Summary
To understand computation in a neural circuit requires a complete synaptic connectivity map and a thorough grasp of the information processing tasks performed by the circuit. Here, we dissect a microcircuit in the mouse retina in which scotopic visual information (i.e., single photon events, luminance, contrast) is encoded by rod bipolar cells (RBCs) and distributed to parallel ON and OFF cone bipolar cell (CBC) circuits via the AII amacrine cell, an inhibitory interneuron. Serial block-face electron microscopy (SBEM) reconstructions indicate that AIIs preferentially connect to one OFF CBC subtype (CBC2); paired whole-cell patch clamp recordings demonstrate that, depending on the level of network activation, AIIs transmit distinct components of synaptic input from single RBCs to downstream ON and OFF CBCs. These findings highlight specific synaptic and circuit-level features that allow intermediate neurons (e.g. AIIs) within a microcircuit to filter and propagate information to downstream neurons.
eTOC Blurb
Graydon et al. dissect the neural circuitry connecting the rod and cone pathways in mouse retina. Anatomical reconstructions and electrophysiological recordings demonstrate how distinct signals are relayed through an intermediary interneuron to different downstream targets. This selective signal transfer changes with background luminance.
Introduction
During night (“scotopic”) vision, light-evoked signals in rod photoreceptors are transmitted primarily to rod bipolar cells (RBCs), “ON” cells that depolarize in response to light increments [1]. RBCs deliver their ON signal via excitatory, glutamatergic ribbon synapses to AII (A2) amacrine cells, compact interneurons that connect the rod pathway to the cone pathway (reviewed by [2]; Figure 1A). AIIs relay RBC signals to ON cone bipolar cell (CBC) axon terminals via electrical synapses [3, 4] and invert the signal via inhibitory glycinergic synapses to OFF CBC axon terminals and some OFF ganglion cells (GCs), which depolarize in response to light decrements [5-10]. AIIs also receive inputs from ribbon synapses in the OFF layer of the inner plexiform layer (IPL; [6, 11, 12], but the physiology and source of these inputs have not been studied in depth.
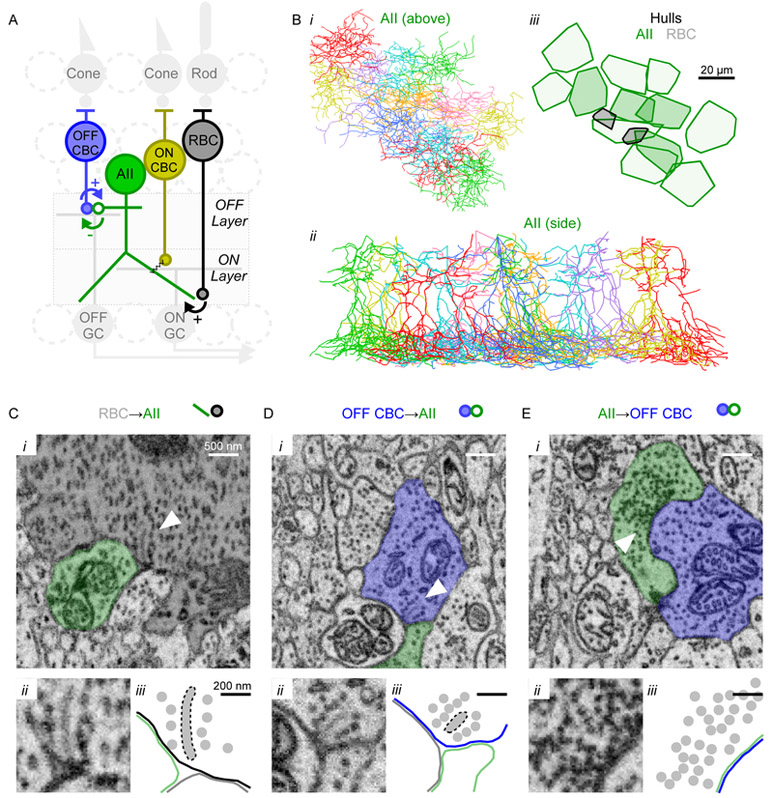
Figure 1 ∣. EM reconstruction of AII circuitry.
(A) Schematic of rod pathway circuitry.
(B) Reconstructed skeletons of 12 AII amacrine cells (i, ii). iii, convex hulls encompassing each AII’s output synapses (darker, “central” AIIs were used to quantify connectivity). Black hulls indicate ribbon synapses from two RBCs.
(C-E) SBEM examples of RBC→AII ribbon synapse, OFF CBC→AII ribbon synapse, and AII→OFF CBC conventional inhibitory synapse. D and E show reciprocal synapses between the same CBC (CBC2)-AII pair. i: arrowheads indicate presynaptic ribbons or vesicle clusters. ii: zoom of ribbon or vesicle cluster. iii: outline of key features in ii, showing ribbons, membranes and vesicles. Also see Figure S1 for sequential EM images above and below those shown in C-E.
Synaptic transmission from RBCs to AIIs comprises transient and sustained components [13] that, during light responses, convey information about visual contrast and luminance, respectively [14, 15]. It is unknown whether AIIs transmit both components to their postsynaptic targets. AIIs might relay transient (contrast) signals directly to OFF GCs (e.g., [7-9]) and sustained (luminance) signals to CBC terminals, leaving CBC ribbon synapses to compute contrast in the same way as RBCs [14]. Additionally, AIIs may transmit distinct signals to ON and OFF CBCs through electrical and chemical synapses, respectively. The input-output characteristics of AIIs also may depend on the level of network activation, which influences analog signaling in the rod pathway [16, 17].
We have examined these issues in mouse retina, first with serial block-face scanning electron microscopy (SBEM; [18]) to examine synaptic connectivity between RBCs, AIIs and OFF CBCs. Consistent with recent reports [12], we found that AIIs make about half of their inhibitory outputs onto one OFF CBC subtype (CBC2). SBEM shows that CBC2 provides, in turn, almost all of the OFF-layer ribbon synaptic input to AIIs, but electrophysiological experiments indicated weak CBC→AII signaling. Simultaneous whole-cell recordings confirmed that RBCs transmit transient and sustained signals to AIIs [13-15]. Paired recordings between AIIs and CBC2s indicated that AIIs are also capable of transient and sustained release, consistent with capacitative exocytosis measurements in AIIs [19]. Finally, we recorded from RBCs and CBCs simultaneously to examine how synaptic signals are transformed by the AIIs in-between. We found that AIIs transmit transient and sustained signals to (ON) CBC5 but primarily transient signals to (OFF) CBC2, depending on the steady-state AII membrane potential. By manipulating the membrane potential of all AIIs to span their voltage range across diverse lighting conditions [16], we found that AII signal transfer varies with the level of network activity: in a relatively depolarized steady-state, AIIs transmit both transient and sustained signals received from individual RBCs. These findings reveal complex synaptic features in the rod pathway that may underlie distinct transformations of ON and OFF signals during night vision.
Results
SBEM reconstruction of AII synaptic connectivity
Recent anatomical analyses indicate that AII amacrine cells contact all types of OFF CBCs in rabbit retina [20] and 4 of 5 OFF CBC types in mouse [12]. Here, we identified pre- and postsynaptic partners of AIIs in a conventionally stained volume of mouse retina imaged by SBEM [21]. The dataset’s resolution precluded reliable identification of gap junctions, so we restricted connectivity analysis to chemical synapses between AII lobular dendrites and OFF CBCs. Tracing the length of each dendritic process, we “skeletonized” 12 AIIs (Figure 1Bi,ii) to visualize each cell’s dendritic arbor and annotated all of the chemical synaptic inputs and outputs in the OFF sublamina (e.g., Figures 1C-E, S1). CBC synaptic ribbons were smaller than those in RBCs, as observed in rabbit [6]. The surface area of individual CBC ribbons was approximately 30% that of RBC ribbons (CBC: 0.022±0.016 μm2 vs. RBC: 0.075±0.052 μm2; p<0.0001; n=43 and 43, respectively) and much smaller than the large presynaptic active zones in AII lobular dendrites (0.91±0.39 μm2; n=30). We also reconstructed the arbors of 49 cells whose axons originated in the inner nuclear layer (INL) and made pre- or postsynaptic contacts in the OFF sublamina of the IPL with any of the 12 AIIs. We quantified connections made by 4 central AIIs (Figure 1Biii), because nearly all of their synaptic partners were contained within the data volume. These AIIs made 64.3±10.6 output synapses (n=4; mean±SD unless indicated otherwise), 89% (57.3±10.2) of which were onto cells whose processes originated in the INL [12]. Only 11% (7.0±2.7) of AII synaptic outputs were made onto GC dendrites, which were not analyzed further.
To identify the subtypes of the 49 INL neurons that contacted AIIs, we first drew convex hulls encompassing all synapses made by each cell to analyze mosaic tiling that facilitates bipolar cell type classification in the retina ([22]; Figure 2). The hulls overlapped substantially, indicating several superimposed cell type mosaics. Cell types were distinguished within this group based on mosaic tiling (Figure 2A-H), axon morphology (Figure 2I-L), stratification characteristics (Figures 2M, S2), and synaptic connectivity (Figure 2N-Q). Stratification within the IPL was measured relative to a band of skeletonized OFF starburst amacrine cell (SAC) dendrites. Cells with axon terminals ramifying in and/or below the OFF SAC layer formed two separate mosaics (Figure 2G,H,K,L) and exhibited features characteristic of CBC3A and CBC3B (Figures 2M, S2; [12, 21, 23]. CBC3s expressed many ribbon synapses (CBC3A: 148±29 (n=7); CBC3B: 110±11 (n=14)), but very few contacted AIIs (CBC3A: 0.6±0.5; CBC3B: 0.6±0.6). Each central AII made conventional synapses onto 1.7±1.7 CBC3As and 1.9±0.8 CBC3Bs but received only 0.5±0.6 and 0.8±1.0 ribbons inputs, respectively, in return (Table S1).
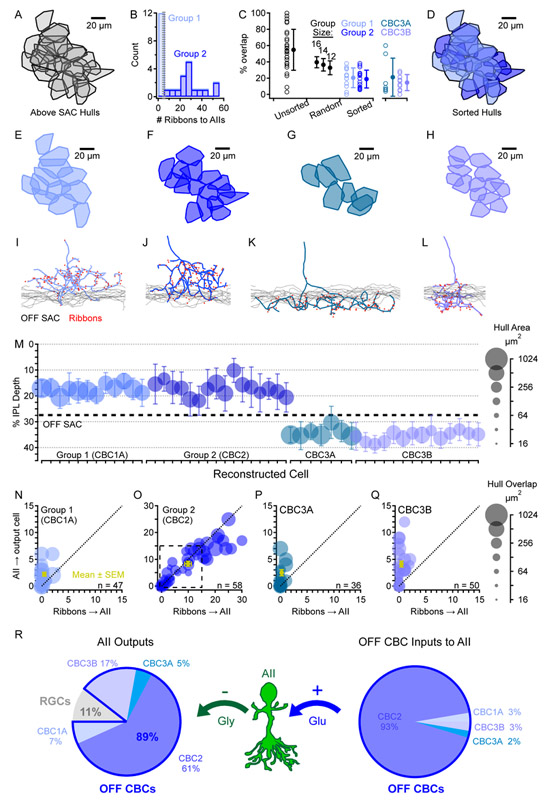
Figure 2 ∣. Synaptic connectivity between AIIs and OFF CBCs.
(A) Hulls encompassing ribbon synaptic inputs onto AIIs from each INL-originating neuron stratifying above the OFF SAC.
(B) A simple criterion – the number of ribbons presynaptic to AIIs – divided the neurons from A into two groups.
(C) Maximum percent overlap from any neighboring hull is plotted for each of the hulls shown in A (Unsorted; n=28). For comparison, to simulate a random sorting of the hulls in A into two separate populations, overlap was also calculated on randomly-chosen subsets of different sizes (Random; n=12,14, or 16; each simulated 106 times). The actual overlap distribution when sorting groups by the criterion in B (Sorted; n=12 and 16 for Group 1 and 2, respectively) shows overlap that is less than “Random” distributions and similar to CBC3s.
(D) Color-coded overlay of sorted Group 1 and Group 2 hulls.
(E-H) Mosaic tiling of hulls for each cell of a given type.
(I-L) Representative example skeletons from each cell type, showing the location of synaptic ribbons (red dots) and stratification depth in relation to OFF SAC dendrites.
(M) Summary data for all reconstructed cells, showing mean±average deviation (see STAR Methods) of ribbon synapse depth in the IPL and lateral hull area. OFF SAC dendrites form a band at an IPL depth of 27.5% (dashed). Also see Figure S2.
(N-Q) Synaptic input/output relationships between each CBC subtype and AIIs in the OFF sublamina of the IPL. All instances of hull overlap between each cell and each AII are shown, with marker area representing the degree of hull overlap. Some overlapping cells were not connected. Dashed box in O indicates the smaller scale of the other graphs.
(R) Summary of SBEM reconstruction synaptic connectivity data. Also see Table S1.
The remaining cells had axon terminals that ramified above the OFF SAC layer; substantial overlap suggested multiple cell types (Figure 2A). These cells exhibited similar hull areas, axonal branching geometry, and axon terminal stratification characteristic of CBC1A and CBC2 [12, 23]. Cells within this group were distinguished most clearly by the number of ribbon synapses made onto AIIs (Figure 2B). One subset (Group 1, n=12) contacted AIIs only very rarely: those overlapping the central AIIs contacted them with only 1.0±1.2 ribbons. By contrast, the second subset (Group 2, n=16) contacted AIIs avidly: those with hulls overlapping the four central AIIs made 47±9 ribbon synapses onto them. The cells in each group expressed similar numbers of ribbon synapses in their axon terminals (155±29 and 177±18, respectively) but, when sorted based on the number of inputs to AIIs, they formed two minimally-overlapping mosaics (Figure 2C,D), suggesting that they constituted two distinct cell types. Group 2 cells, which contacted AIIs most frequently, were also the most common recipients of AII output synapses, receiving 65±8% of the outputs made by the four central AIIs (Figure 2O). For six central Group 2 cells, this translated to 28±6 inputs from AIIs. By contrast, Group 1 cells received only 5.6±3.8% of the outputs from the four central AIIs (4.5±3.1 inputs for four central Group 1 cells), similar to the fraction of AII synapses devoted to CBC3A and CBC3B (Figure 2R). Each Group 1 cell contacted less than one AII on average (0.4±0.5) but was contacted by 1.4±0.7 AIIs. In contrast, each Group 2 cell contacted 2.8±0.9 AIIs and was, in turn, contacted by 2.8±0.9 AIIs (Table S1). Based on the relative connectivity levels with AIIs and close analysis of axonal stratification (Figure S2), Group 1 and Group 2 correspond most closely to recently classified CBC1A and CBC2 [12]. Accordingly, fluorescently labeled cells identified as CBC2 in mice expressing GFP under control of the synaptotagmin-2 (Syt2) promoter [24] exhibited axonal stratification most similar to those designated here as CBC2 (Figures S2, S3A).
Most of our anatomical results, particularly the strong AII↔CBC2 connectivity, agree with a recent study in mouse retina [12], except that those authors reported that AIIs contact CBC4 much more strongly than CBC3A, whereas we observed no AII-CBC4 connectivity, despite analyzing more AIIs. This discrepancy is unlikely due to differences in cell typing, as CBC3A axon terminal morphology observed here agrees closely with their report and is distinct from CBC4 (Figure S2).
Asymmetric synaptic strength between AIIs and CBC2s
To test for functionally bidirectional AII↔CBC2 connectivity, we recorded from CBC2s in acute slices prepared from Syt2-GFP mice. CBC2 recordings exhibited frequent spontaneous events (≤ 170 s−1) that were primarily glycinergic: they reversed near ECl (−45mV; Figure S3); their frequency was greatly reduced (by 77±27%, n=9) and often eliminated by bath application of the glycine receptor (GlyR) antagonist strychnine (Figure S3C,D); large currents were evoked in CBC2s by glycine puffed directly on their axon terminals (Figure S3E); and spontaneous events exhibited exponential decays (τ=3.8±1.9 ms, n=274 events from 10 cells; Figure S3F) similar to previously reported GlyRα1-mediated events between AIIs and OFF CBCs [25].
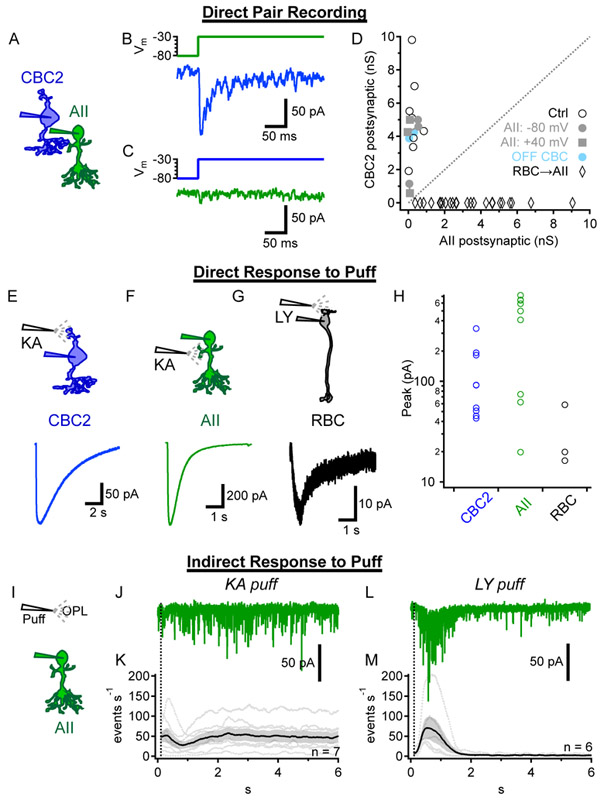
We next made simultaneous whole-cell voltage clamp recordings from synaptically connected AII-CBC2 pairs (Figure 3A) and delivered depolarizing voltage steps to each cell in turn. We consistently observed smaller postsynaptic currents in AIIs compared to CBCs (Figure 3B,C), despite a higher driving force on glutamate receptor conductances in AIIs (Vhold=−80mV, Erev=0mV; all Vm values corrected for liquid junction potentials) compared to GlyR conductances in CBC2s (Vhold=−80mV, ECl=−45mV). Comparing postsynaptic conductances confirmed this asymmetry (P=0.0008, paired t test, n=8 cell pairs; Figure 3D). Attempts to enhance evoked release from CBC2s with weakly calcium-buffered internal solution (0.5 mM EGTA; [13]) did not increase CBC2→AII synaptic strength to levels comparable to AII→CBC2 transmission (Figure 3D). Holding AIIs at +40mV, to relieve Mg2+ block of their NMDA receptors [26-28], did not reveal any increase in synaptic strength (Figure 3D). Two (non-CBC2) OFF CBC↔AII recordings showed a similar asymmetry in postsynaptic conductance, while the overall CBC2 postsynaptic conductance rivaled the AII postsynaptic conductance in RBC→ AII recordings (Figure 3D).
Figure 3 ∣. Asymmetric synaptic strength between AIIs and CBC2s.
(A) Schematic of AII↔CBC2 recordings. Also see Figure S3 for characterization of glycinergic events in CBC2s.
(B-C) Example AII↔CBC2 recordings showing the presynaptic voltage step (top) and evoked postsynaptic current (Vhold = −80mV; bottom). The AII (green) and CBC2 (blue) were stimulated alternately. Averages of 5 postsynaptic responses shown.
(D) Peak evoked postsynaptic conductances for 8 AII↔CBC2 recordings (open circles) following steps depicted in B-C. Three additional pairs were recorded with a low calcium-buffered internal solution (gray circles), with the postsynaptic CBC2 alternately held at Vm = +40mV to test for NMDA conductances (gray squares). AII pairs with two non-CBC2 OFF CBCs (cyan) and presynaptic RBCs (black diamonds, n=24) are shown for comparison.
(E-G) Responses to puff application of KA onto CBC2 dendrites (E) or onto AII lobular dendrites in the OFF sublamina of the IPL (F), and LY onto RBC dendrites (G).
(H)Summary of puff responses for experiments in E-G.
(I)Schematic of experiments for J-M, showing a puff in the OPL while recording from AIIs. Glycinergic and GABAA transmission was blocked (1 μM Strychnine, 100 μM picrotoxin).
(J)AII response to KA puffed in the OPL to drive release from OFF bipolar cells.
(K)Summarized event rates recorded in AIIs during KA puffs in the OPL. Similar results were obtained when TPMPA (50 μM) was added to block GABAC receptors.
(L)Example trace of AII response to LY puffs in the OPL to drive release of ON bipolar cells.
(M)Summarized event rates recorded in AIIs during LY puffs in the OPL.
AIIs might receive stronger inputs from other OFF CBCs. To test this, we activated all OFF CBCs with kainic acid (KA, 1 mM), an exogenous AMPA/kainate receptor agonist, applied via puffer pipette [29, 30]. KA elicited inward currents in CBC2s when puffed in the OPL (Figure 3E,H) and activated glutamate receptors directly on AIIs when puffed into the OFF layer of the IPL (Figure 3F,H) [31]. KA did not, however, evoke strong synaptic responses in AIIs when puffed in the OPL (Figure 3I-K), suggesting that AIIs receive little synaptic input from any OFF CBCs. By contrast, puffing the mGluR6 antagonist LY341495 (100 μM) elicited inward (depolarizing) currents in RBCs (Figure 3G,H; [32]) and vigorous synaptic responses in AIIs (Figure 3L,M), confirming that ON synaptic inputs to AIIs remained intact.
These physiological results suggest that CBC2s contribute little excitatory input to AIIs in mouse retina, despite extensive anatomical connectivity. We therefore focused on synaptic transmission from the AII – specifically, how it relays RBC signals to the cone pathway.
Paired recordings reveal signal transfer from RBCs to CBCs
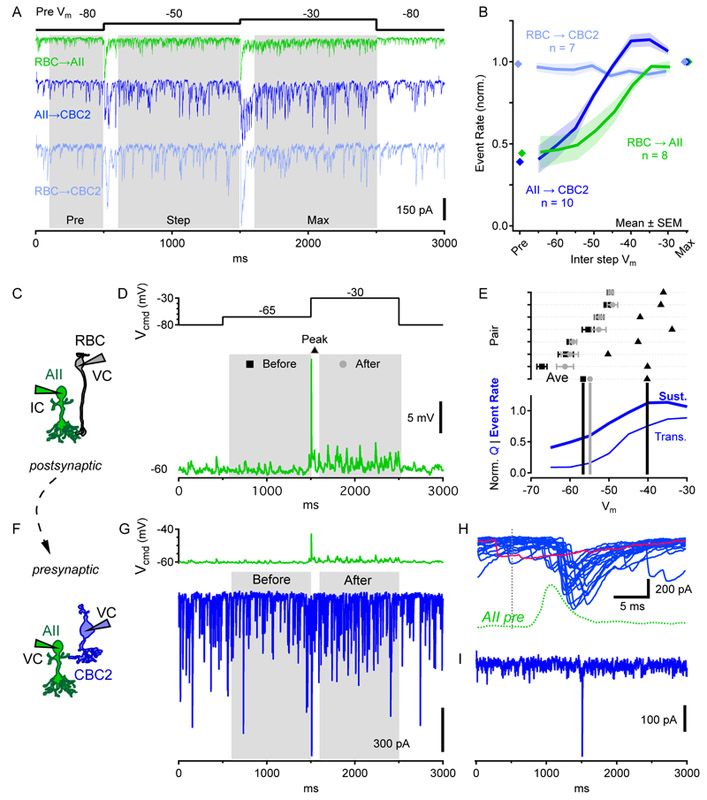
We made paired recordings to examine how inputs from RBCs are relayed by AIIs to their CBC targets. First, we examined RBC→AII transmission between synaptically coupled pairs [13-15, 33, 34]. At the beginning of each trial, both cells were held at -80mV. The RBC was then stepped to one of a range of intermediate potentials (−65mV to −30mV in 5-mV increments) for 1s before stepping to −30mV for 1s (Figure 4D, top). RBC steps from −80mV elicited transient EPSCs in the AII followed by smaller, sustained components (Figure 4D, bottom). Both components exhibited a (presynaptic) voltage dependence similar to L-type voltage-gated calcium (Cav) channels expressed in RBCs ([13, 35-37]; Figures 4G, 5B).
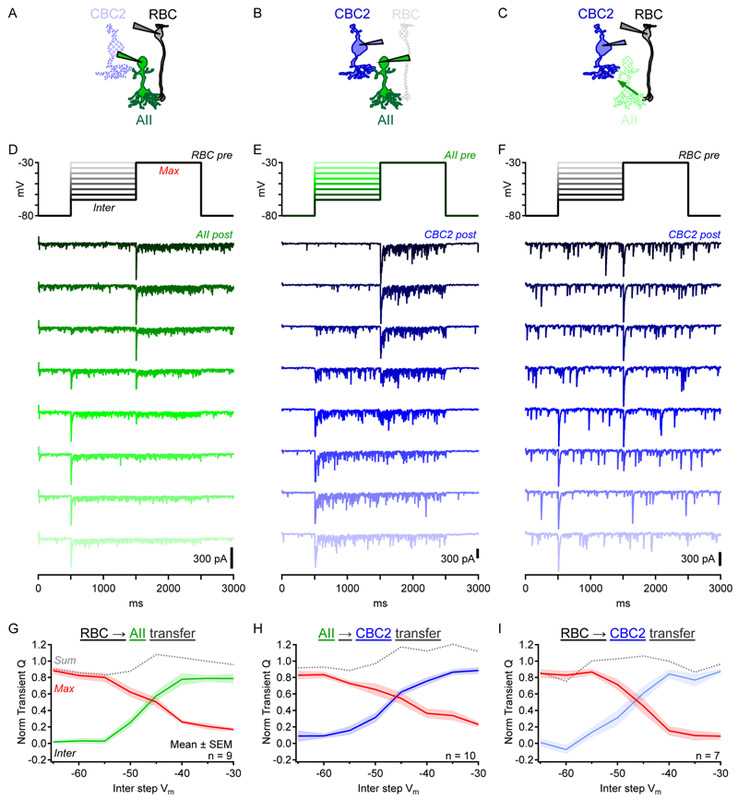
Figure 4 ∣. Exploring RBC→OFF CBC2 signal transfer with paired recordings.
(A-C) Schematics showing different paired recordings.
(D-F) Single postsynaptic currents from individual paired recordings. Under voltage clamp, the presynaptic cell was first stepped from −80mV to an intermediate membrane potential for 1s before being stepped to −30mV for 1s (top). Larger intermediate potential steps are lighter colors. Also see Figure S4, which shows that RBC→ CBC2 responses were blocked by strychnine.
(G-I) Synaptic transfer functions observed during paired recordings. Postsynaptic charge (Q, normalized to a maximum of 1) was calculated during the first 100ms following presynaptic steps and plotted vs. membrane potential (Vm) of the presynaptic step. Green, blue, light blue represent charge evoked by steps to different intermediate potentials. Red represents charge evoked by steps to −30mV from intermediate potentials. Gray = sum of “Max” and “Inter”.
Figure 5 ∣. Breaking down RBC→CBC2 transmission into sequential pairs.
(A) Postsynaptic currents of indicated paired recordings in response to presynaptic voltage steps (black). “Sustained” event rates were measured during the last 0.9s of each 1s step. “Pre” event rates were measured during the 0.4s before the first step and reflects the background rate of non-evoked postsynaptic events.
(B) Comparison of postsynaptic event rates in pairs described in A, normalized to the “Max” event rate for each pair.
(C) Schematic of RBC→ AII paired recordings, with the presynaptic RBC in voltage clamp and the postsynaptic AII in current clamp.
(D) Voltage steps in presynaptic RBC (top) elicited transient EPSPs in current clamped AIIs (bottom). AII membrane potential was measured during the 0.9s preceding the RBC step to −30mV (“Before”, square); at the EPSP peak following the RBC step to −30mV (“Peak”, triangle); and during the last 0.9s of the 1s RBC step to −30mV (“After”, circle).
(E) top, Postsynaptic membrane potentials for 7 pairs at the time points described in D. Data for “Before” and “After” presented as mean±SD across time window, while “Peak” is a single value. Average for all pairs shown on the bottom row. Symbols as in D. Bottom, AII→CBC2 transient (Figure 4H) and sustained (B) transfer functions replotted on the same scale.
(F) Schematic of AII→CBC2 paired recordings (both cells in voltage clamp).
(G) The AII EPSP from D was used as a presynaptic voltage command waveform (top) and IPSCs (Vhold=−80mV) were recorded in the CBC2 (bottom, single trial shown).
(H) Overlaid CBC2 IPSCs from 20 repeats of the AII stimulus in G (15s interval) during a single paired recording. Dotted lines indicate timing of RBC step to −30mV in D (black) and the consequent AII EPSP (green). Red trace indicates synaptic failure.
(I) Average CBC2 IPSC for the 20 trials shown in H.
Using the same presynaptic step protocol, we measured AII→CBC2 synaptic transfer (Figure 4B,E,H; postsynaptic ECl=0mV). AII voltage steps evoked a large increase in synaptic event frequency relative to baseline, indicating that most postsynaptic activity during presynaptic steps reflected release from the AII (Figure 4E). AII step depolarizations evoked both transient and sustained IPSCs in CBC2s, consistent with capacitance measurements of exocytosis from AIIs [19]. The transient transfer function exhibited similar range and shape to RBC→ AII synapses (Figure 4H), perhaps reflecting similar roles for L-type Cav channels in synaptic release from AIIs [19, 38]. The decay following the peak was slower and settled to a larger sustained component than in RBC→AII responses. These differences may have been partially due to AII stimulation being distributed unevenly through gap junctions to coupled AIIs, because AII→CBC2 IPSCs decayed slightly faster in connexin 36 knockout (Cx36−/−) mice, which lack AII gap junctions (Figure S4F) [39].
Next, we recorded simultaneously from RBCs and CBC2s connected via intermediary AIIs (RBC→CBC2; Figure 4C,F,I). RBC voltage steps elicited IPSCs in CBC2s (ECl=0mV) that were blocked by strychnine (Figure S4), confirming that they were glycinergic. Although sustained components of release from both RBCs and AIIs varied with presynaptic membrane potential (Figure 4D,E), sustained RBC→CBC2 signals did not, in WT (Figure 4F) or Cx36−/− mice (Figure S4G-I). RBC→CBC2 responses were unaffected by Nav channel blockade (TTX; Figure S4), which has been shown to shape AII signals [40]. These results suggest that AIIs transmit primarily transient signals from single RBCs to OFF CBCs. The next experiments probed this feature in greater detail.
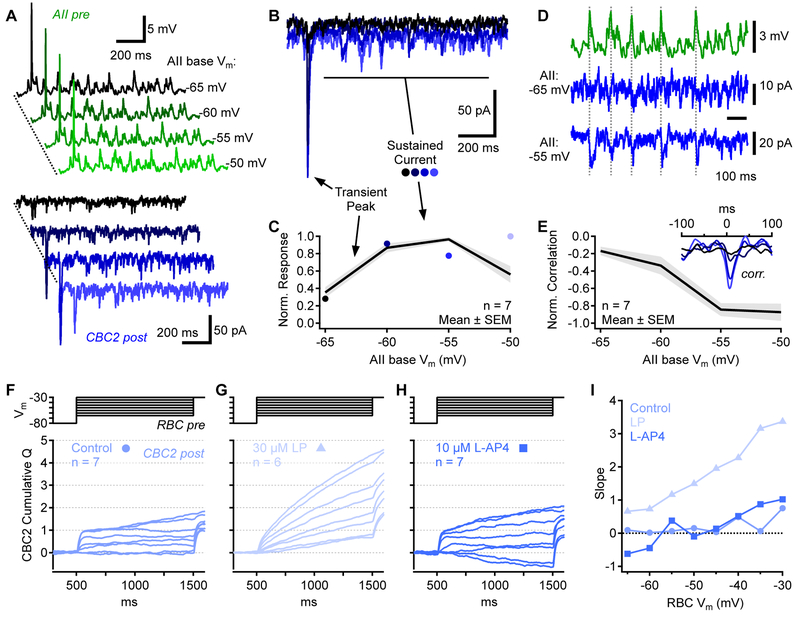
Sustained RBC→AII signals depend on network activity
Our paired recordings suggest that RBC→AII and AII→CBC2 transmission encodes information about sustained release, but RBC→CBC2 transmission does not (Figure 5A,B). We hypothesized that inputs from a single RBC may not depolarize AIIs sufficiently to evoke sustained output. To test this idea, we broke down RBC→CBC2 transmission into sequential components. First, we recorded excitatory postsynaptic potentials (EPSPs) in current-clamped AIIs (with K+-based internal solution) in response to voltage steps in presynaptic RBCs (Figure 5C). RBC steps from −65mV to −30mV elicited large transient EPSPs but relatively small sustained depolarizations in AIIs (Figure 5D). The transient EPSPs reached a peak potential sufficient to evoke robust release from AIIs (−40±5mV, triangles in Figure 5E), then decayed to a steady-state level (−55±5mV) just 2mV, on average, above the resting potential (−57±6mV; Figure 5E). The small membrane depolarization and shallow AII synaptic transfer function near − 55mV (Figure 5E, bottom) suggests that the RBC step would cause little change in AII steady-state release, while the larger transient EPSP would ascend the steep portion of the transfer function and elicit transient IPSCs in CBC2s.
To test this prediction, we used an AII EPSP (Figure 5D) as a presynaptic voltage command in AII→CBC2 pairs (Figure 5F-I). We quantified the steady-state CBC2 event rate before and after the transient EPSP stimulus (Figure 5G) and the reliability of the transient IPSC evoked in between (Figure 5H). Steady-state event rates in postsynaptic CBC2s were similar before and after the AII step response (ratio of “After”/”Before” = 97.5±7.7%, n=5 pairs), as when event rate was measured in the analogous RBC→CBC2 paired recordings (ratio: 103.8±9.5% in 7 pairs; Figure 5B). The transient AII depolarization evoked transient IPSCs in the CBC2 on 81±26% of trials (n=6 pairs; Figure 5H). The average IPSC confirmed that only the transient response was conveyed reliably (Figure 5I).
Previous work indicates that the AII steady-state membrane potential varies across lighting conditions [16]: it is relatively hyperpolarized in the dark, depolarizes by ~5mV in the mid-scotopic range (0.5 R*/rod/s) and then hyperpolarizes again as background luminance approaches mesopic levels. The scotopic steady-state depolarization is likely driven by an increasing barrage of RBC inputs, while hyperpolarization at higher light levels may reflect decreased inputs due to Cav channel inactivation in tonically depolarized RBC terminals [15, 16]. The AII synaptic transfer function derived here (Figure 5E) suggests that changes in steady-state AII membrane potential may influence signal transfer to CBC2s. For example, moderate steady-state depolarization may shift the AII into a steeper part of its transfer curve, potentially enabling it to encode even small sustained depolarizations. To test this, we obtained AII→CBC2 recordings as above and added a bias voltage to the presynaptic EPSP waveform to simulate changes in steady-state membrane potential (Figure 6A). Depolarizing the baseline membrane potential revealed a steady-state component in the AII→CBC2 synaptic transfer (Figure 6B,C), suggesting that AIIs depolarized by ongoing input are able to encode smaller, sustained synaptic signals. Accordingly, as AIIs were depolarized, IPSCs recorded in the CBC2 became more correlated with the presynaptic waveform (Figure 6D,E), indicating that even small, possibly quantal signals were relayed by the AII.
Figure 6 ∣. Sustained signal transfer depends on AII membrane potential.
(A)AII→CBC2 paired voltage clamp recordings. Presynaptic voltage commands (green) comprised the AII EPSP plus a bias voltage to change the “baseline” presynaptic potential (indicated at right). Bottom, corresponding CBC2 IPSCs (Vhold=−80mV).
(B) Four CBC2 IPSCs stimulated by AII EPSPs with baseline potentials between −65mV and − 50mV (5mV increments). Same color scheme as in A.
(C) Peak and sustained components of CBC2 IPSC as a function of AII baseline Vm.
(D) Sustained presynaptic EPSP command waveform (green) and postsynaptic CBC IPSCs (blue). Dashed vertical lines highlight presynaptic/postsynaptic correlations.
(E) Presynaptic-postsynaptic correlations as a function of AII baseline Vm. Inset, average cross correlations from all pairs, color coded as in A. AII depolarization increased correlations. Correlations are negative because AII EPSPs are positive (upward) signals, whereas CBC2 IPSCs are negative (downward) in these conditions.
(F-H) RBC→CBC2 paired voltage clamp recordings. For a range of presynaptic RBC steps (top), the cumulative IPSC charge (Q) is shown (bottom). Charge was normalized so that the maximum evoked postsynaptic transient (synaptic charge accumulated within 100 ms following step onset) was 1. Charge calculated from combined averages of paired recordings.
(I) Average slopes of the traces shown in F-H.
To determine the effect of AII membrane potential on RBC→CBC2 signaling, we bath-applied the Kv channel blocker linopirdine (LP, 30 μM), which depolarizes AIIs by ~6mV [41]. We integrated postsynaptic current over time during presynaptic voltage steps, then subtracted the baseline slope measured prior to the first voltage step; sustained release rate during RBC steps to different intermediate values was compared by measuring the slope of the accumulated charge transfer waveform during the last 900ms of the RBC step. In control conditions, this slope did not vary substantially over the range of RBC steps (Figure 6F,I); by contrast, in the presence of LP the charge transfer slope varied with step magnitude, indicating that AIIs relayed sustained information (Figure 6G,I). Bath application of the mGluR6 agonist L-AP4 (10 μM), which hyperpolarized AIIs by ~5mV (from −55±1mV to −60±1mV, mean±SEM, n=11 cells), had little effect on sustained RBC→CBC2 transmission (Figure 6H,I) confirming that, in our experimental conditions, AIIs rest at relatively hyperpolarized potentials, at the foot of their release range, so that their signal transfer is rectified.
In our paired recordings the presynaptic AII was dialyzed with 10 mM EGTA in the patch pipette, whereas in LP/L-AP4 experiments the AIIs’ cytoplasm was undisturbed. The similar emergence of sustained signaling with AII depolarization in both experiments suggests that exogenous buffers in the whole-cell experiments did not distort significantly the dynamics of AII→CBC2 signaling. Collectively, these data indicate that intermediary AIIs dynamically filter RBC signals to downstream OFF and ON CBCs depending background activity.
Comparing RBC signal transfer to the ON and OFF CBC pathways
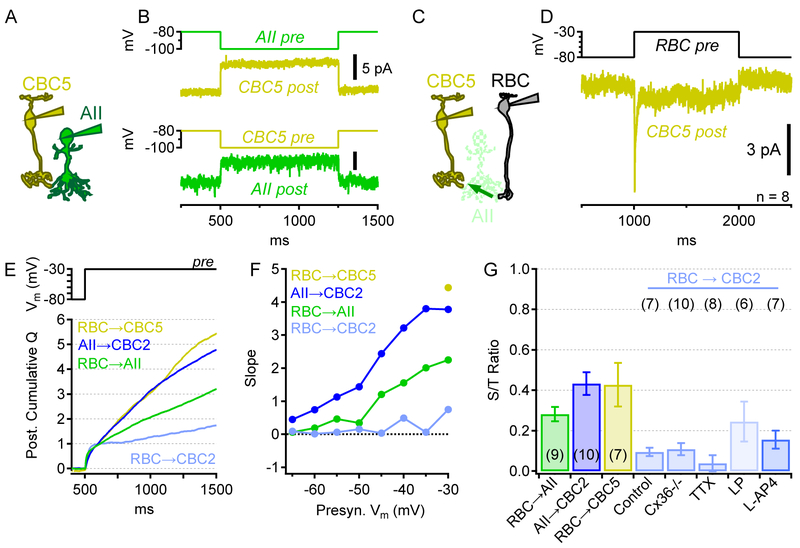
To examine synaptic transfer from AIIs through electrical synapses to ON CBCs, we obtained simultaneous voltage-clamp recordings from AIIs and ON CBCs that were bidirectionally coupled by gap junctions ([12, 42]; Figure 7A,B). CBC5s were targeted in slices from Htr3a-GFP transgenic mice, which express GFP in CBC5 [22]. CBC5s comprise multiple subtypes (CBC5A-D) [23, 43, 44], only a subset of which are labeled in Htr3a-GFP mice [44]. We could not distinguish specific subtypes with epifluorescence imaging, so here we refer to these cells generally as CBC5, although recent anatomical results suggest that most AII↔CBC5 connections involve CBC5A [12], which expresses Htr3a mRNA more than other CBC5 subtypes [44]. In AII↔CBC5 recordings, presynaptic hyperpolarizing voltage steps in either cell elicited square-shaped postsynaptic responses in the other, a hallmark of electrical synapses (Figure 7B). When an EPSP waveform (no bias voltage) was used as a command potential in AIIs, CBC5 responses correlated with fluctuations in the presynaptic stimulus (Figure S5).
Figure 7 ∣. Comparison of synaptic transfer to ON and OFF cone bipolar pathways.
(A) Schematic of AII↔CBC5 recordings.
(B) Postsynaptic currents in a CBC5 (gold) and an AII (green) resulting from alternately hyperpolarizing the other cell. Average of 18 trials.
(C) Schematic of indirect RBC→CBC5 recordings, mediated by chemical (RBC→AII) and electrical (AII↔CBC5) synapses.
(D) Stepping a presynaptic RBC to −30mV evokes transient and sustained postsynaptic currents in CBC5s (average of 8 pairs).
(E) Cumulative charge, computed as in Figure 6F, of responses to a voltage step from −80 to − 30mV, in different recording configurations.
(F) Average slopes vs. presynaptic Vm in different recording configurations. Also see Figures S5 and S6, which compare the timing and correlation of synaptic responses in different recorded pairs.
(G) Sustained-to-transient (S/T) ratios (mean±SEM) for direct pairs (RBC→AII; AII→CBC2) and indirect pairs through unclamped AIIs (RBC→CBC5; RBC→CBC2). Ratios compare the relative rates of charge accumulation during transient (first 100ms after step) and sustained (last 900ms of 1s step) release following a presynaptic step from −80mV to −30mV.
Step depolarizations in voltage-clamped RBCs elicited responses in CBC5s, presumably through intermediary AIIs (Figure 7C,D). Because RBC-elicited EPSCs in CBC5s were small (<13 pA peak responses, Figure 7D), we evoked and analyzed only responses evoked by steps from −80mV to −30mV. (Ambient laboratory lighting conditions of our slice experiments likely minimized the extent of gap junction coupling [45, 46]; AII↔CBC5 connectivity may be stronger under lower light conditions.) Both transient and sustained components of synaptic transmission were evident in postsynaptic CBC5 currents (Figure 7D).
AII→CBC5 responses exhibited no synaptic delay, characteristic of electrical synapses, whereas RBC→AII and AII→CBC2 responses each exhibited delays typical of chemical synapses (Figure S6). Consequently, the synaptic delay in RBC→(AII)→CBC5 responses was similar to that of a single chemical synapse, whereas RBC→AII)→CBC2 delays reflected roughly the sum of two chemical synapses in series (RBC→AII→CBC2; Figure S6B). Synaptic delays in RBCs were slower than those reported in recordings from goldfish bipolar cell terminals at room temperature [47], possibly reflecting species adaptations to meet distinct requirements of homeothermic or poikilothermic systems.
To compare responses in CBC2s and CBC5s driven by RBCs or AIIs, we integrated postsynaptic current over time during presynaptic voltage steps (Figure 7E). In directly-connected RBC→AII and AII→CBC2 pairs, increasing presynaptic depolarizations evoked increasing rates of charge transfer (i.e. slopes; Figure 7F). As shown above, in RBC→CBC2 pairs, cumulative synaptic charge plateaued soon after the onset of a presynaptic step, whereas in RBC→CBC5 pairs presynaptic steps to −30mV evoked continuous accumulation of charge throughout the duration of the step (Figure 7F,G). RBC→CBC2 response amplitudes varied substantially from pair to pair (SD/mean=64%; n=7 pairs), perhaps reflecting the large variability in synaptic connectivity between the component neurons (Figure 2). RBC→CBC5 responses also varied from pair to pair (SD/mean=47%; n=8 pairs).
Discussion
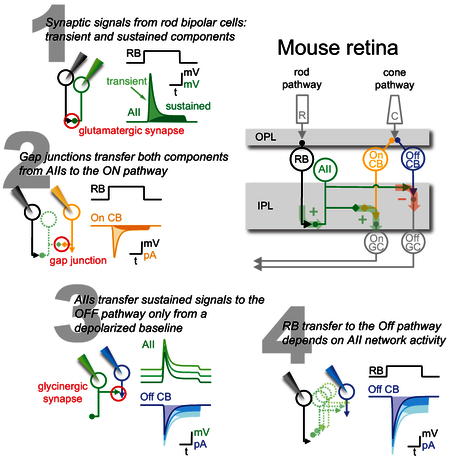
We have dissected local microcircuitry surrounding the mouse AII by detailing the anatomical connectivity between AIIs and OFF CBCs and probing the functional synaptic transfer at each step in the circuitry. Anatomically, we show that AIIs receive ribbon synapse inputs from and make inhibitory synaptic outputs to multiple OFF CBC types but exhibit particularly strong ultrastructural connectivity with CBC2. AII↔CBC2 connections appear functionally asymmetric, however, as AII→CBC2 signals are much larger than those in the CBC2→AII direction. Transient synaptic signals are transmitted from RBCs to CBC2s with high fidelity, while ongoing network activity determines whether sustained signals are also conveyed to CBC2s or filtered out. Meanwhile, CBC5 receives both transient and sustained signal components from RBCs.
Synaptic connectivity in the rod pathway
Our anatomical reconstructions provide information about the convergent/divergent connectivity throughout this RBC→AII→OFF CBC circuitry that builds on previous studies [12, 48, 49]. Each CBC2 made ribbon synapses onto 3.0±0.9 AIIs and received inputs from 3.0±0.9 AIIs (n=6). Therefore, given the divergence of 1 Rod → 2 RBCs [49], 1 RBC → ~3-5 AIIs [48], and 1 AII → ~2-4 CBC2s (the present analysis), the signal from a single rod may diverge to as many as 12-40 CBC2s, not accounting for potential additional spread through gap junctions. Conversely, a single CBC2 may collect the convergent inputs of as many as 432-1680 rods, although accounting for rods shared by RBCs could reduce that number [48]: 1 CBC2 ← ~2-4 AIIs (the present analysis), 1 AII ← ~9-12 RBCs [48], 1 RBC ← 24-35 Rods [48, 49].
Most functional studies of AII influence on the OFF pathway have focused on AII inhibitory inputs to OFF GCs [5-10] (although see [17, 50]), but our anatomical results indicate that AIIs make ~8 times as many synapses onto OFF CBCs than onto GCs (Figure 2R). This bias suggests that AIIs may exert their greatest impact on OFF signaling via presynaptic inhibition, especially for those OFF GCs receiving excitatory input from CBC2, which receives more AII input than all other targets combined (Figure 2R). Moreover, each synapse onto an OFF CBC terminal may influence postsynaptic GC responses more than one directly onto an OFF GC, because GCs operate primarily within a voltage range closer to ECl, where the driving force on excitatory conductances is much larger compared to that on GABAergic and glycinergic inhibitory conductances [51].
Many features of CBC1A and CBC2 axonal arbors are similar to those reported for GluMI (likely CBC1B in [44]), a ribbon-expressing, glutamatergic amacrine cell [52], although GluMI axon terminals ramify more closely to the OFF SAC layer [12, 23]; Figure S2). GluMIs could not be identified positively here by their characteristic lack of OPL dendrites [52], because the EM dataset did not include INL cell bodies. Given their distinct stratification pattern, relatively low number of synaptic ribbons [52] and apparent lack of synaptic contact with AII amacrine cells [12], GluMIs were not likely among the cell types found here to contact AIIs.
OFF CBC inputs to rod pathway remain enigmatic
AIIs receive synaptic inputs from RBCs and OFF CBCs via ribbon synapses and ON CBCs via electrical synapses. RBCs and ON CBCs transmit transient and sustained signals to AIIs, but OFF CBC→ AII synaptic transmission remains poorly understood, with contradictory results awaiting reconciliation. For example, although CBC2s make numerous synaptic inputs onto AIIs (Figure 2) and OFF CBCs provide robust synaptic input to AIIs in rat retina [11], our recordings in mouse indicate functionally weak OFF CBC→ AII connectivity (Figure 3; [53]). Functional glutamate receptors clearly populate AII OFF layer lobules (Figure 3F,H; [31]), but perhaps they do not appose CBC ribbons. Electron microscopy and immunogold labeling are required to address this possibility. Our results are consistent with a model in which AIIs provide primarily ON-driven feedforward inhibition to OFF CBCs (particularly CBC2) and GCs, but more work is needed to clarify functional roles for OFF CBC inputs to AII circuitry.
Predictions for scotopic signaling
Although the basic voltage step waveforms delivered here do not emulate visual signals, our results, in the context of previous work [16, 17, 45, 50], suggest that AIIs may sculpt light-evoked signals differently depending on background luminance. These predicted differences involve changes in gap junction connectivity [45], AII membrane potential [16], AII signaling dynamics (Figures 6) and crossover inhibition from the ON pathway (via AIIs) that rectifies OFF CBC responses [17, 50].
In darkness, ON pathway signaling is relatively low and RBCs and AIIs are hyperpolarized such that their synaptic gains are nearly maximal [14-16, 54]. AII→OFF CBC inhibition would be diminished, so OFF CBCs, depolarized by dark release from cones, would inhabit the central, less rectified region of their release transfer function [17]. Relatively weak gap junction coupling [45] would narrow AII receptive fields, increase AII input resistance and decrease AII↔ON CBC connectivity.
During a single photon response (SPR), 1-2 RBCs provide strong input to the AII(s) with which they are preferentially coupled [48, 55]. This would elicit a strong depolarization in an uncoupled, electronically compact AII, causing it to relay a transient inhibitory signal to OFF CBCs (primarily CBC2) and a subset of OFF GCs [8-10, 17, 50]. SPRs in ON CBCs may be relatively weak due to the reduced gap junction coupling, although the concomitant increase in ON CBC input resistance may enable even small inputs from AIIs to elicit consequential depolarizations. Overall, however, we would predict that SPRs are transmitted primarily via a small number of AIIs to the OFF pathway. A strong transient signal may reflect the most reliable way to broadcast SPRs above the dark noise in the OFF pathway.
At moderate scotopic light levels, AIIs are more depolarized [16], so that they would transmit a substantial sustained component to OFF CBCs (Figure 6), hyperpolarizing them so that their release is rectified and they signal only light decrements [17]. Gap junctional coupling is increased [45], so that ON CBCs receive substantial AII input and signal light increments and decrements. AII→ON CBC signal decrements would be enhanced by transient AII hyperpolarizations at light offset [56]. AII-mediated inhibition decreases high-frequency signaling in OFF CBCs [17], possibly to emphasize sustained inhibition of OFF CBCs. Thus, we would predict that OFF signaling predominates in this range, possibly reflecting adaptation to a natural world that presents mostly negative contrast [17, 57].
In high scotopic conditions, AII hyperpolarization may reflect decreased RBC release due to presynaptic Cav channel inactivation [15, 16], although our paired RBC→AII recordings suggest that even strongly depolarized RBCs are capable of prolonged, sustained release (Figure 7E; [14]). Regardless, hyperpolarized AIIs would therefore transmit primarily transient signals to OFF CBCs (Figure 5). Decreased gap junction coupling [45] would diminish AII↔ON CBC signaling, possibly to prevent saturation and extend the luminance range over which rod pathway signals can be encoded.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeffrey S. Diamond (diamondj@ninds.nih.gov)
Experimental Model and Subject Details
Group housed mice older than postnatal day 40 were used from the following lines: Syt2-EGFP, 5HT3Ra-EGFP, Cx36−/−, C57/BL6. Syt2-EGFP, 5HT3Ra-EGFP, and Cx36−/− lines were crossed into a C57/BL6 background. CBC2s are labeled in Syt-2 mice, making targeted patching of CBC2 easier [22]. For Connexin36−/− recordings, Cx36−/− [39] and Syt2 lines were crossed. Only males were used from Syt2-EGFP lines. Both male and females were used from 5HT3Ra-EGFP, Cx36−/−, C57/BL6 lines. Mice were maintained on a 12-hour light/dark cycle and were provided ad libitum access to food and water. Animal procedures were conducted according to NIH guidelines and were approved by the NINDS Animal Care and Use Committee (ASP 1344).
Method Details
Electron microscopy reconstruction
We analyzed dataset k0725, a ~50 × 210 × 260 μm3 block of fixed mouse retina imaged by SBEM [21]. The voxel size (13.2 × 13.2 × 26 nm) allowed identification of chemical synapses and vesicles, but not gap junctions. Skeletonization was performed in KNOSSOS software [23] (http://www.knossostool.org), beginning at RBC terminals, which were easily identified based on their size and position within the IPL [59]. From RBC dyad synapses, AIIs were traced, and then, from the proximal lobules of AIIs, OFF CBCs were traced. OFF CBC output synapses were identified by the appearance of presynaptic ribbons generally located at dyad synapses in which one of the postsynaptic dendrites had the appearance of a ganglion cell (i.e. a large-diameter dendrite with a clear cytoplasm). AII output synapses were identified by the appearance of vesicle clusters at the presynaptic membrane. Often, these clusters were quite large and multiple clusters were presynaptic to the same postsynaptic process; in such instances, we counted a single AII→postsynaptic cell contact (and, therefore, we might have underestimated the true number of AII→CBC2 synapses). Segmentation of selected presynaptic active zones (bipolar cell ribbons and AII output synapses) was performed in KNOSSOS. Segmentations were converted to polygonal surface meshes by custom-written Python scripts and analyzed using Paraview (http://www.paraview.org): meshes were smoothed and then surface areas measured.Detailed connectivity analysis was performed using custom-written Python scripts. First, voxel coordinates were normalized to the positions of the ON and OFF starburst amacrine cells (SACs) identified in the same dataset [21] and then synaptic contacts were identified by matching proximate (within 1 μm) annotated pre- and postsynaptic sites (e.g. an AII→OFF CBC synapse was identified by matching an annotated AII output synapse with a nearby annotated OFF CBC input). The matching algorithm was checked manually for subsets of AII↔OFF CBC pairs and found to be robust. Average of the absolute deviation from the mean (quantifying the spread of ribbon synapse depth in the IPL, Figure 2M) was calculated as:
Using the Shapely Python Package, cell type mosaics were generated by fitting convex hulls to the annotated synapses associated with single cells, and hull overlap was calculated. For the 28 cells stratifying above the OFF SAC, each cell’s hull was compared to all other hulls, resulting in the “Unsorted” calculation of maximum percent overlap (Figure 2C). The same overlap calculations were performed: 1) on randomly chosen subsets (12, 14, or 16 of the 28 cells) of hulls, repeated 106 times (“Random”); 2) for Groups 1 and 2 that were sorted by the number of ribbons presynaptic to AIIs (“Sorted”); and, 3) for CBC3a and CBC3b hulls.
Electrophysiology
Animal procedures were conducted according to NIH guidelines and were approved by the NINDS Animal Care and Use Committee (ASP 1344). Recordings were made from the following mice lines: Syt2-EGFP, 5HT3Ra-EGFP, Cx36−/−, C57/BL6. Retina slices were prepared from 6 week- to 6 month-old male mice. Retinas were embedded in low melting point agarose and sliced on a Leica VT1000S vibratome. Retina slices were cut, stored and perfused during experiments (2-3 ml/min) with bicarbonate-buffered Ames medium (US Biological) at room temperature and under photopic laboratory lighting conditions. Internal solutions for patch clamp experiments were (280-290 mOsm, pH7.3-7.4): Standard Cs internal (mM): 90 CsCH3SO3, 20 TEA-Cl, 4 MgATP, 0.4 NaGTP, 10 EGTA, 10 Na2 Phosphocreatine, 10 HEPES. High Cl− Cs internal (ECl ~0mV): 120 CsCl, 9 NaCl, 2 MgATP, 1 NaGTP, 1 EGTA, 2 MgCl2, 5 HEPES. K internal: 100 KCH3SO3, 20 KCl, 4 MgATP, 0.4 NaGTP, 2 EGTA, 10 Na2 Phosphocreatine, 10 HEPES. For all experiments patching the AII with a Cs-based solution, 3 mM QX-314 (Alamone Labs) was added to the internal to prevent sodium action potentials. For some experiments testing the bidirectionality of AII↔CBC2 transmission (Figure 3D), the standard Cs internal was modified to include only 0.5 EGTA and 98 CsCH3SO3. Liquid junction potentials were calculated for each internal (~5mV for the High Cl- Cs internal, ~10mV for others) using JPCalcW within pCLAMP (Molecular Devices), were corroborated with 3M KCl salt bridge measurements [60], and adjusted for offline after recordings. Borosilicate glass recording pipettes were pulled to 4-6 MΩ tip resistances on a vertical two-stage puller (PP-830, Narishige). Series resistances, measured at the end of whole-cell recordings, were (mean±SEM): RBC, 42.9±1.9MΩ (n=72); AII, 33.5±1.3MΩ (n=76); CBC2, 42.9±1.1MΩ (n=110); CBC5, 50.3±2.5MΩ (n=9). Series resistance was not compensated. Data was acquired on Axon Multiclamp 700B and Axopatch 1D amplifiers at 10 kHz with a 4 or 5kHz Bessel lowpass filter. Unless otherwise indicated, patched cells were held at −75 to −80mV. The interval between stimulus trials was 15s. A TTL-driven Picospritzer III (Parker Hannifin) was used for puff application (~3 psi) of kainic acid (Tocris, 20 ms puffs) and LY341495 (Tocris, 500 ms puffs) during recordings. Data acquisition and analysis was performed in Igor Pro (Wavemetrics).
QUANTIFICATION AND STATISTICAL ANALYSIS
During whole-cell voltage-clamp paired recordings incorporating the voltage step protocol shown in Figure 4, data was collected > 2 minutes after achieving whole cell configuration (“breaking in”) to allow internal solution to dialyze the cell. Analysis was performed on averages of 3-5 protocol repetitions, with each repetition including 8 different intermediate voltage steps. Synaptic delays were determined visually on average traces following a presynaptic step to −30mV. Postsynaptic conductance measurements (Figure 3D) were calculated on the peak postsynaptic responses (<10 ms from onset of presynaptic step). Synaptic event detection, used in event rate calculations (Figures 3K,M and 5B,E), was performed via a custom first-derivative threshold algorithm written in Igor Pro. Event rates were smoothed with a 200 ms boxcar moving average. Postsynaptic charge (Q) plots were generated by integrating the postsynaptic current recordings and normalized by subtracting the rate of charge accumulation prior to the presynaptic stimulus (to account for spontaneous events). Sustained-to-transient (S/T) ratios (Figure 7G) were calculated as the ratio of the rate of charge accumulation during the last 900 ms of a presynaptic step to −30mV (“Sustained”) to the rate of charge accumulation during the first 100 ms of a presynaptic step to −30mV (“Transient”).
DATA AND SOFTWARE AVAILABILITY
Electron microscopy dataset k0725 is available upon request.
Supplementary Material
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, or recombinant proteins | ||
| Tetrodotoxin (TTX) | Alomone | Cat # T-550 |
| L-AP4 | Tocris | Cat # 0103 |
| LY 341495 | Tocris | Cat # 1209 |
| Kainic Acid | Tocris | Cat # 0222 |
| Picrotoxin | Sigma | Cat # P1675 |
| Strychnine hydrochloride | Sigma | Cat # S8753 |
| SR 95531 hydrobromide (Gabazine) |
Tocris | Cat # 1262 |
| TPMPA | Tocris | Cat # 1040 |
| Linopirdine Dihydrochloride | Tocris | Cat # 1999 |
| Isradipine | Tocris | Cat # 2004 |
| Glutamate | Sigma | Cat # G1251 |
| Glycine | Sigma | Cat # G7126 |
| QX-314 Bromide | Alomone | Cat # Q-100 |
| Deposited Data | ||
| EM dataset: k0725 | Kevin L. Briggman | Ding et al., 2016 |
| Experimental Models: Cell strains | ||
| Mouse:Syt2-EGFP | MMRRC; http://www.mmrrc.org |
RRID:MMRRC_032122-UCD |
| Mouse:5HT3Ra-EGFP | MMRRC; http://www.mmrrc.org |
RRID:MMRRC_000273-UNC> |
| Mouse:Cx36−/− | Deans et al., 2001 | RRID:MGI:3810172 |
| Software and Algorithms | ||
| KNOSSOS | http://www.knossostool.org | RRID:SCR_003582 |
| Python | http://www.python.org | RRID:SCR_008394 |
| pClamp | Molecular Devices | RRID:SCR_011323 |
| Igor Pro vers. 6 and 7 | WaveMetrics; www.wavemetrics.com |
RRID:SCR_000325 |
| Paraview | www.paraview.org | RRID:SCR_002516 |
Highlights.
• AII amacrine cells relay distinct synaptic signals to ON and OFF cone bipolar cells.
• AIIs contact the OFF cone pathway primarily via type 2 cone bipolar cells (CBC2s).
• CBC2-AII connectivity is morphologically symmetric but physiologically asymmetric.
• AII→CBC2 signal transfer depends on the level of activity within the AII network.
Acknowledgements
This work was supported by the NINDS Intramural Research Program (NS003039; J.S.D. and NS003133; K.L.B.), and NIH grant EY017836 to J.H.S. The authors declare no competing financial interests. The authors thank Wei Li and Will Grimes for critical comments during the writing of the manuscript, Kacie Furcolo and Adit Sabnis for assistance with SBEM investigation, and Alexander Baden for assistance with surface mesh visualization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Euler T, and Masland RH (2000). Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol 83, 1817–1829. [DOI] [PubMed] [Google Scholar]
- 2.Demb JB, and Singer JH (2012). Intrinsic properties and functional circuitry of the AII amacrine cell. Vis Neurosci 29, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Famiglietti EV Jr., and Kolb H (1975). A bistratified amacrine cell and synaptic cirucitry in the inner plexiform layer of the retina. Brain Res 84, 293–300. [DOI] [PubMed] [Google Scholar]
- 4.Tsukamoto Y, Morigiwa K, Ueda M, and Sterling P (2001). Microcircuits for night vision in mouse retina. J Neurosci 21, 8616–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pourcho RG, and Goebel DJ (1985). A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol 233, 473–480. [DOI] [PubMed] [Google Scholar]
- 6.Strettoi E, Raviola E, and Dacheux RF (1992). Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol 325, 152–168. [DOI] [PubMed] [Google Scholar]
- 7.Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, and Demb JB (2008). Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci 28, 4136–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy GJ, and Rieke F (2008). Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci 11, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, and Roska B (2009). Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci 12, 1308–1316. [DOI] [PubMed] [Google Scholar]
- 10.van Wyk M, Wassle H, and Taylor WR (2009). Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci 26, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veruki ML, Mørkve SH, and Hartveit E (2003). Functional properties of spontaneous EPSCs and non-NMDA receptors in rod amacrine (AII) cells in the rat retina. J Physiol 549, 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto Y, and Omi N (2017). Classification of Mouse Retinal Bipolar Cells: Type-Specific Connectivity with Special Reference to Rod-Driven AII Amacrine Pathways. Front Neuroanat 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer JH, and Diamond JS (2003). Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci 23, 10923–10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oesch NW, and Diamond JS (2011). Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci 14, 1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarsky T, Cembrowski M, Logan SM, Kath WL, Riecke H, Demb JB, and Singer JH (2011). A synaptic mechanism for retinal adaptation to luminance and contrast. J Neurosci 31, 11003–11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimes WN, Schwartz GW, and Rieke F (2014). The synaptic and circuit mechanisms underlying a change in spatial encoding in the retina. Neuron 82, 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Z, and Freed MA (2010). The ON pathway rectifies the OFF pathway of the mammalian retina. J Neurosci 30, 5533–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denk W, and Horstmann H (2004). Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol 2, e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balakrishnan V, Puthussery T, Kim MH, Taylor WR, and von Gersdorff H (2015). Synaptic Vesicle Exocytosis at the Dendritic Lobules of an Inhibitory Interneuron in the Mammalian Retina. Neuron 87, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marc RE, Anderson JR, Jones BW, Sigulinsky CL, and Lauritzen JS (2014). The AII amacrine cell connectome: a dense network hub. Front Neural Circuits 8, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding H, Smith RG, Poleg-Polsky A, Diamond JS, and Briggman KL (2016). Species-specific wiring for direction selectivity in the mammalian retina. Nature 535, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wässle H, Puller C, Müller F, and Haverkamp S (2009). Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci 29, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, and Denk W (2013). Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174. [DOI] [PubMed] [Google Scholar]
- 24.Fox MA, and Sanes JR (2007). Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol 503, 280–296. [DOI] [PubMed] [Google Scholar]
- 25.Wässle H, Heinze L, Ivanova E, Majumdar S, Weiss J, Harvey RJ, and Haverkamp S (2009). Glycinergic transmission in the Mammalian retina. Front Mol Neurosci 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartveit E, and Veruki ML (1997). AII amacrine cells express functional NMDA receptors. Neuroreport 8, 1219–1223. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Tencerová B, Hartveit E, and Veruki ML (2016). Functional NMDA receptors are expressed by both AII and A17 amacrine cells in the rod pathway of the mammalian retina. J Neurophysiol 115, 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kothmann WW, Trexler EB, Whitaker CM, Li W, Massey SC, and O'Brien J (2012). Nonsynaptic NMDA receptors mediate activity-dependent plasticity of gap junctional coupling in the AII amacrine cell network. J Neurosci 32, 6747–6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalbaugh TL, Zhang J, and Diamond JS (2009). Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells. J Neurosci 29, 1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartveit E (1997). Functional organization of cone bipolar cells in the rat retina. J Neurophysiol 77, 1716–1730. [DOI] [PubMed] [Google Scholar]
- 31.Kim MH, and von Gersdorff H (2016). Postsynaptic Plasticity Triggered by Ca(2)(+)-Permeable AMPA Receptor Activation in Retinal Amacrine Cells. Neuron 89, 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, and Nawy S (2009). A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci 29, 6088–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer JH, and Diamond JS (2006). Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol 95, 3191–3198. [DOI] [PubMed] [Google Scholar]
- 34.Singer JH, Lassova L, Vardi N, and Diamond JS (2004). Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci 7, 826–833. [DOI] [PubMed] [Google Scholar]
- 35.Berntson A, Taylor WR, and Morgans CW (2003). Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J Neurosci Res 71, 146–151. [DOI] [PubMed] [Google Scholar]
- 36.Satoh H, Aoki K, Watanabe SI, and Kaneko A (1998). L-type calcium channels in the axon terminal of mouse bipolar cells. Neuroreport 9, 2161–2165. [DOI] [PubMed] [Google Scholar]
- 37.Matsui K, Hosoi N, and Tachibana M (1998). Excitatory synaptic transmission in the inner retina: paired recordings of bipolar cells and neurons of the ganglion cell layer. J Neurosci 18, 4500–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habermann CJ, O'Brien BJ, Wassle H, and Protti DA (2003). AII amacrine cells express L-type calcium channels at their output synapses. J Neurosci 23, 6904–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, and Paul DL (2002). Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36, 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian M, Jarsky T, Murphy GJ, Rieke F, and Singer JH (2010). Voltage-gated Na channels in AII amacrine cells accelerate scotopic light responses mediated by the rod bipolar cell pathway. J Neurosci 30, 4650–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cembrowski MS, Logan SM, Tian M, Jia L, Li W, Kath WL, Riecke H, and Singer JH (2012). The mechanisms of repetitive spike generation in an axonless retinal interneuron. Cell Rep 1, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veruki ML, and Hartveit E (2002). Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci 22, 10558–10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greene MJ, Kim JS, Seung HS, and Eye Wirers (2016). Analogous Convergence of Sustained and Transient Inputs in Parallel On and Off Pathways for Retinal Motion Computation. Cell Rep 14, 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, Adiconis X, Levin JZ, Nemesh J, Goldman M, et al. (2016). Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 166, 1308–1323 e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bloomfield SA, and Volgyi B (2004). Function and plasticity of homologous coupling between AII amacrine cells. Vision Res 44, 3297–3306. [DOI] [PubMed] [Google Scholar]
- 46.Bloomfield SA, Xin D, and Osborne T (1997). Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci 14, 565–576. [DOI] [PubMed] [Google Scholar]
- 47.von Gersdorff H, Sakaba T, Berglund K, and Tachibana M (1998). Submillisecond kinetics of glutamate release from a sensory synapse. Neuron 21, 1177–1188. [DOI] [PubMed] [Google Scholar]
- 48.Tsukamoto Y, and Omi N (2013). Functional allocation of synaptic contacts in microcircuits from rods via rod bipolar to AII amacrine cells in the mouse retina. J Comp Neurol 521, 3541–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behrens C, Schubert T, Haverkamp S, Euler T, and Berens P (2016). Connectivity map of bipolar cells and photoreceptors in the mouse retina. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Z, and Freed MA (2012). Cross inhibition from ON to OFF pathway improves the efficiency of contrast encoding in the mammalian retina. J Neurophysiol 108, 2679–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Homann J, and Freed MA (2017). A Mammalian Retinal Ganglion Cell Implements a Neuronal Computation That Maximizes the SNR of Its Postsynaptic Currents. J Neurosci 37, 1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Della Santina L, Kuo SP, Yoshimatsu T, Okawa H, Suzuki SC, Hoon M, Tsuboyama K, Rieke F, and Wong RO (2016). Glutamatergic Monopolar Interneurons Provide a Novel Pathway of Excitation in the Mouse Retina. Curr Biol 26, 2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pang JJ, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, and Wu SM (2007). Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol 580, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunn FA, Doan T, Sampath AP, and Rieke F (2006). Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci 26, 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimes WN, Zhang J, Tian H, Graydon CW, Hoon M, Rieke F, and Diamond JS (2015). Complex inhibitory microcircuitry regulates retinal signaling near visual threshold. J Neurophysiol 114, 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson R (1982). AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol 47, 928–947. [DOI] [PubMed] [Google Scholar]
- 57.Brady N, and Field DJ (2000). Local contrast in natural images: normalisation and coding efficiency. Perception 29, 1041–1055. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, and Wassle H (2004). Types of bipolar cells in the mouse retina. J Comp Neurol 469, 70–82. [DOI] [PubMed] [Google Scholar]
- 59.Mehta B, Ke JB, Zhang L, Baden AD, Markowitz AL, Nayak S, Briggman KL, Zenisek D, and Singer JH (2014). Global Ca2+ signaling drives ribbon-independent synaptic transmission at rod bipolar cell synapses. J Neurosci 34, 6233–6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neher E (1992). Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207, 123–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.