Abstract
Cardiovascular Disease (CVD) and related disorders remain a leading cause of health disparities and premature death for African Americans. Hypovitaminosis D is disproportionately prevalent in African Americans and has been linked to CVD and CVD risk factors including hypertension, diabetes, and obesity. Thus, hypovitaminosis D may represent a common pathway influencing CV risk factors in a select subgroup of persons. The purpose of this paper is to report the study design of a prospective eight week prospective double-blind randomized, placebo-controlled trial (n = 330 allocated 2:1 to intervention vs. control) to assess the effect of placebo vs. high-dose oral cholecalciferol (100,000 IU vitamin D3 at baseline and week 2) on 6-week change of select biologic cardiometabolic risk factors (including parathyroid hormone to assess biologic activity, pro-inflammatory/pro-thrombotic/fibrotic markers, insulin sensitivity and vitamin D metabolites) and their relationship to vitamin D administration and modification by vitamin D receptor polymorphisms in overweight, hypertensive African Americans with hypovitaminosis D. Findings from this trial will present insights into potential causal links between vitamin D repletion and mechanistic pathways of CV disease, including established and novel genomic markers.
Keywords: Vitamin D, randomized controlled trial, nutrigenomics, race, disparities, African American, cardiovascular disease
Introduction
Cardiovascular Disease (CVD) and related disorders remain the leading cause of death in the nation1. Hypovitaminosis D has been linked to several CV risk factors including hypertension, diabetes, obesity2,3, in addition to increased rates of CVD2,4,5. Racial/ethnic minorities suffer from increased rates of both premature CV events and hypovitaminosis D6. While hypovitaminosis D is highly prevalent with an estimated 55% of the US adult population having levels at or below 30 ng/ml, of significance over 80% of African Americans have suboptimal values7. Thus, hypovitaminosis D may represent a common pathway for a select subgroup of persons with a clustering of CV risk factors in a profile that is more prevalent among racial/ethnic minorities.
Although there has been an overall decline in cardiovascular disease (CVD) mortality over the past century, racial/ethnic minorities continue to suffer a disproportionate burden of CVD morbidity and mortality1. The explosive increase over the past few decades in the prevalence of many established (e.g., obesity, diabetes, lipid disorders) and putative CV risk factors (e.g., hypovitaminosis D), many of which disproportionately affect minority populations, threatens to maintain the high rates of CV related mortality and widen the racial disparity in CVD.
The transition from an agrarian to a digital society has contributed to both a limited average daily sun exposure and to a relatively unrecognized epidemic of hypovitaminosis D6. Hypovitaminosis D has been linked to several CV risk factors, including hypertension, insulin-resistance, dyslipidemia, and obesity4. Several observational studies have noted that persons with vitamin D levels in the low normal range exhibit a greater prevalence of CVD risk factors as well as CV events compared with those with normal vitamin D levels3,8–10. Prior studies have shown vitamin D analogs lower blood pressure in hypertensive subjects11,12, while a recent Mendelian randomization study found increased plasma 25(OH)D levels might reduce the risk of hypertension13. However, the precise mechanism for this putative antihypertensive effect is unknown although it may be mediated in part through down-regulation of the renin-angiotensin-aldosterone system (RAAS)14, as well as inhibition of pro-inflammatory and pro-fibrotic pathways (5,15).
Even more intriguing is reports suggesting treatment with active vitamin D [1,25(OH)2D3] or vitamin D analogs reduce CV mortality in dialysis patients4,16. However, to date the few controlled intervention studies that have examined the role of vitamin D repletion on CV function or CV mediators have reported mixed results17–24. To better understand the potential mechanism(s) through which Vitamin D repletion may mediate CV risk in persons with hypovitaminosis, we performed a randomized controlled trial to assess the impact of vitamin D repletion on key biologic mediators of CV health, as well as PTH to assess the biologic activity of Vitamin D, in a cohort of participants with hypovitaminosis D and increased risk for premature CVD.
Primary Research Goals
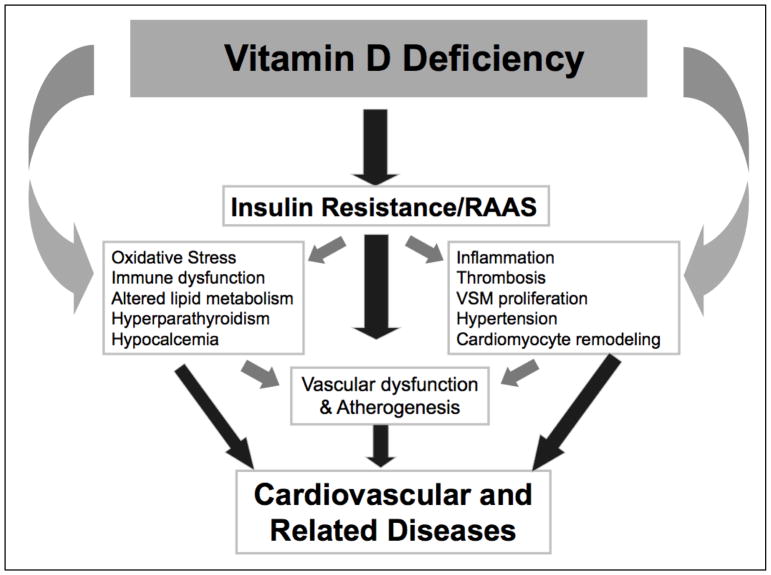
Numerous studies have demonstrated that hypovitaminosis D is associated with activation of select vasconstrictive and hypertrophic mediators that lead to endothelial dysfunction, elevated blood pressure and CVD (Figure 1)18,25,26. Thus, the proposed project tests the hypothesis that in high-risk persons with hypovitaminosis D, administration of vitamin D will improve the profile of biologic markers of CVD and CVD risk (including parathyroid hormone, pro-inflammatory/pro-thrombotic/fibrotic markers, insulin sensitivity and vitamin D metabolites).
Figure 1.
Conceptual model of the relationship between vitamin D and cardiovascular disease. RAAS, renin angiotensin-aldosterone system; VSM, vascular smooth muscle. Adapted from5.
The nuclear Vitamin D Receptor (VDR) regulates parathyroid hormone (PTH) gene transcription. Therefore, the plasma PTH level serves as a sensitive biomarker of the Vitamin D nutri-genomic response. By characterizing the nutri-genomic response of Vitamin D supplementation on the Vitamin D-PTH relationship in African-Americans, we can compare the clinical and laboratory characteristics of the sub-set of African-Americans that exhibit a weak or null response to Vitamin D supplementation to those how manifest a strong response. The secondary goal is to define the biological markers of CV risk as well as the multivariate determinants (covariates such as age, BMI, baseline Vitamin D level and dietary calcium) of the Vitamin D-PTH level relationship in African-Americans.
Rationale for Recruitment of African American Participants
Self-reported “race” is a social construct with a problematic history of using superficial characteristics such as skin color to “categorize” human beings. Race reflects culture, history, socioeconomics, and political status, juxtaposed with epigenetic changes due to differing psychological and physical environmental influences and differing frequencies of select genetic variants that reflect important linkages to ancestral geographic origins27,28. For example, the association of varying frequencies of select gene variants by self-reported race is associated with increased risk for kidney disease in African Americans29,30, as well as the reduced risk for osteoporosis and bone fractures31.
In the U.S., African Americans or Blacks suffer from disproportionately high rates of CV risk factors and premature CV morbidity and mortality32. Analyses from the National Health and Nutrition Examination Surveys (NHANES) have established excess rates of both hypovitaminosis D and cardiovascular risk factors among African Americans using “race/ethnicity” as a self-reported variable3. Self-report of “race” as Black or African Americans identifies a population with increased skin pigmentation, which is known to attenuate the capacity of individuals to generate vitamin D as a result of exposure to UV light6,33. Accordingly, African Americans are particularly predisposed to vitamin D insufficiency6, although the clinical impact of vitamin D repletion in African Americans with low vitamin D levels is unknown.
Study Design
The trial was conducted using a prospective, randomized, double-blind, placebo-controlled approach across two clinical sites, Morehouse School of Medicine and Charles R. Drew University of Medicine and Science, with data management by the National Institutes of Health (NIH) Research Centers in Minority Institutions (RCMI) Translational Research Network (RTRN) Data and Technology Coordinating Center34.
Eligibility and Study Population
Of 2396 potentially eligible prescreened participants, 885 attended the eligibility-screening visit. From these 330 eligible participants were randomized. Based on preliminary results of a prior clinically focused study35, the inclusion values for the serum Vitamin D levels (D2 and D3 level) were set between 5 and 25 ng/ml. The limits of body mass index (BMI) were > 25 kg/m2 and < 45 kg/m2. Subjects not treated for controlled hypertension needed a systolic blood pressure (SBP) ≥ 120 mmHg and ≤ 160 mmHg, and diastolic blood pressure (DBP) ≥ 80 mmHg and ≤ 100 mmHg, while those treated for hypertension SBP needed to be ≤ 160 mmHg and DBP ≤ 100 mmHg. Subjects with diabetes were allowed in the study if HbA1c ≤ 8.5%. Participants were recruited from their local communities at health fairs, churches, university campuses and other community locations, as well as through flyers and direct referrals. A summary of inclusion/exclusion criteria is detailed in Table 1.
Table 1.
Major inclusion/exclusion criteria
| Inclusion | Exclusion |
|---|---|
| Males or females, 18–70 years of age and self-identified as African American or Black | Not African American or Black |
| Screening vitamin D (D2 and D3 level) between 5 and 25 ng/ml (recommended level ≥ 30 ng/ml) | Screening vitamin D (D2 and D3 level) < 5 ng/ml or > 25 ng/ml |
| High normal blood pressure or controlled hypertension: If a potential study patient was not on treatment their systolic blood pressure (SBP) needed to be ≥ 120 mmHg or diastolic blood pressure (DBP) ≥ 80 mmHg, and SBP ≤ 160 mmHg and DBP ≤ 100 mmHg. If a potential study patient was on treatment for hypertension the SBP needed to be ≤ 160 mmHg and DBP ≤ 100 mmHg. |
Poorly controlled high blood pressure on treatment (SBP > 160 mmHg or DBP > 100 mmHg) |
| Subjects requiring treatment with other vitamin D preparations containing more than 400 IU of vitamin D | |
| Patients who, in the opinion of the Investigator, had a condition which would interfere with their evaluation or may experience an unacceptable health risk by participating in this study* | |
| Body mass index ≥ 25 kg/m2 and ≤ 45 kg/m2 | Body mass index < 25 kg/m2 or > 45 kg/m2 |
| HbA1c > 8.5% |
estimated glomerular filtration rate (eGFR) < 45 ml/min, evidence of disease that could result in hypercalcemia, history of kidney stones (less than one year prior to screening), history of drug, alcohol, or illicit substance abuse (within the past 6 months), recent (<6 months) myocardial infarction, stroke, or hospitalization for congestive heart failure and liver function tests (LFTs) greater than twice the upper limit of normal.
Informed Consent
The project was approved by the Morehouse School of Medicine, Charles R. Drew University of Medicine and Science and Jackson State University Institutional Review Boards, and was registered with ClinicalTrials.gov:NCT02802449. To monitor the study progress a Data and Safety Monitoring Board (DSMB) met on quarterly basis via teleconference (and ad hoc as needed) to review/discuss any adverse events. Regardless of causality, each site reported Serious Adverse Events (SAEs) to their IRB and the DSMB within 24 hours of knowledge of the event for all SAEs occurring from screening through the last participant’s assessment. The DSMB made recommendations regarding the continuation status of the protocol and oversaw all unmasking for the trials. Written, signed and dated informed consent to participate in each trial was given by the subject, in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines, before completing any study-related activities/procedures. The original signed and dated consent is kept in the subject’s research file and a copy was given to the subject. A copy was also placed in participant’s source document, while ineligible subject’s consent forms were also filed.
Trial Procedure
Table 2 represents the schedule of major study events per study visit. Pre-screening for eligibility was conducted with a short survey asking potential participants about the general information (name, age, race, gender, contact information, etc.) as well as information about general health, current medications, and past participation in other similar research. After prescreening, participants determined to be potentially eligible attended a screening visit (Visit 1). After signing the informed consent, research staff verified inclusion criteria through assessment of select blood test, BP measurements and anthropometric data. Participants determined to be eligible were randomized to either the vitamin D or placebo group by blocked randomization to achieve balance between study arms and to reduce the opportunity for bias and confounding. Instead of using single block size, we used random block sizes (6, 9, and 12) to keep the investigators blind to the size of each block. For this study, the unblinded biostatistician created a computer-generated list of Randomization Numbers allocating subjects in a 2:1 ratio to receive Vitamin D3 or placebo. For each of the two sites, a separate Randomization Table was developed. The study statistician provided the random allocation sequence to the licensed study pharmacist who prepared the study medication and placebo. Neither the pharmacist nor statistician had contact with participants. Eligible participants underwent a baseline visit (Visit 2) within 2 weeks from visit 1 (screening visit), where a series of baseline measures were obtained, including BP, anthropometry and food frequency questionnaires, urine and fasting blood. Participants were then given either vitamin D3 100,000 IU or blinded placebo under direct observation. Two weeks after the baseline visit (Visit 3), the second dose of study drug or placebo was given to participants again under direct observation. Additional follow-up visits were performed at week 6 (Visit 4), and at the end of study visit on week 8 (Visit 5) where participants were provided their results of general laboratory tests. A visit window of up to 7 days was allowed. Participant recruitment started on March 31, 2014, and the follow-up of the last randomized cohort ended on January 20, 2016.
Table 2.
Schedule of Major Study Events per Visit
| Visit 1 (Screening) | Visit 2 (Baseline) | Visit 3 (Week 2 from baseline visit) | Visit 4 (Week 6 from baseline) | Visit 5 (week 8 from baseline) | |
|---|---|---|---|---|---|
| Informed Consent | X | ||||
| Checking Inclusion/exclusion criteria | X | X | X | X | |
| Demographics | X | ||||
| Medical history and Review of Medications | X | X | X | X | |
| Mini Physical Examination [including Height (cm), Weight (kg), and Waist Circumference (cm) (calculated body mass index)] | X | X | |||
| Vital Signs (Sitting blood pressure, heart rate) | X | X | X | X | X |
| Randomization | X | ||||
| Full Lab Panel (Biochemistry and Complete Blood Count) | X | X | |||
| Fasting blood sugar (mg/dl) or HbA1c (%) | X | X | |||
| Serum intact parathyroid hormone (iPTH) (pg/ml) and 1-84 PTH/7-84 PTH ratio) | X | X | |||
| Blood for serum calcium (mg/dl), phosphorus (mg/dl), albumin (mg/dl) | X | X | X | ||
| C-reactive protein (CRP), interleukin 6 (IL-6), adiponectin, plasminogen activator inhibitor 1 (PAI-1), homocysteine, Homeostatic model assessment insulin resistance (HOMA) | X | X | |||
| Serum vitamin D binding protein (VDBP) | X | X | |||
| Blood and DNA STORED for other biomarkers (e.g. pro-inflammatory/pro-thrombotic/fibrotic markers), vitamin D binding protein polymorphisms | X | X | |||
| Serum vitamin D [25(OH)D = D2 + D3] (ng/ml) | X | X | X | ||
| Spot urine for calcium/creatinine and albumin/creatinine ratios and isoprostanes | X | X | |||
| Pregnancy Test | X (serum) | X (urine) | X (urine) | X (urine) | |
| Study drug dispensed | X | X | |||
| Adverse event | X | X | X | X | |
| Concomitant Medication (including prescription, herbal, and over-the-counter medications) | X | X | X | X | |
| Nutritional Survey | X | ||||
| Drug Accountability | x | x |
Intervention
A prospective eight week double-blind randomized, placebo-controlled trial (n = 330 allocated 2:1 to intervention vs. control to assess the effect of high-dose oral cholecalciferol (one dose of 100,000 IU vitamin D3 at baseline and one dose at week 2 given under direct observation) on select biologic and genetic responses in overweight, hypertensive African Americans with hypovitaminosis D.
Procedure for Clinical and Laboratory Measures
As seen in Table 2, blood and urine samples were collected in Clinical Research Centers at each site at baseline (Visit 2) and week 6 (Visit 4). Quest Diagnostic Laboratory analyzed most specimens including serum vitamin D levels, calcium, phosphate, glucose, insulin, intact parathyroid hormone (iPTH), insulin and spot urine microalbumin/creatinine ratio. The Analytical Core Laboratory of Morehouse School of Medicine analyzed urine 8-isoprostane by using the competitive immunoassay for the quantitative determination of free 8-iso-Prostaglandin F2α (8-iso-PGF2α ELISA kit, Enzo Life Sciences Inc.) and serum homocysteine, and glutathione levels by high-pressure liquid chromatography (HPLC). Additional blood samples (whole blood, serum and plasma) and urine samples were stored for future assessment of select biomarkers such vitamin D binding protein polymorphisms, vitamin D metabolite ratios (e.g. serum 24,25(OH)2D to 25D) and epigenetic analyses.
Routine sitting blood pressure was measured three times at each visit with a Tycos Classic Hand Aneroid device or a validated, automated sphygmomanometer in a standardized fashion as recommended by the American Heart Association. Anthropometric measurements included height, weight, and waist circumference. Both weight and height readings were used to generate body mass index expressed as kg/m2. A self-administered, 126-item semi-quantitative food-frequency questionnaire (Harvard 2007 FFQ) was used to estimate nutrient intake (protein, fats, carbohydrates, vitamins, minerals). (https://regepi.bwh.harvard.edu/health/nutrition.html).
Site clinical research staff were trained in good clinical practices and designated staff were trained by the RTRN Data and Technology Coordinating Center to enter data into the electronic case report form utilizing a web-based electronic data capture system.
Outcome Measures
The primary outcomes include change in key markers of cardiovascular risk including interleukin [IL]-6, adeponectin, C-reactive protein [CRP], homocysteine, urinary isoprostane and plasminogen activator inhibitor-1 [PAI-1], and insulin sensitivity (Homeostatic model assessment insulin resistance (HOMA-IR) using an intent-to-treat design. Secondary analyses include changes in markers of cardiovascular risk, insulin sensitivity within the Vitamin D treatment group stratified by nutrient resistant and nutrient sensitive based on parathroid hormone (1-84 PTH; intact PTH) response. Serum vitamin D level will be measured using high performance liquid chromatography tandem mass spectrometry (LC-MS/MS) which is sensitive and specific for both 25(OH)D2 and 25(OH)D3. Concentrations of each form of Vitamin D will be measured and reported independently as well as summed together to give the total Vitamin D level to allow exporation of any differential responses based on levels of Vitamin D2/D3, D2 or D3.
Statistical Analyses
Baseline characteristics of the study samples will be summarized by the use of two sample t-tests for continuous variables and chi-square tests for categorical variables. In addition to simple statistics (percentage, mean, p-value), we will provide 95% confidence interval, which will enable to better assess the level of evidence for or against the treatment effect since they provide a range of plausible values for the unknown true difference between the groups (Yuko, 2015). Most statistical analyses will be performed using SAS version 9.1.2. To investigate the effect of Vitamin D supplementation on select biomarkers (i.e. C-reactive protein, IL-6, Adiponectin, etc.), we will determine group difference in 6-week change of outcome variables. We will use a generalized mixed model, which is well suited for longitudinal data analysis using repeated measures. In this mixed model, the cluster (or class) variable is subject ID and the random variables are intercept and slope, implying that both the intercept and slope vary across the subjects. If the interaction between study arms and visit is significant, then we can conclude that the change rate of outcome variable (CRP or Adiponectin) differs between groups. We will include Site ID as a covariate in the analytical model to control for potential differential effects across sites.
To characterize nutri-genomic response of Vitamin D supplementation, we will determine the moderating effect of select baseline predictor variables of age, BMI, baseline Vitamin D and dietary calcium on the Vitamin D-PTH relationship. A generalized linear model will be used in which the primary outcome is change in PTH level and the main predictor variable is change in vitamin D level. Moderating effect of select baseline predictor on vitamin D-PTH relation will be investigated by testing the significance of appropriate interactions between each of baseline predictor variables and change of vitamin D level.
The final sample size of 330 was selected to detect at least medium effect size (0.23–0.31) of group difference in changed outcome in a design with 2 repeated measurements having a Compound Symmetry covariance structure when the correlation between observations on the same subject is 0.3, the alpha level is 0.05, and power is assumed to be 80%. Our assumption in conducting power analysis (i.e., covariance structure of compound symmetry and 0.3 of intraclass correlation coefficient), was based on the data from our pilot study.35 Once 40% of the planned samples were collected, we conducted an interim analysis to verify and fine-tune the planned sample size, resulting in the appropriateness of planned samples. The oversampling of the treated arm in the trial (n= 220) was done to ensure appropriate power to determine the interaction effect of select covariates on the Vitamin D-PTH relation (and potentially Vitamin D metabolite ratios) among only those who took the Vitamin D3 supplement, which will be used for the secondary goal to assess the response of CV biomarkers based on repletion of Vitamin D in the setting of physiologic evidence of low Vitamin D. Based on the algorithm proposed by Wahlsten36, the final treated sample of 220 achieved 90% power at an alpha of 0.05 to detect a least assumed effect size of 0.20 (which was based on the results from our pilot study35) in a 2 × 2 factorial design with a hypothesized interaction term between the two factors [e.g., changed vitamin D level (low vs. high) and serum calcium level (low vs. high)]. Although dropouts were infrequent due to the short duration of the intervention, the effect of missing data on the results will be examined by using a pattern mixture model, which includes a variable indicating the missing pattern as a covariate. Before we conduct data analyses, we will define the pattern of missing values and dropouts and then choose the appropriate imputation method. It is known that, for studies with high missing values in repeated measure study design, the mixed model without imputation is more powerful than other imputation methods. The multiple imputation method is also advantageous for dealing with missing values in repeated measure design. Therefore, we will conduct sensitivity analysis to compare each method (mixed model vs. multiple imputation). All statistical models will include Site ID as a covariate to control for the potential of differential effects across sites (i.e., heterogeneity due to unmeasured confounding or effect-modifying factors). If needed, we will conduct analyses stratified by sites to account for potential interactions. Detail statistical analysis plan and approaches to address different methods of analysis, definitions of outcomes, protocol deviations, missing data, sensitivity analyses, and outliers will be documented in the statistical analysis plan (SAP) before data analysis is performed.
The pooled dataset across sites would be expected to increase statistical power, allow for a more refined set of subgroup analyses, enhanced generalizability and the capacity to undertake comparison, cross validation or replication across datasets37,38. We will also assess the impact of key assumptions or variations on the overall conclusions of a study including different methods of analysis, definitions of outcomes, protocol deviations, missing data, and outliers39–41.
Discussion
Hypovitaminosis D is a silent and emerging epidemic42,43 that has been associated with multiple medical conditions, especially cardiometabolic disorders44, which is not surprising given the ubiquitous nature of the vitamin D receptor45. Despite multiple advances in our understanding of the basic mechanisms of vitamin D and mounting clinical data, it seems we know less and less2, while the need for identifying the optimal intake of vitamin D remains ever present33. From a clinical perspective there is a need for more trials to comprehensively examine the clinical impact of vitamin D supplementation in persons with hypovitaminosis D in a prospective, randomized fashion. A recent large study of 100,000 IU of vitamin D or placebo given monthly for a median of 3.3 years found no difference in CV outcomes46. However mean baseline vitamin D levels were only slightly low at 26.5 ng/ml. Consideration of the differential action of Vitamin D by race/ethnicity47–49 and/or acting in concert with or a modifier of other systems (such as inflammatory, fibrotic, oxidative stress or renin-angiotensin pathways) also need to be considered25,50–54. Substantial racial/ethnic heterogeneity was noted in the association of vitamin D and cardiovascular outcomes with a significant correlation found in Whites but not Blacks in the Multi-Ethnic Study of Atherosclerosis47,55. Further, while serum 25D levels in the range of 12–15 to 20ng/ml may lead to adverse outcomes in the general population, it may not have deleterious affects in African Americans possibly due to differences in the prevalence of select VDBP polymorphisms49. However recent advances in our understanding of free 25D levels being associated with similar vitamin D metabolite ratios (e.g. serum 24,25(OH)2D to 25D) in African Americans and Whites suggests vitamin D metabolite ratios may be a better physiologic indicator of vitamin D adequacy56.
In summary, vitamin D supplementation may have differential effects in varying settings and populations. The project described herein will help to move us toward a better understanding of vitamin D in a high risk population, African Americans with hypovitaminosis D. We believe the findings from our trial will provide critical insights into the mechanism of action of vitamin D in a cohort of individuals at greatest risk for untoward effects of hypovitaminosis D and move us closer to a better understanding of what subsets of individuals are most likely to benefit or not from normalization of serum vitamin D levels.
Acknowledgments
Funding/Support:
The authors are supported by the research grants from the NIH including: U54MD008149 (KN, MEB, YM, DM, JL), 3U54RR022762-03S4 (KN, YM, DM, GG), P20-MD000182 (KN, DM), UL1TR000124 (KN), and P30AG021684 (KN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank the Morehouse School of Medicine and Charles R. Drew University of Medicine and Science clinical research nurses and study coordinators including Jan Morgan-Billingslea, Halima Garba, Caroline Farodolu and Laurice Pitts, as well as the National Institutes of Health (NIH) Research Centers in Minority Institutions (RCMI) Translational Research Network (RTRN) Data and Technology Coordinating Center data management team including Alnida M. Ngare, M. Theresa Perry and Andrew L. Dent, II. Finally, a special thanks to Dr. Michael Sayre for his editorial inputs and overall support of the study, and to Dr. Naureen Tareen and all of the patients who agreed to participate in this trial.
Footnotes
Financial Disclosures:
None of the authors declare any relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Scott MG, Gronowski AM, Reid IR, Holick MF, Thadhani R, Phinney K. Vitamin D: the more we know, the less we know. Clinical chemistry. 2015;61(3):462–465. doi: 10.1373/clinchem.2014.222521. [DOI] [PubMed] [Google Scholar]
- 3.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Archives of internal medicine. 2007;167(11):1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 4.Wolf M, Betancourt J, Chang Y, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. Journal of the American Society of Nephrology: JASN. 2008;19(7):1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clinical journal of the American Society of Nephrology: CJASN. 2009;4(9):1515–1522. doi: 10.2215/CJN.02260409. [DOI] [PubMed] [Google Scholar]
- 6.Artaza JN, Contreras S, Garcia LA, et al. Vitamin D and cardiovascular disease: potential role in health disparities. Journal of health care for the poor and underserved. 2011;22(4 Suppl):23–38. doi: 10.1353/hpu.2011.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Archives of internal medicine. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Archives of internal medicine. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 10.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Archives of internal medicine. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. The Journal of clinical endocrinology and metabolism. 2001;86(4):1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 12.Lind L, Lithell H, Skarfors E, Wide L, Ljunghall S. Reduction of blood pressure by treatment with alphacalcidol. A double-blind, placebo-controlled study in subjects with impaired glucose tolerance. Acta medica Scandinavica. 1988;223(3):211–217. [PubMed] [Google Scholar]
- 13.Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. The lancet Diabetes & endocrinology. 2014;2(9):719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang T, Xu C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. Journal of the American Society of Nephrology: JASN. 2017;28(4):1040–1049. doi: 10.1681/ASN.2016070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. The Journal of endocrinology. 2009;200(2):207–221. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(12):2724–2734. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welles CC, Whooley MA, Karumanchi SA, et al. Vitamin D deficiency and cardiovascular events in patients with coronary heart disease: data from the Heart and Soul Study. American journal of epidemiology. 2014;179(11):1279–1287. doi: 10.1093/aje/kwu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy Vanga S, Good M, Howard PA, Vacek JL. Role of vitamin D in cardiovascular health. The American journal of cardiology. 2010;106(6):798–805. doi: 10.1016/j.amjcard.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Chin K, Appel LJ, Michos ED. Vitamin D, Calcium, and Cardiovascular Disease: A”D”vantageous or “D”etrimental? An Era of Uncertainty. Current atherosclerosis reports. 2017;19(1):5. doi: 10.1007/s11883-017-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMullan CJ, Borgi L, Curhan GC, Fisher N, Forman JP. The effect of vitamin D on renin-angiotensin system activation and blood pressure: a randomized control trial. Journal of hypertension. 2017;35(4):822–829. doi: 10.1097/HJH.0000000000001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PloS one. 2012;7(5):e36617. doi: 10.1371/journal.pone.0036617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gepner AD, Colangelo LA, Blondon M, et al. 25-hydroxyvitamin D and parathyroid hormone levels do not predict changes in carotid arterial stiffness: the Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(5):1102–1109. doi: 10.1161/ATVBAHA.113.302605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valina-Toth AL, Lai Z, Zhang S, Flack JM. Vitamin D and Parathyroid Hormone Relationships with Urinary Nitric Oxide Metabolites and Plasma Isoprostanes in African-Americans. Cardiorenal medicine. 2012;2(3):234–242. doi: 10.1159/000339942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valina-Toth AL, Lai Z, Yoo W, Abou-Samra A, Gadegbeku CA, Flack JM. Relationship of vitamin D and parathyroid hormone with obesity and body composition in African Americans. Clinical endocrinology. 2010;72(5):595–603. doi: 10.1111/j.1365-2265.2009.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IM, Norris KC, Artaza JN. Vitamin D and Cardiac Differentiation. Vitamins and hormones. 2016;100:299–320. doi: 10.1016/bs.vh.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and supplementation and relation to cardiovascular health. The American journal of cardiology. 2012;109(3):359–363. doi: 10.1016/j.amjcard.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Laster M, Norris KC. Lessons Learned in Mortality and Kidney Transplant Outcomes Among Pediatric Dialysis Patients. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2017010017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norris KC, Williams SF, Rhee CM, et al. Hemodialysis Disparities in African Americans: The Deeply Integrated Concept of Race in the Social Fabric of Our Society. Seminars in dialysis. 2017 doi: 10.1111/sdi.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science (New York, NY) 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman BI, Divers J, Palmer ND. Population ancestry and genetic risk for diabetes and kidney, cardiovascular, and bone disease: modifiable environmental factors may produce the cures. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013;62(6):1165–1175. doi: 10.1053/j.ajkd.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman BI, Register TC. Effect of race and genetics on vitamin D metabolism, bone and vascular health. Nature reviews Nephrology. 2012;8(8):459–466. doi: 10.1038/nrneph.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. The American journal of clinical nutrition. 2007;85(3):649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 34.Fleming ES, Perkins J, Easa D, et al. Addressing health disparities through multi-institutional, multidisciplinary collaboratories. Ethnicity & disease. 2008;18(2 Suppl 2):S2-161–167. [PMC free article] [PubMed] [Google Scholar]
- 35.Martins D, Meng YX, Tareen N, et al. The Effect of Short Term Vitamin D Supplementation on the Inflammatory and Oxidative Mediators of Arterial Stiffness. Health (Irvine Calif) 2014;6(12):1503–1511. doi: 10.4236/health.2014.612185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahlsten D. Sample size to detect a planned contrast and a one degree-of-freedom interaction effect. Psychological Bulletin. 1991;110(3):587. [Google Scholar]
- 37.Spjuth O, Krestyaninova M, Hastings J, et al. Harmonising and linking biomedical and clinical data across disparate data archives to enable integrative cross-biobank research. European journal of human genetics: EJHG. 2016;24(4):521–528. doi: 10.1038/ejhg.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fortier I, Burton PR, Robson PJ, et al. Quality, quantity and harmony: the DataSHaPER approach to integrating data across bioclinical studies. International journal of epidemiology. 2010;39(5):1383–1393. doi: 10.1093/ije/dyq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Souza RJ, Eisen RB, Perera S, et al. Best (but oft-forgotten) practices: sensitivity analyses in randomized controlled trials. The American journal of clinical nutrition. 2016;103(1):5–17. doi: 10.3945/ajcn.115.121848. [DOI] [PubMed] [Google Scholar]
- 40.Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. The New England journal of medicine. 2012;367(14):1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC medical research methodology. 2013;13:92. doi: 10.1186/1471-2288-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. The Proceedings of the Nutrition Society. 2017:1–8. doi: 10.1017/S0029665117000349. [DOI] [PubMed] [Google Scholar]
- 43.Cashman KD, Dowling KG, Skrabakova Z, et al. Vitamin D deficiency in Europe: pandemic? The American journal of clinical nutrition. 2016;103(4):1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mousa A, Naderpoor N, Teede HJ, De Courten MP, Scragg R, De Courten B. Vitamin D and cardiometabolic risk factors and diseases. Minerva endocrinologica. 2015;40(3):213–230. [PubMed] [Google Scholar]
- 45.Minghetti PP, Norman AW. 1,25(OH)2-vitamin D3 receptors: gene regulation and genetic circuitry. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1988;2(15):3043–3053. doi: 10.1096/fasebj.2.15.2847948. [DOI] [PubMed] [Google Scholar]
- 46.Scragg R, Stewart AW, Waayer D, et al. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA cardiology. 2017 doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norris KC, Williams SF. Race/ethnicity, serum 25-hydroxyvitamin D, and heart disease. JAMA: the journal of the American Medical Association. 2013;310(2):153–155. doi: 10.1001/jama.2013.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA: the journal of the American Medical Association. 2013;310(2):179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. The New England journal of medicine. 2014;370(9):880–881. doi: 10.1056/NEJMc1315850. [DOI] [PubMed] [Google Scholar]
- 50.Canale D, de Braganca AC, Goncalves JG, et al. Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: role of oxidative stress and renin-angiotensin system. PloS one. 2014;9(7):e103055. doi: 10.1371/journal.pone.0103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasing Y, Fenton CG, Jorde R, Paulssen RH. Changes in the human transcriptome upon vitamin D supplementation. The Journal of steroid biochemistry and molecular biology. 2017 doi: 10.1016/j.jsbmb.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Goldsmith D, Thadhani RI. Low Sodium Diet, Vitamin D, or Both for RAASi-Resistant, Residual, Proteinuria in CKD? The ViRTUE Trial Points the Way Forward but Is Not the Last Word. Journal of the American Society of Nephrology: JASN. 2017;28(4):1016–1019. doi: 10.1681/ASN.2016121321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain SK, Kahlon G, Bass P, Levine SN, Warden C. Can L-Cysteine and Vitamin D Rescue Vitamin D and Vitamin D Binding Protein Levels in Blood Plasma of African American Type 2 Diabetic Patients? Antioxidants & redox signaling. 2015;23(8):688–693. doi: 10.1089/ars.2015.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hlaing SM, Garcia LA, Contreras JR, Norris KC, Ferrini MG, Artaza JN. 1,25-vitamin-D3 promotes cardiac differentiation through modulation of the Wnt signaling pathway. Journal of molecular endocrinology. 2014 doi: 10.1530/JME-14-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA: the journal of the American Medical Association. 2013;310(2):179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berg AH, Powe CE, Evans MK, et al. 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans. Clinical chemistry. 2015;61(6):877–884. doi: 10.1373/clinchem.2015.240051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesch Yuko Y. Some common misperceptions about p-values. Stroke. 2014 Dec;45(12):e244–e246. doi: 10.1161/STROKEAHA.114.006138. [DOI] [PMC free article] [PubMed] [Google Scholar]