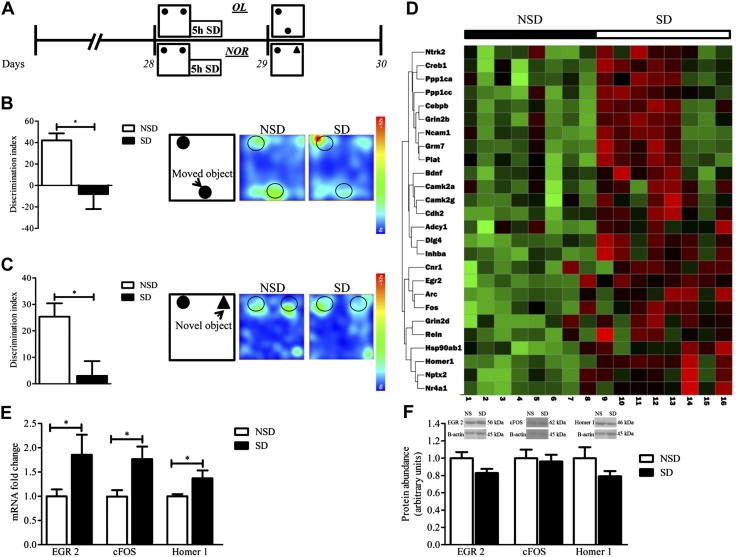
Figure 1.
Acute 5-h SD induces memory impairment. A) Schematic design of the experiment. B, C) Bar graphs of the objects DI of NSD and SD mice. B) OL test (unpaired, 2-tailed Student’s t test, P = 0.01; n = 5/group; left) and representative heat map of the OL (right). C) NOR test (unpaired, 2-tailed Student’s t test, P = 0.02, n = 4–5/group; left) and representative heat map of the NOR test (right). Acute 5-h SD increases gene transcription but not in protein synthesis in vivo. D) Heat map showing the response of 24 synaptic plasticity genes’ expression to acute 5-h SD. Gene expression assessed by quantitative RT-PCR array from hippocampus extracts collected at the end of 5 h of SD in individual samples from NSD and SD mice (n = 8 mice in each condition). Green, low expression; red, high expression. Top panel, condition; left panel, gene tree; bottom panel, sample number. E) Fold change of mRNA expression of Egr2, c-fos, and Homer1 in hippocampal extracts from SD mice relative to those from NSD mice, as assessed by quantitative RT-PCR. Expression was normalized to that of the housekeeping gene, hypoxanthine phosphoribosyltransferase (HPRT). Data are means ± sem of 8–11/group. F) Representative Western blots and quantification of hippocampal extracts protein abundance of EGR2 (n = 5/group), cFOS (n = 8–10/group), and Homer1 (n = 3/group). Abundance was normalized to β-actin loading Ctrl. Data are presented as arbitrary units; means ± sem (unpaired, 2-tailed Student’s t test). *P < 0.05.
To further explore the molecular basis of SD, we examined genome-wide changes in the expression of plasticity-related genes in the hippocampus of SD mice compared with NSD Ctrls. We identified 26 gene products that are differentially expressed in NSD and SD groups, all of them up-regulated in SD compared with NSD with a Wilcoxon rank-sum test (P ≤ 0.05; Supplemental Table 2). Identified gene products were visualized using a heat map (Fig. 1D).
We next used quantitative RT-PCR to validate our microarray studies using hippocampi from separate groups of SD and NSD mice. Studies confirmed the genome-wide up-regulation of mRNA in SD compared with NSD mice (Fig. 1E, mRNA of Egr2, c-fos, Homer1, 2-tailed Student’s t test, SD vs. NSD; P < 0.05; Supplemental Fig. 2). Of interest, we found that validated gene products (Egr2, c-fos, and Homer1) did not demonstrate parallel changes in their protein abundance (Fig. 1F, protein abundance of Egr2, c-Fos, Homer1, 2-tailed Student’s t test, SD vs. NSD; P > 0.05). These findings suggest a disassociation between the transcription of genes related to synaptic plasticity and their relative protein abundance in the hippocampal formation, which supports the hypothesis that SD-mediated deficits in transcription and translation are differentially regulated.
BDPP treatment protects against impairment in hippocampal protein synthesis in SD mice coincidental with the restoration of memory function
To assess whether dietary BDPP can prevent SD-mediated deficits in spatial and recognition memory, C57BL/6J mice were randomly grouped into 3 groups (NSD, SD, and SD + BDPP), fed a polyphenol-free diet, and subjected to treatment with BDPP or drinking water (vehicle). After 2 wk of treatment, we performed behavioral testing for OL and NOR memory tasks. After the training session on d 28, NSD mice were left undisturbed in their home cages and mice in the SD and SD + BDPP groups were kept awake for 5 h by an automated SD apparatus. The next day (d 29), mice were tested for memory performance (Fig. 2A). We found no differences in BW gain, food consumption, or liquid (BDPP/vehicle) consumption among treatment groups in mice that were tested for OL (Supplemental Fig. 3) or in mice that were tested for NOR (Supplemental Fig. 4). The percentage of total object interaction did not differ among treatment groups and ranged from 29.92 to 33.14% in the OL task and from 21.78 to 26.44% in the NOR task (Supplemental Table 3); however, we found that dietary BDPP significantly attenuated the SD-induced impairment of spatial memory assessed by OL test (Fig. 2B, DI: NSD, 36.44 ± 4.36; SD, −2.46 ± 7.95; SD + BDPP, 28.07 ± 3.93; NSD vs. SD, P < 0.0005; SD vs. SD + BDPP, P < 0.005) and restored the SD-impaired object recognition memory as assessed by NOR test (Fig. 2C, DI: NSD, 25.69 ± 5.16; SD, 2.99 ± 4.88; SD + BDPP, 24.75 ± 4.67; NSD vs. SD, P < 0.05; SD vs. SD + BDPP, P < 0.05). Restoration of memory function by BDPP was evident only in SD. BDPP was not found to promote memory performance in mice that were not sleep deprived (Supplemental Fig. 8).
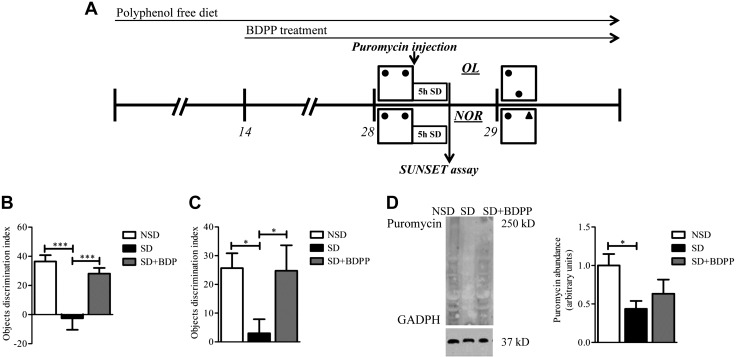
Figure 2.
BDPP treatment prevents memory impairment and hippocampal protein synthesis decrease in response to SD. A) Schematic design of the experiments. B, C) Bar graphs of the objects DI of NSD vehicle-treated mice, SD vehicle-treated mice, and SD mice treated with BDPP. B) OL test (1-way ANOVA, F2,27 = 12.86, P = 0.0001, Bonferroni post hoc test). ***P < 0.0005 NSD vs. SD and SD vs. SD + BDPP, n = 10/group. C) NOR test (1-way ANOVA, F2,25 = 3.512, P = 0.0453, Bonferroni post hoc test). *P < 0.05 NSD vs. SD and SD vs. SD + BDPP, n = 9–10/group. D) Western blot analysis representative figures and analysis for puromycin in hippocampus from male C57BL/6J mice in NSD, SD, or SD + BDPP groups injected intracerebroventricularly with puromycin at the end of 5 h of SD that followed the OL training session. Puromycin signal was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) loading Ctrl and quantified (right). Data are presented as fold change of means ± sem; n = 4–5 mice in each condition (1-way ANOVA, F2,13 = 3.385, P = 0.071, Bonferroni post hoc test). *P < 0.05 NSD vs. SD.
We then assessed whether hippocampal protein synthesis is affected by SD and dietary BDPP using a SUNSET assay that tags nascent proteins with puromycin. Mice were randomly grouped into 3 groups: untreated NSD group, untreated SD group, and BDPP treated SD group. No differences in BW gain, food consumption, or liquid (BDPP/vehicle) consumption were found among all treatment groups throughout the experiment (Supplemental Fig. 5). After 2 wk of treatment, mice received a training session for the OL test followed by 1 infusion of puromycin into the left lateral ventricle. Mice in SD groups were then immediately sleep deprived for 5 h, whereas NSD mice were left undisturbed. Immediately after SD, hippocampi were harvested, and the abundance of proteins tagged with puromycin was measured by Western blot analysis (Fig. 2A). Mice that had undergone 5 h of SD demonstrated a 2-fold reduced amount of puromycin-tagged proteins in the hippocampus compared with mice that had undisturbed sleep (Fig. 2D, puromycin abundance: SD vs. NSD, P < 0.05). SD + BDPP mice revealed a 1.4-fold increase in puromycin abundance compared with the SD group and did not significantly differ compared with the NSD group (Fig. 2D, puromycin abundance: SD + BDPP vs. SD, P < 0.05), which suggests that BDPP protects, in part, against the reduction of protein synthesis in the hippocampus in response to SD.
BDPP treatment restored SD-mediated cognitive deficits, in part, via regulation of mTORC1 signaling pathways
Phosphorylation of mTOR leads to the activation of p70S6K (66–69), which enhances protein translation via phosphorylation of numerous substrates that are involved in translation, including RPS6, a critical ribosomal component that mediates translation initiation (70, 71). Phosphorylation of mTOR also leads to phosphorylation and inactivation of the repressor of mRNA translation, 4E-BP1 (33, 72). To test our hypothesis that dietary BDPP prevents decreased protein synthesis after SD via rescue of the mTORC1 signaling pathway, we trained mice for the OL task, subjected them to SD for 5 h, and examined the abundance of mTOR, p70S6K, RPS6, and 4E-BP1 proteins in hippocampal extracts. Total mTOR, p70S6K, RPS6, and 4E-BP1 protein abundance did not differ among treatment groups (Fig. 3A–D).
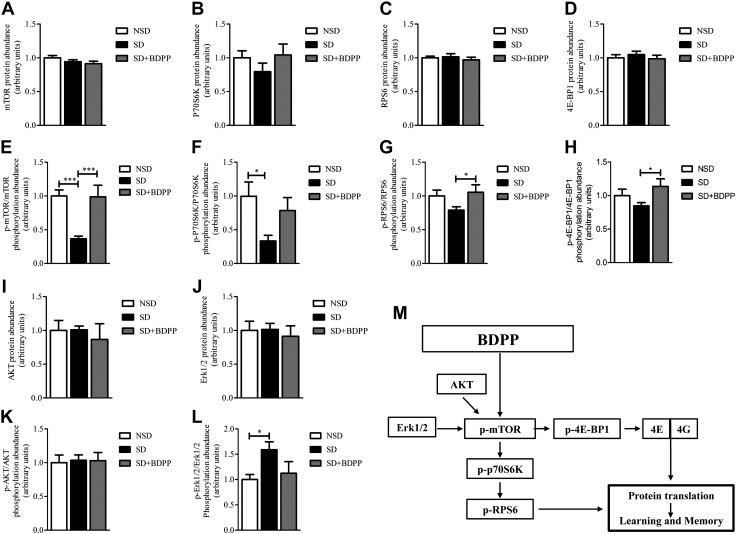
Figure 3.
BDPP activates the mTORC1 signaling pathway in the hippocampus. Hippocampal extracts were collected from NSD vehicle-treated mice, SD vehicle-treated mice, and SD mice treated with BDPP that were trained for OL test and sleep deprived for 5 h. A–D) ELISA-based quantification of protein abundance of mTOR (1-way ANOVA F2,23 = 1.829, P = 0.18; A), p70S6K (1-way ANOVA F2,16 = 0.977, P = 0.40; B), RPS6 (1-way ANOVA F2,21 = 0.387, P = 0.68; C), and 4E-BP1 (1-way ANOVA F2,21 = 0.457, P = 0.63; D). E–H) Quantification of phosphorylation abundance was calculated as the ratio of phosphorylated to total mTOR (p-mTOR, Ser2481, 1-way ANOVA, F2,23 = 10.15, P = 0.0008, Bonferroni post hoc test; E), ***P < 0.0005 NSD vs. SD and SD vs. SD + BDPP; p70S6K (p-p70S6K, Thr421/Ser424, 1-way ANOVA, F2,16 = 3.997, P = 0.04, Bonferroni post hoc test; F), *P < 0.05 NSD vs. SD; RPS6 (p-RPS6, Ser235/236, 1-way ANOVA, F2,21 = 2.487, P = 0.10, Bonferroni post hoc test; G), *P < 0.05 SD vs. SD + BDPP. 4E-BP1 (p-4E-BP1, Thr36, 1-way ANOVA, F2,21 = 2.821, P = 0.08, Bonferroni post hoc test; H), *P < 0.05 SD vs. SD + BDPP. Data are presented as fold change of means ± sem; n = 6–8 mice per condition. I–L) Luminex xMAP multiplexed immunoassays based quantification of total protein abundance of AKT (1-way ANOVA F2,11 = 0.244, P = 0.78; I), ERK1/2 (1-way ANOVA F2,11 = 0.177, P = 0.84; J), and quantification of phosphorylation abundance calculated as the ratio of phosphorylated to total of AKT (p-AKT, Ser473, 1-way ANOVA, F2,11 = 0.035, P = 0.96; K), and ERK1/2 (p-ERK1/2, Thr185/Tyr187, 1-way ANOVA, F2,11 = 3.287, P = 0.08; Bonferroni post hoc test; L). *P < 0.05 NSD vs. SD. Data are presented as fold change of means ± sem; n = 4 mice per condition. M) BDPP enhances protein translation and memory consolidation via activation of mTORC1 and downstream signaling pathway.
To determine the activity of the mTORC1 signaling pathway, we examined the phosphorylation of mTOR, p70S6K, RPS6, and 4E-BP1 and found that phosphorylation of residues that influence activity was significantly impaired by SD coinciding with impairment of memory. Specifically, we found a 64% decrease of mTOR phosphorylation in SD compared with NSD mice (Fig. 3E, p-mTOR, Ser2481: SD vs. NSD, P < 0.005). Consistent with this evidence, we found a 66% reduction of p70S6K phosphorylation in SD mice compared with NSD mice (Fig. 3F, p-p70S6K, Thr421/Ser424: SD vs. NSD, P < 0.05). Phosphorylation of RPS6 was decreased by 21% in SD mice compared with NSD mice, although values did not reach statistical significance (Fig. 3G, p-RPS6, Ser235/236). We also detected a nonsignificant 16% reduction of 4E-BP1 phosphorylation in SD mice compared with NSD mice (Fig. 3H, p-4E-BP1, Thr36).
On the basis of this evidence, we tested the hypothesis that dietary BDPP may rescue SD-mediated cognitive impairment by restoring mTOR, p70S6K, RPS6, and 4E-BP1 activation as assessed by phosphorylation. We found that dietary BDPP in SD mice fully rescued mTOR phosphorylation, normalizing it to the levels found in NSD mice (Fig. 3E, p-mTOR, Ser2481: SD vs. SD + BDPP, P < 0.005).
This finding was consistent with a partial rescue of p70S6K phosphorylation in BDPP-treated SD mice compared with vehicle-treated SD mice, although values did not reach statistical significance (Fig. 3F, p-p70S6K, Thr421/Ser424: SD vs. SD + BDPP, P > 0.05); however, dietary BDPP increased the phosphorylation of RPS6 by 21.5% (Fig. 3G, p-RPS6, Ser235/236: SD vs. SD + BDPP P < 0.05) and that of 4E-BP1 by 29% (Fig. 3H, p-4E-BP, Thr36; SD vs. SD + BDPP, P < 0.05) compared with untreated SD mice.
Investigation of mTOR upstream activators, AKT and ERK1/2, revealed no differences in total protein abundance (Fig. 3I, J). We found no effect of SD or SD + BDPP treatment on AKT phosphorylation (Fig. 3K). We also detected a 58% increase in ERK1/2 phosphorylation in the hippocampus of SD mice compared with NSD mice, which was reduced by 46% in BDPP-treated SD mice (Fig. 3L, p-ERK1/2, Thr185/Tyr187: SD vs. NSD, P < 0.05). Together, these results suggest that dietary BDPP restores SD-induced memory impairment via mechanisms that involve hippocampal mTORC1 activation rather than expression, which results in the activation of its downstream effectors, 4E-BP1 and p70S6K (Fig. 3M).
Specific polyphenol metabolites activate mTORC1 signaling in primary neurons
Previous high-throughput bioavailability studies have indicated that select BDPP-derived polyphenolic metabolites accumulate in the brain after dietary BDPP treatment (Supplemental Table 4) (36, 73). To screen for metabolites that activate the mTORC1 signaling pathway, we treated primary embryonic mouse cortico-hippocampal neuron cultures with brain bioavailable polyphenol metabolites in a dose-dependent manner and measured mRNA expression of Mtor, P70s6k, Eif4g, and Eif4e. We calculated EC50 concentrations for each of the genes and averaged the EC50 from the mRNA expression of the 4 genes that demonstrated a reproducible dose response in response to treatment with metabolites (Supplemental Table 5).
Of 6 brain bioactive polyphenols previously found to accumulate in the brain of BDPP-treated mice, we found CYA and HPP had a positive dose response on the mRNA expression of all 4 genes, whereas DEL and HBA had a negative dose response (Supplemental Table 5). We then examined the effect of these specific metabolites on promoting mTORC1 signaling in primary neurons.
We treated primary neurons with 0.1 nM CYA, 0.15 μM HPP, or a combination of 0.1 nM CYA with 0.15 μM HPP. In comparison, we treated primary neurons with 0.1 nM DEL, 0.03 μM HBA, or a combination of 0.1 nM DEL and 0.03 μM HBA. Doses were determined on the basis of EC50 values and were within the range of concentrations found in the brain. No cytotoxic effect of compounds were found compared with vehicle-treated cells (Supplemental Fig. 6).
Consistent with our findings with in vivo dietary BDPP studies, we found that none of the metabolites when delivered individually or in combination influenced the protein abundance of mTOR (Fig. 4A), p70S6K (Fig. 4B), RPS6 (Fig. 4C), and 4E-BP1 (Fig. 4D); however, treatment with CYA + HPP significantly increased the ratio of phosphorylated over total mTOR by 56% compared with Ctrl (Fig. 4E, p-mTOR, Ser2481: CYA + HPP vs. Ctrl, P = 0.009) and over p70S6K by 39% (Fig. 4F, p-p70S6K, Thr421/Ser424: CYA + HPP vs. Ctrl, P = 0.039).
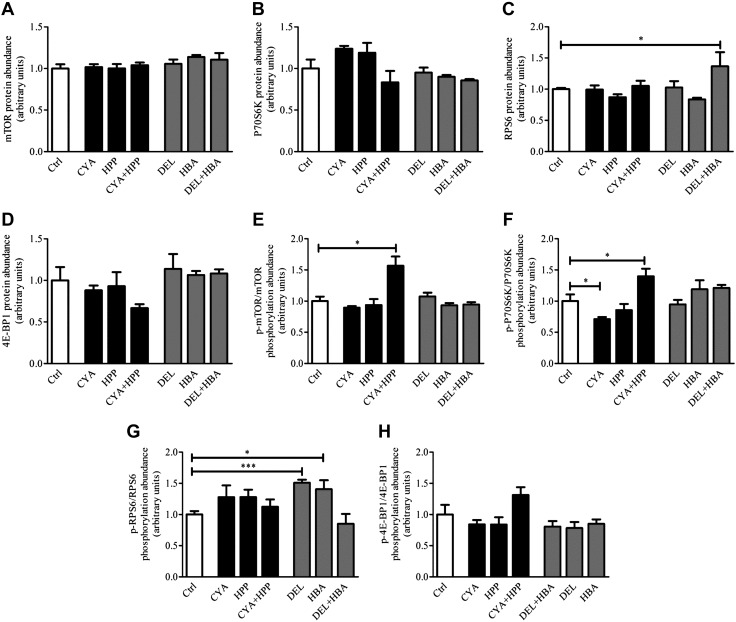
Figure 4.
Specific phenolic metabolites promote mTORC1 signaling pathway activation. Primary neuronal cultures were treated with phenolic metabolites, either alone or in combination, to examine their effect on the mTORC1 signaling pathway. Polyphenols and doses were chosen on the basis of the slope direction (positive/negative) of dose-response curves and calculated EC50 (Supplemental Table 5). For promoting effect, cultures were treated with CYA (0.1 nM), HPP (0.15 μM), or a combination of CYA (0.1 nM) +HPP (0.15 μM). For Ctrl, effect cultures were treated with DEL (0.1 nM), HBA (0.03 μM), or a combination of DEL (0.1 nM) + HBA (0.03 μM). Ctrl cells were treated with DMSO. After 24 h of treatment, cells were washed once with ice-cold PBS and protein was extracted. ELISA-based assay was used for quantification of fold change in total protein abundance of mTOR (A), p70S6K (B), RPS6 (C), 4E-BP1 (D), and quantification of fold of change in phosphorylated protein abundance calculated as ratio of phosphorylated to total protein of mTOR (p-mTOR, Ser2481; E), p70S6K (p-p70S6K, Thr421/Ser424; F), RPS6 (p-RPS6, Ser235/236; G), and 4E-BP1 (p-4E-BP1, Thr36; H). Data are presented as means ± sem; n = 4–7 samples/condition. *P < 0.05, unpaired, 2-tailed Student’s t test, treatment vs. Ctrl.
This response was compound specific, as treatment with CYA, HPP, DEL, or HBA delivered individually, or the combinations of DEL + HBA, had no effect on mTOR or p70S6K phosphorylation compared with Ctrl (Fig. 4E, F). In contrast, cells that were treated with CYA showed a decrease of 29% in p70S6K phosphorylation compared with Ctrl (Fig. 4F, p-p70S6K phosphorylation abundance: CYA vs. Ctrl, P = 0.032).
Neuronal cultures that were treated with CYA + HPP demonstrated an increased ratio of phosphorylated over total RPS6 by 15% and of 4E-BP1 by 30%, although the phosphorylation ratio did not reach statistical significance (Fig. 4G, p-RPS6, Ser235/236; Fig. 4H, p-4E-BP1, Thr36, P > 0.05). Surprisingly, primary embryonic cortico-hippocampal neuronal cultures that were treated with DEL and HBA demonstrated a 51 and 40% increase, respectively, in RPS6 phosphorylation (Fig. 4G, p-RPS6, Ser235/236: DEL vs. Ctrl, P = 0.0004; HBA vs. Ctrl, P = 0.037) but not when combined. The observation that each of the polyphenols, when administered separately [CYA (0.1 nM)/HPP (0.15 μM)/DEL (0.1 nM)/HBA (0.03 μM)] resulted in contradicting effects on mTORC1 signaling pathways suggests that the interaction among the different polyphenols is critical for the overall effect on modulating the mTORC1 signaling pathway.
Consistent with our in vivo dietary BDPP treatment studies, we found a modest, nonsignificant increase in protein synthesis in cells that were treated with CYA + HPP compared with untreated cells in the nonradioactive SUNSET assay (Supplemental Fig. 7), which suggests that other combinations of polyphenol metabolites might be necessary to increase total protein synthesis in primary neurons.
Select polyphenol metabolite combinations reverse the effect of 4EGI-1 on 4E-BP1 phosphorylation
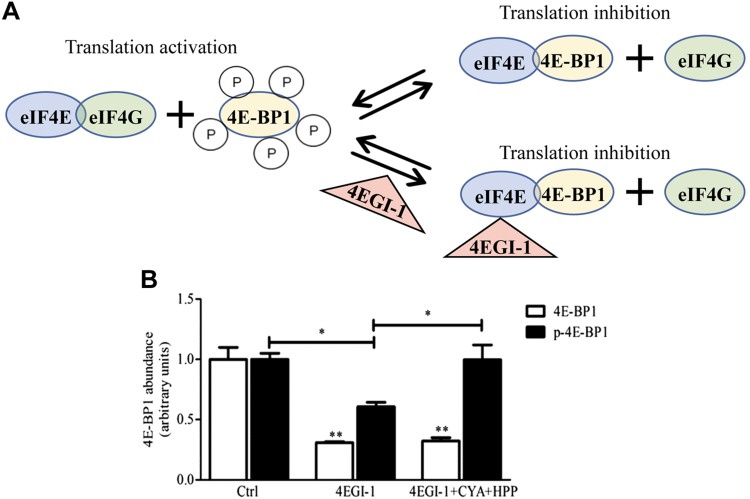
We next determined whether the activation of mTORC1 signaling by a combination of polyphenolic compounds can promote eIF4G–eIF4E interaction to initiate translation by investigating their effect on the inhibition of the eIF4G–eIF4E interaction. 4EGI-1 is a specific inhibitor of cap-dependent translation that selectively disrupts the interaction between eIF4E and eIF4G by stabilizing the eIF4E–4E-BP1 interaction (74). In contrast, hyperphosphorylation of 4E-BP1 decreases its affinity for eIF4E, which enables eIF4G to interact with eIF4E (Fig. 5A).
Figure 5.
Treatment with select phenolic metabolites reverses the specific 4E-4G interaction inhibitor (4EGI-1) -induced impairment of 4E-BP1 phosphorylation. A) Model of the action of 4EGI-1 in cells. Phosphorylated 4E-BP1 cannot bind eIF4E, which frees eIF4E to bind with eIF4G to stimulate translation. 4EGI-1 dissociates eIF4G from eIF4E by binding to eIF4E and stabilizing the binding of unphosphorylated 4E-BP1, which inhibits translation. Primary neuronal cultures were treated with DMSO (Ctrl) or a combination of CYA (0.1 nM) and HPP (0.15 μM), or with the specific 4E-4G interaction inhibitor 4EGI-1 either alone (4EGI-1) or combined with CYA (0.1 nM) +HPP (0.15 μM). After 24 h treatment, cells were washed once with ice-cold PBS and protein was extracted. B) ELISA-based assay was used to quantify protein abundance of total 4E-BP1 (white bars, unpaired, 2-tailed Student’s t test, 4EGI-1 vs. Ctrl; and 4EGI-1 + CYA + HPP vs. Ctrl) and phosphorylated 4E-BP1 (black bars, p-4E-BP1, Thr36, 4EGI-1 vs. Ctrl; and 4EGI-1 + CYA + HPP vs. 4EGI-1; unpaired, 2-tailed Student’s t test). Data are presented as means ± sem; n = 3–6 samples/group. *P < 0.05, **P < 0.005.
To investigate the role of polyphenolic metabolites in promoting eIF4E–eIF4G interaction, we treated primary neuronal cells with a combination of CYA (0.1 nM) + HPP (0.15 μM) or 4EGI-1 alone or in combination with CYA (0.1 nM) + HPP (0.15 μM) and examined the abundance of total and phosphorylated 4E-BP1. We found that, compared with Ctrl, treatment with 4EGI-1 either alone or in combination with CYA + HPP reduced the abundance of total 4E-BP1 protein by 70 and 68%, respectively (Fig. 5B, 4E-BP1: 4EGI-1 vs. Ctrl, P = 0.0021; 4EGI-1 + CYA + HPP vs. Ctrl, P = 0.0025).
In contrast, we found a 40% reduction in the phosphorylation of 4E-BP1 in 4EGI-1–treated neuronal cultures compared with vehicle-treated neuronal cultures (Fig. 5B, p-4E-BP1, Thr36: 4EGI-1 vs. Ctrl, P = 0.0015), which was rescued by treatment with CYA + HPP before 4EGI-1 treatment (Fig. 5B, p-4E-BP1, Thr36: 4EGI-1 CYA + HPP vs. 4EGI-1, P = 0.037). These results suggest a role for CYA + HPP in stabilizing a phosphorylated form of 4E-BP1, which promotes dissociation of 4E-BP1 from eIF4E to allow eIF4E–eIF4G interaction that is necessary to initiate translation.
DISCUSSION
Previous studies have suggested that SD affects mechanisms that regulate protein synthesis by specifically impairing the mTORC1-dependent translation initiation pathway, thereby disrupting the mechanisms of memory consolidation in the hippocampus (34, 35). Consistent with other studies, we have demonstrated that 5 h of SD impairs hippocampal-dependent spatial and recognition memory (11, 18). We found up-regulation of the transcription of a large number of genes that are related to synaptic plasticity in the hippocampus of SD mice. This evidence is consistent with a number of gene-screening studies (34, 75–77) that found a wide range of genes whose expression is up-regulated by SD; however, this is thought to be a stress response triggered by SD (78) and fails to explain the mechanism by which SD impairs hippocampal function. Our work found disassociation between the transcription of genes related to synaptic plasticity and their relative protein abundance in the hippocampal formation. As the transcription of mRNA from DNA and the translation of protein from mRNA are subjected to complex regulation by a variety of mechanisms (79–81), often leading to separate regulation of mRNA and protein levels that are related to the same gene (82), mRNA assays do not necessarily reflect protein abundance. Changes in protein levels are more indicative of an effective genomic response than of changes in the amount of mRNA transcripts (82). Among up-regulated genes, c-fos, Arc, Creb1, and Camk2g (14, 83) are associated with positive regulation of transcription. In line with the hypothesis that sleep promotes translation, but not transcription, of specific proteins that are essential for synaptic plasticity (84), these findings suggest SD disrupts the signaling mechanisms that regulate translation. Impaired translation of the transcribed mRNA of plasticity-related genes disrupts the experience-dependent synaptic plasticity necessary for long-lasting changes in synaptic strength and memory consolidation (16).
Using a nonradioactive in vivo translation assay, we demonstrated that 5 h of SD after behavioral training impairs experience-based de novo protein synthesis in the hippocampus. This work supports the hypothesis that sleep facilitates protein synthesis (85, 86), which is consistent with previous findings (35). Our previous work suggested that dietary supplementation with BDPP significantly improved contextual memory deficit caused by SD (38). Here, we demonstrate that BDPP rescues SD-induced spatial and recognition memory impairments. Using the nonradioactive in vivo translation assay, we found that BDPP protects, in part, against SD-impaired protein synthesis, which is consistent with our previous findings that BDPP treatment promotes the phosphorylation of p70S6K, a direct target of mTORC1 signaling, in the hippocampus of SD mice (38). Partial rescue of protein synthesis by BDPP could be explained by a ceiling effect wherein depressed translation can be rescued by BDPP, but translation could not be facilitated beyond the physiologic abundance of the expression of initiation factors. Our finding that the prevention of protein synthesis impairment is sufficient to prevent SD-induced spatial and recognition memory deficits demonstrates the functional importance of the impact of BDPP on hippocampal protein synthesis. In future experiments, it would be interesting to assess whether BDPP affects other SD-impaired behaviors by up-regulating protein synthesis in other brain regions.
Previous studies have demonstrated that intracortical administration of rapamycin during sleep abolishes consolidation by inhibiting mTOR (87). Tudor et al. (35) demonstrated that SD attenuated mTOR abundance and activity by reducing both total protein and phosphorylated mTOR, which reduced the abundance of phosphorylated 4EBP2, but not of phosphorylated S6K in the hippocampus. Consistent with these results, we found that SD specifically attenuated mTOR phosphorylation, and thus, activation, but did not affect mTOR protein abundance. Conversely, we found that impaired mTOR activity reduced phosphorylated p70S6K and, to a lesser extent, RPS6 or 4E-BP1 in the mouse hippocampus and did not affect their protein abundance. These inconsistencies might stem from the different experimental designs. We and Tudor et al. collected hippocampi at the end of 5 h of SD; however, in our study, SD followed cognitive stimulation in an OL training trial. It is possible that mTORC1-dependent translation is differently affected by SD when experienced-based synaptic plasticity is taking place. This is supported by previous results that show that sleep promoted phosphorylation but not protein abundance of protein synthesis regulators (i.e., 4E-BP1 and eEF2) and the translation, but not transcription, of key synaptic plasticity-related mRNAs (Arc and Bdnf) when occurring after cognitive stimulation with ocular dominance plasticity (87).
A possible explanation for the specificity of the impact of SD on p70S6K but not RPS6 or 4E-BP1 phosphorylation may be as a result of the action of specific protein phosphatases that have been demonstrated to have increased expression in the hippocampus after SD (34). In addition, although 4E-BP1 is typically phosphorylated by mTORC1 (88, 89), it is possible that the rescue of 4E-BP1 in SD by BDPP treatment is via 4E-BP1 kinases that are independent of mTORC1 signaling. For example, PI3K, which serves as an upstream activator of mTOR and is activated upon cognitive stimulation (90), may also directly phosphorylate 4E-BP1 (91) in our mouse model; however, whether 4E-BP1 is phosphorylated by mTORC1-independent kinases is beyond the scope of the current study.
Supported by our previous finding (38), we show here that BDPP attenuates SD-induced hippocampus-dependent memory impairments as a result of the activation of mTORC1-dependent protein translation in the hippocampus. BDPP rescues the SD-induced impairment of mTOR and p70S6K phosphorylation and promotes both RPS6 and 4E-BP1 phosphorylation in the hippocampus of SD mice without affecting protein abundance. We did not find any effect of SD or BDPP on the phosphorylation or protein abundance of the mTOR upstream regulator, AKT. Impairment of AKT phosphorylation in the rat whole brain was shown only after rapid eye movement–specific SD (92). In contrast, we found elevated ERK1/2 phosphorylation (but not protein abundance) after SD that was normalized by BDPP. This is in contrast to a study that demonstrated no effect of SD on ERK1/2 phosphorylation or protein abundance in rat hippocampi, although rats were not cognitively stimulated before SD (9). These results highlight the specificity of SD after cognitive stimulation and the beneficial effect of BDPP on mTORC1 signaling by impairing the activation of mTORC1 downstream effectors (summarized in Fig. 3M). Restoring memory in SD mice by BDPP treatment clearly demonstrated the importance of the activation of mTORC1 downstream signaling for protein synthesis and subsequent memory impairment after SD.
We showed that up-regulation of mTOR activation and subsequent protein synthesis by BDPP was sufficient to prevent the memory deficits that were associated with SD. Furthermore, we demonstrated that a select combination of the brain-bioavailable phenolic metabolites, CYA and HPP, can promote the phosphorylation of mTOR and p70S6K and, to a lesser extent, RPS6 and 4E-BP1 signaling in primary neuronal cultures. When separately administered, these polyphenolic metabolites had either none or the opposite effect on mTORC1 pathway activation. Contradictory effects of polyphenols that targeted the mTORC1 signaling pathway have been reported. For example, it has been found that resveratrol inhibits inflammation in microglial cells via mTORC1 activation (93). In contrast, application of piceatannol in prostate cancer cells resulted in down-regulation of mTORC1 and its upstream and downstream effector proteins, AKT and 4E-BP1 (94). Other combinations of polyphenol metabolites should be investigated to better understand the roles of their interactions in protein translation and cognitive function.
Phosphorylation of 4E-BP1 prevents its binding to eIF4E, which frees eIF4E to bind with eIF4G to stimulate translation (33). The recently discovered 4EGI-1 dissociates the eIF4E–eIF4G complex and inhibits cap-dependent translation. 4EGI-1 displaces eIF4G from eIF4E, effectively enhancing eIF4E–4E-BP1 association, both in vitro and in cells (74). Here, we show for the first time the reduced abundance of total and phosphorylated 4E-BP1 protein in primary neuronal cultures that were treated with 4EGI-1. Results are aligned with the effect of rapamycin and its analogs, which decrease eIF4E–eIF4G association by inhibiting mTORC1-dependent phosphorylation of 4E-BP1 (95, 96), and are further supported by recent models of 4EGI-1 activity that suggest that 4EGI-1 dissociates eIF4G from eIF4E and stabilizes the binding of 4E-BP1 to eIF4E (summarized in Fig. 5A). We found that treatment with the combination of the brain-bioavailable polyphenol metabolites CYA + HPP recovered mTORC1-dependent 4E-BP1 phosphorylation in 4EGI-1–treated cells, likely as a result of stabilizing phosphorylated 4E-BP1. Phosphorylation of 4E-BP1 decreases its affinity for eIF4E, which enables eIF4G binding and thus shifts toward the initiation of translation. These results further support mTORC1 pathway activation by treatment with the polyphenol metabolite combination, CYA + HPP. Future studies that explore the effect of CYA + HPP on eIF4E–eIF4G interaction, protein synthesis, and cognitive function in a mouse model of SD are required for further validation of their role in protein translation–mediated synaptic plasticity.
In summary, our findings demonstrate that activation of the mTORC1 signaling pathway in the regulation of protein synthesis is a critical mediator of BDPP in promoting resilience to SD-induced memory deficits. Given the safety and tolerability of BDPP, our preclinical study has provided a basis for potential translational application of dietary polyphenol compounds in promoting resilience to SD-induced cognitive deficits by targeting the mTORC1 signaling pathway.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This study was supported by Grant P50-AT008661-01 from the U.S. National Institutes of Health (NIH) National Center for Complementary and Integrative Health (NCCIH), and the Office of Dietary Supplements (ODS). G.M.P. holds a Senior Veterans Affairs Career Scientist Award. The contents of this study do not represent the views of the NCCIH, the ODS, the NIH, the U.S. Department of Veterans Affairs, or the U.S. Government. The authors declare no conflicts of interest.
Glossary
- 4E-BP1
eukaryotic translation initiation factor 4-binding protein 1
- BDNF
brain-derived neurotrophic factor
- BDPP
bioactive dietary polyphenol preparation
- BW
body weight
- CREB
cAMP-response element binding protein
- Ctrl
control
- CYA
cyanidin-3-O-glucoside
- DEL
delphinidin-3-O-glucoside
- DI
discrimination index
- eIF4
eukaryotic translation initiation factor 4
- GSPE
grape seed polyphenol extract
- HBA
3-hydroxybenzoic acid
- HPP
3-(3′-hydroxyphenyl) propionic acid
- mTORC1
mammalian target of rapamycin complex 1
- NOR
novel object recognition
- NSD
non-sleep deprived
- OL
object location
- p70S6K
ribosomal protein S6 kinase β-1
- RPS6
ribosomal protein S6
- SD
sleep deprivation
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. Frolinger wrote the paper and conducted research and analyzed data; C. Smith and C. F. Cobo performed research; and S. Sims, J. Brathwaite, S. de Boer, and J. Huang contributed to supplementary research and recorded experiments.
REFERENCES
- 1.Hublin C., Kaprio J., Partinen M., Koskenvuo M. (2001) Insufficient sleep—a population-based study in adults. Sleep 24, 392–400 [DOI] [PubMed] [Google Scholar]
- 2.Banks S., Dinges D. F. (2007) Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 3, 519–528 [PMC free article] [PubMed] [Google Scholar]
- 3.Curcio G., Ferrara M., De Gennaro L. (2006) Sleep loss, learning capacity and academic performance. Sleep Med. Rev. 10, 323–337 [DOI] [PubMed] [Google Scholar]
- 4.Abbott S. M., Videnovic A. (2016) Chronic sleep disturbance and neural injury: links to neurodegenerative disease. Nat. Sci. Sleep 8, 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson K. N., Bradley A. J. (2013) Sleep disturbance in mental health problems and neurodegenerative disease. Nat. Sci. Sleep 5, 61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abel T., Lattal K. M. (2001) Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 11, 180–187 [DOI] [PubMed] [Google Scholar]
- 7.Morris R. G., Moser E. I., Riedel G., Martin S. J., Sandin J., Day M., O’Carroll C. (2003) Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves L. A., Heller E. A., Pack A. I., Abel T. (2003) Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 10, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan Z., Peng X., Fang J. (2004) Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 1018, 38–47 [DOI] [PubMed] [Google Scholar]
- 10.Kumar T., Jha S. K. (2012) Sleep deprivation impairs consolidation of cued fear memory in rats. PLoS One 7, e47042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havekes R., Park A. J., Tudor J. C., Luczak V. G., Hansen R. T., Ferri S. L., Bruinenberg V. M., Poplawski S. G., Day J. P., Aton S. J., Radwańska K., Meerlo P., Houslay M. D., Baillie G. S., Abel T. (2016) Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife 5, e13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp C., Longordo F., Nicholson J. R., Lüthi A. (2006) Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J. Neurosci. 26, 12456–12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecsey C. G., Baillie G. S., Jaganath D., Havekes R., Daniels A., Wimmer M., Huang T., Brown K. M., Li X. Y., Descalzi G., Kim S. S., Chen T., Shang Y. Z., Zhuo M., Houslay M. D., Abel T. (2009) Sleep deprivation impairs cAMP signalling in the hippocampus. Nature 461, 1122–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez P. J., Abel T. (2008) The role of protein synthesis in memory consolidation: progress amid decades of debate. Neurobiol. Learn. Mem. 89, 293–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold P. E. (2008) Protein synthesis inhibition and memory: formation vs amnesia. Neurobiol. Learn. Mem. 89, 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S., Greenberg M. E. (2008) Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu. Rev. Cell Dev. Biol. 24, 183–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bramham C. R., Wells D. G. (2007) Dendritic mRNA: transport, translation and function. Nat. Rev. Neurosci. 8, 776–789 [DOI] [PubMed] [Google Scholar]
- 18.Palchykova S., Winsky-Sommerer R., Meerlo P., Dürr R., Tobler I. (2006) Sleep deprivation impairs object recognition in mice. Neurobiol. Learn. Mem. 85, 263–271 [DOI] [PubMed] [Google Scholar]
- 19.Bourtchouladze R., Abel T., Berman N., Gordon R., Lapidus K., Kandel E. R. (1998) Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn. Mem. 5, 365–374 [PMC free article] [PubMed] [Google Scholar]
- 20.Igaz L. M., Bekinschtein P., Vianna M. M. R., Izquierdo I., Medina J. H. (2004) Gene expression during memory formation. Neurotox. Res. 6, 189–204 [DOI] [PubMed] [Google Scholar]
- 21.Igaz L. M., Vianna M. R., Medina J. H., Izquierdo I. (2002) Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J. Neurosci. 22, 6781–6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliss T. V., Collingridge G. L. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 [DOI] [PubMed] [Google Scholar]
- 23.Zagaar M. A., Dao A. T., Alhaider I. A., Alkadhi K. A. (2016) Prevention by regular exercise of acute sleep deprivation-induced impairment of late phase LTP and related signaling molecules in the dentate gyrus. Mol. Neurobiol. 53, 2900–2910 [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa A., Kanayama Y., Matsumura H., Tsuchimochi H., Ishida Y., Nakamura S. (2006) Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur. J. Neurosci. 24, 243–248 [DOI] [PubMed] [Google Scholar]
- 25.Sweatt J. D. (1999) Toward a molecular explanation for long-term potentiation. Learn. Mem. 6, 399–416 [DOI] [PubMed] [Google Scholar]
- 26.Frey U., Huang Y. Y., Kandel E. R. (1993) Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260, 1661–1664 [DOI] [PubMed] [Google Scholar]
- 27.Kelleher R. J., III, Govindarajan A., Tonegawa S. (2004) Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 44, 59–73 [DOI] [PubMed] [Google Scholar]
- 28.Groppo R., Richter J. D. (2009) Translational control from head to tail. Curr. Opin. Cell Biol. 21, 444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa-Mattioli M., Sonenberg N. (2008) Translational control of gene expression: a molecular switch for memory storage. Prog. Brain Res. 169, 81–95 [DOI] [PubMed] [Google Scholar]
- 30.Banko J. L., Klann E. (2008) Cap-dependent translation initiation and memory. Prog. Brain Res. 169, 59–80 [DOI] [PubMed] [Google Scholar]
- 31.Ma X. M., Blenis J. (2009) Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 32.Pene F., Claessens Y. E., Muller O., Viguié F., Mayeux P., Dreyfus F., Lacombe C., Bouscary D. (2002) Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene 21, 6587–6597 [DOI] [PubMed] [Google Scholar]
- 33.Hoeffer C. A., Klann E. (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 33, 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vecsey C. G., Peixoto L., Choi J. H., Wimmer M., Jaganath D., Hernandez P. J., Blackwell J., Meda K., Park A. J., Hannenhalli S., Abel T. (2012) Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol. Genomics 44, 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tudor J. C., Davis E. J., Peixoto L., Wimmer M. E., van Tilborg E., Park A. J., Poplawski S. G., Chung C. W., Havekes R., Huang J., Gatti E., Pierre P., Abel T. (2016) Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci. Signal. 9, ra41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Bi W., Cheng A., Freire D., Vempati P., Zhao W., Gong B., Janle E. M., Chen T. Y., Ferruzzi M. G., Schmeidler J., Ho L., Pasinetti G. M. (2014) Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 6, 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasinetti G. M. (2012) Novel role of red wine-derived polyphenols in the prevention of Alzheimer’s disease dementia and brain pathology: experimental approaches and clinical implications. Planta Med. 78, E24. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W., Wang J., Bi W., Ferruzzi M., Yemul S., Freire D., Mazzola P., Ho L., Dubner L., Pasinetti G. M. (2015) Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 89, 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J., Ferruzzi M. G., Ho L., Blount J., Janle E. M., Gong B., Pan Y., Gowda G. A., Raftery D., Arrieta-Cruz I., Sharma V., Cooper B., Lobo J., Simon J. E., Zhang C., Cheng A., Qian X., Ono K., Teplow D. B., Pavlides C., Dixon R. A., Pasinetti G. M. (2012) Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J. Neurosci. 32, 5144–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Ho L., Zhao W., Ono K., Rosensweig C., Chen L., Humala N., Teplow D. B., Pasinetti G. M. (2008) Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 28, 6388–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Santa-Maria I., Ho L., Ksiezak-Reding H., Ono K., Teplow D. B., Pasinetti G. M. (2010) Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 22, 653–661 [DOI] [PubMed] [Google Scholar]
- 42.Ho L., Ferruzzi M. G., Janle E. M., Wang J., Gong B., Chen T. Y., Lobo J., Cooper B., Wu Q. L., Talcott S. T., Percival S. S., Simon J. E., Pasinetti G. M. (2013) Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 27, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flowers A., Lee J. Y., Acosta S., Hudson C., Small B., Sanberg C. D., Bickford P. C. (2015) NT-020 treatment reduces inflammation and augments Nrf-2 and Wnt signaling in aged rats. J. Neuroinflammation 12, 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dias G. P., Cavegn N., Nix A., do Nascimento Bevilaqua M. C., Stangl D., Zainuddin M. S., Nardi A. E., Gardino P. F., Thuret S. (2012) The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid. Med. Cell Longev. 2012, 541971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J. E., Janle E. M., Lobo J., Ferruzzi M. G., Davies P., Marambaud P. (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 285, 9100–9113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blount J. W., Ferruzzi M., Raftery D., Pasinetti G. M., Dixon R. A. (2012) Enzymatic synthesis of substituted epicatechins for bioactivity studies in neurological disorders. Biochem. Biophys. Res. Commun. 417, 457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 48.Krikorian R., Nash T. A., Shidler M. D., Shukitt-Hale B., Joseph J. A. (2010) Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 103, 730–734 [DOI] [PubMed] [Google Scholar]
- 49.Hines D. J., Schmitt L. I., Hines R. M., Moss S. J., Haydon P. G. (2013) Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatry 3, e212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaghouby F., Schildt C. J., Donohue K. D., O’Hara B. F., Sunderam S. (2014) Validation of a closed-loop sensory stimulation technique for selective sleep restriction in mice. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 3771–3774 [DOI] [PubMed] [Google Scholar]
- 51.Vogel-Ciernia A., Wood M. A. Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci. 2014. 69, 8.31.1–8.31.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taglialatela G., Hogan D., Zhang W. R., Dineley K. T. (2009) Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav. Brain Res. 200, 95–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt E. K., Clavarino G., Ceppi M., Pierre P. (2009) SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 [DOI] [PubMed] [Google Scholar]
- 54.Hoeffer C. A., Tang W., Wong H., Santillan A., Patterson R. J., Martinez L. A., Tejada-Simon M. V., Paylor R., Hamilton S. L., Klann E. (2008) Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron 60, 832–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Ho L., Chen L., Zhao Z., Zhao W., Qian X., Humala N., Seror I., Bartholomew S., Rosendorff C., Pasinetti G. M. (2007) Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 117, 3393–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta CT) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 57.Mumby D. G., Gaskin S., Glenn M. J., Schramek T. E., Lehmann H. (2002) Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 9, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haettig J., Stefanko D. P., Multani M. L., Figueroa D. X., McQuown S. C., Wood M. A. (2011) HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn. Mem. 18, 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bermudez-Rattoni F., Okuda S., Roozendaal B., McGaugh J. L. (2005) Insular cortex is involved in consolidation of object recognition memory. Learn. Mem. 12, 447–449 [DOI] [PubMed] [Google Scholar]
- 60.Balderas I., Rodriguez-Ortiz C. J., Salgado-Tonda P., Chavez-Hurtado J., McGaugh J. L., Bermudez-Rattoni F. (2008) The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn. Mem. 15, 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winters B. D., Forwood S. E., Cowell R. A., Saksida L. M., Bussey T. J. (2004) Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J. Neurosci. 24, 5901–5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akirav I., Maroun M. (2006) Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb. Cortex 16, 1759–1765 [DOI] [PubMed] [Google Scholar]
- 63.Rossato J. I., Bevilaqua L. R., Myskiw J. C., Medina J. H., Izquierdo I., Cammarota M. (2007) On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn. Mem. 14, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antunes M., Biala G. (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tully T., Bourtchouladze R., Scott R., Tallman J. (2003) Targeting the CREB pathway for memory enhancers. Nat. Rev. Drug Discov. 2, 267–277 [DOI] [PubMed] [Google Scholar]
- 66.Dann S. G., Selvaraj A., Thomas G. (2007) mTOR complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 13, 252–259 [DOI] [PubMed] [Google Scholar]
- 67.Janus A., Robak T., Smolewski P. (2005) The mammalian target of the rapamycin (mTOR) kinase pathway: its role in tumourigenesis and targeted antitumour therapy. Cell. Mol. Biol. Lett. 10, 479–498 [PubMed] [Google Scholar]
- 68.Richter J. D., Sonenberg N. (2005) Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433, 477–480 [DOI] [PubMed] [Google Scholar]
- 69.Hay N., Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 70.Biever A., Valjent E., Puighermanal E. (2015) Ribosomal protein S6 phosphorylation in the nervous system: from regulation to function. Front. Mol. Neurosci. 8, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choo A. Y., Yoon S. O., Kim S. G., Roux P. P., Blenis J. (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. USA 105, 17414–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hara K., Yonezawa K., Kozlowski M. T., Sugimoto T., Andrabi K., Weng Q. P., Kasuga M., Nishimoto I., Avruch J. (1997) Regulation of eIF-4E BP1 phosphorylation by mTOR. J. Biol. Chem. 272, 26457–26463 [DOI] [PubMed] [Google Scholar]
- 73.Wang D., Ho L., Faith J., Ono K., Janle E. M., Lachcik P. J., Cooper B. R., Jannasch A. H., D’Arcy B. R., Williams B. A., Ferruzzi M. G., Levine S., Zhao W., Dubner L., Pasinetti G. M. (2015) Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 59, 1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moerke N. J., Aktas H., Chen H., Cantel S., Reibarkh M. Y., Fahmy A., Gross J. D., Degterev A., Yuan J., Chorev M., Halperin J. A., Wagner G. (2007) Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 128, 257–267 [DOI] [PubMed] [Google Scholar]
- 75.Cirelli C., Tononi G. (2000) Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J. Neurosci. 20, 9187–9194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cirelli C., Tononi G. (2000) Gene expression in the brain across the sleep-waking cycle. Brain Res. 885, 303–321 [DOI] [PubMed] [Google Scholar]
- 77.Cirelli C., Tononi G. (2000) On the functional significance of c-fos induction during the sleep-waking cycle. Sleep 23, 453–469 [PubMed] [Google Scholar]
- 78.Da Cousta Souza A., Ribeiro S. (2015) Sleep deprivation and gene expression. Curr. Top. Behav. Neurosci. 25, 65–90 [DOI] [PubMed] [Google Scholar]
- 79.Maas S. (2010) Gene regulation through RNA editing. Discov. Med. 10, 379–386 [PubMed] [Google Scholar]
- 80.McManus C. J., Graveley B. R. (2011) RNA structure and the mechanisms of alternative splicing. Curr. Opin. Genet. Dev. 21, 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filipowicz W., Bhattacharyya S. N., Sonenberg N. (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114 [DOI] [PubMed] [Google Scholar]
- 82.Vogel C., Marcotte E. M. (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gal-Ben-Ari S., Kenney J. W., Ounalla-Saad H., Taha E., David O., Levitan D., Gildish I., Panja D., Pai B., Wibrand K., Simpson T. I., Proud C. G., Bramham C. R., Armstrong J. D., Rosenblum K. (2012) Consolidation and translation regulation. Learn. Mem. 19, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seibt J., Frank M. G. (2012) Translation regulation in sleep: making experience last. Commun. Integr. Biol. 5, 491–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wisor J. P. (2013) The sleep-deprived hippocampus: a loss in translation. Physiol. Genomics 45, 26–27 [DOI] [PubMed] [Google Scholar]
- 86.Grønli J., Soulé J., Bramham C. R. (2014) Sleep and protein synthesis-dependent synaptic plasticity: impacts of sleep loss and stress. Front. Behav. Neurosci. 7, 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seibt J., Dumoulin M. C., Aton S. J., Coleman T., Watson A., Naidoo N., Frank M. G. (2012) Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr. Biol. 22, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brunn G. J., Hudson C. C., Sekulić A., Williams J. M., Hosoi H., Houghton P. J., Lawrence J. C., Jr., Abraham R. T. (1997) Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 [DOI] [PubMed] [Google Scholar]
- 89.Beretta L., Gingras A. C., Svitkin Y. V., Hall M. N., Sonenberg N. (1996) Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15, 658–664 [PMC free article] [PubMed] [Google Scholar]
- 90.Lin C. H., Yeh S. H., Lin C. H., Lu K. T., Leu T. H., Chang W. C., Gean P. W. (2001) A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron 31, 841–851 [DOI] [PubMed] [Google Scholar]
- 91.Foukas L. C., Shepherd P. R. (2004) eIF4E binding protein 1 and H-Ras are novel substrates for the protein kinase activity of class-I phosphoinositide 3-kinase. Biochem. Biophys. Res. Commun. 319, 541–549 [DOI] [PubMed] [Google Scholar]
- 92.Somarajan B. I., Khanday M. A., Mallick B. N. (2016) Rapid eye movement sleep deprivation induces neuronal apoptosis by noradrenaline acting on alpha1 adrenoceptor and by triggering mitochondrial intrinsic pathway. Front. Neurol. 7, 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhong L. M., Zong Y., Sun L., Guo J. Z., Zhang W., He Y., Song R., Wang W. M., Xiao C. J., Lu D. (2012) Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS One 7, e32195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsieh T. C., Lin C. Y., Lin H. Y., Wu J. M. (2012) AKT/mTOR as novel targets of polyphenol piceatannol possibly contributing to inhibition of proliferation of cultured prostate cancer cells. ISRN Urol. 2012, 272697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Tourneau C., Faivre S., Serova M., Raymond E. (2008) mTORC1 inhibitors: is temsirolimus in renal cancer telling us how they really work? Br. J. Cancer 99, 1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abraham R. T., Gibbons J. J. (2007) The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin. Cancer Res. 13, 3109–3114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.