Abstract
T cell receptor (TCR) gene-modified T cells are a promising immunotherapy but require refinement to improve clinical responses and limit off-target toxicities. A variety of TCR and gene-delivery vector modifications have been developed to enhance introduced TCR expression and limit introduced/endogenous TCR chain mispairing, improving target antigen recognition and minimizing mispairing-induced cross-reactivity. Using our well-characterized HCV1406 TCR, we previously compared the impact of various chain pairing enhancing modifications on TCR expression and cognate antigen recognition. HCV1406 TCR is also natively cross-reactive against naturally occurring altered peptide ligands (APLs), which was shown to be dependent on high TCR surface density. In this report, we observed in a Jurkat model that absent TCR chain pairing competition alleviated CD8-dependent APL recognition and induced novel cross-reactivity of HCV1406 TCR. We then compared chain pairing enhancing modifications’ effects on TCR cross-reactivity in Jurkat and T cells, showing C-terminal leucine zippers and constant region murinization alleviated CD8-dependence and induced novel APL recognition. While modifications enhancing TCR chain pairing intend to avoid cross-reactivity by limiting mispairing with the endogenous TCR, these data suggest they may also enhance natural cross-reactivity and reduce dependence on CD8. These observations have significant implications on the design/implementation of TCR gene-modified T cells.
Keywords: T cell, T cell receptor, gene-modified T cells, chain pairing, adoptive cell therapy, altered peptide ligands
Summary Sentence:
Evaluation of how TCR modifications used to enhance TCR chain pairing in gene-modified T cells can increase antigen cross-reactivity and eliminate CD8 dependence
INTRODUCTION
The use of T cell receptor (TCR) gene-modified T cells to treat malignancies is a promising immunotherapy, but refinements in their design and implementation are needed to improve clinical outcomes and minimize adverse events1. For instance, a variety of clinical trials displayed a range of on- and off-target adverse events. These included: toxicities in the eye and inner ear using a gp100-reactive TCR for melanoma2; inflammatory colitis targeting CEA+ colon cancer due to recognition of CEA-expressing normal colon tissue3; neurotoxicity caused by cross-reactivity against MAGE-A12 by a MAGE-A3-reactive TCR4; and cardiogenic shock and death due to unexpected cross-reactivity against titin expression in cardiac tissue by another MAGE-A3-reactive TCR5, 6. Another safety concern is the potential for novel self-reactivity caused by mispairing between introduced and endogenous TCR α and β chains. One study demonstrated novel allo-HLA-reactivity by mixed TCR dimers in vitro7, and a mouse model demonstrated GVHD in five TCR systems8. While to date there has been no clinical evidence of GVHD in over 100 patients treated with TCR gene-modified T cells9, it remains a possibility.
To help prevent adverse events of TCR mispairing and improve targeted antigen recognition, many groups have used a variety of techniques to alter gene delivery vectors, TCR sequence, or TCR structure to promote efficient transgene translation, augment surface expression, and/or facilitate proper pairing and of introduced TCRs. These strategies include codon optimization of transgenes10, 11, addition of cysteine residues in the α and β constant regions to promote disulfide bridge formation12, addition of C-terminal leucine zippers13, substituting human constant regions with portions of or the entire mouse constant regions (murinization)14–18, or engineering Vα-Vβ single chain TCRs19–21. Recently, we directly compared the ability of each of the above modifications to augment surface expression and increase reactivity of T cells engineered to express a hepatitis C virus (HCV)-reactive TCR (HCV1406 TCR). We found that leucine zipper and constant region murinization with Cβ2 modifications induced the greatest increase in properly paired TCR expression and T cell reactivity22. We have also shown, however, that this HCV1406 TCR cross-reacts against naturally occurring mutant immune escape variants23, and that the surface density of introduced TCRs in gene-modified T cells has a critical impact on the recognition of cognate and altered peptide ligands (APLs)24. Because we and others have shown that TCR chain pairing enhancing modifications increase surface expression and relative surface density, they could potentially induce recognition of low levels of cognate antigen or cross-reactivity against APLs. In this study, we utilize our well-characterized HCV1406 TCR and APL model, to determine if TCR pairing-enhancing strategies impact cross-reactivity against related ligands.
We show in a Jurkat model that the absence of an endogenous TCR alleviates dependence on the CD8 co-receptor for recognition of low affinity APLs by HCV1406 TCR, and induces novel cross-reactivity against a previously unrecognized APL. Interestingly, leucine zipper and Cβ2 constant region murinization modifications of HCV1406 TCR allowed CD4+ PBL to recognize previously CD8-dependent ligands, CD8+ PBL to novelly recognize an additional APL. These observations have significant implications for the use of such enhanced TCR chain pairing strategies on the safety in patients, but also might be useful in generating polyclonal reagents where recognition of immune escape variants may be critical for eliminating genetically unstable diseases, including HCV.
MATERIALS AND METHODS
Cells and media.
HEK293GP, PG13, T2, Jurkat E6.1, and Jurkat76 cell lines were obtained from the American Type Culture Collection (Rockford, MD). All medium components were obtained from Corning Life Sciences (Corning, NY), unless otherwise noted. HEK293GP cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen Life Technologies, Carlsbad, CA). PG13 cells were maintained in Iscove’s DMEM supplemented with 10% FBS. Jurkat E6.1 and Jurkat76 cells were engineered to express CD8αβ using retroviral vectors as previously described23. T2, Jurkat E6.1, and Jurkat76 cells were maintained in RPMI 1640 medium supplemented with 10% FBS. PBMC were purchased as de-identified apheresis products from Key Biologics (Memphis, TN) and isolated using Ficoll-Paque (General Electric Company, Fairfield, CT) density gradient centrifugation. PBMC were stimulated prior to transduction with 50 ng/mL anti-CD3 mAb (Miltenyi Biotec, Bergisch Gladbach Germany) in AIM-V medium supplemented with 5% human AB serum (hAB; Valley Biomedical, Inc., Winchester, VA), 300 IU/mL IL-2 (Novartis Pharmaceuticals Corporation, East Hanover, NJ), and 100 ng/mL IL-15 (Biological Resources Branch, National Cancer Institute, Bethesda, MD). T cells were maintained in AIM-V medium supplemented with 5% hAB, 300 IU/mL IL-2 and 100 ng/mL IL-15.
Peptides.
The generation and rationale for naturally occurring mutant HCV NS3:1406–1415 epitopes has been previously described23. Wildtype (WT) and variant 1406–1415 peptides as well as tyrosinase:368–376 (used as a negative control) were purchased from Synthetic Biomolecules (San Diego, CA) at 95% purity.
Retroviral transduction.
Generation of WT, disulfide bridge (DSB), leucine zipper (LZ), constant region substitution with the murine Cα and Cβ2 (mCβ2) modified TCR retroviral vectors were previously described22. Retroviral supernatants were prepared using stable, high-titer producer PG13 cells expressing WT and pairing-modified TCRs in modified SAMEN retroviral vectors each containing TCR α chain linked to β chain and a truncated CD34 molecule by a P2A or T2A self-cleaving sequence, respectively25. Generation of PG13 retroviral producer cell lines, preparation and collection of retrovirus, and TCR-transduction by spinoculation have been described elsewhere25. Cultures were enriched for high and uniform transgene expression by positive selection using anti-CD34 mAb-coated immunomagnetic beads (Miltenyi Biotec).
Immunofluorescence.
Expression of T cell surface markers was determined by immunofluorescence staining using fluorochrome conjugated mAb’s: anti-CD3-APC/Cy, anti-CD4-PE/Cy7, anti-CD8-PerCP/Cy5.5, anti-CD34-PE; Biolegend, San Diego, CA). Expression of Jurkat cell surface markers was determined by immunofluorescence staining using mAb’s: anti-Vβ8 TCR-FITC (Beckman Coulter, Brea, CA), anti-CD8-PerCP/Cy5.5 (Biolegend), and anti-CD34-PE (Biolegend). HCV1406 TCR does not bind well against commercially available TCR Vβ antibodies. Thus, to determine relative surface density of the TCR, cells were stained with HLA-A*0201 tetramer folded around HCV NS3:1406–1415 peptide (provided by the NIH Tetramer Core Facility, Atlanta, GA). Flow cytometry was performed using an LSRFortessa flow cytometer (BD Biosceinces, San Jose, CA) and data was analyzed with FlowJoX software (Treestar, Ashland, OR).
Cytokine release assays.
Peptide-loaded T2 cells were used as stimulators. HCV1406 TCR-transduced Jurkat E6.1, Jurkat76, or PBL-derived normal human T cells were used as effectors. T2 cells were pulsed with 10 ug/mL peptide for 2 hr. 1×105 stimulators and 1×105 effectors were co-cultured in 96-well U-bottom tissue-culture plates overnight at 37°C, 5% CO225. 10 ng/mL PMA (Sigma-Aldrich, St. Louis, MO) was added to Jurkat E6.1 and Jurkat76 cell co-cultures to enhance sensitivity of stimulation23. Supernatants were harvested and the amount of IL-2 or IFNγ released by 1×105 Jurkat or T cells, respectively was measured by ELISA (Biolegend).
Intracellular IFNγ/degranulation bi-functional assay.
CD107a expression was used a surrogate marker for lytic function/degranulation. 3×105 TCR-transduced T cells were co-cultured with 3×105 peptide-loaded T2 cells in 96-well U-bottom issue culture plates at 37°C, 5% CO2 for 5 hr25. 250 ng anti-CD107a mAb, 5.0 ng/mL brefeldin-A, and 2.0 nM monensin (all Biolegend) were added at the beginning of co-culture26. After 5 hr, cells were stained for surface markers (described above) for 20 min at room temperature. Cells were fixed, permeabilized, and counterstained for intracellular IFNγ (anti-IFNγ-Brilliant Violet 421 mAb) according to manufacturer’s protocols (Biolegend). Data were acquired using an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA).
Statistical Analysis.
Log transforms were taken as needed to symmetrize data prior to statistical analysis. Comparisons of mean cytokine release by Jurkat E6.1 and Jurkat76 cells were performed using two-sample t-tests with unequal variances. To evaluate the differences in T cell reactivity with multiple donors and replicates, linear regressions were fit per cell type (CD4+ total reactivity, CD4+ bi-functional, CD8+ total reactivity, or CD8+ bi-functional) and per peptide condition. Covariates in the models included main effects of donor and main effects of ‘treatment group’ (where groups are WT, DSB, LZ, and mCβ2). Wald tests were used for each peptide condition and cell type to test for difference between treatment groups and WT.
Ethics Statement.
All recombinant DNA and retroviral transduction work was done under approved Loyola University Chicago Institutional Biosafety Committee protocols. Human PBMC used were derived from de-identified apheresis products purchased from commercial sources. Therefore, these studies are not considered Human Subjects Research and did not require Institutional Review Board approval.
RESULTS & DISCUSSION
Absence of TCR-pairing competition enhances HCV1406 TCR cross-reactivity and can alleviate CD8-dependence.
In order to first determine if competition for TCR pairing has a direct impact on antigen recognition, we evaluated the cross-reactivity of TCR+ Jurkat E6.1 and TCR- Jurkat76 cells against our panel of NS3:1406–1415 APLs. Because Jurkat E6.1 cells (like T cells) express an endogenous TCR (Vβ8), introduced TCR surface expression is limited by competition for CD3-association and chain mispairing between the endogenous and introduced TCRs. Since Jurkat76 cells lack an endogenous TCR27 (Fig. 1a), TCR gene-modified Jurkat76 cells do not encounter the normal limitations for surface expression that occur with Jurkat E6.1 and PBL-derived T cells. We also engineered both Jurkat cell lines to express the CD8 co-receptor to evaluate the effects of TCR chain pairing competition on CD8-dependent APL recognition (Fig. 1b). Together, this Jurkat system serves as useful tool to evaluate the effects of TCR chain mispairing and TCR pairing-enhancing modifications on APL recognition and CD8-dependence.
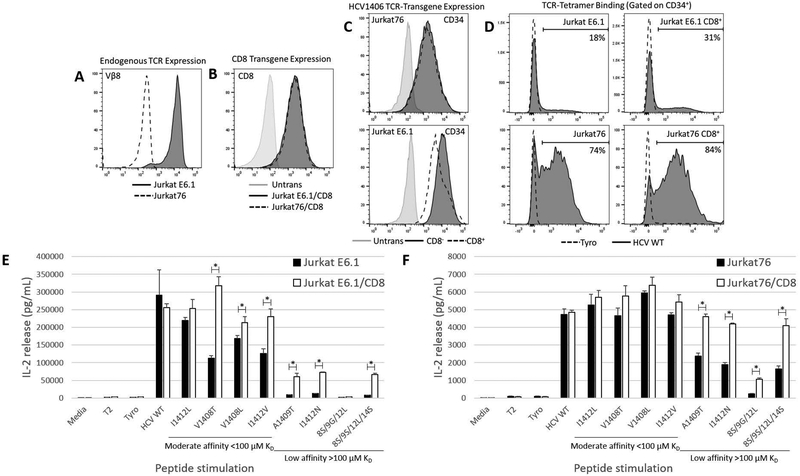
Figure 1. Absent TCR-pairing competition enhances HCV1406 TCR cross-reactivity and alleviates CD8-dependent recognition of APLs.
(a) Jurkat E6.1 (TCR/Vβ8+) or Jurkat76 (TCR/Vβ8-) cells (natively CD8-) were engineered to express (b) CD8αβ and/or (c) HCV1406 TCR as measured by transgene expression marker CD34. (d) Each group was evaluated for relative TCR surface expression levels by their ability to bind HLA-A*0201 tetramer folded around either HCV NS3:1406–1415 or tyrosinase:368–376 as a negative control. T2 cells were loaded with 10 ug/mL WT or variant HCV NS3:1406–1415 peptides or tyrosinase:368–376 as a negative control. Peptide-loaded T2 cells were co-cultured with HCV1406 TCR-transduced (d) Jurkat E6.1 or (f) Jurkat76 cells for 18 hr. IL-2 release by CD8- (black bars) and CD8+ (white bars) cells was determined by ELISA. Mean and standard deviation of triplicate measurements are shown. APL are organized from by decreasing TCR-pMHC affinity, left to right. Significant differences in IL-2 release between CD8- and CD8+ Jurkat cells are denoted *p<0.05. These data are representative of three independent experiments.
We previously reported that HCV1406 TCR is cross-reactive against a variety of naturally occurring immune escape variant epitopes of HCV NS323. We also demonstrated that recognition of HCV1406 TCR-APL interactions cluster into ranges of TCR-pMHC affinity that tend to dictate CD8-dependence. These affinity ranges are useful to differentially evaluate the effects of TCR chain pairing competition. For our purposes here, APLs cluster into moderate (<100 μM) and low (>100 μM) affinity, compared to WT TCR-pMHC interaction (17 μM)24. Jurkat E6.1 and Jurkat76 cells were transduced to express the HCV1406 TCR and sorted for high and uniform transgene expression using our CD34 marker (Fig. 1c). Despite similar transgene expression, tetramer staining suggested TCR-transduced Jurkat76 cells had dramatically higher HCV1406 TCR surface expression than TCR-transduced Jurkat E6.1 cells due to absent chain pairing competition (Fig. 1d). Reactivity of HCV1406 TCR-transduced Jurkat E6.1 cells recapitulated our previous observations that recognition of WT and moderate affinity mutants V1408L, I1412L, I1412V, and V1408T are recognized by both CD8+ and CD8- Jurkat E6.1 cells, and recognition of low affinity mutants A1409T, I1412N, and 8S/9S/12L/14S is dependent on the expression of the CD8 co-receptor (Fig. 1e). We have previously shown that the dependence on CD8 is influenced by on both TCR-pMHC structural stabilization as well as lck-recruitment to the TCR/CD3 complex24, 28. Surprisingly, CD8- HCV1406 TCR-transduced Jurkat76 cells secreted significant amounts of IL-2 against low affinity variants A1409T, I1412N, and 8S/9S/12L/14S, indicating that enhanced TCR pairing can lead to reversal of CD8 dependence or an apparent increase in TCR-pMHC affinity. The differences in IL-2 release between CD8- and CD8+ Jurkats against moderate affinity APL were also much smaller in the absence of an endogenous TCR. Additionally, the absence of TCR chain pairing competition enhanced the cross-reactivity of HCV1406 TCR as CD8+ Jurkat76 cells recognized 8S/9G/12L (Fig. 1f). This APL was not recognized in previous studies by TCR-transduced Jurkat E6.1 cells, TCR-transduced PBL-derived T cells, or even the parent T cell clone from which HCV1406 TCR was isolated. The absence of recognition by the T cell clone is exceptionally interesting because there was only an endogenous TCR. However, in our first characterization we showed that the parent clone exhibited aberrant allelic exclusion expressing two alpha chains, only one of which gave HCV1406 TCR its specificity29. This unique endogenous TCR chain pairing competition effectively reduced the relative density of the HCV-specific TCR and was presumably below expression levels of T cells with proper allelic exclusion. This is a critical observation because it highlights the importance of TCR chain pairing competition and its impact on cross-reactivity against related ligands. Together, these data suggest that TCR chain pairing in TCR gene-modified T cells can impact TCR cross-reactivity, and that alleviation of an endogenous TCR (eliminating chain pairing and limitations on surface expression) directly enhances cross-reactivity and reduces the requirements for antigen recognition.
Recently, some investigators have attempted to eliminate the expression of endogenous TCRs using siRNA, designer zinc-finger nucleases, or TAL effector nucleases to augment expression and pairing rather than modifying the introduced TCR30–32. In light of our observations with HCV1406 TCR, it is imperative that careful measures are taken to evaluate the consequences of absent pairing competition on the requirements for antigen recognition and cross-reactivity in other TCR gene-modified T cells as these strategies become more widespread.
Constant region murinization using Cβ2 enhances cross-reactivity and alleviates CD8-dependence in TCR-transduced Jurkat E6.1 cells.
We have previously compared the impact of various TCR chain pairing enhancing strategies on relative surface expression and T cell reactivity against cognate antigen22. Because TCR chain pairing competition has a direct impact on TCR cross-reactivity, we wanted to evaluate if TCR chain pairing enhancing strategies alter the cross-reactivity against naturally occurring APLs. In our previous report, we determined that addition of a constant region disulfide bridge (DSB), C-terminal leucine zippers (LZ), and murinization of TCR constant regions specifically using Cβ2 (mCβ2) had the greatest impact on transgene expression, surface density, and/or reactivity by IFNγ release and degranulation. Because these modifications had the greatest effect on surface density, we focused on these modifications.
CD8+ and CD8- Jurkat E6.1 cells were engineered to express WT HCV1406 TCR, DSB, LZ, or mCβ2 modifications, and enriched for high and uniform transgene expression using our CD34 marker (Fig. 2a). We previously showed that there is a direct relationship between CD34+ expression and antigen-specific multimer binding by our TCR-transduced cells22. Therefore, we know that enhanced tetramer binding demonstrates enhanced surface pairing of the modified TCRs compared to WT (Fig. 2b). Gated on CD34+ (TCR-transduced) cells, percentages of tetramer-positive cells reflect the relative density of properly paired introduced TCR in cells expressing the TCR transgene22. While DSB had modestly improved tetramer binding by CD8- Jurkat E6.1 cells (9% compared to 5% of WT TCR), LZ and mCβ2 modifications facilitated 3–4 fold enhanced tetramer binding compared to WT. Additionally, LZ and mCβ2 modified TCRs dramatically enhanced tetramer binding from 33% (WT) to 51% and 58%, respectively. These data demonstrate that while all groups have relatively similar TCR transgene expression levels (using CD34 as a surrogate marker), modified TCRs augment the relative surface expression of the introduced TCR evaluated by tetramer binding, as previously described22.
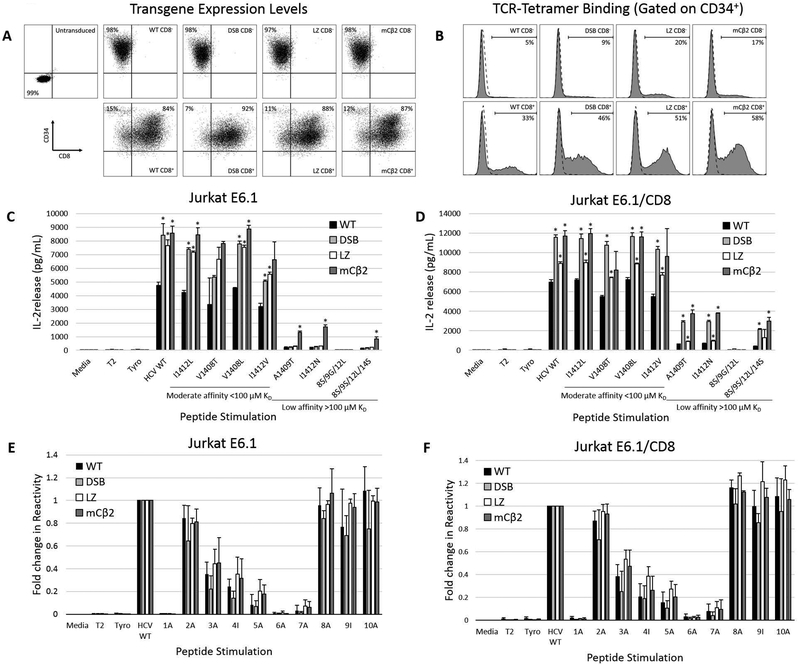
Figure 2. TCR pairing modifications enhance APL recognition by HCV1406 TCR-transduced Jurkat E6.1 cells.
(a) CD8- or CD8+ Jurkat E6.1 cells were engineered to express WT, DSB, LZ, or mCβ2 modified HCV1406 TCRs and immunomagnetically enriched for expression marker CD34 to ensure high and uniform transgene expression between groups. (b) Relative introduced HCV1406 TCR density was evaluated by tetramer binding. (c) CD8- or (d) CD8+ Jurkat E6.1 cells were co-cultured with WT and naturally occurring mutant NS3 peptide-loaded T2 cells. (e) CD8- or (f) CD8+ Jurkat E6.1 cells were co-cultured with WT and alanine-substituted NS3 peptide-loaded T2 cells. Fold change in cytokine release of each peptide substitution compared to WT sequence peptide is displayed. IL-2 release by WT (black bars), DSB (light gray bars), LZ (white bars), or mCβ2 (dark gray bars) TCR-transduced Jurkats was determined by ELISA. Mean and standard deviation of triplicate measurements are shown. APL are organized from by decreasing TCR-pMHC affinity, left to right. Significant differences in IL-2 release induced by modified TCRs compared to WT TCR are denoted *p<0.05. Data are representative of three independent experiments.
In order to evaluate the impact of TCR chain pairing enhancing modifications on cross-reactivity and CD8-depedence, TCR-transduced Jurkat E6.1 cells were co-cultured with WT and APL-loaded T2 cells. All three TCR chain pairing modifications increased the amount of IL-2 released against WT peptide by nearly two-fold and significantly augmented cytokine release against most moderate affinity APLs compared to Jurkat E6.1 cells expressing the WT HCV1406 TCR (p<0.05; Fig. 2c). Interestingly, the mCβ2 modification facilitated novel CD8-independent recognition of low affinity mutants A1409T, I1412N, and 8S/9S/12L/14S, compared to Jurkat E6.1 cells engineered to express the WT, DSB, or LZ-modified TCR. CD8+ Jurkat E6.1 cells engineered to express DSB, mCβ2, LZ TCR modifications also enhanced IL-2 release against nearly all WT and APL peptide-loaded T cells, but to a lesser degree than CD8- Jurkat E6.1 cells (Fig. 2d). Interestingly, the LZ-modified TCR exhibited lower IL-2 release compared to DSB or mCβ2 against all APLs, and was comparable to WT TCR against low affinity APLs. We previously speculated that leucine zippers may interfere with signaling scaffolding at the TCR/CD3 complex22, and may be responsible for only modest increases in IL-2 release here as well. Interestingly, none of the modified TCRs recognized 8S/9G/12L even with CD8 present. This suggests that while mCβ2 increases CD8-independent cross-reactivity in Jurkat E6.1 cells, it can promote proper chain pairing, but cannot fully eliminate competition for CD3 or surface expression with the endogenous TCR. An alternative hypothesis is that either TCR modifications or the presence of CD8 may structurally alter the TCR-pMHC interface, inducing novel mechanisms for cross-reactivity or CD8 independence. To evaluate for changes in pMHC interface, we measured fold-change in reactivity of TCR-transduced Jurkat E6.1 cells in the absence (Fig. 2e) or presence (Fig. 2f) of CD8 against a panel of alanine-substituted NS3:1406–1415 peptides. Interestingly, while certain peptide residues are key for antigen recognition (i.e. K1406 and G1411), the pattern of recognition is uniform among all TCRs regardless of the presence of CD8, suggesting that these modifications do not alter the way in which the TCR engages with pMHC. Together, these data suggest that reducing TCR chain mispairing and enhancing relative density of the introduced TCR can impact cross-reactivity and eliminate the requirement for CD8.
Additions of C-terminal leucine zippers or Cβ2 constant region murinization enhance cross-reactivity, alleviate CD8-dependence, and increase bi-functionality in TCR gene-modified T cells.
We wanted to next evaluate if these same modifications alter cross-reactivity or CD8-dependence in TCR gene-modified PBL-derived T cells to more accurately mimic the potential effects of TCR chain pairing enhancing strategies in T cells used for patient treatment. We also expanded our functional evaluation to assess for both IFNγ production and lysis, which are common and important parameters when assessing T cell reactivity. This also gives a broader evaluation of the functional potential of T cells engineered with chain pairing enhanced TCRs. We engineered PBL-derived T cells from three normal donors with WT, DSB, LZ, and mCβ2 modified HCV1406 TCRs, and measured intracellular IFNγ production and degranulation by CD107a expression (gated on TCR-transduced CD34+ T cells) using immunofluorescence to evaluate T cell function after APL stimulation on a per (transduced) cell basis. Experiments were performed three times using three independent PBL donor sources (n=9). Figure 3a-b is a representative experiment displaying functional differences between CD4+ and CD8+ T cells, respectively. Tables 1–2 summarize averages among the 9 experiments, which are referenced below and were used to test for statistically significant differences among TCRs.
Figure 3. TCR pairing modifications augments cross-reactivity against APL by HCV1406 TCR-transduced PBL.
Peptide-loaded T2 cells were co-cultured with PBL-derived T cells engineered to express WT, DSB, LZ, or mCβ2 modified HCV1406 TCRs. Intracellular IFNγ production and degranulation (CD107a expression) by (a) CD4+ and (b) CD8+ TCR-transduced T cells was determined by immunofluorescence. Pie wedges indicate frequency of CD34+ (TCR-transduced) cells expressing IFNγ only (yellow), CD107a only (red), are bi-functional (IFNγ+CD107a+; green), or are non-reactive (gray). APL are arranged by decreasing TCR-pMHC affinity, top to bottom. Data are a representative of 3 independent experiments performed with 3 donors. Average percent reactive cells in each category for all experiments (n=9) can be found in Tables 1–2.
Table 1.
Percentages of IFNγ producing and/or degranulating CD4+ T cells expressing chain pairing enhancing modified TCRs after stimulation with HCV APLsa
| TCR | |||||
|---|---|---|---|---|---|
| Peptide | Phenotype | Wildtype | Disulfide Bridge | Leucine Zipper | mCβ2 |
| Tyrosinase | non-reactive | 93.3 | 93.7 | 89.9 | 88.8 |
| IFNγ | 2.7 | 3.5 | 5.0 | 4.8 | |
| CD107a | 3.0 | 1.9 | 3.9 | 4.4 | |
| IFNγ & CD107a | 1.0 | 0.9 | 1.2 | 2.1 | |
| HCV WT | non-reactive | 61.5 | 57.9 | 36.8* | 35.3* |
| IFNγ | 21.0 | 23.9 | 34.5 | 29.9 | |
| CD107a | 6.9 | 5.2 | 9.3 | 11.8 | |
| IFNγ & CD107a | 10.6 | 13.0 | 19.4 | 23.0 | |
| I1412Lb | non-reactive | 63.9 | 69.5 | 49.2* | 41.0** |
| IFNγ | 19.3 | 17.3 | 26.7 | 30.3 | |
| CD107a | 7.0 | 6.6 | 9.2 | 9.6 | |
| IFNγ & CD107a | 9.8 | 9.2 | 14.9 | 19.1 | |
| V1408Tb | non-reactive | 76.7 | 81.5 | 64.1* | 57.8* |
| IFNγ | 12.9 | 13.4 | 19.3 | 21.3 | |
| CD107a | 4.8 | 2.8 | 6.0 | 8.8 | |
| IFNγ & CD107a | 5.6 | 5.2 | 10.5 | 12.1 | |
| V1408Lb | non-reactive | 59.5 | 65.8 | 45.0* | 36.9* |
| IFNγ | 22.3 | 20.5 | 30.3 | 32.1 | |
| CD107a | 7.0 | 4.0 | 8.6 | 10.0 | |
| IFNγ & CD107a | 11.2 | 9.7 | 16.2 | 20.7 | |
| I1412Vb | non-reactive | 75.7 | 81.0* | 64.0* | 53.5** |
| IFNγ | 12.8 | 10.5 | 18.8 | 25.7 | |
| CD107a | 5.1 | 3.0 | 6.9 | 7.9 | |
| IFNγ & CD107a | 6.3 | 5.5 | 10.3 | 12.8 | |
| A1409Tc | non-reactive | 89.6 | 91.4 | 83.9* | 72.9** |
| IFNγ | 4.5 | 4.7 | 9.3 | 17.0 | |
| CD107a | 3.7 | 2.0 | 3.9 | 4.9 | |
| IFNγ & CD107a | 2.2 | 1.8 | 2.9 | 5.2 | |
| I1412Nc | non-reactive | 86.6 | 88.6 | 75.7* | 70.3* |
| IFNγ | 7.2 | 7.3 | 14.5 | 18.8 | |
| CD107a | 3.7 | 2.0 | 5.1 | 5.1 | |
| IFNγ & CD107a | 2.5 | 2.1 | 4.8 | 5.8 | |
| 8S/9G/12Lc | non-reactive | 91.4 | 92.8 | 87.0 | 82.2* |
| IFNγ | 4.5 | 4.3 | 7.7 | 9.9 | |
| CD107a | 2.7 | 1.8 | 3.6 | 4.2 | |
| IFNγ & CD107a | 1.5 | 1.2 | 1.7 | 3.7 | |
| 8S/9S/12L/14Sc | non-reactive | 91.6 | 91.7 | 83.3* | 78.9* |
| IFNγ | 3.7 | 5.0 | 8.6 | 11.8 | |
| CD107a | 3.1 | 1.8 | 4.2 | 4.9 | |
| IFNγ & CD107a | 1.6 | 1.4 | 3.8 | 4.5 | |
Averages of 3 independent experiments using 3 PBL donors (n=9)
Moderate affinity APL;
Low affinity APL;
Significantly greater total percent reactive cells compared to WT TCR (significantly less non-reactive percentage) is denoted
p<0.05,
p<0.001.
Table 2.
Percentages of IFNγ producing and/or degranulating CD8+ T cells expressing chain pairing enhancing modified TCRs after stimulation with HCV APLsa
| TCR | |||||
|---|---|---|---|---|---|
| Peptide | Phenotype | Wildtype | Disulfide Bridge | Leucine Zipper | mCβ2 |
| Tyrosinase | non-reactive | 96.0 | 96.6 | 92.6 | 91.8 |
| IFNγ | 1.1 | 1.0 | 2.6 | 2.9 | |
| CD107a | 2.3 | 1.9 | 3.3 | 3.4 | |
| IFNγ & CD107a | 0.6 | 0.4 | 1.9 | 1.9 | |
| HCV WT | non-reactive | 28.7 | 35.4* | 9.5** | 7.9** |
| IFNγ | 15.5 | 11.0 | 13.6 | 14.3 | |
| CD107a | 13.4 | 27.0 | 12.5 | 10.1 | |
| IFNγ & CD107a | 42.5 | 26.7† | 64.3† | 67.7†† | |
| I1412Lb | non-reactive | 30.7 | 36.7 | 10.7** | 8.4** |
| IFNγ | 15.2 | 11.7 | 12.9 | 14.8 | |
| CD107a | 15.6 | 24.5 | 13.1 | 9.1 | |
| IFNγ & CD107a | 38.9 | 27.1† | 63.4† | 67.7†† | |
| V1408Tb | non-reactive | 34.5 | 41.7* | 12.9** | 9.6** |
| IFNγ | 14.1 | 11.0 | 14.1 | 14.7 | |
| CD107a | 14.5 | 22.9 | 12.8 | 10.3 | |
| IFNγ & CD107a | 36.8 | 24.5† | 60.2† | 65.5†† | |
| V1408Lb | non-reactive | 30.0 | 36.0* | 10.4** | 8.0** |
| IFNγ | 15.0 | 11.9 | 13.3 | 14.5 | |
| CD107a | 15.1 | 24.6 | 12.5 | 9.3 | |
| IFNγ & CD107a | 39.9 | 27.5† | 63.9† | 68.2†† | |
| I1412Vb | non-reactive | 33.7 | 41.9* | 10.6** | 9.1** |
| IFNγ | 14.6 | 12.3 | 13.7 | 14.7 | |
| CD107a | 13.7 | 21.1 | 12.7 | 10.0 | |
| IFNγ & CD107a | 38.0 | 24.8† | 62.9† | 66.2†† | |
| A1409Tc | non-reactive | 51.5 | 63.7* | 23.0** | 17.0** |
| IFNγ | 12.2 | 7.8 | 16.9 | 18.5 | |
| CD107a | 12.7 | 15.8 | 12.8 | 10.0 | |
| IFNγ & CD107a | 23.7 | 12.6†† | 47.3† | 54.6†† | |
| I1412Nc | non-reactive | 45.4 | 55.0* | 16.0** | 12.6** |
| IFNγ | 13.2 | 9.8 | 16.0 | 16.4 | |
| CD107a | 12.6 | 17.9 | 12.6 | 10.3 | |
| IFNγ & CD107a | 28.8 | 17.4† | 55.3†† | 60.7†† | |
| 8S/9G/12Lc | non-reactive | 85.2 | 88.6 | 71.4* | 64.0* |
| IFNγ | 4.3 | 3.4 | 9.2 | 11.1 | |
| CD107a | 5.6 | 5.4 | 7.5 | 8.3 | |
| IFNγ & CD107a | 4.9 | 2.6 | 11.9† | 16.6† | |
| 8S/9S/12L/14Sc | non-reactive | 51.3 | 62.7* | 24.4** | 19.3** |
| IFNγ | 11.6 | 8.1 | 16.0 | 16.6 | |
| CD107a | 13.1 | 15.9 | 13.4 | 11.7 | |
| IFNγ & CD107a | 24.0 | 13.2† | 46.3† | 52.4†† | |
Averages of 3 independent experiments using 3 PBL donors (n=9)
Moderate affinity APL;
Low affinity APL;
Significantly greater total percent reactive cells compared to WT TCR (significantly less non-reactive percentage) is denoted
p<0.05,
p<0.001;
Significantly greater frequency of bi-functional cells compared to WT TCR is denoted
p<0.05,
p<0.001.
In CD4+ T cells, the LZ and mCβ2 modifications greatly increased the total average number of total percent reactive cells against WT peptide (63.2% and 64.7%, respectively) compared to WT (38.5%) and DSB (42.1%) TCR-transduced T cells (Fig. 3a; Table 1). LZ and mCβ2 modifications also elicited significant increases in total percent reactive cells against moderate affinity APL: I1412L (1.4 and 1.6-fold increase, respectively), V1408T (2.0 and 1.8-fold increase), V1408L (1.4 and 1.6-fold increase), and I1412V (1.5 and 1.9-fold increase) compared to WT TCR-engineered CD4+ T cells. Interestingly, both LZ and mCβ2 also facilitated robust IFNγ production and degranulation by CD4+ PBL against previously CD8-dependent, low affinity APL A1409T and I1412N. Modest, but significant, IFNγ production and degranulation was also seen against 8S/9S/12L/14S and 8S/9S/12L. Additionally, the LZ and mCβ2 modifications often induced a greater proportion of lytic activity in CD4+ T cells, which could be useful in eliciting a more diverse immune response (Fig. 3a).
DSB, and to a much greater extent LZ and mCβ2 modifications also significantly enhanced IFNγ production and degranulation by CD8+ T cells against WT antigen and both moderate and low affinity APL (Fig. 3b; Table 2). 65–70% of WT TCR-transduced CD8+ T cells produced IFNγ and/or degranulated against WT and moderate affinity APL, which increased to generally over 90% when engineered with LZ or mCβ2 modified TCRs. Additionally, 8S/9G/12L was recognized by CD8+ T cells engineered with LZ or mCβ2 modifications, which as mentioned before had never been observed. Interestingly, these modifications also significantly enhanced the frequency of bi-functional IFNγ+CD107a+ CD8+ T cells against all APLs, suggesting that TCR chain pairing enhancing modifications may also induce more potent effectors. Together, these data support that LZ and mCβ2 chain pairing modifications can effectively enhance TCR cross-reactivity by alleviating CD8-dependence in CD4+ T cells and inducing robustly more reactive CD8+ effectors against APLs.
It is important to highlight that LZ and mCβ2 modifications facilitated CD4+ T cell recognition of previously CD8-dependent APL exhibiting ~10-fold lower affinity than WT peptide24. Because these modifications did not directly alter the TCR-pMHC interface, they may provide an alternative way to facilitate antigen recognition by CD4+ T cells with natively CD8-dependent TCRs without modifying TCR affinity. Although enhancing TCR affinity has generated more avid CD4+ and CD8+ effectors, affinity (as well as target antigen choice) has played a role in facilitating on- and off-target adverse events discussed earlier2–6. Additionally, enhanced TCR-pMHC affinity can cause activation-induced cell death upon antigen encounter, potentially reducing therapeutic efficacy33, 34. The ability to modify the TCR without changing its biophysical properties with pMHC may be useful in generating novel CD4+ effectors for helper cytokine support, facilitating cross-priming or epitope spreading, or engineering CD4+ regulatory T cells to help control autoimmune diseases. Thus, pairing-enhancing modifications may offer an alternative approach to generate MHC-I-restricted CD4+ T cell help.
However, because LZ and mCβ2 modifications enhanced the cross-reactivity of HCV1406 TCR gene-modified T cells, it is important to consider the safety implications when using chain pairing enhanced TCRs in patients. Augmenting the relative density of these introduced TCRs and alleviation of CD8 requirements could potentially initiate recognition of unwanted related epitopes, as seen in clinical studies using high-affinity TCRs targeting MAGE-A34–6. Before this study, it had not been evaluated how or if these pairing-enhancing strategies alter TCR cross-reactivity. Thus, in light of these results, it is still important to consider other effects of alternative TCR pairing strategies on T cell reactivity other than cytokine release including, target antigen expression, kinetic strength of TCR-pMHC engagement, downstream signaling patterns, as well as other co-activating/inhibiting surface molecules.
Taken together, augmenting the pairing efficiency of introduced TCRs in TCR gene-modified T cells is an attractive strategy to improve surface expression and minimize mispairing with endogenous TCRs. However, it must be considered how these strategies could impact cross-reactivity and other requirements for recognition including CD8-dependence. While it may offer new CD4+ helper support without modifying the TCR affinity, unintended consequences of enhanced cross-reactivity could be costly for patients. While HCV1406 TCR provides a model to evaluate the effects of enhanced TCR chain pairing on cross-reactivity, it is nevertheless important to consider the potential for enhanced recognition or cross-reactivity on a case-by-case basis. As pairing-modification strategies become more refined, their introduction into the clinic should proceed with caution
ACKNOWLEDGEMENTS
Funding for these studies was supported by grants issued by the National Cancer Institute: R01 CA104947 (MIN), R01 AI129543 (MIN), P01 CA154778 (MIN), and F30 CA180731 (TTS). Tetramers were provided by the NIH Tetramer Core Facility.
ABBREVIATIONS
- APL
Altered peptide ligand
- DSB
Disulfide bridge
- FBS
Fetal bovine serum
- hAB
Human AB serum
- HCV
Hepatitis C virus
- LZ
Leucine zipper
- mCβ2
Murinized constant region β2 locus
- TCR
T cell receptor
- WT
wildtype
Footnotes
Co-first authors
AUTHORSHIP
TTS: Conception/design; acquisition/analysis/interpretation; drafting/revising; final approval
KCF: Conception/design; acquisition/analysis/interpretation; drafting/revising; final approval
EG: Acquisition/analysis/interpretation; drafting/revising; final approval
MIN: Conception/design; acquisition/analysis/interpretation; drafting/revising; final approval
CONFLICT OF INTEREST DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Spear TT, Nagato K, and Nishimura MI (2016). Strategies to genetically engineer T cells for cancer immunotherapy. Cancer Immunol Immunother 65: 631–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. (2011). T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19: 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. (2013). Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36: 133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. (2013). Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5: 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. (2013). Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, et al. (2010). Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci U S A 107: 10972–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, et al. (2010). Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med 16: 565–570, 561p following 570. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA (2010). Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol Ther 18: 1744–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leisegang M, Turqueti-Neves A, Engels B, Blankenstein T, Schendel DJ, Uckert W, et al. (2010). T-cell receptor gene-modified T cells with shared renal cell carcinoma specificity for adoptive T-cell therapy. Clin Cancer Res 16: 2333–2343. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhuber P, Conrad H, Starck L, Leisegang M, Busch DH, Uckert W, et al. (2010). Targeting the epidermal growth factor receptor (HER) family by T cell receptor gene-modified T lymphocytes. J Mol Med (Berl) 88: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 12.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, et al. (2007). Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109: 2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HC, Bao Z, Yao Y, Tse AG, Goyarts EC, Madsen M, et al. (1994). A general method for facilitating heterodimeric pairing between two proteins: application to expression of alpha and beta T-cell receptor extracellular segments. Proc Natl Acad Sci U S A 91: 11408–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blank U, Boitel B, Mege D, Ermonval M, and Acuto O (1993). Analysis of tetanus toxin peptide/DR recognition by human T cell receptors reconstituted into a murine T cell hybridoma. Eur J Immunol 23: 3057–3065. [DOI] [PubMed] [Google Scholar]
- 15.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, and Morgan RA (2006). Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res 66: 8878–8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastings AE, Hurley CK, Robinson ED, Salerno K, Hernandez E, and Richert JR (1996). Molecular interactions between transfected human TCR, immunodominant myelin basic protein peptide 152–165, and HLA-DR13. J Immunol 157: 3460–3471. [PubMed] [Google Scholar]
- 17.Roszkowski JJ, Yu DC, Rubinstein MP, McKee MD, Cole DJ, and Nishimura MI (2003). CD8-independent tumor cell recognition is a property of the T cell receptor and not the T cell. J Immunol 170: 2582–2589. [DOI] [PubMed] [Google Scholar]
- 18.Sommermeyer D, and Uckert W (2010). Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J Immunol 184: 6223–6231. [DOI] [PubMed] [Google Scholar]
- 19.Aggen DH, Chervin AS, Schmitt TM, Engels B, Stone JD, Richman SA, et al. (2012). Single-chain ValphaVbeta T-cell receptors function without mispairing with endogenous TCR chains. Gene Ther 19: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss RH, Thomas S, Pfirschke C, Hauptrock B, Klobuch S, Kuball J, et al. (2010). Coexpression of the T-cell receptor constant alpha domain triggers tumor reactivity of single-chain TCR-transduced human T cells. Blood 115: 5154–5163. [DOI] [PubMed] [Google Scholar]
- 21.Stone JD, Harris DT, Soto CM, Chervin AS, Aggen DH, Roy EJ, et al. (2014). A novel T cell receptor single-chain signaling complex mediates antigen-specific T cell activity and tumor control. Cancer Immunol Immunother 63: 1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foley KC, Spear TT, Murray DC, Nagato K, Garrett-Mayer E, Nishimura MI (2017). HCV T Cell Receptor Chain Modifications to Enhance Expression, Pairing, and Antigen Recognition in T Cells for Adoptive Transfer. Mol Ther Oncolytics 17: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear TT, Riley TP, Lyons GE, Callender GG, Roszkowski JJ, Wang Y, et al. (2016). Hepatitis C virus-cross-reactive TCR gene-modified T cells: a model for immunotherapy against diseases with genomic instability. J Leukoc Biol 100: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear TT, Wang Y, Foley KC, Murray DC, Scurti GM, Simms PE, et al. (2017). Critical biological parameters modulate affinity as a determinant of function in T-cell receptor gene-modified T-cells. Cancer Immunol Immunother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spear TT, Callender GG, Roszkowski JJ, Moxley KM, Simms PE, Foley KC, et al. (2016). TCR gene-modified T cells can efficiently treat established hepatitis C-associated hepatocellular carcinoma tumors. Cancer Immunol Immunother 65: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spear TT, Callender GG, Roszkowski JJ, Moxley KM, Simms PE, Foley KC, Murray DC, Scurti GM, Li M, Thomas JT, Langerman A, Garrett-Mayer E, Zhang Y, and Nishimura MI (2016). TCR gene-modified T cells can efficiently treat established hepatitis C-associated hepatocellular carcinoma tumors Cancer Immunology, Immunotherapy In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heemskerk MH, Hoogeboom M, de Paus RA, Kester MG, van der Hoorn MA, Goulmy E, et al. (2003). Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood 102: 3530–3540. [DOI] [PubMed] [Google Scholar]
- 28.Lyons GE, Moore T, Brasic N, Li M, Roszkowski JJ, and Nishimura MI (2006). Influence of human CD8 on antigen recognition by T-cell receptor-transduced cells. Cancer Res 66: 11455–11461. [DOI] [PubMed] [Google Scholar]
- 29.Callender GG, Rosen HR, Roszkowski JJ, Lyons GE, Li M, Moore T, et al. (2006). Identification of a hepatitis C virus-reactive T cell receptor that does not require CD8 for target cell recognition. Hepatology 43: 973–981. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto S, Mineno J, Ikeda H, Fujiwara H, Yasukawa M, Shiku H, et al. (2009). Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res 69: 9003–9011. [DOI] [PubMed] [Google Scholar]
- 31.Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, Reik A, et al. (2012). Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med 18: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berdien B, Mock U, Atanackovic D, and Fehse B (2014). TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther 21: 539–548. [DOI] [PubMed] [Google Scholar]
- 33.Combadiere B, Reis e Sousa C, Trageser C, Zheng LX, Kim CR, and Lenardo MJ (1998). Differential TCR signaling regulates apoptosis and immunopathology during antigen responses in vivo. Immunity 9: 305–313. [DOI] [PubMed] [Google Scholar]
- 34.Lenardo MJ, Boehme S, Chen L, Combadiere B, Fisher G, Freedman M, et al. (1995). Autocrine feedback death and the regulation of mature T lymphocyte antigen responses. Int Rev Immunol 13: 115–134. [DOI] [PubMed] [Google Scholar]