Abstract
Emotions are at the core of human cognition and behavior. Traditionally, emotions have been classified dichotomously as being either positive or negative. However, recent behavioral research An et al (2017) suggests that emotions contain both positivity and negativity. The current work investigated neural correlates of experiencing positive and negative emotions in response to happy and sad photos. Functional MRI revealed the precuneus and posterior cingulate cortex showed stronger activation when experiencing positivity compared to negativity of sadness, but not happiness, whereas the bilateral cerebellum showed greater response to positivity than negativity regardless of emotion. Results suggest that there are similarities and differences in the neural activation of positivity and negativity of happiness and sadness, consistent with previous findings An et al (2017). Emotion from both the neural and psychological perspectives were investigated. Further implications are discussed.
Keywords: emotion, fMRI, positivity, negativity, precuneus, cerebellum
INTRODUCTION
A fundamental assumption about emotion is that emotions are dichotomous, and must be categorized as either positive or negative, regardless of variations in activation or arousal [2,3,4]. However, recent behavioral findings suggest that experiencing each emotion, regardless of traditional emotion dichotomies, can produce both favorable and unfavorable consequences [5,6,7]. Cacioppo and colleagues [8] proposed a model whereby positivity and negativity can exist simultaneously. Recent behavioral findings [1] corroborate this, showing that each emotion contains both positivity and negativity, and there are cross-cultural differences. Yet, we know little about how the human brain processes positivity and negativity of different emotions. To clarify, this issue has at least two important implications for understanding the nature of emotional processing.
First, neural responses to emotional stimuli have been identified in multiple brain regions that play different roles in emotional processing; the amygdala is activated during coding the emotional significance of salient stimuli [9,10], and the medial prefrontal cortex (mPFC) is engaged in a volitional act of appraising the extent of personal associations with emotional stimuli [11]. Activation in these brain regions is usually observed when viewing emotional expression (e.g. facial expression). Thus an important question concerns whether other brain regions might be activated in response to the emotional content of stimuli (which would induce such an emotion). Second, since previous behavioral findings suggest that participants experienced positivity/negativity of different emotions (e.g. happiness, and sadness), it is pivotal to clarify whether there are common and distinct neural substrates of processing positivity/negativity for different emotions.
The current work examined the neurocognitive processes involved in the evaluating of the two sides of emotions by scanning healthy adults using functional magnetic resonance imaging (fMRI) when viewing photos depicting happiness and sadness. Participants rated their feelings of positivity and negativity in response to each photo; participants’ neural activity was examined while both viewing photos and performing ratings to separate brain activations related to evaluating positivity/negativity. This design allowed us to examine neural responses to emotional content by contrasting the blood oxygenation level-dependent responses valence of happiness and sadness.
Candidates for the neural underpinnings of positivity evaluation of happy and sad photos include the posterior cingulate cortex (PCC) and precuneus, which have been associated with positive emotions (e.g., happiness, [12]) and retrieval of episodic memory [13,14]. This can be tested by comparing the brain activity of positivity versus negativity, and testing whether emotion (happiness/sadness) modulates such activity.
Candidates for the neural underpinnings of negativity evaluation of happy and sad photos include the cerebellum, frontal cortex, superior temporal gyrus. These areas are known to relate to sadness, and negative emotions in general [15,16,17]. This can be tested by comparing brain activity of negativity versus positivity of both happy and sad photos. Female adults were tested to avoid the confounding of both sex differences in feeling and reporting emotion [18] and distinct brain activity in response to emotion in the two sexes [19,20].
METHODS
Participants
An a priori power analysis using G*power software [21] revealed 27 participants were necessary to detect a moderate effect size with 80% power for the rating task. A sample of this size is in line with typical fMRI studies. Thus, we recruited 30 Chinese female adults (M=22.38, SD=2.08 years) who were paid 100RMB for participation. All participants were right-handed, had normal or corrected-to-normal vision, and reported no neurological or psychiatric history. Four participants were excluded from further fMRI data analyses because their functional images showed head movement larger than one voxel (3.75 mm). Participants provided informed consent prior to fMRI scanning. This study was approved by the local ethics committee of Peking University.
Stimuli
We gathered over 100 online photos representing each emotion (i.e., sadness and happiness). We then presented 45 pre-selected photos for each emotion to Americans (N=51; M=19.82 years, SD=1.84) and Chinese (N=51; M=21.98 years, SD=2.22) in a lab setting and asked them to rate each photo for the six basic emotions (i.e., sadness, happiness, surprise, disgust, fear, and anger) with 0=not at all, 6=very much. We next selected photos of sadness or happiness to which rating scores were matched in the two cultural groups (in order to conduct cross-cultural comparisons in future research). 16 sad (M=4.08, SD=0.38) and 16 happy (M=4.52, SD=0.43) photos showing humans in social situations that were perceived to reflect sadness or happiness were ultimately chosen for the current fMRI study. Thus, a total of 32 photos, 16 photos for each emotion (happiness and sadness) were used in the fMRI study. The cursor started at 0 at all times.
Procedure
We employed a 2 (Emotion: happiness vs. sadness) × 2 (Valence: negativity and positivity) within-subjects design. After participants granted consent, we checked their eligibility for the fMRI experiment, their demographic information, and the Self Construal Scale (SCS). For the fMRI session, participants were presented with photos of sadness and happiness and had to rate the positivity and negativity of each photo. Each photo was presented twice in a random order and participants rated each photo in terms of positivity or negativity. There were 4 functional runs, and 16 photos (8 happy, and 8 sad) were presented in each run. Each photo subtended a visual angle of 3.6°×3.6° (width × height) at a viewing distance of 80 cm. Fig. 1 illustrates the stimuli and procedure of a single trial during fMRI scanning. On each trial, the question “How positive (or negative) is this photo to you?” was first presented for 2000 ms, which was followed by a photo for 2000 ms. After an inter-stimulus interval of 1, 3, or 5 s, a rating scale appeared for 6000 ms and participants rated each photo from 0 (not at all) to 6 (extremely) by pressing the left or right button on a response keyboard using their right index or middle finger. An inter-trial interval varying among 2, 4, or 6 s followed the rating scale. All questions, photos, and ratings were presented in the center of the screen.
Figure 1.
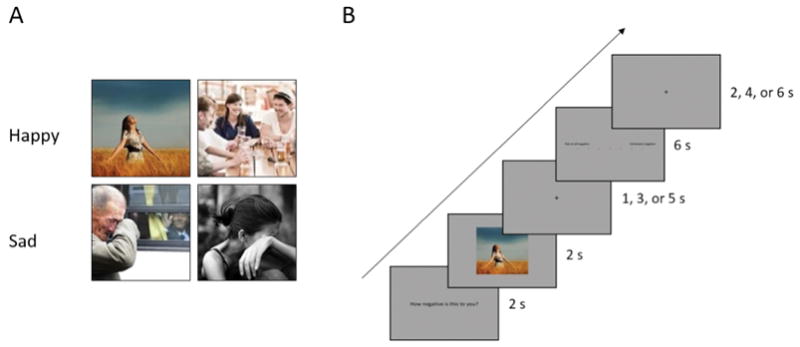
Sample stimuli and procedure. (A) Two samples of happy and sad photos. (B) The structure of a trial during fMRI scanning. Each photo was presented twice for positivity and negativity.
Imaging Parameters
One hundred and fifty-six functional images were acquired during each functional run using a 3.0 T GE Signa MR750 scanner (GE Healthcare; Waukesha, WI) with a standard head coil. Functional images were acquired using a T2-weighted, gradient-echo, echo-planar imaging sequence (64 × 64 × 32matrix with 3.75 × 3.75 × 5 mm3 resolution, repetition time=2000 ms, echo time=30 ms, flip angle=90°, field of view=24 × 24 cm2). A high-resolution T1-weighted structural image (512 × 512 × 180matrix with a spatial resolution of 0.47 × 0.47 × 1.0 mm3, repetition time=8.204 ms, echo time=3.22 ms, flip angle=12°) was acquired before the functional runs.
Imaging Data Analysis
Images were preprocessed using SPM 8 software (Wellcome Trust Centre for Neuroimaging, London, UK). The first three volumes were removed to allow for T1 equilibration effects. Images were adjusted for slice timing by correcting all to the first slice, realigned to the first scan to correct for head motion, normalized into stereotactic Montreal Neurological Institute (MNI) space with 3-mm cubic voxels, and spatially smoothed by a Gaussian filter with full-width/half-maximum parameter (FWHM) set to 8 mm. We then modeled trials of each condition by convolving the canonical hemodynamic response function (HRF) and its time derivative at the onset of the presentation of photos and onset of the presentation of ratings. Six motion parameters (translation: x, y, z; rotation: pitch, roll, yaw) were also included in the model to account for effects of no interest. Low-frequency signal drifts were removed by high-pass filtering (cutoff 128 s), and temporal autocorrelations were corrected by using an autoregressive AR(1) function. We conducted individual-level analysis to identify neural responses that showed main effects of Valence and Emotion and Valence-by-Emotion interaction. Specifically, the contrast [1 −1 −1 1] and its reverse contrast were applied to the four conditions (happy_positive, happy_negative, sad_positive, sad_negative) to test for Valence-by-Emotion interaction; [1 −1 1 −1] and its reverse contrast were used to test for the main effect of Valence; and [1 1 −1 −1] and its reverse contrast tested for the main effect of Emotion. These contrasts were conducted separately for brain activity during presentation of photos and during rating. Group-level analysis was then conducted based on the contrast images from individual participants to allow population inference. Significant activations were identified using a voxel threshold of p < 0.001 and a cluster threshold of p < 0.05 (FDR corrected for multiple comparisons). In order to inspect the direction of the neural activities that showed significant Valence-by-Emotion interaction, regions of interest were defined as a spherical region of 5-mm radius centered at the peak voxel of the activated cluster, and post-hoc analysis was performed on the parameter estimates extracted from those regions.
RESULTS
Behavioral Results
Rating scores of the stimuli during the fMRI scanning were subject to ANOVA with Emotion (happiness vs. sadness) and Valence (positivity vs. negativity) as within-subjects independent variables. There were significant main effects of Emotion (F(1, 25)=17.81, p < .001, η2=.42) and Valence (F(1, 25)=47.51, p < .001, η2=.66) and a significant interaction between Emotion and Valence (F(1, 25)=179.10, p < .001, η2==.88).The positivity for happy photos was rated stronger (M=4.76, SD=.85) compared to the positivity for sad photos (M=1.37, SD=.65). The negativity for sad photos (M=3.47, SD=.82) was rated stronger compared to the negativity for happy photos (M=.80, SD=.98). The simple effect tests revealed that all the ratings were significantly different from each other (ps<.001). The SCS score was −6.19, indicating participants were more interdependent than independent.
Brain Activity during the Presentation of Photos
The whole-brain 2 × 2 ANOVA did not reveal any significant main effect of Valence or Emotion. Significant Valence-by-Emotion interaction was revealed by the contrast [−1 1 1 −1] in the precuneus (k=121, Z=4.42, x/y/z=12/-64/46), right middle frontal gyrus (MFG) (k=127, Z=4.33, x/y/z=42/35/34), and the PCC (k=186, Z=3.86, x/y/z=3/-22/31) (Fig. 2A).
Figure 2.
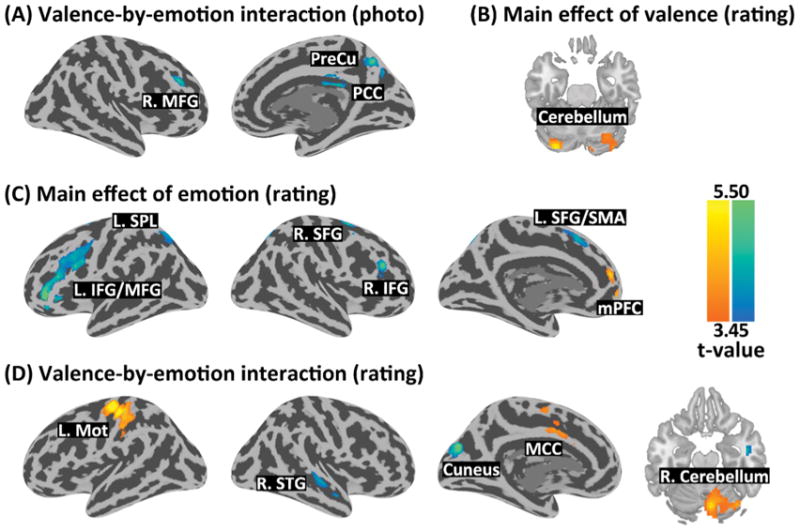
Brain regions showing1: (A) valence-by-emotion interaction during photo display; (B) main effect of valence during rating; (C) main effect of emotion during rating; and (D) valence-by-emotion interaction during rating.
Post-hoc analyses showed that the precuneus activity did not differ in response to happy_positivity vs. happy_negativity (t(25)= −0.59, p=.560), but was significantly greater in response to sad_positivity compared to sad_negativity (t(25)=2.83, p=.009). The right MFG showed marginally weaker response to happy_positivity compared to happy_negativity (t(25)= −1.93, p=.064), and significantly stronger response to sad_positivity compared to sad_negativity (t(25)=2.29, p=.031). The PCC activity did not differ in response to happy_positivity vs. happy_negativity (t(25)=−1.31, p=.203), but was marginally greater in response to sad_positivity compared to sad_negativity (t(25)=1.76, p=.090).
Brain Activity during Rating
The whole-brain 2 × 2 ANOVA revealed a significant main effect of Valence at the bilateral cerebellum which showed greater activity in response in the positivity vs. negativity conditions (k=279, Z=4.81 & 3.72, x/y/z=−30/−82/−23 & 21/−73/−20) (Fig. 2B). There was a main effect of Emotion in the medial prefrontal cortex (mPFC), which showed greater response in the happy vs. sad conditions (k=301, Z=5.09, x/y/z=0/56/1). A main effect of Emotion was also found in the left inferior frontal gyrus (k=544, Z=4.82, x/y/z=−42/8/28), right inferior/middle frontal gyrus (k=106, Z=4.49, x/y/z=51/32/25), left superior frontal gyrus/supplementary motor area (k=154, Z=4.20, x/y/z=−27/2/61), right superior frontal gyrus (k=121, Z=4.55, x/y/z=27/−1/61), left superior parietal lobule (k=141, Z=3.74, x/y/z=−21/−61/52), and right superior parietal lobule (k=126, Z=3.97, x/y/z=15/−70/49), which showed greater response in the sad vs. happy conditions (Fig. 2C). A significant Valence-by- Emotion interaction was revealed by the contrast [1 −1 −1 1] in the left motor cortex (k=663, Z=5.36, x/y/z=−36/−22/70), right cerebellum/cerebellar vermis (k=380, Z=4.48, x/y/z=6/−73/−17), and mid-cingulate cortex (k=140, Z=3.95, x/y/z=−6/2/43). The reverse contrast [−1 1 1 −1] showed interaction in the cuneus (k=236, Z=4.85, x/y/z=−9/−79/31) and the right superior temporal gyrus (k=167, Z=3.80, x/y/z=51/−19/−5) (Fig. 2D).
Post-hoc analyses showed that the left motor cortex activity was significantly stronger in response to happy_positivity vs. happy_negativity (t(25)=4.43, p < .001), and did not differ in response to sad_positivity and sad_negativity (t(25)=−1.09, p=.286). The right cerebellar activity was significantly stronger in response to happy_positivity vs. happy_negativity (t(25)=4.95, p < .001), and was marginally weaker in response to sad_positivity vs. sad_negativity (t(25)=−1.76, p=0.091). The mid-cingulate activity was significantly stronger in response to happy_positivity vs. happy_negativity (t(25)=3.10, p=.005), and was marginally weaker in response to sad_positivity and sad_negativity (t(25)=−1.77, p=.089). The cuneus activity was significantly weaker in response to happy_positivity vs. happy_negativity (t(25)=−4.70, p < .001), and did not differ in response to sad_positivity and sad_negativity (t(25)=1.11, p=.278). The right superior temporal activity was significantly weaker in response to happy_positivity vs. happy_negativity (t(25)=−5.03, p < .001), and did not differ in response to sad_positivity and sad_negativity (t(25)=0.64, p=.527).
DISCUSSION
The present research aimed to understand how the human brain encodes positivity and negativity of emotions via the emotion induced by photo stimuli, and the rating processes in response to them. While the behavioral results in the current work are consistent with previous findings [1] and support the claim that individuals can process both positivity and negativity when viewing happy and sad photos, the fMRI results further revealed the underlying neural substrates.
First, the imaging results during the photo viewing revealed an interaction between Valence and Emotion at the PCC/precuneus and right middle frontal gyrus (MFG). The PCC/precuneus plays a key role in experiencing positivity is consistent with the findings of previous neuroimaging studies of emotion; both the PCC and precuneus were found to be associated with subjective happiness when participants were asked to rate subjective feelings of happiness and life satisfaction [12]. The PCC/precuneus also plays an important role in episodic memory retrieval [22,23]. Recollecting one’s own experiences might be even more critical when participants tried to assess their feelings of positivity when viewing sad photos, because intuitive emotional responses to sad photos are negative. Altogether, this explains why and how the PCC/precuneus was only activated for positivity of sadness in the current paradigm.
The right MFG is related to attention and working memory [24]. This suggests that the right MFG was activated in order for the participants to control and pay more attention towards their feelings of contradicting emotions, negativity of happiness, and positivity of sadness. However, the effect was only marginally significant for sadness, since the difference between valences of sadness was smaller compared to happiness. Also, this supports the notion that East Asians tend to have stronger positivity of sadness compared to Westerners [1].
There was no significant activation for negativity minus positivity for neither happiness nor sadness. Neural structures such as the amygdala and insula are frequently activated by facial expressions of others [25,26,27]. This is not surprising because we contrasted positivity minus negativity (or the reverse) judgments, and this analysis might have reduced the effect of arousal on brain activity to a minimum degree.
The imaging results for rating showed more complex activation, because it involved more cognitive attention. The bilateral cerebellum was activated in response to positivity relative to negativity for a main effect of Valence. Cerebellum is associated with higher emotion processing [28,29]. The mPFC was activated in response to happiness relative to sadness for a main effect of Emotion. The mPFC is known for appraising the extent of personal association with emotional stimuli [11].
The bilateral inferior frontal gyrus, left middle frontal gyrus, right superior frontal gyrus, and bilateral superior parietal lobule were activated in response to sadness relative to happiness for a main effect of emotion. The bilateral inferior frontal gyrus is frequently activated in response to sad and negative emotions in general, and triggers other areas related to memory retrieval and empathy [30,31]. The left middle frontal gyrus and the right superior frontal gyrus are found to be related to emotion regulation [32] as well as depression [33]; it might be possible that sadness requires more complex neurocognitive processing compared to happiness.
The main effects were qualified by an interaction between Valence and Emotion; this involved the left motor cortex, right cerebellum, mid-cingulate cortex, cuneus, and right superior temporal gyrus activations. The left motor cortex was activated most strongly for positivity of happiness compared to negativity of happiness. There was no difference between the positivity and negativity of sadness, which could be due to the relatively strong positivity of sadness, while differences of positivity and negativity of happiness were much stronger. A simple account of the left motor activity was that participants pressed buttons more frequently to produce the rating scores. The cerebellum is again known for higher emotion processing [28,29]. On the other hand, mid-cingulate activation was stronger for positivity of happiness and negativity of sadness compared to negativity of happiness and positivity of sadness, respectively. The mid-cingulate was activated when there was unpleasant affect and motor interaction when unpleasantness and neutral stimuli were examined [34]. However, we found that this also was the case for happiness, suggesting that the mid-cingulate area might be related to the salience of emotions. The reverse interaction showed cuneus and right superior temporal gyrus activation. The cuneus was found to be related to negative emotion, particularly relevant to negative autobiographical memory [35], and negative emotion regulation processes [17,36]. Also, recent findings showed that the superior temporal gyrus is activated in response to some negative emotions such as sadness and depression [15,37].
There are caveats to overcome. First, we tested only two emotions (i.e., happiness and sadness) in the current work. Although many studies use happiness as a representative positive emotion and sadness as a representative negative emotion, including additional emotions could be useful. Second, it is possible that the results are due to emotion regulation; determining what extent emotion regulation was involved could be informative. Lastly, the current study only scanned female participants. Given the sex differences in subjective feelings of emotion [38] and brain activity in response to emotion [19,20], future research should test male participants.
Finally, the previous fMRI findings suggest cross-cultural differences in brain responses to emotion such that European Americans showed greater activity in circuits associated with affect and reward (bilateral ventral striatum, left caudate) while viewing excited versus calm expressions than did Chinese [39]. This can be addressed by employing cross-cultural comparisons.
In summary, ourt brain imaging findings provide evidence for neural activity underlying the processing of positivity and negativity when viewing happy and sad photos, and rating processes. There was stronger activation in PCC/precuneus for positivity than negativity of sadness, but no differences in PCC/precuneus for positivity and negativity of happiness. The cerebellum was activated during the rating processes for positivity for both happiness and sadness. The current findings help us to understand and better explain the mixed feelings that we often experience.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Projects 31470986; 31421003; 91332125) to S.H. S.A., X.H., and B.W. contributed equally to the experiment. Z.S. was supported by the NIDAT32 Translational Addiction Research postdoctoral fellowship (DA028874).
Footnotes
Regions in red/yellow were identified by the following contrasts applied to happy_positive, happy_negative, sad_positive, and sad_negative: (A)&(D) [1 −1 −1 1]; (B) [1 −1 1 −1]; and (C) [1 1 −1 −1]. Regions in blue/green were identified by the reverse contrasts. L/R.: left/right; SFG/MFG/IFG: superior/middle/inferior frontal gyrus; PreCu: precuneus; PCC/MCC: posterior/mid-cingulate cortex; SPL: superior parietal lobule; SMA: supplementary motor area; mPFC: medial prefrontal cortex; Mot: motor cortex; STG: superior temporal gyrus.
DECLARATIONS OF INTEREST
None.
References
- 1.An S, Ji LJ, Marks MJ, Zhang Z. Two sides of emotion: exploring positivity and negativity in six basic emotions across cultures. Frontiers in Psychology. 2017;8:610. doi: 10.3389/fpsyg.2017.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekman P. Are there basic emotions? Psychological Review. 1992;99:550–553. doi: 10.1037/0033-295x.99.3.550. [DOI] [PubMed] [Google Scholar]
- 3.Plutchik RE, Conte HR. Circumplex models of personality and emotions. American Psychological Association; Washington, DC: 1997. [Google Scholar]
- 4.Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. [Google Scholar]
- 5.DeNeve KM. Happy as an extraverted clam? The role of personality for subjective well-being. Current Directions in Psychological Science. 1999;8:141–144. [Google Scholar]
- 6.Diener E, Seligman ME. Very happy people. Psychological Science. 2002;13:81–84. doi: 10.1111/1467-9280.00415. [DOI] [PubMed] [Google Scholar]
- 7.Gruber J, Mauss IB, Tamir M. A dark side of happiness? How, when, and why happiness is not always good. Perspectives on Psychological Science. 2011;6:222–233. doi: 10.1177/1745691611406927. [DOI] [PubMed] [Google Scholar]
- 8.Cacioppo JT, Berntson GG, Norris CJ, Gollan JK. The evaluative space model. Handbook of Theories of Social Psychology: Volume One. 2011;1:50. [Google Scholar]
- 9.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Sato W, Yoshikawa S, Kochiyama T, Matsumura M. The amygdala processes the emotional significance of facial expressions: an fMRI investigation using the interaction between expression and face direction. Neuroimage. 2004;2:1006–1013. doi: 10.1016/j.neuroimage.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;2:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 12.Sato W, Kochiyama T, Uono S, Kubota Y, Sawada R, Yoshimura S, Toichi M. The structural neural substrate of subjective happiness. Scientific Reports. 2015;5 doi: 10.1038/srep16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human brain mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson AE, Voyer D. Sex differences in the ability to recognise non-verbal displays of emotion: A meta-analysis. Cognition and Emotion. 2014;28:1164–1195. doi: 10.1080/02699931.2013.875889. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Fan Y, Mao L. Gender difference in empathy for pain: an electrophysiological investigation. Brain Research. 2008;1196:85–93. doi: 10.1016/j.brainres.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 20.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 22.Francis MM, Hummer TA, Vohs JL, Yung MG, Liffick E, Mehdiyoun NF, Radnovich AJ, McDonald BC, Saykin AJ, Breier A. Functional neuroanatomical correlates of episodic memory impairment in early phase psychosis. Brain Imaging and Behavior. 2016;10:1–11. doi: 10.1007/s11682-015-9357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 24.Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Frontiers in systems neuroscience. 2015:9. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. NeuroImage. 2007;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Fan Y, Xu X, Qin J, Wu B, Wang X, Aglioti SM, Mao L. Empathic neural responses to others’ pain are modulated by emotional contexts. Human Brain Mapping. 2009;30:3227–3237. doi: 10.1002/hbm.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng F, Liu Q, Li H, Fang F, Han S. Task modulations of racial bias in neural responses to others’ suffering. NeuroImage. 2014;88:263–270. doi: 10.1016/j.neuroimage.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Schutter DJ, Van Honk J. The cerebellum on the rise in human emotion. The Cerebellum. 2005;4:290–294. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- 29.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Cognitive Brain Research. 2003;17:613–620. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- 31.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 32.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. The Journal of neuroscience. 2001 doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KRR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. American Journal of Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 34.Pereira JD, Sansom SN, Smith J, Dobenecker MW, Tarakhovsky A, Livesey FJ. Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proceedings of the National Academy of Sciences. 2010;107(36):15957–15962. doi: 10.1073/pnas.1002530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biological psychiatry. 2009;65:361–366. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sander D, Grandjean D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, Vuilleumier P. Emotion and attention interactions in social cognition: brain regions involved in processing anger prosody. Neuroimage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Habel U, Klein M, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26:206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt DP, Realo A, Voracek M, Allik J. Why can’t a man be more like a woman? Sex differences in. Big Five personality traits across 55 cultures. Journal of Personality and Social Psychology. 2008;94:168–182. doi: 10.1037/0022-3514.94.1.168. [DOI] [PubMed] [Google Scholar]
- 39.Park B, Tsai JL, Chim L, Blevins E, Knutson B. Neural evidence for cultural differences in the valuation of positive facial expressions. Social Cognitive and Affective Neuroscience. 2016;11:243–252. doi: 10.1093/scan/nsv113. [DOI] [PMC free article] [PubMed] [Google Scholar]


