Abstract
Recent publications have described epithelial cytoplasmic vacuoles and inclusions incidentally noted within gallbladder epithelium and concluded that they represent coccidian parasite infection, in particular, Cystoisospora belli. We identified eight gallbladder specimens from our institution in the past three years in which this diagnosis was suggested or in which similar epithelial alterations were prominent. Molecular analysis was performed on the eight gallbladder specimens and on three positive control specimens: small bowel biopsies from acquired immunodeficiency syndrome patients with diarrhea. PCR using primers designed to amplify an internal transcribed spacer (ITS2) in the C. belli ribosomal gene cluster was performed on the DNA samples. All eight gallbladder specimens were negative for amplification, while a product consistent with C. belli was amplified from all three positive controls. Histologically, the gallbladder cytoplasmic inclusions stained diffusely positive for GMS and PAS with diastase. In contrast, sections from a positive control small bowel biopsy demonstrated organisms that were negative for GMS and showed a distinct capsular and punctate internal staining on PAS/D in various parasite forms. Together, the lack of molecular evidence of C. belli, and the distinct morphologic and special staining patterns in these gallbladders compared with positive control small bowel suggest that these epithelial changes do not represent true C. belli infection. Our results suggest that gallbladders of immunocompetent patients may occasionally show epithelial changes that can morphologically mimic C. belli infection. Pathologists should be aware of this histologic variant to minimize unnecessary treatment, testing, and patient anxiety.
Keywords: Cystoisospora belli, gallbladder, immunocompetent
Introduction
Cystoisospora belli (C. belli, formerly known as Isospora belli) is a coccidian parasite that is known to cause enteric disease in humans(1). The organisms have a global distribution, mostly within tropical and sub-tropical locations. Transmission occurs via ingestion of food or water contaminated with the mature oocysts. Typical infections occur in children in endemic areas and outbreaks may also occur, predominantly in developing countries. Immunosuppressed persons, including acquired immunodeficiency syndrome (AIDS) patients, are at particular risk for C. belli infection. Clinical manifestations typically include sudden-onset watery, non-bloody diarrhea, fever, nausea and vomiting. Patients predominantly present with intestinal manifestations; however extra-intestinal infections have been reported (1–5). Cystoisosporiasis in immunocompromised patients can lead to chronic diarrhea and malabsorption, and these patents may develop cachexia(6).
The diagnosis of C. belli can be made by the identification of ovoid oocysts that taper at the ends, have a smooth double-layered wall, and measure 20-33 by 10-19 microns in concentrated stool wet mounts(7). The presence of oocysts in the stool may be transient, requires special staining (modified acid fast or modified safranin) or UV autofluorescence microscopy, and multiple stool samples may need to be examined to detect the organisms.
C. belli can also be identified in histologic sections from infected tissues. Biopsies from the small bowel of infected individuals may show villous blunting and in cases with heavy parasite burden can show mucosal atrophy(8). Sporozoites, schizonts, merozoites, and gametocyte forms may be seen in the intestinal epithelial cells. These developmental stages are seen within vacuoles which can aid in the identification of C. belli. Routine hematoxylin and eosin (H&E) staining is adequate for identification(9), although the organisms can be sparse(10).
Recent publications have described epithelial alterations within gallbladder mucosa, which have been attributed to C. belli infection occurring in immunocompetent patients (11–13). The cytoplasmic inclusions were noted to be supra- and sub-nuclear, and the characteristic oval- to banana-shaped intraepithelial parasites within perinuclear parasitiphorous vacuoles were described. The background mucosa was described as showing epithelial disarray with rare intraepithelial lymphocytosis, without associated eosinophilia. None of the patients were immunocompromised, and none were reported to have diarrhea or constitutional symptoms of infection. Special stains including PAS with diastase and GMS were performed to confirm the presence of organisms. No reported molecular testing was performed on these tissues to confirm infection. In addition, recent abstracts with larger series presented at the United States and Canadian Academy of Pathology meeting (2018) concluded that C. belli infections were seen in 6.1% of pediatric cholecystectomy specimens(14), and 9.7% of all immunocompetent cholecystectomy patients(15). An additional abstract described the utility of Pax8 in identifying C. belli inclusions(16).
C. belli infection can occur in gallbladders and has been reported in patients with immunosuppression and associated diarrhea. The first description of C. belli infection of the gallbladder was reported in 1994 in a 39-year-old man with AIDS(17). Another report of 107 cholecystectomy specimens from patients with AIDS demonstrated one case of C. belli infection (18). A subsequent report of 101 cholecystectomy specimens from AIDS patients reported that forty percent of the gallbladders showed evidence of infectious organisms, including Cryptosporidium, cytomegalovirus, Mycobacterium avium/intracellulare complex, or some combination of these infections(19), however, no cases of C. belli infection were identified in this cohort.
A case report describing a patient with C. belli infection of the gallbladder, that included molecular confirmation of infection, described a West African patient with HIV who presented with abdominal pain, vomiting and diarrhea(5). DNA was isolated from the paraffin block and PCR was performed using primers for the internal transcribed spacer (ITS2) in the C. belli ribosomal gene cluster, amplifying a band of the expected size from the patient and not in control tissue. This report was the first to demonstrate molecular confirmation of biliary C. belli infection, and was the basis of our testing strategy. Stool studies in this patient demonstrated oocysts of Cystoisospora, additionally confirming the infection.
We identified eight cases at our institution in which a diagnosis of C. belli infection in the gallbladder, in accordance with the published studies, was suggested by histology. Given the lack of clinical concern for parasite infection, absence of diarrhea, and lack of underlying risk factors for infection in these cases, we retrospectively examined them for molecular evidence and histologic features of parasite infection. In parallel, for positive controls, we examined small bowel biopsies from acquired immunodeficiency syndrome (AIDS) patients with C.belli infection and profuse diarrhea.
Materials and Methods
Patient selection
With approval from the Institutional Review Board from the University of Utah, the pathology database was searched for gallbladder specimens with a diagnosis of Cystoisospora infection, which identified six cases between 2015 and 2017. An additional two cholecystectomy cases were identified that showed similar prominent epithelial alterations that suggested C. belli infection, so that a total of eight cholecystectomy cases were examined. As controls, small bowel biopsies with prior diagnoses of C. belli infection along with normal small bowel biopsies were identified from within the University of Utah and Cedars-Sinai Medical Center archives. Electronic medical records and pathology reports were examined to determine clinical indications for cholecystectomy or biopsy, associated symptoms including fever and diarrhea, history of immunosuppression, stool studies, and subsequent treatment and follow up.
Molecular analysis
Formalin-fixed paraffin-embedded (FFPE) tissue scrolls were cut at 50 microns from each block, and DNA was isolated from tissue by the Biorepository and Molecular Pathology Core Laboratory at the University of Utah. Two rounds of quantitative PCR (qPCR) were performed on all gallbladder samples, each using 100 ng of DNA template, 0.5 μM each of forward and reverse primers, and Quanta bio PerfeCTa SYBR Green FastMix (cat. Number 95072-012) amplified on an Eppendorf Masterplex realcycler EPgradient with fluorescence readings taken every cycle. Two or three technical replicates (wells) were performed for each reaction.
PCR was performed as described previously (5, 20). For round one, we used previously published (5) ITS2 primers (forward CCGAACGTCATCCGAAATAG; reverse ACTAGGAGCTGACGATACAC), β-actin (ACTB) primers (forward TATGGTAATAACGCGGCCGG; reverse CACGATGGAGGGGAAGACG), or β-globin (HBB) primers (forward ACACAACTGTGTTCACTAGC; reverse = CAACTTCATCCACGTTCACC) and the following cycle conditions: 1) 95°C for 10 minutes; 2) forty cycles of 95°C for 1 minute, 60°C for 1 minute, 72°C for 2 minutes; 3) 72°C for 10 minutes; 4) 10°C hold. For round two, we used previously published (20) ITS1 primers (forward TCCGTAGGTGAACCTGCGG; reverse GCTGCGTTCTTCATCGATGC) or β-actin primers and the same cycling conditions as above.
For each round, one representative replicate for each sample was loaded and run on a 1.1% agarose LE gel alongside a New England Biolabs N3231S 100 bp ladder and imaged using a BioRad Quantity One ChemiDoc XRS. For round two, bands were cut out from the gel, gel purified using a Qiagen gel extraction kit 28704, and submitted for Sanger sequencing by GeneWiz (South Plainfield, NJ). The resulting sequences were analyzed using NCBI BLAST.
Histologic analysis and special staining
Hematoxylin-and-eosin-stained slides were re-reviewed by pathologists specializing in gastrointestinal (E.A.S., F.C., and K.J.E.) and infectious (F.C., M.R.C.) pathology. Giemsa, GMS, PAS/D, and mucicarmine special stains were performed on representative FFPE tissue blocks following standard protocols at ARUP Laboratories (Salt Lake City, UT). Special stains were reviewed by three pathologists (E.A.S., F.C., and K.J.E.) and one pathology resident (J.M.).
Results
Demographic and clinical background
Electronic medical records were reviewed for the cholecystectomy patients (Table 1). One gallbladder was from a donor liver, and detailed clinical information was not available. The mean age of the patients whose gallbladders were examined was 43 years of age (range 26-68). They included 4 males and 3 females. One patient had a history of immunosuppression due to a renal transplant 6 months prior secondary to systemic lupus erythematosus. Indications for cholecystectomy included biliary colic, choledocholithiasis, gallstone pancreatitis, biliary dyskinesia, and incidental resection during hepatic resection of a hepatocellular carcinoma. None of the patients reported a clinical history of diarrhea.
Table 1.
Clinical and Pathologic Information on Patients with Gallbladder Resections and Epithelial Inclusions
| Age/Gender | Significant clinical history |
Pathologic findings |
Cholelithiasis | Reason for surgery | Intact with bile |
Allograft | Immunosupressed | Diarrhea | Treated for parasite infection |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 F | None | Chronic cholecystitis | Yes | Choledocholithiasis | Yes | No | No | No | No, discussed with patient |
| 2 | 63 M | Coronary artery disease | Acute cholecystitis | No | Gallstone pancreatitis | Yes | No | No | No | No |
| 3 | 43 F | SLE, Kidney transplant | Chronic cholecystitis | No | Biliary colic | not specified | No | Yes | No | Yes, ID consult |
| 4 | 31 M | None | Chronic cholecystitis | Yes | Chronic cholecystitis with cholelithiasis | Yes | No | No | No | Yes, ID consult |
| 5 | 35 M | None | Chronic cholecystitis | No | Biliary dyskinesia | Yes | No | No | No | Yes |
| 6 | 34 F | None | Chronic cholecystitis | Yes | Chronic cholecystitis with cholelithiasis | Yes | No | No | No | No (not mentioned in path report) |
| 7 | 68 M | UC, PSC, HCC | Chronic cholecystitis | No | HCC resection | Yes | No | No | No | No (not mentioned in path report) |
| 8 | N/A | Not available | No significant abnormality | No | Transplant | not specified | Yes | Not available | Not available | Yes (liver recipient) |
Abbreviations: M (male), F (female), SLE (systemic lupus erythematosus), UC (ulcerative colitis), PSC (primary sclerosing cholangitis, HCC (hepatocellular carcinoma)
Molecular testing
DNA from the eight gallbladders was subjected to real-time PCR with primers designed to amplify the ITS2 internal transcribed spacer (ITS2) in the C. belli ribosomal gene cluster (5). Using previously reported reaction conditions (5), none of the eight gallbladder samples showed amplification of C. belli template (Figure 1). In contrast, we did see amplification of a fragment of the expected size using DNA isolated from three small bowel biopsies from two different patients with AIDS and diarrhea (Figure 1). All gallbladder samples and all positive controls showed amplification of internal control products of the expected size using primers designed against human beta-actin (ACTB) and/or beta-globin (HBB) to monitor for adequate DNA quality and reaction conditions (Figure 1).
Figure 1. PCR of DNA isolated from gallbladder samples does not amplify C. belli DNA.

A. Gel shows PCR reactions performed in parallel using primers specific for ITS2 (left side) or human housekeeping genes (right side). No product is seen for PCR reactions using ITS2 primers and DNA isolated from gallbladder cases 1- 8 (lanes 1-8), water/no template control (lane W), or DNA isolated from small bowel cases with no significant abnormality (lanes A and B). A 176 bp band is amplified using DNA isolated from small bowel biopsies from AIDS patients (lanes C-E). All samples except water/no template control showed amplification of a 257 bp or 110 bp fragment using primers specific for human β-actin or β-globin, respectively. L = 100 bp ladder.
An additional extended range PCR screening assay was also performed using ITS1 primers, which recognize conserved DNA in eukaryotic organisms and allows specific discrimination for many fungi and protozoa (20). ITS1 primers amplified a product of the expected size (626 bp) in a positive control sample, but did not amplify this fragment in any of the eight gallbladder specimens (Supplemental Digital Figure 1). The 626 bp product was sequenced and showed 100% sequence identity to Cystoisospora belli gene for small subunit (18S) ribosomal RNA. At greater than 30 cycles, several minor products were amplified from the gallbladder samples (Supplemental Digital Figure 1). To investigate the possibility that these bands could represent parasitic infection of the gallbladder(s), we purified and sequenced seven of the most concentrated amplicons. These included lanes 1 through 5 as well as 7 and 8. Sequencing of these minor products matched fungal and plant material sequences. No protozoal sequence was identified in these database queries.
Gross and Microscopic examination
Gallbladders were received in the gross room intact with luminal bile in 6 of the cases, while the gross description did not specify if luminal bile was present in the remaining 2 cases. The gross examination noted cholelithiasis in 3 cases. No masses were grossly observed. Pathologic diagnoses included acute and chronic cholecystitis, cholelithiasis, and epithelial inclusions that were attributed to C. belli infection in 6 of the cases.
Histologic re-examination of these cases demonstrated the aforementioned epithelial changes including cytoplasmic vacuolization with eosinophilic inclusions within some of the vacuoles (Figure 2). The findings were focal in some cases, and in others diffusely involved the majority of the epithelium. Crypt epithelium was a frequent location for the findings. The eosinophilic inclusions were mostly small (5-10 microns, with occasional forms up to 15 microns) and without discernable internal structures. The forms were relatively monomorphic without wide variation in appearance. The inclusions were ovoid to elongate in shape, with some demonstrating banana-shaped forms. GMS, PAS with diastase, and mucicarmine stains highlighted the inclusions as well as small mucin droplets throughout the remaining epithelium. These stains were relatively homogenous across the structures (Figure 2).
Figure 2. Gallbladder from an immunocompetent patient showing prominent epithelial inclusions.

A. H&E 40X. Gallbladder epithelium shows bubbly cytoplasm and epithelial disarray. B-C. H&E, 400x. Cytoplasmic vacuolization and eosinophilic inclusions are seen as oval to banana shaped inclusions with a glassy and frequently homogenous staining pattern (arrows). D. H&E 1000x. Some inclusions show some uneven eosinophilic staining, mimicking internal structures of parasite forms (arrows).
In contrast, slides from small bowel with C. belli infection showed a background of epithelial damage as well as cytoplasmic inclusions with a variety of distinctive life stages of the organisms (Figure 3). The organisms were seen as ovoid structures with variable shape and size. Eosinophilic to basophilic internal structures were noted within the organisms, as well as occasional filamentous refractile material. Some epithelial cells contained multiple elongated banana shaped merozoites packaged contained in a schizont within the epithelium. Organisms were also focally present within the lamina propria. The organisms were negative on GMS special stain and were subtly highlighted by Giemsa stain. PAS/D stain occasionally highlighted the organism capsule in the lamina propria, as well as some punctate perinuclear internal staining in other forms (Figure 4). Diffuse PAS/D staining was not observed in the organisms, in contrast to the gallbladder inclusions.
Figure 3. Small bowel mucosa from an HIV-infected patient with severe diarrhea showing numerous stages of parasite development in the epithelium and lamina propria.

A-D. H&E 1000X. Sporozoites, schizonts, merozoites, and gametocyte forms (arrows). Note the variety in the shape and size of the forms as well as the staining of internal structures.
Figure 4. PAS/D staining pattern in gallbladder inclusions is distinct from the pattern in small bowel with molecularly confirmed C. belli infection.
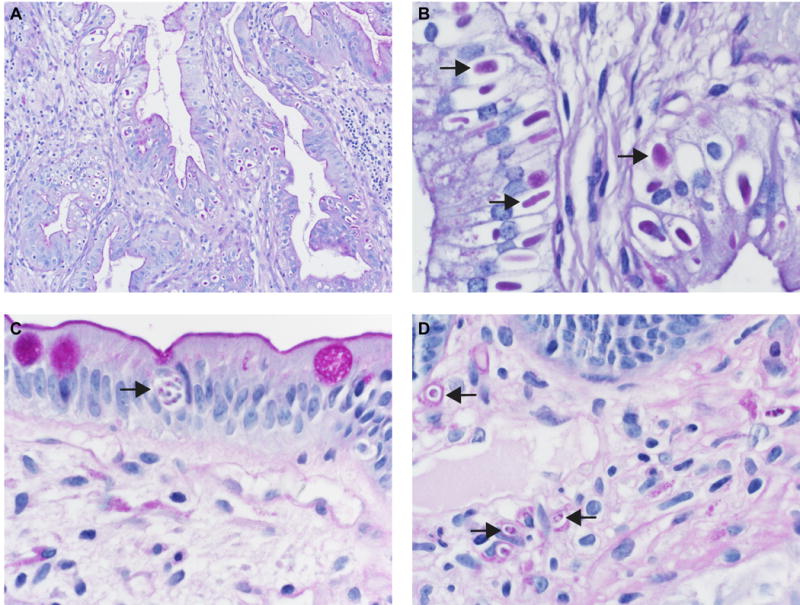
A-B: PAS/D, 40X and 1000X. Sections from gallbladders with cytoplasmic inclusions (Case 8). The inclusions stain diffusely positive for PAS/D (arrows). C-D: PAS/D, 1000X. Sections from small bowel with C. belli infection. PAS/D stain shows punctate internal staining, predominantly perinuclear, as well as some staining of capsules in the lamina propria forms (arrows).
Clinical Follow-up
Four of the six patients in which the possibility of C. belli infection was mentioned in the pathology report received antibiotic treatment based on the pathology report (trimethoprim-sulfamethoxazole). Two patients had subsequent stool ova and parasite studies which were negative. Two patients were referred to infectious disease clinicians for further clinical investigation.
Discussion
The recent reports of C. belli gallbladder infection in routine specimens from immunocompetent hosts has raised the possibility of parasite infection to explain the histologic finding of vacuolated/bubbly cytoplasm with eosinophilic inclusions and epithelial disarray, as well as the clinical impression of biliary dyskinesia(11–16). Our detailed morphologic analysis showed distinct differences in the morphology of the true organisms in small bowel compared with the inclusions seen in gallbladder epithelium. The true organisms show a wide variety of morphologic forms including rounded trophozoites with central nuclei and cytoplasmic inclusions, multiple/clustered banana-shaped merozoites compartmentalized within schizonts, granular macrogametocytes, and microgameotcytes(10).
We found that special stains, including PAS/D, highlighted the epithelial inclusions in our gallbladder samples as previously reported in cholecystectomy specimens of immunocompetent patients with presumed C. belli infection. We also found that the epithelial inclusions in gallbladder showed positive staining for GMS, and mucicarmine, while true organisms identified in the small bowel were negative for GMS and mucicarmine, and only focally positive for PAS/D with a distinctive punctate internal staining (Figure 3). Similar focally positive PAS/D staining patterns of C. belli were noted in a report of AIDS patient with disseminated C. belli infection. The morphologic description noted that the enclosed trophozoite contained PAS positive granules, corresponding to the presence of amylopectin bodies (2, 3). Another report of extraintestinal C. belli infection had noted a PAS-positive cyst wall in intracellular trophozoites(4). However, none of the reports of C. belli infection in immunocompromised patients describe diffuse, homogenous PAS staining as is seen in the gallbladder. In summary, special stains to highlight possible C. belli organisms should be interpreted with caution, and the most useful stain to evaluate for C. belli is a routine H&E stained section.
Molecular testing confirmed an absence of C. belli organisms all of our gallbladder samples. The ITS2 primer set confirmed the presence of C. belli DNA in all three small bowel infection cases, while no amplification was seen in any of the eight gallbladder samples. Primer sets for housekeeping genes amplified expected product in all cases to control for the PCR reaction and DNA quality. An ITS1 extended-range PCR assay confirmed the small bowel C. belli infection and showed no amplification of protozoal sequences in our gallbladder samples.
It is likely that the gallbladder findings represent an artifact of epithelial cytolysis related to tissue preservation. Bile is a known cytolytic agent, and tissue can be rapidly degraded when devitalized and left exposed. Experiments performed in the early 1900s on tissues exposed to bile noted that nuclei lost position, form, and capacity to take up hematoxylin(21). Additionally, the cytoplasm is noted to stain with eosin, but most peripherally becomes pale or even disappears altogether, which likely explains the pseudo-parastiphorous vacuole. The majority of the gallbladders in our series were intact upon grossing, and the epithelium was exposed to bile without adequate formalin fixation for a significant period of time (ranging from 6.5 to 21 hours in these cases). Thus, the gallbladders inclusions may represent condensed epithelial cytoplasm and nuclear degradation.
Epithelial inclusions in gallbladder epithelium should be interpreted with caution and attempts should be made at confirmation by alternative methods such as stool examination, molecular studies, or consultation with expert parasitologists. Additionally, correlating with a history of immunosuppression, fever, and diarrhea would be helpful in prompting evaluation for infection. The improper pathologic diagnosis of C. belli may lead to unnecessary sequelae including antibiotic administration, infectious disease consultation, immunodeficiency testing, and patient anxiety. The additional cost and potential harm in treating the infection may be significant. It is important recognize the spectrum of non-specific changes in the gallbladder epithelium and to use strict criteria when suggesting a parasitic infection.
Supplementary Material
Supplemental Digital Figure 1: PCR of DNA isolated from gallbladder samples does not amplify C. belli DNA. Gel shows reactions performed in parallel using primers specific for ITS1 (left side) or human β-actin (right side). Only small, faint products (arrows) are seen using DNA isolated from gallbladder cases 1- 8 (lanes 1-8), while a 626 band is amplified using DNA isolated from a small bowel biopsy from an AIDS patient (lane E, arrowhead). All samples showed amplification of a 257 bp band using primers specific for human β-actin. L = 100 bp ladder.
Acknowledgments
Source of Funding: KJE is partially supported by NIH/NCI K08CA172288 and NIH/NCI R01CA222570.
Footnotes
Conflicts of Interest: The authors have disclosed that they have no significant financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
References
- 1.Legua P, Seas C. Cystoisospora and cyclospora. Curr Opin Infect Dis. 2013;26:479–83. doi: 10.1097/01.qco.0000433320.90241.60. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay DS, Dubey JP, Toivio-Kinnucan MA, et al. Examination of extraintestinal tissue cysts of Isospora belli. J Parasitol. 1997;83:620–5. [PubMed] [Google Scholar]
- 3.Michiels JF, Hofman P, Bernard E, et al. Intestinal and extraintestinal Isospora belli infection in an AIDS patient. A second case report. Pathol Res Pract. 1994;190:1089–93. doi: 10.1016/S0344-0338(11)80908-8. discussion 94. [DOI] [PubMed] [Google Scholar]
- 4.Restrepo C, Macher AM, Radany EH. Disseminated extraintestinal isosporiasis in a patient with acquired immune deficiency syndrome. Am J Clin Pathol. 1987;87:536–42. doi: 10.1093/ajcp/87.4.536. [DOI] [PubMed] [Google Scholar]
- 5.Walther Z, Topazian MD. Isospora cholangiopathy: case study with histologic characterization and molecular confirmation. Hum Pathol. 2009;40:1342–6. doi: 10.1016/j.humpath.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenberg Fv. Pathology of infectious diseases. New York: Raven Press; 1991. [Google Scholar]
- 7.Ash LR, Orihel TC. Atlas of human parasitology. Chicago: American Society of Clinical Pathologists; 1997. [Google Scholar]
- 8.Binford CH, Connor DH, Ash JE, et al. Pathology of tropical and extraordinary diseases. Vol. 1. Washington D.C: Armed Forces Institute of Pathology; 1976. [Google Scholar]
- 9.Lindsay DS, Dubey JP, Blagburn BL. Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev. 1997;10:19–34. doi: 10.1128/cmr.10.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clayton F, Clayton CH. Gastrointestinal pathology in HIV-infected patients. Gastroenterol Clin N. 1997;26:191–240. doi: 10.1016/s0889-8553(05)70293-4. [DOI] [PubMed] [Google Scholar]
- 11.Lai KK, Goyne HE, Hernandez-Gonzalo D, et al. Cystoisospora belli Infection of the Gallbladder in Immunocompetent Patients: A Clinicopathologic Review of 18 Cases. Am J Surg Pathol. 2016;40:1070–4. doi: 10.1097/PAS.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 12.Martelli MG, Lee JY. Parasitic Infection of the Gallbladder: Cystoisospora belli Infection as a Cause of Chronic Abdominal Pain and Acalculous Cholecystitis. J Miss State Med Ass. 2016;57:174–6. [PubMed] [Google Scholar]
- 13.Takahashi H, Falk GA, Cruise M, et al. Chronic cholecystitis with Cystoisospora belli in an immunocompetent patient. BMJ Case Rep [serial online] 2015;2015:bcr2015209966. doi: 10.1136/bcr-2015-209966. Available from . . Accessed 05/04/2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose G, Badizadegan K, Carter C, et al. Cystoisospora is a Frequent Finding in Pediatric Cholecystectomy Specimens. Lab Invest. 2018;98:687. [Google Scholar]
- 15.Mushal Noor CW-M, Lamps LW, Gonzalez RS, et al. Unexpectedly High Prevalence of Cystoisospora belli in Acalculous Gallbladders of Younger Patients. Lab Invest. 2018;98:580. doi: 10.1093/ajcp/aqy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mubeen A, Alkhasawneh A, Monteiro C, et al. PAX-8 Antibody as a Useful Adjunct in Detection of Cystoisospora in Formalin Fixed Paraffin Embedded Tissue: A Study on Cholecystectomies with Cystoisospora Infection. Lab Invest. 2018;98(suppl 1):580. [Google Scholar]
- 17.Benator DA, French AL, Beaudet LM, et al. Isospora belli infection associated with acalculous cholecystitis in a patient with AIDS. Ann Intern Med. 1994;121:663–4. doi: 10.7326/0003-4819-121-9-199411010-00006. [DOI] [PubMed] [Google Scholar]
- 18.French AL, Beaudet LM, Benator DA, et al. Cholecystectomy in patients with AIDS: clinicopathologic correlations in 107 cases. Clin Infect Dis. 1995;21:852–8. doi: 10.1093/clinids/21.4.852. [DOI] [PubMed] [Google Scholar]
- 19.Leiva JI, Etter EL, Gathe J, Jr, et al. Surgical therapy for 101 patients with acquired immunodeficiency syndrome and symptomatic cholecystitis. Am J Surg. 1997;174:414–6. doi: 10.1016/s0002-9610(97)00118-9. [DOI] [PubMed] [Google Scholar]
- 20.Murphy SC, Hoogestraat DR, Sengupta DJ, et al. Molecular diagnosis of cystoisosporiasis using extended-range PCR screening. J Mol Diagn. 2011;13:359–62. doi: 10.1016/j.jmoldx.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatum AL. The Influence Of Bile On Autolysis. J Biol Chem. 1916:xxvii. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Figure 1: PCR of DNA isolated from gallbladder samples does not amplify C. belli DNA. Gel shows reactions performed in parallel using primers specific for ITS1 (left side) or human β-actin (right side). Only small, faint products (arrows) are seen using DNA isolated from gallbladder cases 1- 8 (lanes 1-8), while a 626 band is amplified using DNA isolated from a small bowel biopsy from an AIDS patient (lane E, arrowhead). All samples showed amplification of a 257 bp band using primers specific for human β-actin. L = 100 bp ladder.


