Abstract
Traditionally low-grade B-cell lymphomas have been excluded from the category of monomorphic post-transplant lymphoproliferative disorders (PTLD). However, recent reports identified Epstein-Barr virus-positive (EBV+) extranodal marginal zone lymphomas (MZL), almost exclusively seen in the post-transplant setting. Some reported cases responded to reduced immunosuppression, suggesting that they should be considered as a form of PTLD. We identified 10 cases of EBV+ MZL, 9 in extranodal sites and 1 presenting in lymph node. Two cases arose following solid organ transplantation, but other settings included iatrogenic immunosuppression for rheumatoid arthritis (2); prior chemotherapy (2); congenital immune deficiency (1); and increased age (3), as the only potential cause of immune dysfunction. There were 4 males and 6 females; age range 18–86. The atypical plasmacytoid and/or monocytoid B cells were positive for EBV in all cases, with either latency I or II in all cases tested. Monotypic light chain expression was shown in all with 6 cases positive for IgG, and 2 for IgM, undetermined in 2. Clonal immunoglobulin gene rearrangement was positive in all cases with successful amplification. MYD88 L265P was wild type in the 6 cases tested. We show that EBV+ MZLs can arise in a variety of clinical settings, and are most often extranodal. Treatment varied, but most patients had clinically indolent disease with response to reduction of immune suppression, or immuno-chemotherapy.
Keywords: Epstein Barr virus, immunodeficiency, post-transplant lymphoproliferative disease, immunosenescence, marginal zone lymphoma, MALT lymphoma, immunosuppression
Introduction
Post-transplantation lymphoproliferative disorders (PTLD) refer to a heterogeneous group of lymphoid proliferations associated with immunosuppression following solid organ or hematopoietic stem cell transplantation. The incidence of PTLD among solid organ transplant recipients is variable, between 1–20%, with risk in a specific individual determined mainly by patient age, organ type, immunosuppression regimens, and pre-transplant EBV-status1.
Most PTLDs are of B-cell origin and EBV-driven, comprising a spectrum of morphologically evolving lesions ranging from infectious mononucleosis-like or plasmacytic hyperplasia-type early lesions, polymorphic PTLD, to monomorphic more aggressive B-cell lymphomas. Additionally, it is well established that EBV+ B-cell lymphoproliferative disorders (LPD) clinically and pathologically resembling PTLD may occur in other immunocompromised settings such as immunosenescence due to advanced age 2, patients receiving immunosuppressive or cytotoxic therapy for autoimmune diseases3 or malignancy4, or individuals with inherited immunodeficiency syndromes5
The 2008 World Health Organization (WHO) Tumor Classification specifically excluded low-grade B-cell lymphomas, including marginal zone lymphomas (MZL), from the category of monomorphic PTLD6. However, EBV-positive B-cell lymphomas resembling MZL, particularly extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue type (MALT lymphoma), were recently reported to occur in the post-transplant setting7–10, and rarely in other forms of immunodeficiency3, 5, 11. Some of the reported EBV+ MALT lymphoma cases responded to reduced immunosuppression, highlighting the need to distinguish them from EBV-negative cases, so that they could be managed properly. Thus, the 2017 revision of the 4th Edition of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues recognized EBV+ extranodal MZL as a rare form of PTLD12.
We report ten cases of EBV+ MZL, making it the largest series of such cases to date. Nine cases arose in a variety of extranodal sites (MALT lymphoma), while one case was exclusively nodal. Significantly, the clinical settings were diverse, a finding not appreciated in prior reports. Patients presented following solid organ transplantation, iatrogenic immune suppression, prior multiagent chemotherapy, and with congenital immune deficiency, and advanced age without other cause of immune suppression.
Materials and methods
Selection of cases
The pathology archives of two pathology departments were searched, and 10 EBV+ MZL cases were identified. Slides were reviewed by ESJ, SG, WX, and SP. Cases resembling polymophic post-transplant lymphoproliferative disorder (PTLD) were excluded based on the presence of necrosis, prominent apoptotic bodies, a large cell or immunoblastic component, and cytologically immature plasmacytoid cells. The clinical history prior to diagnosis, treatment following diagnosis, and clinical outcome were collected from the original medical record whenever possible, or public birth/death database (if lost follow-up).
Immunohistochemistry and EBER in situ hybridization
Immunohistochemical stains were performed on formalin-fixed, paraffin-embedded (FFPE) tissue sections following routine procedures on a Ventana Benchmark automated stainer (Roche Ventana Medical Systems, Tucson, AZ), using the antibodies against CD20 (L26, Roche Diagnostics), CD3 (2GV6, Ventana), CD79a (SP18, Roche Diagnostics), PAX-5 (SP34, Roche Diagnostics), LMP1 (CS 1–4, Dako), EBNA2 (PE2, Abcam), (CD138 (B-838, Roche Diagnostics), kappa (rabbit, Roche Diagnostics), lambda (rabbit, Roche Diagnostics), CD38 (SPC32, Vector), MUM-1 (MRQ-43, Roche Diagnostics), Ki-67 (MIB-1, Dako), IgG (rabbit, Dako), IgM (rabbit, Dako), and IgA (rabbit, Dako). Epstein-Barr virus-encoded small RNA (EBER) in situ hybridization was performed as previously described 13.
Molecular Studies
DNA was extracted from FFPE tissue and PCR amplified for detection of immunoglobulin gene (IGH and IGκ loci) rearrangements (8 cases), and MYD88 gene mutation (6 cases). For the IGH locus, two separate reactions were performed, the first using consensus primers to framework region III and the joining region of the immunoglobulin heavy chain gene (FRIII-IGH PCR), and the second using consensus primers to framework region II and the joining region of the immunoglobulin heavy chain gene (FRII-IGH PCR), as described by Ramasamy et al 14. For both reactions the joining region primer was covalently linked to a fluorescent dye FAM to allow for fluorescence detection. The products were analyzed by capillary electrophoresis on an ABI 3130xl Genetic Analyzer, and electropherograms were analyzed using GeneMapper software version 4.0 (ABI). Two additional reactions were performed for the IGκ locus using the BIOMED-2 primer set described by van Dongen et al15, and supplied by InVivoScribe Technologies (IGK Gene Clonality Assay - ABI Fluorescence Detection). These reactions interrogate rearrangements involving the Vκ loci and Jκ (Tube A), the Vκ locus and the κDE locus (Tube B), and the κ intron RSS locus and the κDE locus (Tube B). The products were analyzed by capillary electrophoresis on an ABI 3130xl Genetic Analyzer, and electropherograms were analyzed using GeneMapper software version 4.0 (ABI).
For detection of the lymphoid malignancy-associated MYD88 (c.794T>C; p.L265P) mutation, the DNA was subjected to a mutant allele enrichment PCR technique using a single primer set encompassing codon 265 of the MYD88 gene16. Enrichment of the mutant allele is achieved through the addition of a modified oligonucleotide (containing several bridged nucleic acids) to the PCR reaction that reduces the amplification efficiency of the normal allele as compared to mutant sequences that may be present. The product was then subjected to pyrosequencing on a Qiagen PyroMark Q24 system.
Results
Clinical findings
Clinical Presentation and Risk Factors
The 10 patients included 4 males and 6 females (ages 18–86, Table 1). Patient 1 (18-year old [y.o.] female) was the only patient diagnosed with nodal disease. She had received a heart/kidney combined transplant for mitochondrial complex IV deficiency 7 years prior to her presentation with inguinal lymphadenopathy. All other patients presented with extranodal MALT lymphomas, 5 involving skin and subcutaneous tissue, with other sites being parotid gland (2 cases), lung (1 case), and breast (1 case).
Table 1.
Summary of patient demographics, clinical history, treatment, and follow-up of EBV+ MZL
| Patient | Anatomic Site | Age/Sex | History | Treatment | Follow-up |
|---|---|---|---|---|---|
| 1 | Left inguinal LN | 18 F | Heart/kidney combined transplant in 2002 | Reduced immunosuppression; riituximab at relapse one year later. | Relapsed 6 yrs after diagnosis, with similar histology, and clonality proven by Ig PCR. In continuous CR following rituximab. |
| 2 | Subcutaneous masses, arm, abdomen | 78 M | Classical Hodgkin lymphoma in 2001, s/p chemotherapy; advanced Parkinson’s disease at the time of diagnosis. Subcutaneous masses in 2008 and 2009 | Unknown | Received ABVD for CHL in 2001; R-ICE recommended in 2008 but patient lost to follow-up; died in 2014 |
| 3 | Skin, flank, abdomen | 70 M | No significant PMH | Unknown | Died in 2013 |
| 4 | Skin, arm | 69 M | Subcutaneous nodules, mediastinal and cervical LAD (2008) | R-CHOP and local radiation | CHL (EBV+) was diagnosed 5 months later. Recurrence of EBV+ cutaneous MZL in 2011 and 2014. EBV+ B-cell proliferation in oropharynx in 2016. |
| 5 | Periparotid soft tissue | 63 F | Rheumatoid arthritis and Sjogren syndrome, treated with methotrexate; Presented with parotid mass (2014) | Unknown | Alive |
| 6 | Lung | 26 M | Anterior chest wall mass in 2010, patient treated as Ewing Sarcoma; developed right lung nodule in 2011 which had been enlarging; lung wedge resection performed in 2016 | Unknown | Alive |
| 7 | Breast | 31 F | Status post liver transplant in 2004 for Wilson’s disease; developed multiple right breast lesions in 2011, 2015, residual breast mass was resected in 2016 | DA R-EPOCH × 6 cycles in 2011; reduction of immunosuppression. Subsequent chemorx with bortezomib, dexamethasone, and valganciclovir HCL. | Currently stable |
| 8 | Skin, arm | 86F | No significant PMH; presented with skin nodules on bilateral forearms, face, and trunk; she was also noted to have peripheral blood and bone marrow involvement (2016) | Received prednisone but no response | Currently stable |
| 9 | Parotid gland | 54F | History of rheumatoid arthritis, Sjogren syndrome, treated with methotrexate, etanercept, infliximab since 2014. Presented with parotid mass in 2017. | Discontinued immunosuppression. Rituximab induction with rituximab maintenance | Currently stable and clinically improved with resolution of parotid symptoms. |
| 10 | Parotid gland | 18F | History of chronic active EBV infection in 2015; parotid mass in 2017. Genetic testing showed mutation in RNA Component of Mitochondrial RNA Processing Endoribonuclease (RMRP) gene and congenital immune deficiency. Elevated viral load at 3382 copies/ml. | Stem cell translant to correct immune deficiency | Complete response, NED. |
Abbreviations: LN, lymph node; CR, complete remission; s/p, status-post; PMH, past medical history; LAD, lymphadenopathy; chemorx, chemotherapy; DA-R-EPOCH, Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab; R-ICE, rituximab, ifosfamide, carboplatin, etoposide; ABVD, adriamycin, bleomycin, vincristine, doxorubicin; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; NED, no evidence of disease; CHL, classical Hodgkin lymphoma.
The clinical settings and underlying risk factors varied. A second patient with PTLD (case 7) was status post-liver transplant for Wilson’s disease, and 7 years later developed multiple lesions in the right breast. Two patients had a history of rheumatoid arthritis and Sjogren syndrome, and had been treated with methotrexate, (plus additionally etanercept and infliximab in 1). Both patients developed lesions associated with the parotid gland (case 9) and peri-parotid soft tissue (case 5). Two patients (case 2, case 6) developed EBV+ MALT lymphoma involving soft tissue and lung, respectively, following chemotherapy for a malignant diagnosis, classical Hodgkin lymphoma (CHL) in one, and Ewing’s sarcoma in the second. Three cases (cases 3, 4 and 8) had no underlying risk factors other than age, with presumed immune senescence, presenting with EBV+ MALT lymphoma at ages 69, 70 and 86.
Finally, one patient (case 10), had a history of chronic EBV infections, presenting with an EBV+ MALT lymphoma of the parotid at age 18. This patient had cartilage hair hypoplasia syndrome, short stature, and lymphopenia of both B and T-cells. Genetic testing showed a pathogenic mutation in RNA Component of Mitochondrial RNA Processing Endoribonuclease (RMRP) gene as a cause of underlying immune deficiency.
Treatment and Clinical Outcome
The two patients with EBV+ MZL of PTLD type both initially received only reduced immune suppression. Case 1 developed a clonally related PTLD 6 years later with similar histology, which was treated with 6 cycles of rituximab with no subseqent evidence of recurrent disease. The second (case 7) received DA R-EPOCH in 2011 but relapsed in 2015. Immunosuppression was reduced but soon full immunosuppression was reinstituted because she developed graft rejection. She then received 5 cycles of bortezomib and dexamethasone and valganciclovir hydrochloride and showed partial response. The residual mass was resected and she is currently stable.
Patients 3 (70 y.o. male) and 8 (86 y.o. male) were elderly and had no history of immunosuppressive therapy or prior malignancy. They both presented with multiple skin nodules, which were biopsied and diagnosed as EBV+ MZL. Patient 8 also was noted to have peripheral blood and bone marrow involvement. Patient 3 was lost to follow-up after the diagnosis, but records show that he died in 2013. Patient 8 received prednisone but without response. She is currently stable. Case 4, who also presented with only age as a risk factor, subsequently developed CHL. He subsequently received chemotherapy and radiation and is currently alive and in remission.
Two patients developed EBV+ MZL following chemotherapy. Case 2, a 78 y.o. male, had received ABVD for CHL in 2001. At the time of his second diagnosis of EBV+ MZL in 2008, he was treated with further chemotherapy, was lost to follow-up and died in 2014. Case 6, a 26 y.o. male, was treated with chemotherapy for a presumptive diagnosis of Ewing’s sarcoma in 2010. This diagnosis was reversed on consultation at the time he presented with EBV+ MALT lymphoma of the lung in 2011. The pulmonary lesion persisted, and in 2016, was resected. The patient is currently stable.
Cases 5 and 9 developed iatrogenic EBV+ MZL following immunosuppressive therapy for rheumatoid arthritis and Sjogren syndrome. Case 9 was managed with discontinuation of therapy and treatment with rituximab. Patient is clinically improved. Case 5 is alive, with uncertain status.
Case 10, the patient with congenital immune deficency, received a stem cell transplant. She has had a complete remission of her EBV-related disease, with reconstitution of immune status.
Morphologic, immunohistochemical, and molecular features
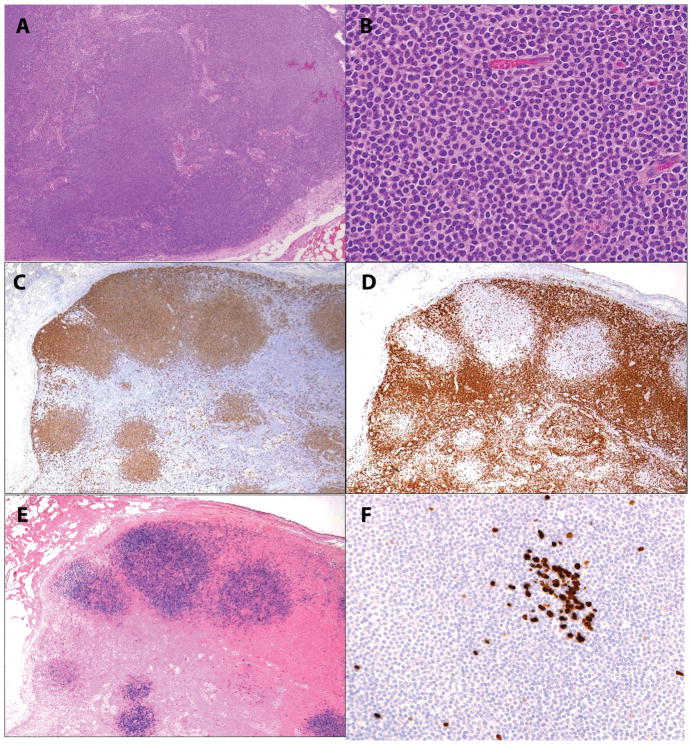
In this case series of EBV+ MZL, 9 of 10 patients had extranodal MZL of MALT type, and only 1 patient had nodal MZL (Table 2). The single nodal case (Case 1) contained nodular aggregates of monocytoid appearing cells, with round to oval nuclei, finely clumped chromatin, and a rim of pale cytoplasm (Figure 1). Normal follicles were absent. The nodules were surrounded by residual paracortical T-cells. The atypical cells were positive for CD20, CD79a, showed dim expression of IgGk, and had a low proliferative rate with Ki67. The cells were only focally positive for CD138 and CD38. EBER positivity was confined to the nodules of atypical monocytoid B-cells.
Table 2.
Summary of pathologic and major laboratory findings of EBV+ MZL cases
| Patient | Anatomic Site | H&E | CD20 | CD79a | CD138 or CD38 | MUM-1 | κ/λ | IgG/IgM/IgA | EBER/LMP1 | Ki-67 | MyD88 | Ig PCR Clonality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Left inguinal LN | Nodular infiltrate of monocytoid cells. | Pos | Pos | Both focally pos | Pos | Kappa | IgG | Pos/Neg | Low | WT | IgH pos |
| 2 | Subcutaneous masses, arm, abdomen | Lymphocytic/plasmacellular, Biphasic pattern | Pos | Pos | CD38 pos, CD138 neg | Pos | Kappa | IgG | Pos/Neg | Low | WT | Poor DNA |
| 3 | Skin, flank, abdomen | Lymphocytic/plasmacellular, Polymorphic | Partially pos | Pos | CD138 pos | Pos | Lambda | IgG | Pos/Neg | Moderate | WT | IgH and IgK pos |
| 4 | Skin, arm | Lymphocytic/plasmacellular, Biphasic pattern | Partially pos | Pos | CD138 partially pos | Pos | Lambda | IgG | Pos/Neg | Moderate | N.D. | IgH and IgK pos |
| 5 | Periparotid soft tissue | Lymphocytic/plasmacellular, Biphasic pattern | Partially pos | Pos | CD138 variably pos | Pos | Kappa | All neg | Pos/Neg | Low | WT | IgH pos |
| 6 | Lung | Lymphocytic/plasmacellular, Biphasic pattern | Partially pos | Pos | CD138 pos | Pos | Lambda | IgG | Pos/Pos | Moderate | N.D. | IgH and IgK pos |
| Lymph node (2nd Bx) | Classical Hodgkin lymphoma, EBV-positive, LD | IgH and IgK Pos (unrelated) | ||||||||||
| 7 | Breast | Monocytoid/plasmacellular, BiphasicMott cells and Russell bodies | Partially pos | N.D. | Pos | N.D. | Kappa | IgG | Pos/N.D. | Low | N.D. | N.D. |
| 8 | Skin, arm | Lymphocytic/plasmacellular, Polymorphic | Partially pos | Pos | N.D. | Pos | Lambda | N.D. | Pos/N.D. | Moderate | N.D. | N.D. |
| 9 | Parotid gland | Monocytoid/plasmacellular, Biphasic | Pos | N.D. | CD138 partially pos | Pos | Kappa | IgM | Pos/Neg | Moderate | WT | IgH and IgK pos |
| 10 | Parotid gland | Monocytoid/plasmacellular, Biphasic | Pos | N.D. | CD138 partially pos | Pos | Kappa | IgM | Pos/Pos | Low | WT | IgH and IgK pos |
Abbreviations: LN, lymph node; Pos, positive; N.D., not done; WT, wild type; LD, lymphocyte depleted.
Figure 1.
EBV-positive nodal marginal zone lymphoma in an 18-year old female with heart/kidney combined transplant (case 1). A and B, Nodal architecture is effaced by nodular infiltrates of monocytoid cells with a rim of pale cytoplasm C. Monocytoid cells are positive for CD20, while CD3 [D] surrounds and delineates the tumor nodules. E. EBER shows a similar distribution to CD20. F. Ki-67 demonstrates a very low proliferation index in the monocytoid cells, while being positive in a small residual germinal center.
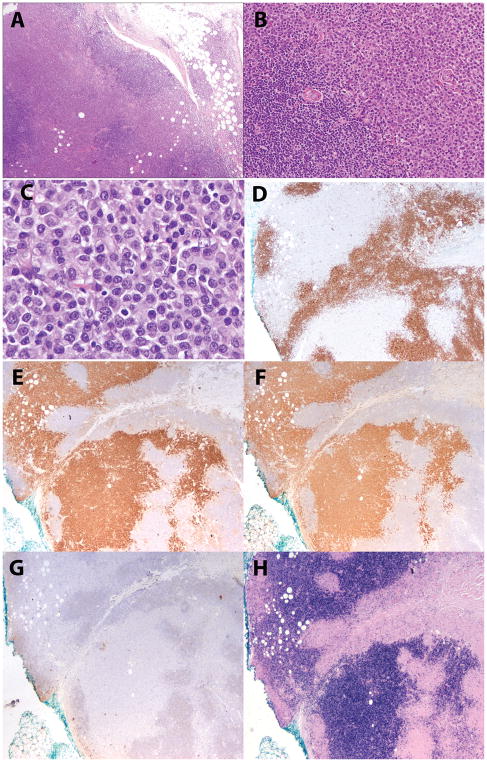
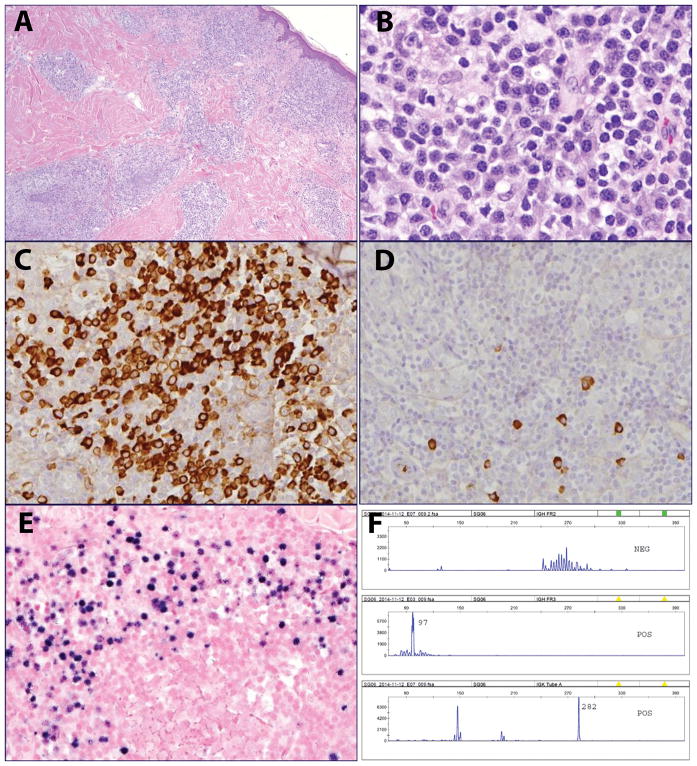
Nine extranodal cases all demonstrated more marked plasmacytoid differentiation, generally associated with adjacent areas composed of sheets or aggregates of small lymphocytes (in six cases) or monocytoid cells (in three cases). Seven of the nine extranodal cases had a biphasic pattern, with segregation of the plasmacytoid cells from the adjacent small lymphocytic or monocytoid cells (Figure 2). However, in two of the cases, both of which involved the skin (cases 3, 8), the plasmacytoid and lymphoid components were more intermixed, with a polymorphous cellular composition (Figure 3). None of the cases showed evidence of necrosis or significant apoptotic activity, helping to rule out polymorphic PTLD. Residual lymphoid follicles were sometimes present, but prominent reactive germinal centers were not seen. EBER in situ hybridization was predominant in the plasmacytoid cells in those cases showing plasmacytoid differentiation, while residual B-cell areas tended to be negative.
Figure 2.
EBV-positive MZL lymphoma in a 78-year old male (case 2). A, B. The subcutaneous mass is composed of sheets of monotonous plasmacytoid cells rimmed by small lymphocytes. C. Plasmacytoid features are shown at high power. D. CD20-positive small lymphocytes surround the atypical plasmacytoid cells, which are CD20-negative. E. Plasmacytoid cells are positive for kappa and F. also positive for CD38. G. Lambda is negative, while H. EBER is positive.
Figure 3.
EBV-positive MALT lymphoma in a 70-year old male with no significant past medical history (case 3). A. The infiltrate involves the dermis and subcutaneous tissue with a perivascular and periadnexal distribution. B. The infiltrate is composed of monocytoid and plasmacytoid cells. C. The plasmacytoid cells are positive for lambda and D. negative for kappa light chain. E. EBER shows a similar distribution to lambda. F. PCR for immunoglobulin gene rearrangements revealed a clonal pattern, with peaks in IGH Fr III, and Kde.
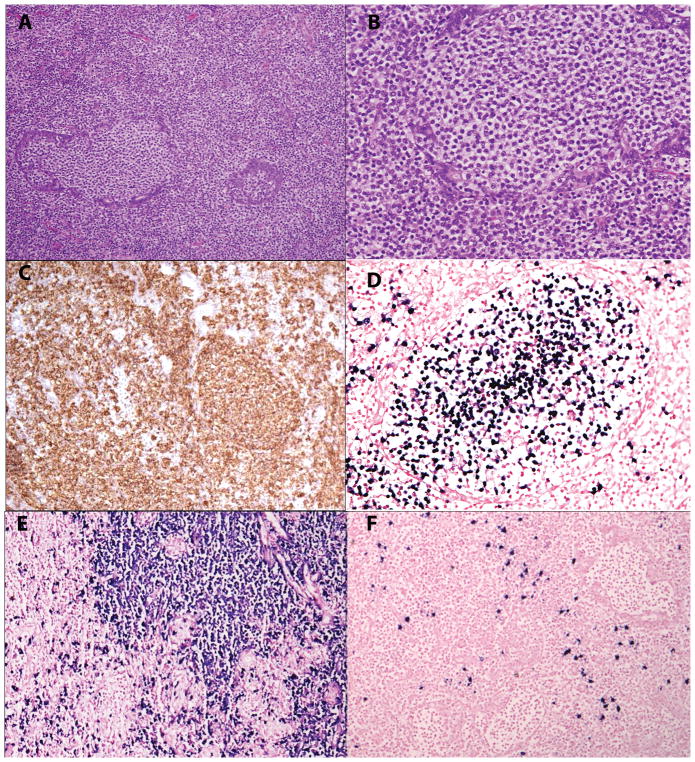
Case 9 involving the parotid gland showed a slightly different pattern, and was composed of nodules and sheets of monocytoid cells, adjacent to an infiltrate of cells with marked plasmacytic differentiation (Figure 4). The monocytoid cells infiltrated the residual ducts, with prominent lymphoepithelial lesions. In contrast to the other extranodal cases, EBER positivity was particularly associated with the monocytoid cells.
Figure 4.
EBV-positive MALT lymphoma in a 54-year old female with history of rheumatoid arthritis and Sjogren syndrome (case 9). A. The parotid ducts are filled with monocytoid appearing cells. B. The cells have a rim of pale cytoplasm. C. CD20 is positive. D. EBER is positive within the monocytoid cells filling the altered duct. E. In situ hybridization for kappa stains the plasmacytoid cells surrounding the glands, while lambda is positive in only few plasma cells [F].
EBV latency was addressed in 7 of the 10 cases. Two cases contained cells positive for LMP1, while the other cases were negative. All cases studied were negative for EBNA2; no case demonstrated a Latency III phenotype.
All 10 cases demonstrated light chain restriction (6 kappa 4 lambda). Six cases expressed IgG heavy chain, whereas both cases involving the parotid gland expressed IgM. None of the cases showed staining for IgA. Ki-67 revealed variable proliferation rates from low to moderate.
Immunoglobulin gene rearrangement studies showed clonal peaks in all cases with successful amplification (7 cases); one case failed PCR amplification due to poor DNA quality and in two cases material for testing was not available. MYD88 gene was wild type in all 6 cases tested.
Discussion
The EBV virus has the potential to transform B-cells, and less commonly NK-cells and T-cells, leading to a broad spectrum of EBV-positive lymphomas and lymphoproliferative disorders. EBV-positive B-cell lymphomas are most often high grade, and include EBV-positive diffuse large B-cell lymphoma (DLBCL), NOS, Burkitt lymphoma, and classical Hodgkin lymphoma, among others. EBV-positive lymphomas occur sporadically, but many show greater prevalence in the setting of immune deficiency.
EBV-positive low-grade B-cell lymphomas, in particular, EBV+ extranodal MZLs, are rare and were described recently in the post-transplant setting.8 The revised 4th Edition of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues now recognizes EBV-positive extranodal MZL as a rare form of PTLD.12 The report from Gibson, et al. identified four such cases, all of which occurred in the setting of iatrogenic immune suppression following solid organ transplant. All patients presented with subcutaneous nodules, and all expressed IgA8. Their review of the literature identified 9 additional cases, all but two of which involved cutaneous sites. Two patients presented with EBV-positive MALT lymphomas of the stomach and small bowel, both arising following kidney transplant.17, 18
The major differential diagnosis for EBV+ MZL in the post-transplant setting is EBV-positive PTLD,19 but morphologically similar lesions can be seen in other immune deficiency states.20 The EBV+ MZLs in this series lacked the necrosis and apoptosis that are key features of polymorphic PTLD. While both types of lesions show evidence of plasmacytoid differentiation, the infiltrates in polymorphic PTLD are typically very polymorphic, with a wide range of cellular differentiation. Notably, most of the cases in our series had a biphasic pattern, with the plasmacytoid cells segregated from the other cellular components, monocytoid cells and small lymphocytes. Only two cases, both of which involved skin, had a more mixed, polymorphous cellular composition. In those cases showing plasmacytoid differentiation, EBER was most evident in the plasmacytoid component, with the exception of cases 1 and 9, in which the EBER localized to monocytoid cells. Thus, while the dominant population was positive for EBER in all cases, there were admixed B-cells present that appeared negative for EBV.
While the histological appearance may raise a differential diagnosis with polymorphic PTLD, all of the cases studied for EBNA2 were negative. Polymorphic PTLD usually demonstrates a Latency III phenotype (EBNA2-positive). LMP1 was focally positive in two of the cases studied (Latency II) but the predominat pattern was Latency I. This profile is helpful in excluding polymorphic PTLD, and is similar to the pattern reported by Gibson et al.8
Our report expands significantly the spectrum of EBV+ MZLs. We report 10 new cases, only two of which arose in patients who were immunosuppressed following organ transplantation. Two cases arose in patients being treated with methotrexate +/− further therapy with etanercept and infliximab for rheumatoid arthritis and Sjogren syndrome. Two additional patients had received combination chemotherapy for other malignant diagnoses. Three cases arose in older patients with no specific risk factors, other than advanced age (69, 79 and 86 years of age) as a probable cause of immune senescence. Age also may have played a role in Case 2, a 78-year old male who was in complete remission following treatment for CHL 8 years earlier. Immune senescence has been identified as a significant risk factor for EBV+ DLBCL, NOS, and EBV+ mucocutaneous ulcer.2, 13, 21 In addition, epidemiological studies have identified a late age peak for EBV-positive CHL that is likely related to immune senescence.22
The last case arose in an 18-y.o. female with a pathogenic mutation in RNA Component of Mitochondrial RNA Processing Endoribonuclease (RMRP) gene as a cause of underlying immune deficiency. She had long-standing lymphopenia of both B-cells and T-cells, as well as evidence of persistent EBV viral infection prior to the development of an EBV+ MALT lymphoma in the parotid gland. Following stem cell transplant, she had reconstitution of immune function and no recurrence of EBV-related disease. Another report in the literature identified an EBV-positive MALT lymphoma in the parotid of a patient with ataxia telangiectasia.5
It is notable that three of our cases arose in the parotid gland, the other two both in patients with rheumatoid arthritis and Sjogren syndrome. The association of Sjogren syndrome and MALT lymphoma of the salivary gland is well-recognized,23, 24 but EBV is only rarely studied in this setting.25 Diss et al. using PCR amplification to search for EBV-related sequences found EBV-DNA in 3/45 cases of myoepithelial sialadenitis or MALT lymphoma of the salivary gland. However, PCR studies are not able to determine the prevalence and distribution of the EBV-positive cells. Notably, in our cases, the histological features were not suspicious for an EBV-related lesion, but EBER was strongly and diffusely positive in monocytoid B-cells forming lymphoepithelial lesio within salivary gland ducts. These data warrant further investigation of EBV in MALT lymphomas arising in patients with Sjogren syndrome, especially if they are receiving iatrogenic immune suppression.
Our series also expands the anatomic localization of EBV-positive MZL. Similar to the report from Gibson et al., skin and subcutaneous tissue were common sites, being involved in 4 of the 10 cases. As noted, three cases involved the parotid gland and adjacent soft tissue. Two cases involved the lung and breast, both of which are commonly affected by MALT lymphoma. Notably, one post-transplant case was nodal in origin, a site not previously reported for EBV+ MZL.
EBV+ MZL needs to be distinguished from plasmacytoma, which can occur as a form of PTLD. None of our cases consisted of a pure population of plasma cells, and all had evidence of other B-cell components, either monocytoid cells or small lymphoid cells. CD20 and/or PAX5 were expressed, at least partially in all cases. The prior report from Gibson et al. reported expression of IgA in all four of their cases.8 However, we were not able to identify IgA in a single case. Six cases expressed IgG heavy chain, whereas two cases involving the parotid gland expressed IgM. In two cases the heavy chain class could not be determined. The differential diagnosis also includes lymphoplasmacytic lymphoma (LPL). Because of significant plasma cell differentiation seen in 9/10 cases, we performed PCR for evidence of MYD88 L265P mutation. All six cases tested exhibited MYD88 in the wild type state.
It has been questioned whether EBV-negative MALT or nodal MZL might occur with increased incidence in the post-transplant setting or other forms of immune deficiency 26–29. Many of these early cases arose in stomach, and often were associated with Helicobacter pylori infection. MZLs also appear to occur in the setting of HIV infection with increased frequency (relative risk 2.4), and can be seen in both adults and children.30, 31 The role of immune suppression in the pathogenesis of HIV-associated MZL is uncertain, but may be related to chronic antigenic stimulation. Whether EBV-positive MZLs are more common than initially expected requires further investigation.
Based on the observations in published studies, post-transplant associated EBV+ MZLs often respond to reduced immunosuppression8, 9, 32. Although we had difficulty in obtaining the details of treatment and clinical outcomes, at least 1 patient (case 1) in our series was treated by reduction in immunosuppression, with initial response. Her EBV+ nodal MZL recurred in 2015, but a complete remission that is maintained to the present time was induced following a course of rituximab. Immuno-chemotherapy was also employed in some patients, but the addition of chemotherapy may add to immune compromise in this immunosuppressed patient population.
In summary, we report 10 cases of EBV+ MZL that arose in various settings of immune compromise. In most of the cases the histological features would not have suggested an EBV-related lesion, and thus the incidence of EBV+ MZL may be underestimated in the literature, particularly in the salivary gland where the lesions closely resembled classical EBV-negative MZL.
Acknowledgments
This work was supported by the Intramural Research Budget of the Center for Cancer Research, National Cancer Insitute, National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Mucha K, Foroncewicz B, Ziarkiewicz-Wroblewska B, et al. Post-transplant lymphoproliferative disorder in view of the new WHO classification: a more rational approach to a protean disease? Nephrol Dial Transplant. 2010;25:2089–2098. doi: 10.1093/ndt/gfq231. [DOI] [PubMed] [Google Scholar]
- 2.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima M, Nakamura S, Futamura N, et al. Malignant lymphoma in patients with rheumatic diseases other than Sjogren’s syndrome: a clinicopathologic study of five cases and a review of the Japanese literature. Jpn J Clin Oncol. 1997;27:84–90. doi: 10.1093/jjco/27.2.84. [DOI] [PubMed] [Google Scholar]
- 4.Abruzzo LV, Rosales CM, Medeiros LJ, et al. Epstein-Barr virus-positive B-cell lymphoproliferative disorders arising in immunodeficient patients previously treated with fludarabine for low-grade B-cell neoplasms. Am J Surg Pathol. 2002;26:630–636. doi: 10.1097/00000478-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JA, Bayerl MG. Epstein-barr virus-associated extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT Lymphoma) arising in the parotid gland of a child with ataxia telangiectasia. J Pediatr Hematol Oncol. 2015;37:e114–117. doi: 10.1097/MPH.0b013e31829f3496. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 7.Salama S, Todd S, Cina DP, et al. Cutaneous presentation of post-renal transplant lymphoproliferative disorder: a series of four cases. J Cutan Pathol. 2009;37:641–653. doi: 10.1111/j.1600-0560.2009.01449.x. [DOI] [PubMed] [Google Scholar]
- 8.Gibson SE, Swerdlow SH, Craig FE, et al. EBV-Positive Extranodal Marginal Zone Lymphoma of Mucosa-associated Lymphoid Tissue in the Posttransplant Setting: A Distinct Type of Posttransplant Lymphoproliferative Disorder? Am J Surg Pathol. 2011;35:807–815. doi: 10.1097/PAS.0b013e3182190999. [DOI] [PubMed] [Google Scholar]
- 9.Nassif S, Ozdemirli M. EBV-positive low-grade marginal zone lymphoma in the breast with massive amyloid deposition arising in a heart transplant patient: A report of an unusual case. Pediatr Transplant. 2013;17:E141–145. doi: 10.1111/petr.12111. [DOI] [PubMed] [Google Scholar]
- 10.Cassidy DP, Vega F, Chapman JR. Epstein-Barr Virus-Positive Extranodal Marginal Zone Lymphoma of Bronchial-Associated Lymphoid Tissue in the Posttransplant Setting: An Immunodeficiency-Related (Posttransplant) Lymphoproliferative Disorder? Am J Clin Pathol. 2017;149:42–49. doi: 10.1093/ajcp/aqx134. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Frambach GE, Seilstad KH, et al. Epstein--Barr virus-associated B-cell lymphoma in the setting of iatrogenic immune dysregulation presenting initially in the skin. J Cutan Pathol. 2005;32:474–483. doi: 10.1111/j.0303-6987.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 13.Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol. 1992;45:770–775. doi: 10.1136/jcp.45.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 16.Gachard N, Parrens M, Soubeyran I, et al. IGHV gene features and MYD88 L265P mutation separate the three marginal zone lymphoma entities and Waldenstrom macroglobulinemia/lymphoplasmacytic lymphomas. Leukemia. 2012;27:183–189. doi: 10.1038/leu.2012.257. [DOI] [PubMed] [Google Scholar]
- 17.Bates WD, Gray DW, Dada MA, et al. Lymphoproliferative disorders in Oxford renal transplant recipients. J Clin Pathol. 2003;56:439–446. doi: 10.1136/jcp.56.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oertel SH, Verschuuren E, Reinke P, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD) Am J Transplant. 2005;5:2901–2906. doi: 10.1111/j.1600-6143.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow SH. Post-transplant lymphoproliferative disorders: a morphologic, phenotypic and genotypic spectrum of disease. Histopathology. 1992;20:373–385. doi: 10.1111/j.1365-2559.1992.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 20.Natkunam Y, Goodlad JR, Chadburn A, et al. EBV-Positive B-Cell Proliferations of Varied Malignant Potential: 2015 SH/EAHP Workshop Report-Part 1. Am J Clin Pathol. 2017;147:129–152. doi: 10.1093/ajcp/aqw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong AA, Alexander FE, Cartwright R, et al. Epstein-Barr virus and Hodgkin’s disease: further evidence for the three disease hypothesis. Leukemia. 1998;12:1272–1276. doi: 10.1038/sj.leu.2401097. [DOI] [PubMed] [Google Scholar]
- 23.Falzon M, Isaacson P. The natural history of benign lymphoepithelial lesion of the salivary gland in which there is a monoclonal population of B cells: a report of two cases. Am J Surg Pathol. 1991;15:59–65. doi: 10.1097/00000478-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hyjek E, Smith W, Isaacson P. Primary B cell lymphoma of salivary gland and its relationship to myoepithelial sialadentiis (MESA) Hum Pathol. 1988;19:766–776. doi: 10.1016/s0046-8177(88)80259-4. [DOI] [PubMed] [Google Scholar]
- 25.Diss TC, Wotherspoon AC, Speight P, et al. B-cell monoclonality, Epstein Barr virus, and t(14;18) in myoepithelial sialadenitis and low-grade B-cell MALT lymphoma of the parotid gland. Am J Surg Pathol. 1995;19:531–536. doi: 10.1097/00000478-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Wotherspoon AC, Diss TC, Pan L, et al. Low grade gastric B-cell lymphoma of mucosa associated lymphoid tissue in immunocompromised patients. Histopathology. 1996;28:129–134. doi: 10.1046/j.1365-2559.1996.292338.x. [DOI] [PubMed] [Google Scholar]
- 27.Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766–775. [PubMed] [Google Scholar]
- 28.Hsi ED, Singleton TP, Swinnen L, et al. Mucosa-associated lymphoid tissue-type lymphomas occurring in post-transplantation patients. Am J Surg Pathol. 2000;24:100–106. doi: 10.1097/00000478-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Kim MJ, Yun SH, Chun HK, et al. Post-transplant lymphoproliferative disorder localized in the colon after liver transplantation: report of a case. Surg Today. 2009;39:1076–1079. doi: 10.1007/s00595-008-3981-6. [DOI] [PubMed] [Google Scholar]
- 30.Teruya-Feldstein J, Temeck BK, Sloas MM, et al. Pulmonary malignant lymphoma of mucosa-associated lymphoid tissue (MALT) arising in a pediatric HIV-positive patient. Am J Surg Pathol. 1995;19:357–363. doi: 10.1097/00000478-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Gibson TM, Morton LM, Shiels MS, et al. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. Aids. 2014;28:2313–2318. doi: 10.1097/QAD.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempf W, Kazakov DV, Mitteldorf C. Cutaneous lymphomas: an update. Part 2: B-cell lymphomas and related conditions. Am J Dermatopathol. 2014;36:197–208. doi: 10.1097/DAD.0b013e318289b20e. quiz 209–110. [DOI] [PubMed] [Google Scholar]