Abstract
Pathologic examination of hepatic metastasectomies from patients with metastatic small intestinal or pancreatic neuroendocrine tumor frequently reveals micrometastases undetectable by radiologic or macroscopic gross examination. This finding raises the possibility that undetectable micrometastases remain in these patients after metastasectomy. Here we examined liver resections for micrometastases and assessed their impact on prognosis. Hepatic metastasectomies from 65 patients with neuroendocrine tumor of the small intestine (N=43) or pancreas (N=22) were reviewed for the presence of micrometastases, which were defined as microscopic tumor foci ≤1 mm in greatest dimension. Medical records were also reviewed for patient demographics, clinical history, and follow-up data. Micrometastasis was identified in 36 (55%) of 65 hepatic resection specimens. More hepatic micrometastases were seen in small intestinal cases than in pancreatic cases (29/43, 67% versus 7/22, 32%; P<0.01). They were typically present within portal tracts, sometimes with extension into the periportal region or sinusoidal spaces away from the portal tracts. Patients without hepatic micrometastases had fewer macrometastases or more R0 hepatic resections than those with micrometastases. The presence of hepatic micrometastases was associated with poor overall survival both before (hazard ratio [HR] 3.43; 95% CI 1.14–10.30; P=0.03) and after accounting for confounding variables in stratified Cox regression (HR 4.82; 95% CI 1.06–21.79; P=0.04). In conclusion, hepatic micrometastases are common in patients with metastatic small intestinal or pancreatic neuroendocrine tumor and are independently associated with poor prognosis. These data suggest that surgical resection of hepatic metastases is likely not curative in these patients.
Keywords: Micrometastases, liver metastasis, small intestine, pancreas, neuroendocrine tumor
1. 1 INTRODUCTION
The digestive tract is the most common site of origin of well-differentiated neuroendocrine tumors (NETs)[1, 2]. Digestive tract NETs can arise anywhere in the gastrointestinal tract, including the pancreatobiliary tract, and have historically been divided into foregut, midgut, and hindgut NETs[3]. Most digestive tract NETs arise in the small intestine (midgut), rectum (hindgut), or pancreas (foregut). While rectal NETs are frequently small and diagnosed incidentally, up to 50% of small intestinal or pancreatic NETs are metastatic at presentation, usually involving the liver[2–10]. Most patients with digestive tract NETs have an indolent clinical course. Even after liver metastasis, many patients live for an additional 5–10 years. Therefore, treatment of hepatic metastasis is an important aspect of the clinical management of patients with small intestinal and pancreatic NETs.
Current therapeutic modalities for NET patients with liver metastasis can be classified as 1) systemic (e.g. somatostatin analogs, targeted therapy) or 2) local (e.g. hepatic artery chemoembolization, hepatic surgery)[10–14]. Debulking surgery, either by wedge resection or partial hepatectomy, is commonly used to reduce tumor volume and decrease the possibility of tumor-associated syndromes, such as carcinoid syndrome[15]. Curative surgery is sometimes attempted when patients have limited disease potentially curable with aggressive resection. Despite these efforts, many patients suffer disease recurrence or progression after surgery. In this study, we reviewed hepatic resection specimens from patients with metastatic pancreatic and small intestinal NETs to evaluate the prevalence of micrometastases and their impact on patient outcome.
2.1 MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board at Vanderbilt University Medical Center (IRB#101735). The requirement for informed consent was waived.
The Surgical Pathology archives at Vanderbilt University Medical Center were searched for cases of pancreatic and small intestinal NET in which hepatic metastases were surgically excised between July 2002 and August 2016. Eighty-two cases were identified. Cases were excluded if hematoxylin and eosin (H&E)-stained slides were unavailable for review (N=13) or if there was insufficient background liver to evaluate for the presence of micrometastases (N=4), leaving 65 cases for further study. For major hepatectomy specimens (resection of 3 or more segments), representative sections were submitted for histologic examination, whereas for minor hepatectomy or wedge resections, specimens were either submitted totally or subtotally. The average number of slides reviewed per case was 10 (range 1–28 slides).
Two gastrointestinal pathologists (WEG and CS) reviewed H&E-stained slides of liver resection specimens and recorded the presence or absence of micrometastases. Micrometastasis was defined as microscopic tumor foci ≤1 mm in greatest dimension (Figures 1 and 2), as 2 mm was the smallest size that could be identified grossly in the hepatic metastastectomies seen in this cohort. When one or more micrometastases were present in the same section with a grossly visible lesion, they were considered as “micrometastases adjacent to macroscopic metastases,” whereas those in sections without gross liver tumors were considered “micrometastases in background liver.”
Figure 1.
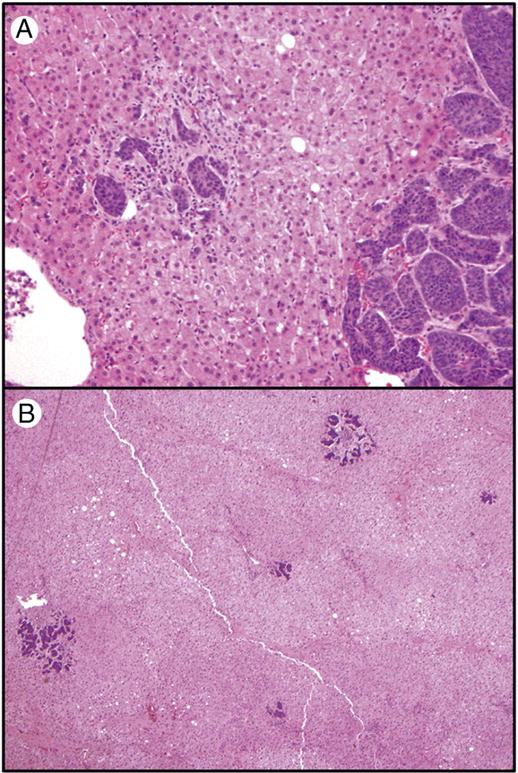
Representative sections of hepatic micrometastases. A. A hepatic micrometastasis (left) adjacent to a macroscopic hepatic metastasis (right) (original magnification 40×); B. Scattered hepatic micrometastases in background liver without macroscopically evident tumor (original magnification 20×).
Figure 2.
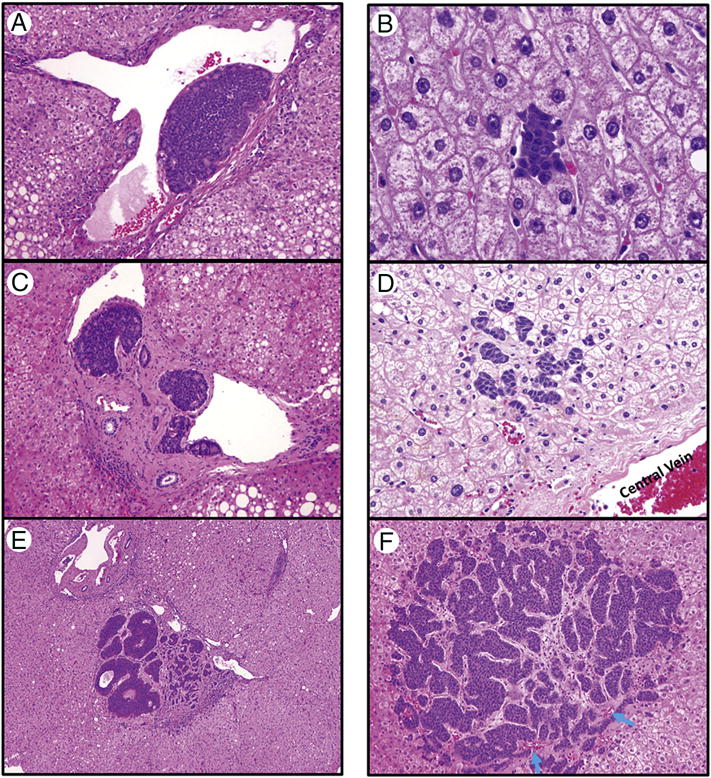
Hepatic micrometastases arising within the portal tracts (A–C) or within the lobules (D–F). A. Vascular invasion showing a tumor cluster within a portal vein (original magnification 200×); B. Tumor cluster within a portal vein and invading the portal tract stroma (original magnification 100×); C. Portal tract micrometastases causing portal expansion and invading periportal hepatic parenchyma (original magnification 40×); D. A small tumor cluster within a sinusoidal space (original magnification 400×); E. Several tumor clusters within sinusoidal spaces (original magnification 200×); F. A large lobular micrometastasis (1 mm) causing desmoplastic change (original magnification 100×). Blue arrows: residual central vein.
Electronic medical records were reviewed for patient demographics, clinical history, and follow-up data, including pre- and post-resection systemic treatment history. The presence of liver metastasis was documented by review of radiology and pathology reports. The surgical margin status (R0, R1, and R2) was determined after reviewing pathology reports of liver resections and operative notes. Pathologic reports and slides were also reviewed for tumor size, focality, primary tumor (pT) category, lymph node (pN) category, lymphovascular invasion, and mesenteric tumor deposits (MTDs). MTDs are a frequent finding in patients with small intestinal NET [16, 17]. Patients were staged according to the 8th edition of the AJCC Cancer Staging Manual[18]. The Ki67 proliferative index of the liver metastases and the primary tumors was available for 63 and 58 of the 65 cases, respectively, via immunohistochemical stains for Ki67 (MIB-1, 1:100, Dako, Carpinteria, CA) performed as part of routine clinical care on representative formalin-fixed, paraffin-embedded tumor sections. This information, along with mitotic rate, was used to grade NETs according to 2010 World Health Organization (WHO) criteria[19].
Fisher’s exact test and Student’s t-test were used to compare clinicopathologic variables between patients with hepatic micrometastases and those without micrometastases, as well as patients with pancreatic NET versus those with small intestinal NET. Associations between micrometastases and other clinicopathologic variables and overall survival after hepatic resection were assessed by log rank tests. Stratified Cox proportional hazard regression was subsequently performed to account for significant confounding variables identified in univariable analysis (WHO grade, margin status, and pre- and post-resection medical therapy). All hypothesis tests were two-sided, with α=0.05. All statistical analyses were performed using Stata v13.1 (Stata Corporation, College Station, TX).
3.1 RESULTS
3.1.1 General clinicopathologic features
The 65 cases included 39 (60%) females and 26 (40%) males, with ages ranging from 19 to 82 years (median age of 59). General clinicopathologic features are listed in Table 1. Most patients presented with hepatic metastasis at initial presentation (55/65, 84%); 69% of them (38/55) underwent concurrent primary resection and metastasectomy. R0 hepatic resection was achieved in approximately half (49%, 32/65) of the patients; 25 of 32 (78%) had intrahepatic recurrence after resection. The seven cases without hepatic recurrence included three small intestinal and four pancreatic NETs; six had a single liver lesion, and one had four liver lesions. Overall, there were nine cases with a single liver lesion at the time of liver metastatecomy; a third of them developed recurrent liver disease. While most pancreatic primaries were non-functional, there were two functional tumors (one gastrinoma and the other secreting adrenocorticotropic hormone). Two patients with pancreatic NET had a hereditary syndrome (one with multiple endocrine neoplasia type 1 and the other with Von Hippel–Lindau disease).
Table 1.
Clinicopathologic features of small intestinal and pancreatic neuroendocrine tumor patients with and without liver micrometastasis
| All cases (n=65) |
Pan-NET (n=22) |
SI-NET (n=43) |
P-value | No micrometastasis (n=29) |
Micrometstasis (n=36) |
P-value | ||
|---|---|---|---|---|---|---|---|---|
| Sex | Female | 39 (60%) | 17 (77%) | 22 (51%) | 0.06 | 18 (62%) | 21 (58%) | 0.80 |
| Male | 26 (40%) | 5 (23%) | 21 (49%) | 11 (38%) | 15 (42%) | |||
| Age (mean ± SD) | 57.5±12.3 | 56.8±13.7 | 57.9±11.8 | 0.74 | 57.5±12.3 | 59.8±11.4 | 0.22 | |
| Stage at diagnosis | III | 10 (15%) | 6 (27%) | 4 (9%) | 0.08 | 5 (17%) | 5 (14%) | 0.74 |
| IV | 55 (85%) | 16 (73%) | 39 (91%) | 24 (83%) | 31 (86%) | |||
| Primary tumor size (mean±SD; cm) (n=56) | 3.1±2.8 | 5.3±3.9 | 2.0±0.8 | <0.01 | 4.2±3.7 | 2.2±1.2 | <0.01 | |
| Primary tumor | Single | 44 (76%) | 17 (89 %) | 27 (69%) | 0.11 | 19 (76%) | 25 (76%) | 1.00 |
| Focality (n=58) | Multiple | 14 (24%) | 2 (11%) | 12(31%) | 6 (24%) | 8 (24%) | ||
| pT category (n=59)* | T1-2 | 9 (15%) | 6 (32%) | 3 (7%) | 0.01 | 5 (20%) | 4 (12%) | 0.10 |
| T3 | 39 (66%) | 13 (68%) | 26 (65%) | 18 (72%) | 21 (62%) | |||
| T4 | 11 (19%) | 0 | 11 (28%) | 2 (8%) | 9 (26%) | |||
| pN category (n=58) | N0 | 8 (14%) | 3 (18%) | 5 (10%) | 0.68 | 5 (21%) | 3 (9%) | 0.26 |
| N1/2 | 50 (86%) | 14 (82%) | 36 (90%) | 19 (79%) | 31 (91%) | |||
| MTD size (cm) (n=35) | 2.4±1.9 | 2.4±1.9 | 3.0±2.7 | 2.1±1.4 | 0.18 | |||
| Primary tumor LVI (n=59) | No | 5 (8%) | 5 (36%) | 0 (0%) | <0.01 | 5 (20%) | 0 (0%) | 0.01 |
| Yes | 54 (92%) | 14 (64%) | 40 (100%) | 20 (80%) | 34 (100%) | |||
| Primary tumor** WHO grade (n=58) | 1 | 37 (64%) | 5 (26%) | 32 (82%) | <0.01 | 14 (56%) | 23 (70%) | 0.41 |
| 2 | 14 (24%) | 7 (37%) | 7 (18%) | 6 (24%) | 8 (24%) | |||
| 3 | 7 (12%) | 7 (37%) | 0 (0%) | 5 (20%) | 2 (6%) | |||
| Liver lesion*** WHO grade (n=63) | 1 | 26 (41%) | 7(32%) | 19 (46%) | 0.03 | 11 (38%) | 15 (44%) | 0.87 |
| 2 | 21 (34%) | 5 (23%) | 16 (39%) | 10 (34%) | 11 (32%) | |||
| 3 | 16 (25%) | 10 (45%) | 6 (15%) | 8 (28%) | 8 (24%) | |||
| Number of liver lesions | ≤5 | 26 (40%) | 12 (54%) | 14 (33%) | 0.11 | 19 (66%) | 7 (19%) | <0.01 |
| >5 | 39 (60%) | 10 (46%) | 29 (67%) | 10 (34%) | 29 (81%) | |||
| Largest liver lesion (cm) | 2.6±2.1 | 2.7±2.8 | 2.5±1.6 | 0.69 | 1.9±0.8 | 3.1±2.6 | 0.02 | |
| Margin status | R0 | 32 (49%) | 15 (68%) | 17 (40%) | 0.04 | 19 (66%) | 13 (36%) | 0.03 |
| R1/R | 2 | 33 (51%) | 7 (32%) | 26 (60%) | 10 (34%) | 23 (64%) | ||
| Systemic treatment | No | 21 (32%) | 4 (18%) | 17 (40%) | 0.04 | 10 (34%) | 11 (31%) | 0.79 |
| Pre | 9 (14%) | 5 (23% | 4 (9%) | 6 (21%) | 3 (8%) | |||
| Post | 22 (34%) | 11 (50%) | 11 (60%) | 11(48%) | 11 (31%) | |||
| Both | 13 (20%) | 2 (9%) | 11 (26%) | 2 (7%) | 11 (31%) | |||
| Micrometast asis | Yes | 36 (55%) | 7 (32%) | 29 (67%) | 0.01 | |||
| No | 29 (45%) | 15 (68%) | 14 (33%) | |||||
PanNET: Pancreatic neuroendocrine tumor; SI-NET: small intestinal neuroendocrine tumor; MTD: mesenteric tumor deposit; LVI: lymphovascular invasion.
: comparing between T1–2 and T3–4;
: comparing WHO grade 1 and WHO grade 2–3;
: comparing WHO grade 1–2 and WHO grade 3.
The pathologic features of the primary tumors are also shown in Table 1. While pancreatic primaries were larger in size (P<0.01) and higher in WHO tumor grade (p<0.01) than small intestinal primaries, small intestinal primaries had higher T categories (P=0.01) and more lymphovascular invasion (P<0.01) than pancreatic primaries. Compared to the small intestinal group, pancreatic liver metastases were more likely to be WHO grade 3 (10/22 [45%] versus 6/41 [15%]; P=0.03). In this series, more patients with small intestinal NET had numerous liver metastases (more than 15–20 lesions) than those with pancreatic NET (16/43 [37%] versus 1/22 [5%]; P=0.01), whereas more patients with pancreatic NET had a single liver lesion than those with small intestinal NET (7/22 [32%] versus 2/43 [5%]; P=0.01).
3.1.2 Hepatic micrometastasis
Hepatic micrometastases were identified in 36 of the 65 (55%) study cases. Micrometastases were seen adjacent to macroscopic metastases (Figure 1A) or in background liver (Figure 1B). Among the 36 cases with micrometastases, 23 had one or more sections from background unremarkable liver without grossly visible lesions. Of these 23 cases, micrometastases were present adjacent to a macroscopic lesion in 13 (56%), micrometastases were seen both adjacent to a grossly visible lesion and in background liver in 8 (35%) cases, and micrometastases were only observed in macroscopically normal background liver in the remaining 2 cases (9%).
Micrometastases were observed within the portal tracts (Figure 2A–C) or in the lobules (Figure 2D–F). Some micrometastases in the portal tracts consisted of tumor cells within the portal vein and those extending beyond the portal vein and invading portal tract stroma (Figure 2B), whereas larger micrometastases could invade the periportal hepatic lobules (Figure 2C). Adjacent to these micrometastases in the portal tracts, tumor clusters were frequently observed within the portal veins (Figure 2A). Lobular micrometastases varied in size even within the same tissue sections, with smaller lesions showing tumor clusters only in sinusoidal spaces (Figure 2D–E) and larger lesions frequently displaying desmoplastic reaction and invading across several hepatic plates (Figure 2F).
In this series, more hepatic micrometastases was seen in small intestinal NET cases (29/43, 67%) than in pancreatic NET cases (7/22, 32%; P=0.01). There were no significant differences in sex, age, AJCC stage at initial diagnosis, primary tumor focality, pT category, pN category, or WHO grade (both primary and metastasis) between patients with or without micrometastases (Table 1). Interestingly, these patients without micrometastasis had larger primary tumors (4.2±3.7cm versus 2.2±1.2 cm; P<0.01), which is probably due to larger primary tumor size of pancreatic NET and more patients with pancreatic primary in the group. In addition, patients with hepatic micrometastasis more likely had lymphovascular invasion identified in the primary tumor (P=0.01) compared to those without.
When comparing pathologic features of primary tumors within the group with small intestinal NET, there were no differences in tumor focality, tumor size, pT category, pN category, presence or absence of MTD, MTD sizes, lymphovascular invasion and WHO tumor grade between patients with and without hepatic micrometastasis.
Patients with hepatic micrometastases had more macroscopically identifiable liver lesions than those without micrometastases (P<0.01; Table 1). The largest tumor resected in patients with hepatic micrometastasis was larger than that from patients without micrometastasis (P=0.02; Table 1). In addition, negative (R0) surgical resection margins were more often achieved in patients without hepatic micrometastases than those with micrometastases (P=0.03; Table 1).
3.1.3 Association of hepatic micrometastases with patient outcome
Log-rank tests for equality of survivor functions showed no differences for anatomic site or origin, sex, or AJCC tumor stage at presentation (Table 2). Statistically significant differences were observed for presence/absence of hepatic micrometastases, WHO histologic grade, hepatic resection margin status, and administration of systemic adjuvant therapy. In univariable analysis, the presence of hepatic micrometastases was associated with poor overall survival (hazard ratio [HR] 3.43; 95% CI 1.14–10.30; P=0.03). The presence of hepatic micrometastases retained independent statistical significance after stratified Cox regression adjusting for WHO histologic grade, surgical resection margin status, and history of systemic therapy (hazard ratio, 4.82 95% CI 1.06–21.79; P=0.04; Figure 3).
Table 2.
Log-rank test for equality of survivor functions
| Events observed | Events expected | P value | ||
|---|---|---|---|---|
| Site | Pan-NET | 6 | 6 | 1.00 |
| SI-NET | 15 | 15 | ||
| Sex | Female | 11 | 11.64 | 0.77 |
| Male | 10 | 9.36 | ||
| Micrometastasis | No | 4 | 9.20 | 0.02 |
| Yes | 17 | 11.80 | ||
| Tumor stage at diagnosis | III | 3 | 1.68 | 0.28 |
| IV | 18 | 19.32 | ||
| WHO grade (liver lesion) | 1 | 5 | 7.17 | <0.01 |
| 2 | 6 | 9.11 | ||
| 3 | 9 | 3.72 | ||
| Margin status | R0 | 8 | 14.06 | <0.01 |
| R1/2 | 13 | 6.94 | ||
| Systemic treatment | Yes | 13 | 16.63 | 0.04 |
| No | 8 | 4.37 | ||
Figure 3.
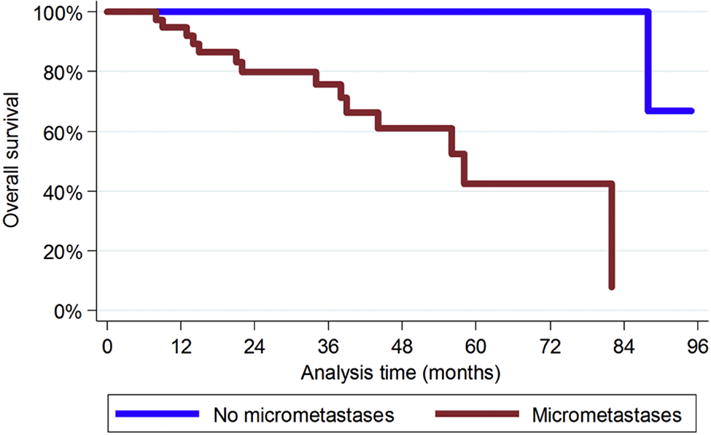
Kaplan-Meier overall survival curves for patients with or without hepatic micrometastases adjusted for WHO grade, surgical resection margin status, and administration of adjuvant therapy.
4.1 DISCUSION
Hepatic metastasis is one of the most significant prognostic factors for patients with small intestinal and pancreatic NETs. Optimal management of metastatic disease may prolong survival and improve quality of life. While debulking surgery is sometimes performed to decrease tumor-related symptoms in patients with metastasis involving both hepatic lobes or diffuse metastasis, curative partial hepatectomy with or without adjuvant radiological-directed therapies is only possible in patients with metastasis within one hepatic lobe or confined to two adjacent segments[13]. In this series, 32 cases underwent margin-negative hepatic resection of between one to ten liver lesions; only seven (22%) had no hepatic recurrence after resection. Almost all patients with more than one liver lesion suffered recurrent hepatic disease after surgical resection.
Through careful pathologic examination of thin slices of hepatic metastasectomy specimens, Elias et al. reported frequent detection of many more lesions than detected preoperatively[20], which corroborates the results of our study. We documented hepatic micrometastases in more than half of all patients who had undergone metastasectomy for metastatic pancreatic or small intestinal NET. These micrometastases were not detected by preoperative imaging studies or by gross examination of the surgical specimens. The presence of micrometastases might explain the clinical observation of frequent tumor recurrence following hepatic resection. In this study, 11 of 25 (44%) patients with intrahepatic recurrence after margin-negative hepatectomy had hepatic micrometastasis, whereas micrometastases were not identified in any of the seven patients who remained disease-free after surgical resection during the follow-up period.
In this study, we observed that more patients with small intestinal NET had numerous liver lesions or hepatic micrometastases, whereas more patients with pancreatic NET had single liver lesions. The differences between two groups may partially contribute to the different criteria for hepatectomy in these patients; palliative liver resection is not commonly done for non-functional pancreatic NETs but it is relatively common in patients with metastatic small intestinal NETs to relieve carcinoid syndrome[21]. The 2016 European Neuroendocrine Tumor Society guidelines recommend surgery with curative intent for patients with “simple” pattern of liver metastasis located in one or two contiguous lobes. Debulking surgery can also be considered in patients with uncontrolled functional tumors, such as carcinoid syndrome, or other symptoms related to tumor burdens [22].
The mechanism of NET micrometastasis in the liver may differ from that of similar patterns of spread of other malignancies. Microscopic tumor growth has been reported in approximately 10% of liver resections from patients with metastatic colorectal cancer, and has been attributed to intrabiliary spread[23]. The frequent observation of tumor clusters in portal veins and sinusoidal spaces in this study instead suggests that hepatic NET metastases are primarily mediated by hematogenous mechanisms. The indolent growth pattern of metastatic NETs allows for identification of these lesions at various stages of development, including micrometastases. In our study, the size of metastatic liver lesions varied greatly within the same patient, ranging from <1 mm to >5 cm.
The frequent presence of hepatic micrometastases has important therapeutic implications. Among the patients in our series undergoing curative surgery, only seven patients (22%) with a limited number (1 to 4) of macroscopic liver lesions achieved complete eradication of liver disease, at least during the observed follow-up intervals. All seven patients had no hepatic micrometastasis. This suggests that surgical resection may not be sufficient, even for patients with an apparently limited extent of disease, especially when hepatic micrometastasis is present in resection specimens. To control tumor growth and prevent new liver lesions from developing, systemic treatment may be more effective. While small liver metastases and micrometastases cannot be detected by somatostatin-receptor targeted imaging studies, they do express somatostatin receptor type 2A[24]. Therefore, peptide receptor radionucleotide therapies targeting somatostatin receptor type 2A may be a promising systemic treatment for metastatic NETs.
In conclusion, hepatic micrometastases are common in patients with metastatic NETs of the pancreas and small intestine and are associated with poor prognosis. These data suggest that complete surgical resection of hepatic metastases is likely impossible in the majority of these patients. Other approaches, such as systemic molecular targeted therapy (e.g. somatostatin-receptor based therapy), may be a more effective alternative.
Highlights.
Hepatic micrometastases are common in liver metastasis from digestive NETs.
Patients with hepatic micrometastases had more liver lesions than those without.
R0 resection was more likely achieved in patients without hepatic micrometastases.
Hepatic micrometastases are associated with poor overall survival.
Acknowledgments
Funding resources: This study is supported by NIH/NIDDK 5P30 DK058404-13 (CS) and NIH/NCI 5P50 CA095103-13 (CS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Viudez A, De Jesus-Acosta A, Carvalho FL, Vera R, Martin-Algarra S, Ramirez N. Pancreatic neuroendocrine tumors: Challenges in an underestimated disease. Crit Rev Oncol Hematol. 2016;101:193–206. doi: 10.1016/j.critrevonc.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Mocellin S, Nitti D. Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n = 25 531) Ann Oncol. 2013;24:3040–4. doi: 10.1093/annonc/mdt377. [DOI] [PubMed] [Google Scholar]
- 5.Viudez A, Carvalho FL, Maleki Z, Zahurak M, Laheru D, Stark A, Azad NZ, Wolfgang CL, Baylin S, Herman JG, et al. A new immunohistochemistry prognostic score (IPS) for recurrence and survival in resected pancreatic neuroendocrine tumors (PanNET) Oncotarget. 2016;7:24950–61. doi: 10.18632/oncotarget.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan AT, Halperin DM, Chan JA, Fogelman DR, Hess KR, Malinowski P, Regan E, Ng CS, Yao JC, Kulke MH. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol. 2015;16:695–703. doi: 10.1016/S1470-2045(15)70136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464–73. doi: 10.1111/j.1572-0241.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 8.Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:691–703. doi: 10.1016/j.bpg.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013;31:420–5. doi: 10.1200/JCO.2012.44.5924. [DOI] [PubMed] [Google Scholar]
- 10.Chan JA, Kulke MH. Neuroendocrine Tumors–Current and Future Clinical Advances. Hematol Oncol Clin North Am. 2016;30:xiii–xiv. doi: 10.1016/j.hoc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Farley HA, Pommier RF. Treatment of Neuroendocrine Liver Metastases. Surg Oncol Clin N Am. 2016;25(1):217–25. doi: 10.1016/j.soc.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases. Cancer. 2015;121:1172–86. doi: 10.1002/cncr.28760. [DOI] [PubMed] [Google Scholar]
- 13.Zappa M, Abdel-Rehim M, Hentic O, Vullierme MP, Ruszniewski P, Vilgrain V. Liver-directed therapies in liver metastases from neuroendocrine tumors of the gastrointestinal tract. Target Oncol. 2012;7:107–16. doi: 10.1007/s11523-012-0219-8. [DOI] [PubMed] [Google Scholar]
- 14.Auernhammer CJ, Goke B. Therapeutic strategies for advanced neuroendocrine carcinomas of jejunum/ileum and pancreatic origin. Gut. 2011;60:1009–21. doi: 10.1136/gut.2009.204453. [DOI] [PubMed] [Google Scholar]
- 15.John BJ, Davidson BR. Treatment options for unresectable neuroendocrine liver metastases. Expert Rev Gastroenterol Hepatol. 2012;6:357–69. doi: 10.1586/egh.11.60. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez RS, Liu EH, Alvarez JR, Ayers GD, Washington MK, Shi C. Should mesenteric tumor deposits be included in staging of well-differentiated small intestine neuroendocrine tumors? Mod Pathol. 2014;27:1288–1295. doi: 10.1038/modpathol.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fata CR, Gonzalez RS, Liu E, Cates JM, Shi Cl. Mesenteric Tumor Deposits in Midgut Small Intestinal Neuroendocrine Tumors Are a Stronger Indicator Than Lymph Node Metastasis for Liver Metastasis and Poor Prognosis. Am J Surg Pathol. 2017;41:128–133. doi: 10.1097/PAS.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, editors. AJCC cancer staging manual. 8th. Springer; New York, NY S: 2017. [Google Scholar]
- 19.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of Tumours of the Digestive System. 4th. IARC; Lyon, France: 2010. [Google Scholar]
- 20.Elias D, Lefevre JH, Duvillard P, Goere D, Dromain C, Dumont F, Baudin E. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann Surg. 2010;251:307–10. doi: 10.1097/SLA.0b013e3181bdf8cf. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Cheow PC, Teo JY, Ooi LL. Surgical treatment of neuroendocrine liver metastases. Int J Hepatol. 2012;2012:146590. doi: 10.1155/2012/146590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, Öberg K, all other Vienna Consensus Conference participants ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172–85. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 23.Estrella JS, Othman ML, Taggart MW, Hamilton SR, Curley SA, Rashid A, Abraham SC. Intrabiliary growth of liver metastases: clinicopathologic features, prevalence, and outcome. Am J Surg Pathol. 2013;37:1571–9. doi: 10.1097/PAS.0b013e318293ddf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charoenpitakchai M, Liu E, Zhao Z, Koyama T, Huh WJ, Berlin J, Hande K, Walker R, Shi C. In liver metastases from small intestinal neuroendocrine tumors, SSTR2A expression is heterogeneous. Virchows Arch. 2017;470:545–52. doi: 10.1007/s00428-017-2093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


