Abstract
Coenzyme Q10 (CoQ10) protects retinal ganglion cells (RGCs) in experimental retinal ischemia and glaucoma by scavenging reactive oxygen species. We tested whether a diet supplemented with ubiquinol, the reduced form of CoQ10, promotes RGC survival and blocks the apoptotic pathway in ischemic mouse retina induced by acute high intraocular pressure (IOP) elevation. Ubiquinol (1%) treatment significantly promoted RGC survival at 2 weeks after ischemia/reperfusion. The ubiquinol treatment significantly blocked activation of astroglial and microglial cells in the ischemic retina at 2 weeks. While the ubiquinol treatment significantly decreased active Bax protein expression in the ischemic retina, phosphorylation of Bad at serine 112 and Bcl-xL protein expression were preserved in the ubiquinol-treated ischemic retina at 12 hours. Consistently, the ubiquinol treatment prevented apoptotic cell death by blocking caspase-3 cleavage. These results suggest that the ubiquinol enhances RGC survival by modulating the Bax/Bad/Bcl-xL-mediated apoptotic pathway in the ischemic retina. Ubiquinol has therapeutic potential for ameliorating elevated IOP-induced ischemic retinal degeneration.
Keywords: Ubiquinol, Retina, Ischemia, Retinal ganglion cell, Apoptosis
1. Introduction
Elevated intraocular pressure (IOP) is an important risk factor for retinal ganglion cell (RGC) death and optic nerve degeneration in retinal ischemia and glaucoma [1, 2]. Retinal ischemia is a relatively frequent event occurring several pathological processes [3–5]. The accessibility of the retina for the manipulation of blood flow has facilitated the use of different experimental models to investigate neuronal responses following ischemia-reperfusion injury [3, 6]. Importantly, our previous studies have demonstrated that IOP elevation induced apoptotic cell death by modulating the Bax/Bad pathway in the ischemic retina [7, 8].
Coenzyme Q10 (CoQ10), which is also known as ubiquinone, is a small lipid-soluble molecule that is endogenously synthesized and localized within the mitochondrial inner membrane [9–11]. CoQ10, an essential cofactor of the electron transport chain, acts by maintaining the mitochondrial membrane potential, supporting ATP synthesis and inhibiting reactive oxygen species generation for protecting neuronal cells in neurodegenerative diseases [12, 13]. As potent antioxidant and neurotherapeutic agents, CoQ10 and ubiquinol, the reduced form of CoQ10, are attractive and useful supplements to test efficacy in retinal ischemia and glaucoma because the published evidence supports their effectiveness in many neurodegenerative diseases including Alzheimer’s and Parkinson’s diseases [12, 14–18].
Since the dose of CoQ10 supplementation correlated well with plasma CoQ10 level and CoQ10 in large doses was taken up in all tissues, including brain [19], this suggests that CoQ10 taken up by the retina leads to a beneficial effect in retinal degeneration [6, 20]. There is accumulating evidence that CoQ10 protects retinal cells in vivo and in vitro against elevated IOP or oxidative stress [21–23]. Also, our recent studies have demonstrated that CoQ10 ameliorated glutamate excitotoxicity or oxidative stress, and prevented mitochondrial alteration in mouse models of retinal ischemia and glaucoma in vivo, as well as in primary optic nerve head (ONH) astrocytes in vitro [6, 20, 24]. Furthermore, CoQ10 inhibited astroglial and microglial activation in ischemic mouse retina as well as astroglial activation in the retina and ONH in glaucomatous DBA/2J mice, accompanied by the preservation of RGC axon integrity [6, 20, 24]. Collectively, these results suggest that CoQ10 supplementation could be a promising therapeutic strategy to protect RGCs against ischemic or glaucomatous neurodegeneration.
Emerging evidence indicates that ubiquinol is neuroprotective in several neurodegenerative diseases including Alzheimer’s disease, multiple system atrophy, and traumatic brain injury [15–18]. In the current study, we tested whether a diet supplemented with ubiquinol promotes RGC survival, prevents glial activation, and blocks apoptotic cell death in the transient ischemic mouse retina.
2. Materials and methods
2.1. Animals
Female, 4-month-old C57BL/6J mice (20 – 25 g in weight; The Jackson Laboratory, US) were housed in covered cages, fed with a standard rodent diet ad libitum, and kept on a 12 h light/12 h dark cycle. All procedures concerning animals were performed in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and under protocols approved by institutional IACUC committees at the University of California San Diego.
2.2. Induction of transient retinal ischemia
The mice were anesthetized with a mixture of ketamine (100 mg/kg, Ketaset; Fort Dodge Animal Health, US) and xylazine (9 mg/kg, TranquiVed; Vedeco, Inc., US) by intraperitoneal (IP) injection. Eyes were also treated with 1% proparacaine drops. A 30-gauge needle was inserted into the anterior chamber of the right eye that was connected by flexible tubing to a saline reservoir. By raising the reservoir, IOP was elevated to 70–80 mmHg for 50 min. Sham treatment was performed in the contralateral eyes by the insertion of a needle in the anterior chamber without saline injection. Retinal ischemia was confirmed by observing the whitening of the iris and loss of the retina red reflex. IOP was measured with a tonometer (TonoLab, Finland) during ischemia. Non-ischemic contralateral control retinas were used as a control.
2.3. Pharmacological treatment
Ubiquinol was provided from Kaneka Nutrients (US) as a gift. AIN-93G purified control or a diet supplemented with ubiquinol were formulated by Harlan Laboratories (US). Four groups of mice were studied: a group of non-ischemic C57BL/6J mice treated with control diet (n = 20 mice), a group of ischemic C57BL/6J mice treated with control diet (n = 30 mice), a group of non-ischemic C57BL/6J mice treated with 1% Ubiquinol diet [(v/v), which equals a daily dose of 1600–2000 mg/kg body weight in 25–30 g mice, n = 20 mice] and a group of ischemic C57BL/6J mice treated with 1% Ubiquinol diet (n = 30 mice).
2.4. Tissue preparation
Following acute IOP elevation, mice were anesthetized with IP injection of a mixture of ketamine/xylazine, as described, and then mice were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in 1X phosphate buffer saline (PBS, pH 7.4). Both eyes enucleated and fixed in 4% paraformaldehyde in PBS for 4 h at 4°C. The retinas were dissected as flattened whole-mounts at 2 weeks for immunohistochemical analysis or used immediately at 12 h for Western blot analysis after ischemia/reperfusion
2.5. Whole-mount immunohistochemistry
The retinas were blocked in PBS containing 3% donkey serum, 1% bovine serum albumin, 1% fish gel and 0.1% Triton X-100 for 1 h. Primary antibodies included Brn3a (1:500; Santa Cruz Biotechnology, US) for RGCs, glial fibrillary acidic protein (GFAP) (1:500; Advanced ImmunoChemical, US) for astrocytes and Iba-1 (1:2000; Wako Chemical, US) for microglial cells, and the retinas were incubated with antibodies for 3 days at 4°C. After several wash steps, the retinas were incubated with the secondary antibodies, Alexa Fluor-568 donkey anti-goat IgG antibody or Cy5-conjugated anti-guinea pig IgG antibody (Invitrogen, US) for 24 h, and subsequently washed with PBS. Images were acquired with confocal microscopy (Olympus FluoView1000; Olympus, Japan). ImageJ s (http://rsb.info.nih.gov/ij/, National Institute of Health, US) was used to measure the fluorescence intensity in pixels and number per area in GFAP and Iba1 images from retinas. In the image acquisition, all imaging parameters remain the same and mean pixel intensity was measured in this 179,721 square pixel area.
2.6. Quantitative analysis for RGC counting
To count RGCs labeled with Brn3a, each retinal quadrant was divided into three zones by central, middle, and peripheral retina [one-sixth (~400 μm), three-sixths (~1,200 μm), and five-sixths (~2000 μm) of the retinal radius from the optic nerve head]. Images were taken at 20x, covering an area of 0.344 mm2, and then the number of RGCs were normalized per mm2. RGC densities were measured in 24 distinct areas (two areas at central, middle, and peripheral per retinal quadrant) per condition by two investigators in a masked fashion, and the scores were averaged. To further examine RGC survival between control and ubiquinol diet-treated nonischemic retinas, RGC densities were automatically measured using ImageJ cell counting analysis.
2.7. Western blot analysis
The retinas were homogenized in a glass-Teflon Potter homogenizer in RIPA lysis buffer (150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, 1 mM DTT, 0.5% sodium deoxycholate and 50 mM Tri-Cl, pH 7.6) containing complete protease inhibitors (Roche Biochemicals, US). Each sample (10 μg; n = 3 retinas/group) was separated by PAGE and electrotransferred to polyvinylidenedifluoride membrane. The membrane was blocked with 5% nonfat dry milk and 0.1% Tween-20 in PBS for 1 h. The primary antibodies included Bax (1:500; Santa Cruz Biotechnology), phospho-Bad at serine 112 (p-Bad S112, 1:2000; Cell Signaling, US), cleaved caspase-3 (1:3000; Cell Signaling) and actin (1:10,000, Millipore, US) for 16 h at 4°C. The images were captured and quantified by using ImageQuant™ LAS 4000 system (GE Healthcare Bio-Science) and the band densities were normalized to the band densities for actin.
2.8. Statistical analysis
Data were presented as the mean ± SD. Comparison of two or three experimental conditions was evaluated using the unpaired, two-tailed Student’s t-test or one-way analysis of variance and the Bonferroni t-test. P < 0.05 was considered to be statistically significant.
3. Results
3.1. The effect of ubiquinol in IOP and body weight in the transient ischemic retina
We began either unsupplemented control or ubiquinol (1%)-supplemented diet treatment daily for 1 month before the induction of transient retinal ischemia and then continued diet treatment for 12 h or 2 weeks (Fig. 1A). We found that the daily dose for ubiquinol per mouse was 22 ± 3 mg. Transient retinal ischemia was induced by acute IOP elevation to 69.5 ± 0.4 mmHg in mice treated with control diet and 70.3 ± 0.3 mmHg in mice treated with ubiquinol diet for 50 min (Fig. 1B). The mean IOP of the contralateral control eyes was 8.7 ± 0.1 mmHg (Fig. 1B). In addition, no difference was found in body weight between control and ubiquinol diet-treated mice during the experimental period (Fig. 1B).
Fig. 1.
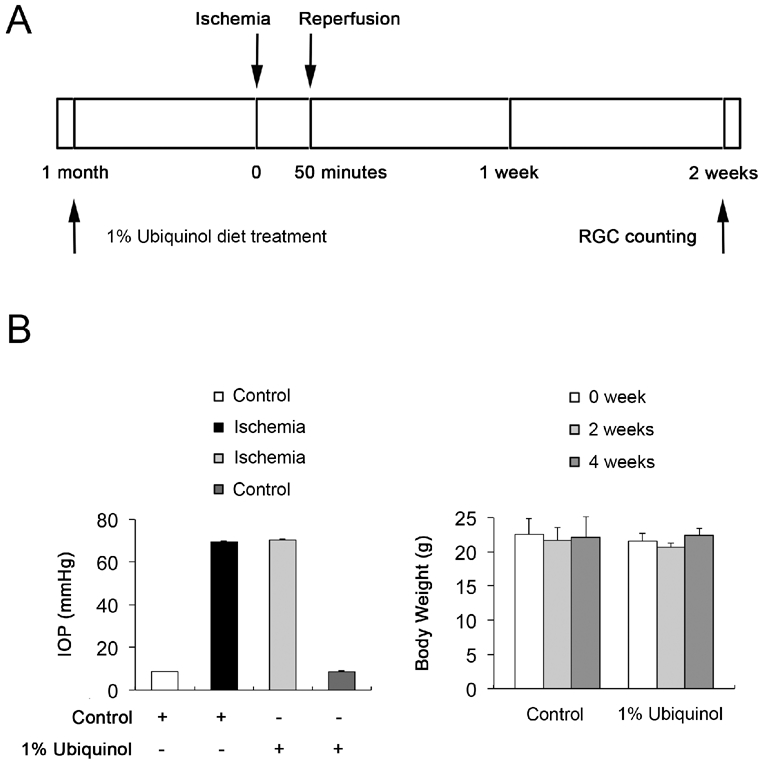
Ubiquinol supplementation and induction of the transient retinal ischemia. (A) Diagram for control and ubiquinol (1%) supplementation before and after ischemic injury. Unsupplemented control or ubiquinol diet were daily treated for 1 month before the induction of transient retinal ischemia and continued for 12 h or 2 weeks. (B) IOP elevation in the mouse eyes and body weight in control diet- and ubiquinol diet-treated mice following transient retinal ischemic injury.
3.2. Ubiquinol promotes RGC survival in the transient ischemic retina
We first determined whether ubiquinol treatment promotes RGC survival in the ischemic retina using whole-mount immunohistochemistry for Brn3a, a marker for RGCs. Non-ischemic control mouse retina had an average of 4144 ± 293 in the central, 3492 ± 662 in the middle and 2827 ± 684 RGCs in the peripheral areas (Fig. 2). In comparison with non-ischemic control retina treated with the control diet, ischemic retina treated with control diet treatment showed about 30% of RGC loss as an average of 2960 ± 361 in the central, 2593 ± 551 in the middle and 1510 ± 484 RGCs in the peripheral areas (Fig. 2). In contrast, ubiquinol treatment significantly promoted RGC survival by an approximate 17% as an average of 3323 ± 424 in the central, 3015 ± 588 in the middle and 1917 ± 453 RGCs in the peripheral areas compared with control diet-treated ischemic retina (Fig. 2). In addition, non-ischemic control mouse retina treated with ubiquinol diet had an average of 4128 ± 295 s in the central, 3463 ± 491 in the middle and 2440 ± 580 RGCs in the peripheral areas (Fig. 2).
Fig. 2.
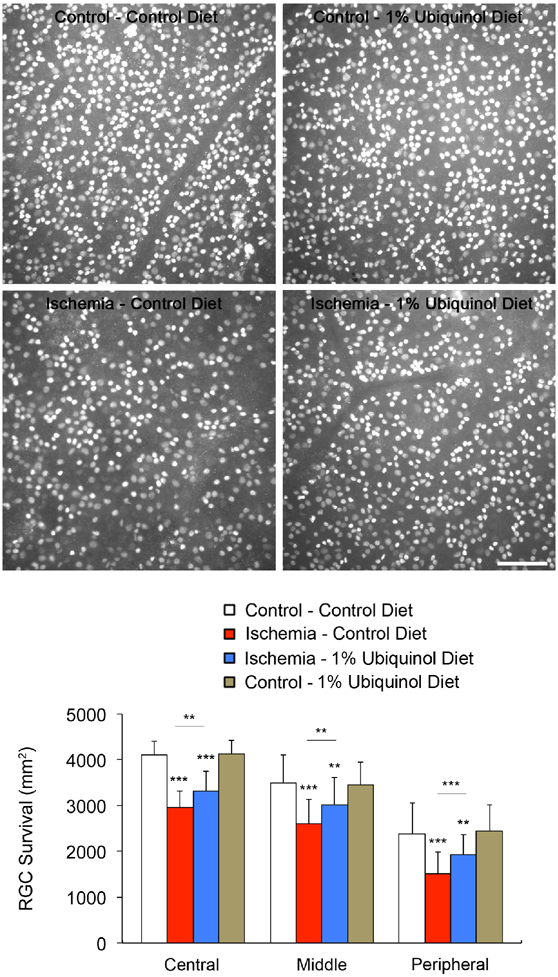
Ubiquinol-mediated protection of RGC survival in the transient ischemic retina. Brn3a whole-mount immunohistochemistry at 2 weeks after transient retinal ischemia. High magnification showed representative images from the middle area of retinas. Quantitative analysis of RGC survival. Values are mean ± SD (n = 5 retinas/group). Significant at *P < 0.05; **P < 0.01; *** P < 0.001compared with control diet-treated non-ischemic control retina or control diet-treated ischemic retina. Scale bar, 100 μm.
3.3. Ubiquinol blocks astroglial and microglial activation in the transient ischemic retina
To investigate whether ubiquinol treatment blocks the activation of astroglial and microglial cells in the ischemic retina, we performed whole-mount immunohistochemistry at 2 weeks and then measured immunoreactive intensity for GFAP and cell number for Iba1-positive microglial cells. In comparison with control diet-treated non-ischemic control retina (50.9 ± 8.13/mm2), the intensity of GFAP immunoreactivity was significantly increased (70.3 ± 7.44/mm2) in the ganglion cell layer (GCL) and nerve fiber layer of control diet-treated ischemic retinas at 2 weeks (Fig. 3A and B). Indeed, control diet-treated ischemic retina showed GFAP-positive hypertrophic cell bodies in astrocytes. However, ubiquinol treatment significantly decreased the intensity of GFAP immunoreactivity (59.5 ± 8.81/mm2) compared with control diet-treated ischemic retina (Fig. 3B). Also, there was no statistically significant difference between control mice treated with control and ubiquinol diet (Fig. 3B). In comparison with control diet-treated non-ischemic control retina (161 ± 19/mm2), the number of Iba1-positive active microglial cells, which show thickened processes and swollen cell body, in the GCL was significantly increased (327 ± 37/mm2) in control diet-treated ischemic retina at 2 weeks (Fig 3A and C). In contrast, ubiquinol treatment significantly decreased the number of Iba1-positive microglial cells (250 ± 17/mm2) compared with control diet-treated ischemic retina (Fig 3A and C). Also, there was no statistically significant difference between control mice treated with control and ubiquinol diet (Fig. 3C).
Fig. 3.
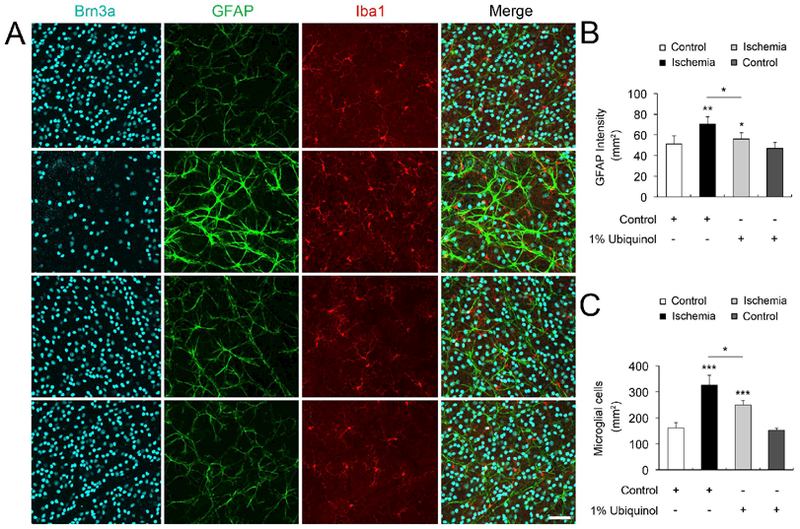
Ubiquinol-mediated blockade of astroglial and microglial activation in the transient ischemic retina. (A) Triple whole-mount immunohistochemistry for Brn3a, GFAP, and Iba-1 in ischemic retinas. Representative images with higher magnification showed a preservation of astroglial and microglial activation in the GCL of the ubiquinol-treated ischemic retina. Scale bars, 50 μm. (B and C) Quantification analyses of the intensity of GFAP immunoreactivity and the number of Iba-1-positive microglial cells. Values are mean ± SD (n = 5 images/group). Significant at *P < 0.05; **P < 0.01; *** P < 0.001compared with control diet-treated nonischemic control retina or control diet-treated ischemic retina.
3.4. Ubiquinol blocks Bax/Bad/caspase-3-mediated apoptotic cell death in the transient ischemic retina
To determine whether ubiquinol treatment prevents apoptotic cell death in the ischemic retina at 12 h, we performed Western blot analyses using apoptotic cell death-related antibodies raised against active Bax, p-Bad S112, Bcl-xL, and cleaved caspase-3. In comparison with control diettreated non-ischemic control retina, control diet treatment showed a significant increase of active Bax, p-Bad S112 and cleaved caspase-3 protein expression by 7.6 ± 0.79-, 5.2 ± 0.72- and 24.1 ± 5.62-fold at 12 h in the ischemic retina, respectively (Fig. 4). However, control diet treatment showed a significant decrease of Bcl-xL protein expression by 0.6 ± 0.04-fold in the ischemic retina at 12 h (Fig. 4). In comparison with control diet-treated ischemic retina, ubiquinol treatment showed a significant decrease of active Bax, p-Bad S112 and cleaved caspase-3 protein expression by 2.6 ± 0.09-, 2.1 ± 0.26-, and 1.5 ± 1.62-fold in the ischemic retina at 12 h, respectively (Fig. 4). However, ubiquinol treatment showed a significant increase of Bcl-xL protein expression by 0.8 ± 0.04-fold in the ischemic retina at 12 h (Fig. 4).
Fig. 4.
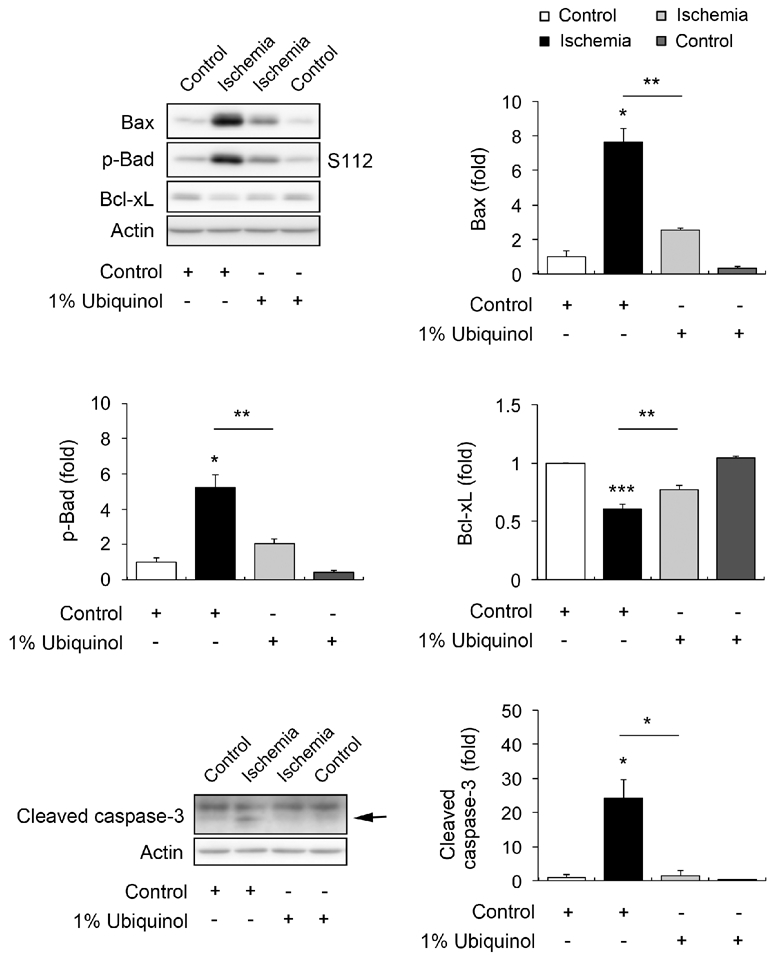
Ubiquinol-mediated blockade of apoptotic pathway in transient ischemic retina. Western blot analyses for Bax, p-Bad S112, Bcl-xL and cleaved caspase-3 protein expression at 12 h after ischemia. Values are mean ± SD (n = 4 retinas/group). Significant at *P < 0.05; **P < 0.01; *** P < 0.001compared with control diet-treated non-ischemic control retina or control diet-treated ischemic retina.
4. Discussion
In the current study, we addressed the question of whether a ubiquinol-supplemented diet promotes RGC survival, prevents glial activation and blocks the apoptotic cell death pathway in the ischemic mouse retina. Our current findings showed for the first time that ubiquinol treatment significantly promoted RGC survival, accompanied by the reduction of astroglial and microglial activation in the late stage of the ischemic retina. This is consistent with our earlier work demonstrating that CoQ10 enhanced RGC survival, accompanied by the blockade of the activation of astroglial or microglial cells in the early stage of ischemic and glaucomatous mouse retinas [6, 20]. We have also previously reported that CoQ10 protected rat ONH astrocytes against oxidative stress in vitro [24]. In addition, ubiquinol decreased apoptotic cell death and GFAP serum level in the rat brain following traumatic brain injury [18]. Collectively, the current findings raised the possibility that ubiquinol has therapeutic potential not only to promote RGC survival but also to preserve glial cells against ischemic damage.
On the other hand, our previous findings have demonstrated that CoQ10 ameliorated mitochondrial dysfunction by preserving mitochondrial transcription factor A or oxidative phosphorylation complex in ischemic and glaucomatous retinas [6, 20]. Intriguingly, we have also demonstrated that CoQ10 increased mitochondrial mass and improved mitochondrial bioenergetic function in cultured ONH astrocytes against oxidative stress [24]. Based on these observations, we believe that further studies will be necessary to examine ubiquinol-mediated functional preservation of mitochondria and its protective mechanisms in both RGCs and glial cells in the ischemic retina.
Bax is a pro-apoptotic member of the Bcl-2 family that is essential in many apoptotic pathways [25, 26] as well as directly interacts with the component forming the mitochondrial permeability transition pore (MPTP) that allows proteins to escape from the mitochondria into the cytosol to initiate apoptosis [27–29]. Bax is counteracted by Bcl-xL that forms heterodimers with dephosphorylation of Bad, which inactivates Bcl-xL; and p-Bad eliminates this dimerization, which activates Bcl-xL [30, 31]. Our previous study has demonstrated that CoQ10 blocked the mitochondria-related apoptotic pathway in the ischemic retina by decreasing Bax activation and increasing p-Bad expression [6], suggesting the possibility that CoQ10 may block the Bax-mediated increase of MPT and preserves mitochondrial homeostasis, as well as enhance p-Bad-mediated endogenous repair mechanism against apoptotic pathway in ischemic retina. Our current findings consistently showed that ubiquinol significantly prevented the increase of active Bax expression, but preserved the expression level of Bcl-xL and p-Bad S112, leading to the blockade of caspase-3-mediated apoptotic cell death in ischemic retina. Our findings indicate the evidence that ubiquinol protects RGCs by modulating the Bax/Bad/Bcl-xL pathway and by inhibiting caspase-3 cleavage in ischemic retinal degeneration.
In summary, these results provide evidence that ubiquinol promotes RGC survival by inhibiting the Bax/Bad-mediated apoptotic pathway and preserves the reaction of astroglial and microglial cells by reducing GFAP and Iba1 expression in the ischemic mouse retina. Based on these observations, our findings raise the possibility that ubiquinol has therapeutic potential for ameliorating elevated IOP-induced ischemic retinal injury as well as glaucoma and other optic neuropathies.
Highlights.
Ubiquinol treatment promotes RGC survival in the ischemic retina.
Ubiquinol treatment blocks astroglial and microglial activation in the ischemic retina.
Ubiquinol treatment prevents apoptotic cell death by modulating the Bax/Bad/Bcl-xL pathway in the ischemic retina.
Acknowledgements
This work was supported, in part, by NIH grants EY018658 (WKJ) and P30EY022589 (Vision Research Core Grant), and a research grant from Kaneka Corporation (Osaka, Japan; WKJ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Weinreb RN, Khaw PT, Primary open-angle glaucoma, Lancet, 363 (2004) 1711–1720. [DOI] [PubMed] [Google Scholar]
- [2].Ju WK, Kim KY, Lindsey JD, Angert M, Duong-Polk KX, Scott RT, Kim JJ, Kukhmazov I, Ellisman MH, Perkins GA, Weinreb RN, Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve, Invest Ophthalmol Vis Sci, 49 (2008) 4903–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ju WK, Kim KY, Hofmann HD, Kim IB, Lee MY, Oh SJ, Chun MH, Selective neuronal survival and upregulation of PCNA in the rat inner retina following transient ischemia, J Neuropathol Exp Neurol, 59 (2000) 241–250. [DOI] [PubMed] [Google Scholar]
- [4].Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J, Retinal ischemia: mechanisms of damage and potential therapeutic strategies, Prog Retin Eye Res, 23 (2004) 91–147. [DOI] [PubMed] [Google Scholar]
- [5].Hayreh SS, Ischemic optic neuropathy, Prog Retin Eye Res, 28 (2009) 34–62. [DOI] [PubMed] [Google Scholar]
- [6].Lee D, Kim KY, Shim MS, Kim SY, Ellisman MH, Weinreb RN, Ju WK, Coenzyme Q10 ameliorates oxidative stress and prevents mitochondrial alteration in ischemic retinal injury, Apoptosis, 19 (2014) 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ju WK, Lindsey JD, Angert M, Patel A, Weinreb RN, Glutamate receptor activation triggers OPA1 release and induces apoptotic cell death in ischemic rat retina, Mol Vis, 14 (2008) 2629–2638. [PMC free article] [PubMed] [Google Scholar]
- [8].Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK, A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina, Invest Ophthalmol Vis Sci, 52 (2011) 2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frei B, Kim MC, Ames BN, Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations, Proc Natl Acad Sci U S A, 87 (1990) 4879–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Do TQ, Schultz JR, Clarke CF, Enhanced sensitivity of ubiquinone-deficient mutants of Saccharomyces cerevisiae to products of autoxidized polyunsaturated fatty acids, Proc Natl Acad Sci U S A, 93 (1996) 7534–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fernandez-Ayala DJ, Lopez-Lluch G, Garcia-Valdes M, Arroyo A, Navas P, Specificity of coenzyme Q10 for a balanced function of respiratory chain and endogenous ubiquinone biosynthesis in human cells, Biochim Biophys Acta, 1706 (2005) 174–183. [DOI] [PubMed] [Google Scholar]
- [12].Beal MF, Shults CW, Effects of Coenzyme Q10 in Huntington’s disease and early Parkinson’s disease, Biofactors, 18 (2003) 153–161. [DOI] [PubMed] [Google Scholar]
- [13].Beal MF, Matthews RT, Coenzyme Q10 in the central nervous system and its potential usefulness in the treatment of neurodegenerative diseases, Mol Aspects Med, 18 Suppl (1997) S169–179. [DOI] [PubMed] [Google Scholar]
- [14].Beal MF, Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases, Biofactors, 9 (1999) 261–266. [DOI] [PubMed] [Google Scholar]
- [15].Mitsui J, Koguchi K, Momose T, Takahashi M, Matsukawa T, Yasuda T, Tokushige SI, Ishiura H, Goto J, Nakazaki S, Kondo T, Ito H, Yamamoto Y, Tsuji S, Three-Year Follow-Up of High-Dose Ubiquinol Supplementation in a Case of Familial Multiple System Atrophy with Compound Heterozygous COQ2 Mutations, Cerebellum, 16 (2017) 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pierce JD, Shen Q, Peltzer J, Thimmesch A, Hiebert JB, A pilot study exploring the effects of ubiquinol on brain genomics after traumatic brain injury, Nurs Outlook, 65 (2017) S44–S52. [DOI] [PubMed] [Google Scholar]
- [17].Frontinan-Rubio J, Sancho-Bielsa FJ, Peinado JR, LaFerla FM, Gimenez-Llort L, Duran-Prado M, Alcain FJ, Sex-dependent co-occurrence of hypoxia and beta-amyloid plaques in hippocampus and entorhinal cortex is reversed by long-term treatment with ubiquinol and ascorbic acid in the 3xTg-AD mouse model of Alzheimer’s disease, Mol Cell Neurosci, (2018). [DOI] [PubMed] [Google Scholar]
- [18].Pierce JD, Gupte R, Thimmesch A, Shen Q, Hiebert JB, Brooks WM, Clancy RL, Diaz FJ, Harris JL, Ubiquinol treatment for TBI in male rats: Effects on mitochondrial integrity, injury severity, and neurometabolism, J Neurosci Res, 96 (2018) 1080–1092. [DOI] [PubMed] [Google Scholar]
- [19].Bhagavan HN, Chopra RK, Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics, Free Radic Res, 40 (2006) 445–453. [DOI] [PubMed] [Google Scholar]
- [20].Lee D, Shim MS, Kim KY, Noh YH, Kim H, Kim SY, Weinreb RN, Ju WK, Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma, Invest Ophthalmol Vis Sci, 55 (2014) 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nakajima Y, Inokuchi Y, Nishi M, Shimazawa M, Otsubo K, Hara H, Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo, Brain Res, 1226 (2008) 226–233. [DOI] [PubMed] [Google Scholar]
- [22].Russo R, Cavaliere F, Rombola L, Gliozzi M, Cerulli A, Nucci C, Fazzi E, Bagetta G, Corasaniti MT, Morrone LA, Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection, Prog Brain Res, 173 (2008) 575–582. [DOI] [PubMed] [Google Scholar]
- [23].Nucci C, Tartaglione R, Cerulli A, Mancino R, Spano A, Cavaliere F, Rombola L, Bagetta G, Corasaniti MT, Morrone LA, Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat, Int Rev Neurobiol, 82 (2007) 397–406. [DOI] [PubMed] [Google Scholar]
- [24].Noh YH, Kim KY, Shim MS, Choi SH, Choi S, Ellisman MH, Weinreb RN, Perkins GA, Ju WK, Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes, Cell death & disease, 4 (2013) e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ, Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death, Science, 292 (2001) 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wasiak S, Zunino R, McBride HM, Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death, J Cell Biol, 177 (2007) 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC, Inhibition of Bax channel-forming activity by Bcl-2, Science, 277 (1997) 370–372. [DOI] [PubMed] [Google Scholar]
- [28].Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ, Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2, Proc Natl Acad Sci U S A, 94 (1997) 11357–11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Desagher S, Martinou JC, Mitochondria as the central control point of apoptosis, Trends Cell Biol, 10 (2000) 369–377. [DOI] [PubMed] [Google Scholar]
- [30].Oltvai ZN, Milliman CL, Korsmeyer SJ, Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death, Cell, 74 (1993) 609–619. [DOI] [PubMed] [Google Scholar]
- [31].Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ, Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death, Cell, 80 (1995) 285–291. [DOI] [PubMed] [Google Scholar]


