Abstract
A better understanding of the underlying pathophysiology of obesity and its comorbidities is needed to develop more effective therapeutics. Although several studies have observed differences in CNS activation/deactivation patterns between obese and lean individuals when viewing food cues, few studies have examined whether the same holds true among individuals with type 2 diabetes. We examined differencescross-sectionally in brain activation to food cues between obese (n=6) vs. non-obese (n=5) individuals with type 2 diabetes using functional magnetic resonance imaging (fMRI). Obese individuals with type 2 diabetes demonstrate less activation of the salience- and reward-related insula while fasting and increased activation of the amygdala to highly desirable foods after a meal. Our findings in type 2 diabetes suggest a persistence of differences between obese versus non-obese individuals. Future, larger studies should confirm this differential activation between lean and obese individuals with and without type 2 diabetes.
Obesity and type 2 diabetes (T2D) are rapidly growing in prevalence worldwide. The human brain is critical to understand the underlying mechanisms of obesity and T2D. Obese individuals show changes in insula, orbitofrontal cortex (OFC), amygdala, striatum, somatosensory cortices, and anterior cingulate cortex as compared to lean individuals while viewing food cues [1, 2]. A recent study observed increased activation of the insula, amygdala and orbitofrontal cortex in obese patients with T2D as compared to lean, healthy controls [3]. Although it is difficult to draw conclusions regarding the effects of T2D versus those of obesity, another study comparing individuals with T2D with age- and body mass index (BMI)-matched healthy controls, observed that patients with T2D demonstrate increased activation to food images in the insula, orbitofrontal cortex and striatum [4]. Given that subjects with either obesity and/or diabetes may activate different central nervous system (CNS) areas when viewing food cues, it remains to be determined whether among subjects with T2D, obesity status may alter CNS activity to visual food cues.
We examined cross-sectionally, using fMRI, how neural responses to food cues differed between individuals with T2D who are obese vs. non-obese. Since we hypothesized that changes would be in reward- or saliency- related brain areas (based on previous studies, where these changes are the most significant, particularly [3]) and we desired to focus this pilot, we used small volume corrections to test for changes in the regions of interest- namely, the insula, striatum (e.g. putamen, caudate), orbitofrontal cortex, and amygdala.
Methods
Eleven men and women with T2D (defined as fasting plasma glucose≥126mg/dL and/or HbA1c>6.5%) provided written informed consent to participate in this study to compare obese (BMI≥30kg/m2) and non-obese (BMI<30 kg/m2) T2D, approved by the Beth Israel Deaconess Medical Center (BIDMC) Institutional Review Board. Based on a study which examined patients with T2D who were obese vs. healthy, lean controls [3], we would need only 3 people per group to have 80% power to detect changes in the insula, amygdala or OFC at α=0.05. Data analyzed herein comes from a larger study which examined the effects of liraglutide on fMRI [5] but analyzes only scans which came from participants who had the placebo condition first, and thus, were not currently and/or had previously been exposed to liraglutide. Participants had an overnight visit (with an overnight fast of at least 12 hours) followed by fMRI scans. Please see Supplementary Data S1 for more information.
fMRI protocol and analysis
Participants viewed food and non-food items at the Center for Biomedical Imaging, Boston University School of Medicine, using a 3T Philips Intera whole-body MRI (Philips Medical Systems, Best, The Netherlands) in both the fasting and fed states. Imaging parameters: TR=3s, TE=25ms, matrix=128×128, FOV=23×23cm, gap=0.8mm, bandwidth=83.33kHz, slice thickness=2mm. The fMRI protocol consisted of six runs, during which subjects viewed blocks of highly desirable (high calorie or high fat images such as cakes, onion rings, and other similar foods), less desirable (low calorie or low fat images such as vegetables and fruits), or non-food images (examples include flowers, rocks, and trees).
fMRI analysis
The contrast images [highly desirable>less desirable food images and all food (both highly and less desirable food cues)>non-food images] of the first-level analysis were used for the second-level group statistics. To compare obese and non-obese T2D, two sample t-tests were used. We then utilized small volume corrections (SVC) on the resultant fMR images for the regions of interest as previously described in order to be able to compare our results to those which were previously found between individuals with diabetes who were obese and healthy, lean controls [3]. Briefly, spherical regions of interest were created in marsbar with radii 5-mm (for amygdala) or 10-mm (for insula, putamen, and OFC) for SVC, and results which passed p<.05, family-wise error (FWE) corrected for multiple comparisons for peak are reported.
Results
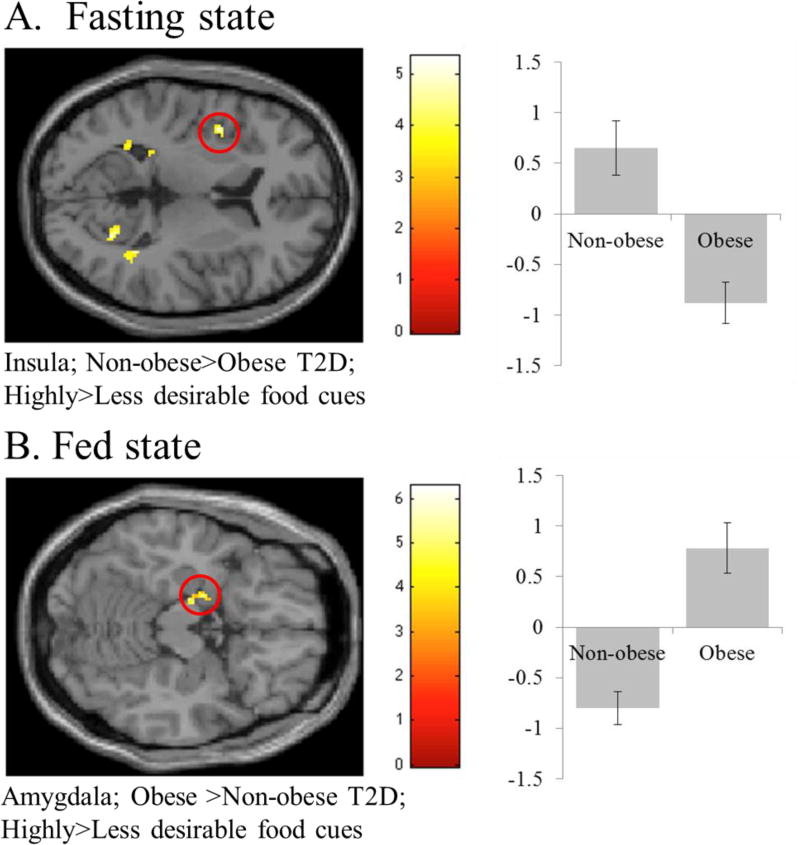
There were no differences between the patients with T2D who are obese (n=6) and non-obese (n=5) in parameters aside from anthropometric characteristics including BMI (p<.001) and weight (p<.001). Notably, HbA1c and glucose were not different between the two groups (Supplementary Table S1). Patients with T2D who are obese show less activation in the insula to highly as compared to less desirable food cues during the fasting state (Supplementary Table S2; Figure 1a). After a meal, patients with T2D who are obese show greater activation in the amygdala to highly as compared to less desirable food cues (Supplementary Table S2; Figure 1b).
Figure 1.
Results from small volume correction (SVC) analysis for reward and saliency-related regions of interest to compare individuals with type 2 diabetes (T2D) who are obese vs. non-obese in the fasting (A) and fed (B) states (see Supplementary Table 2). Areas significant at p<.05, FWE corrected for multiple comparisons are circled in red. The y-axis represents effect size of the activation (z scores). BOLD contrasts are superimposed on a T1 structural image in neurological orientation. The color bar represents voxel T value.
Discussion
In this pilot, we observed changes in the insula in the fasting state and in the amygdala in the fed state for obese vs. non-obese patients with T2D. In the past, several studies have examined differences between obese and lean individuals without diabetes which have been combined through meta-analyses, observing increased activation of the insula, striatum, amygdala, hippocampus, thalamus, dorsolateral prefrontal cortex, cingulate gyrus, and OFC in individuals with obesity while fasting and increased activation of the cingulate gyrus, striatum, OFC, other areas of prefrontal cortex, insula, amygdala, and precuneus when sated [1, 2]. Studies in T2D show similarly increased activation in some of these areas [3, 4], suggesting that this may be in part due to accompanying obesity status. Herein, individuals with T2D who are obese show decreased activation in the insula while fasting and increased response of amygdala to highly desirable food cues after a meal, suggesting that we may be seeing persistent differences of individuals with T2D who are obese vs. lean.
In the fasting state of our pilot study, obese as compared to non-obese patients with diabetes show less activation in the insula to highly versus less desirable food cues. The insula is involved in attention and the processing salient stimuli [6–8]. More specifically, the insula has been implicated in reward-based attention [3, 9]. Additionally, the insula is known to be involved in satiety, or the feeling of fullness or hunger. Ratings of hunger and prandial state, i.e. whether before or after a meal, impact the activation of the insula indicating a role in satiety and the craving of foods [7, 8, 10]. While many studies observe increased activation of the insula to food cues amongst individuals with obesity, there may be differences when accounting for fasting/fed state, where individuals who are obese or prone to becoming obese have lower activation of the insula in the fasting state but higher in the fed state in some studies [11, 12]. Additionally, individuals with prediabetes who are obese also show decreased activation of the insula as compared to individuals without diabetes who are obese, which may also indicate some interactions between blood glucose and obesity [13]. Overall, this altered activation to highly desirable foods while fasting needs to be examined more closely in future studies to determine how it impacts food intake.
In the fed state of our pilot study, patients with T2D who are obese show greater activation in the amygdala to highly desirable food cues. The amygdala responds to emotionally salient and to rewarding stimuli [14–16] and may be particularly activated for individuals who engage in eating when feeling strong emotions, be it stress, happiness, or anger [17, 18]. This may indicate an enhanced response to highly desirable foods in particular in individuals with T2D who are obese, similar to that seen in obese individuals more generally.
Thus, in this pilot study, we observed changes in the central nervous system processing of food cues with individuals with T2D who are obese versus non-obese, which are suggestive of the typical changes between obese and lean individuals. Whether T2D may be an effect modifier for the representation of obesity in the brain (e.g. obese individuals with vs. without T2D) remains to be seen in future, larger studies. These findings suggest that the comorbidities of obesity should be carefully controlled in future and interventional fMRI studies of food cue processing, as differences between individuals who are obese vs. lean may persist despite similar diagnosis of T2D as suggested in this pilot. Of note, the sample size for our populations is moderately small as there are relatively few non-obese individuals with T2D but still demonstrate significance for areas in which we would expect to see changes. These data may be used for power calculations for future, larger studies, which could confirm and extend our findings to explore how obese vs. lean diabetic patients may compare to obese vs. lean non-diabetic patients. Until differences are clearly quantitated, studies should carefully control for BMI and/or diabetes.
Supplementary Material
Acknowledgments
These projects were supported by Harvard Clinical and Translational Science Center Grant UL1 RR025758 from the National Center for Research Resources. This study was also supported in part by NIH DK081913. Olivia M. Farr is supported by training grant 5T32HD052961. This study was part of a larger study funded by Novo Nordisk who supported the study through an Investigator-Initiated Study grant. They approved the design of the study, but had no role in study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Disclosures: The authors have nothing to disclose.
Author Contributions
OMF and CSM designed the study, collected the data, analyzed the data, and wrote the manuscript. OMF is the guarantor of this work.
The authors have no conflicts of interest to declare.
References
- 1.Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Frontiers in nutrition. 2014;1:7. doi: 10.3389/fnut.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PloS one. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015;58:2688–2698. doi: 10.1007/s00125-015-3754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chechlacz M, Rotshtein P, Klamer S, et al. Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: a functional magnetic resonance imaging study. Diabetologia. 2009;52:524–533. doi: 10.1007/s00125-008-1253-z. [DOI] [PubMed] [Google Scholar]
- 5.Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;65:2943–2953. doi: 10.1007/s00125-016-3874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell metabolism. 2011;14:700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32:1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 8.St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135:1014–1018. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Yu H, Hu J, et al. Reward breaks through center-surround inhibition via anterior insula. Human brain mapping. 2015 doi: 10.1002/hbm.23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornier MA, McFadden KL, Thomas EA, et al. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiology & behavior. 2013;110–111:122–128. doi: 10.1016/j.physbeh.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery JA, Powell JN, Breslin FJ, et al. Obesity is associated with altered mid-insula functional connectivity to limbic regions underlying appetitive responses to foods. Journal of psychopharmacology (Oxford, England) 2017;31:1475–1484. doi: 10.1177/0269881117728429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farr OM, Mantzoros CS. Obese individuals with more components of the metabolic syndrome and/or prediabetes demonstrate decreased activation of reward-related brain centers in response to food cues in both the fed and fasting states: a preliminary fMRI study. International journal of obesity (2005) 2017;41:471–474. doi: 10.1038/ijo.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berns GS, Capra CM, Moore S, Noussair C. Three studies on the neuroeconomics of decision-making when payoffs are real and negative. Advances in health economics and health services research. 2008;20:1–29. doi: 10.1016/s0731-2199(08)20001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrido MI, Barnes GR, Sahani M, Dolan RJ. Functional evidence for a dual route to amygdala. Current biology : CB. 2012;22:129–134. doi: 10.1016/j.cub.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature neuroscience. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich-Lai YM, Christiansen AM, Wang X, Song S, Herman JP. Statistical modeling implicates neuroanatomical circuit mediating stress relief by 'comfort' food. Brain structure & function. 2015 doi: 10.1007/s00429-015-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Bloemendaal L, Veltman DJ, Ten Kulve JS, et al. Emotional eating is associated with increased brain responses to food-cues and reduced sensitivity to GLP-1 receptor activation. Obesity (Silver Spring, Md) 2015;23:2075–2082. doi: 10.1002/oby.21200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.