Abstract
Background
Membrane transport protein organic anion transporting polypeptide (OATP) 1B3 mediates the cellular uptake of many drugs including anti-cancer drugs (e.g. paclitaxel). In addition to the well-recognized hepatic expression and function of OATP1B3 [herein named liver-type (Lt) OATP1B3], OATP1B3 also expresses in cancers and has been postulated to play a role in cancer therapy presumably by facilitating the influx of anti-cancer drugs. Recently, a cancer type (Ct)-OATP1B3 mRNA variant was identified in colon and lung cancer tissues, which encodes truncated Ct-OATP1B3 with negligible transport activity. Other than colon and lung cancers, reports on mRNA expression of OATP1B3 in other cancers cannot distinguish between the Lt- and Ct-OATP1B3.
Objective
The current studies were designed to characterize the expression of Lt- and Ct-OATP1B3 mRNA in ovarian, prostate, bladder, breast, and lung tissues.
Methods
Lt- and Ct-OATP1B3 isoform-specific PCR primers were utilized to determine the mRNA levels of Lt- and Ct-OATP1B3, respectively. An expression vector expressing green fluorescent protein (GFP)-tagged Lt-OATP1B3 was transiently transfected into the ovarian cancer cell line SKOV3. Confocal live-cell microscopy was utilized to determine the localization of GFP-Lt-OATP1B3 in SKOV3 cells.
Results
For the first time, Lt-OATP1B3 mRNA was detected in ovarian, prostate, bladder and breast cancers. The GFP-Lt-OATP1B3 expressed in the ovarian cancer cell line SKOV3 has a plasma membrane localization pattern as shown by confocal microscopy.
Conclusion
Our findings are supportive of the potential role of Lt-OATP1B3 in cancer therapy.
Keywords: Organic anion transporting polypeptide (OATP) 1B3, mRNA, transport protein, Cancer, Ovary, Prostate, Bladder, Breast, Lung
1. Introduction
Transport proteins are transmembrane proteins that mediate the translocation of endogenous compounds as well as many xenobiotics across cell membranes. Over 400 membrane transport proteins have been recognized in the human genome [1]. ATP binding cassette (ABC) and solute carrier (SLC) are the two major transporter super families. Though transport proteins are distributed ubiquitously, they are primarily expressed in organs that have barrier function such as the intestine, liver, brain, kidney, and placenta [2]. The organic anion transporting polypeptides (OATPs) are a family of transporters and a subgroup of the solute carrier (SLC) transporter superfamily [3]. Although anionic compounds are preferentially transported by OATPs, there are some exceptions. For example, neutral compounds such as ouabain [4] and digoxin [5] and zwitter ionic compounds such as fexofenadine [6] are also transported by OATPs. The OATP-mediated transport process has been found to be Na+-independent; however, the exact transport mechanism(s) is still unclear [7].
Under normal physiological conditions, organic anion transporting polypeptide (OATP) 1B3 is specifically expressed in the human liver [designated as liver-type (Lt)-OATP1B3) in current study] [8]. Lt-OATP1B3 is a transmembrane protein composed of 702 amino acids [8]. It is localized to the basolateral membrane of the hepatocytes and mediates uptake of numerous drugs, including anticancer drugs such as paclitaxel [9], docetaxel [10], methotrexate [11], SN-38 (active metabolite of irinotecan) [12], platinum-based drugs (e.g., cisplatin, carboplatin, oxaliplatin) [13], and some tyrosine kinase inhibitor drugs (e.g., dasatinib, gefitinib, imatinib, sorafenib) [14], from the blood into the liver. Recently, a cancer-type (Ct) OATP1B3, an mRNA variant of the Lt-OATP1B3, was identified in colon, lung, and pancreatic cancer tissues and cell lines [15–17]. Compared with the Lt-OATP1B3, the transcription start site of Ct-OATP1B3 mRNA is located within the second intron of the SLCO1B3 gene [16]. As a result, the predicted open reading frames of Ct-OATP1B3 lack the N-terminal coding region corresponding to Lt-OATP1B3 [15–18].
Lt- and Ct-OATP1B3 have distinct transport function and membrane localization characteristics. First, Lt-OATP1B3 has significantly higher transport activity than Ct-OATP1B3. Endogenous Lt-OATP1B3 in human hepatocytes demonstrates efficient hepatic uptake function, as reported in the literature [1, 19]. When Lt-OATP1B3 was transiently expressed in the SKOV3 ovarian cancer cell line, it efficiently mediated uptake of paclitaxel, a substrate of OATP1B3, into the cells [20]. In contrast, Ct-OATP1B3 has been reported to have minimal or negligible transport activity. The HCT116, HCT8, and LST118 colon cancer cell lines and the Panc-1 pancreatic cell line express endogenous Ct-OATP1B3, but not Lt-OATP1B3 [16, 17]. In these cell lines, transport of CCK-8, a specific substrate of OATP1B3 [21], was negligible [16, 17]. Further, the transport activity of Ct-OATP1B3 that is exogenously expressed in cancer cell lines was ~15–20 fold lower than that of Lt-OATP1B3 [17]. Second, Lt-OATP1B3 is expressed primarily on the plasma membrane, while Ct-OATP1B3 primarily remained in the cytosol. Lt-OATP1B3 is expressed on the plasma membrane in human liver tissue [8], in cultured human hepatocytes [22], and in the HCT8 colon cancer cell line, when expressed exogenously [17]. However, Ct-OATP1B3 has been shown to be predominantly expressed in the cytosol when transfected into HCT8, MDCK II and HEK293T cells [17, 23].
OATP1B3 transport activity has been associated with increased cytotoxic sensitivity of chemotherapy drugs (e.g., paclitaxel). In vitro, transfection of Lt-OATP1B3 in an ovarian cancer cell line resulted in significantly increased accumulation of paclitaxel in the cancer cells, leading to reduced IC50 of paclitaxel to exert cytotoxicity [20]. In endometrial cancer, high expression levels of OATP1B3 protein localized on the plasma membrane, as determined through immunohistochemical staining [24], were significantly associated with longer disease-free survival after chemotherapy involving paclitaxel [24]. These findings suggest that the transport activity of OATP1B3 may have significant clinical relevance for cancer therapy, particularly for drugs that are OATP1B3 substrates. Because it is Lt-OATP1B3, not Ct-OATP1B3, that has high transport activity, it is critical to characterize the specific expression of Lt-OATP1B3 in cancer tissues to understand the role of OATP1B3 transport activity in cancers.
The mRNA of OATP1B3 has been detected in many cancer tissues, including the pancreas, lung, colon, uterus, bladder, thyroid, testis, stomach, prostate, ovary, gastroesophageal, esophagus, endometrium, and cervix [13, 16, 17, 20, 25]. However, other than the recent report in colon and lung cancers [15, 17], reports on mRNA expression of OATP1B3 in other cancers cannot distinguish between the Lt- and Ct-OATP1B3 as the PCR primers can detect the common region of the Lt- and Ct-OATP1B3 [13, 20, 25]. The identification of Ct-OATP1B3 mRNA expression in cancers confounded the understanding of transport function of Lt-OATP1B3 in cancer therapy as it becomes unknown if the Lt-OATP1B3 indeed expresses in cancers other than colon and lung.
The aim of the current study is to specifically characterize the unidentified specific Lt- and Ct-OATP1B3 expression in normal and cancerous tissues, including ovarian, prostate, bladder and breast tissues, using primer sets that distinguish between these two variants of OATP1B3. The expression of Lt- and Ct-OATP1B3 in lung cancer and normal tissues will also be characterized as a comparison.
2. Materials and Methods
Materials
Transfecting agent GenJet™ was purchased from SignaGen Laboratories (Gaithersburg, MD). McCoy's 5A Medium was obtained from American Type Culture Collection (ATCC® 30-2007™, Manassas, VA). Fetal bovine serum (FBS) and antibiotic antimycotic solution were procured from Sigma-Aldrich (St. Louis, MO, USA). Four-compartment 35 × 10 mm CELLVIEW cell culture dishes with integrated glass bottoms were purchased from Greiner Bio One (Monroe, NC).
Human-derived materials
De-identified normal and cancerous human tissues were purchased from the University of North Carolina at Chapel Hill Tissue Procurement Core Facility. Snap-frozen tissue samples were collected from patients undergoing routine primary surgery at the University of North Carolina Hospital. Cancerous and normal tissue characteristics were pathologically determined from representative formalin-fixed paraffin-embedded (FFPE) slides by a certified pathologist from the Department of Pathology and Laboratory Medicine at UNC Health Care at Chapel Hill.
Cell culture
The Human embryonic kidney (HEK) 293 stable cell line expressing Lt-OATP1B3 (HEK293-OATP1B3) was a gift from Dr. Dietrich Keppler [8]. The HEK293 stable cell line expressing a GFP-tagged OATP1B3 was published established previously [26]. The DLD-1 colon cancer cell line was purchased from the University of North Carolina at Chapel Hill Tissue Culture Facility. The SKOV3 ovarian cancer cell line was purchased from American Type Culture Collection. The HEK293-OATP1B3, DLD-1, and SKOV3 cells were cultured in DMEM, RPMI-1640, and McCoy's 5A medium, respectively; all media contained 10% FBS and 1% antibiotic and antimycotic solution. HEK293 stable cell lines were supplemented with Geneticin at 600 µg/ml. All cells were cultured in a humidified atmosphere (95% O2, 5% CO2) at 37 °C.
Transfection and confocal live cell imaging
Transfection of SKOV3 with the construct of pCMV6-GFP-OATP1B3 [26] was conducted using GenJet (SignaGen Laboratories, Rockville, MD) in vitro DNA transfection reagent following the manufacturer’s instructions. Expression of GFP-Lt-OATP1B3 in transiently transfected SKOV3 cells was determined using an Olympus FluoView FV10i-LIV confocal laser scanning microscope (Olympus, Tokyo, Japan), similar to the approach published previously [26].
RNA Isolation and TaqMan real-time reverse transcription (RT) polymerase chain reaction (RT-PCR)
Total RNA from human tissues, HEK293-OATP1B3, and DLD-1 was extracted using the ABI RNA isolation system (Applied Biosystems, Foster City, CA), similar to the approach published previously [27, 28]. TaqMan real-time RT-PCR was conducted using an ABI Prism 7700 system (Applied Biosystems, Foster City, CA) to determine the mRNA levels of Lt- and Ct-OATP1B3, as described previously [27, 28]. The PCR primers were designed to distinguish the cDNA of Lt- from Ct-OATP1B3, based on a previous publication [17]. GAPDH was used as internal control for ovarian tissues, whereas 18S ribosomal RNA was used as internal control for prostate, breast, bladder, and lung tissues. The TaqMan probe and primers sequences (5’−3’) are summarized in Table 1. Fold changes in mRNA levels of examined tissues and cells were evaluated after normalizing the gene expression levels by their respective internal control (2−ΔΔCt method), as previously described [29].
Table 1.
Primers and probe used in TaqMan real-time RT-PCR
| Forward primer (5’-3’) |
Reverse primer (5’-3’) |
Probe (5’-3’) |
|
|---|---|---|---|
| Lt-OATP1B3 | GCTGCAATGGATTCAAGATGT | CATAATGATTCCACCTAGTGCT | TGGCAGCCCTGTCATTCAGCTATA |
| Ct-OATP1B3 | GCAAGAGAAAAACTAGCAGATGT | CATAATGATTCCACCTAGTGCT | TGGCAGCCCTGTCATTCAGCTATA |
| GAPDH | ACCTCAACTACATGGTTTAC | GAAGATGGTGATGGGATTTC | CAAGCTTCCCGTTCTCAGCC |
| 18S rRNA | AGAAACGGCTACCACATCCA | CTCGAAAGAGTCCTGTATTGT | AGGCAGCAGGCGCGCAAATTAC |
Data analysis
McNemar’s test was used to assess the difference in expression frequency of Lt- and Ct-OATP1B3 mRNA by tissue type (cancerous and normal) and origin. The Fisher/Chi-square test or McNemar’s test was used to assess the difference in the expression frequency of Lt- and Ct-OATP1B3 mRNA between cancerous and normal tissues, depending on whether data were paired or not. When a test was based on both paired and unpaired data (bladder), Fisher’s method for combining P values from independent tests was used. A P value of <0.05 was considered statistically significant. The SAS software (version 9.4, Cary, NC) was used for statistical analyses.
3. Results
Specific detection of Ct-OATP1B3 by TaqMan real-time (RT)-PCR
In a study that used a primer pair to detect the common region, Lt- and Ct-OATP1B3 mRNA was below the detection limit in HEK293 cells [30]. The DLD-1 colon cancer cell line has been reported to express Ct-OATP1B3, but not Lt-OATP1B3 [17]. As shown in supplementary Fig. S1 A, PCR primers designed to specifically determine Lt-OATP1B3 detected high expression of Lt-OATP1B3 mRNA in HEK293-OATP1B3 cells. However, Lt-OATP1B3 mRNA was below the detection limit in the DLD-1 cells. Using the primer pair designed to specifically detect Ct-OATP1B3, Ct-OATP1B3 mRNA was detected in DLD-1 cells (supplementary Fig. S1 B) and SKOV3 ovarian cancer cells (supplementary Fig. S1 C), but not in HEK293-OATP1B3 cells. Lt-OATP1B3 mRNA levels in HEK293-OATP1B3 were therefore used as the 100% control to normalize the Lt-OATP1B3 mRNA levels determined in all tissues. Ct-OATP1B3 mRNA levels in DLD-1 were used as the 100% control to normalize the Ct-OATP1B3 mRNA levels determined in prostate, bladder, breast, and lung tissues. Ct-OATP1B3 mRNA levels in SKOV3 were used as the 100% control to normalize the Ct-OATP1B3 mRNA levels determined in ovarian tissues.
Expression profile of Lt- and Ct-OATP1B3 mRNA in human ovarian tissues
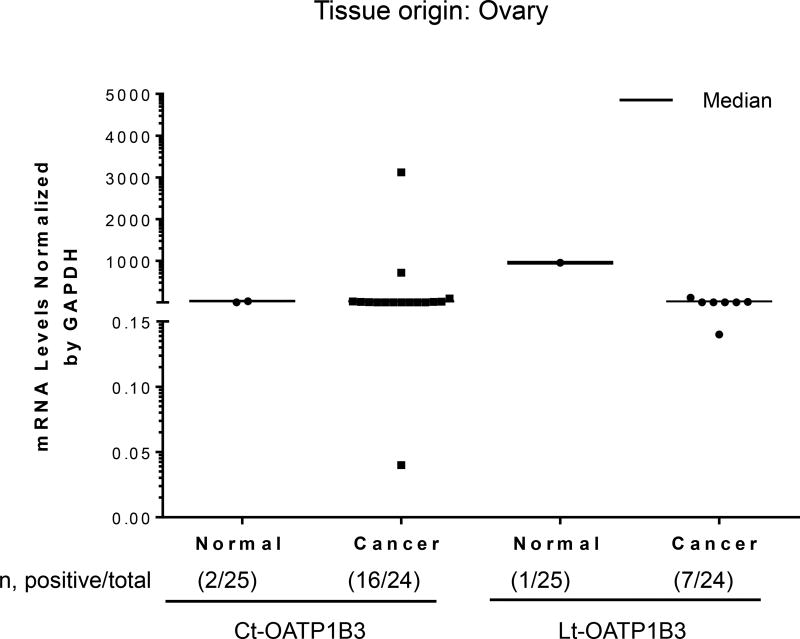
A total of 24 samples of cancerous tissue and 25 samples of normal tissues of ovarian origin were analyzed for the specific expression of Lt- and Ct-OATP1B3 mRNA. Expression of Ct-OATP1B3 mRNA was detected in 66.7% (16/24) of cancerous tissues and 8% (2/25) of normal tissues (Figure 1). Expression of Lt-OATP1B3 mRNA was detected in 29.2% (7/24) of cancerous tissues and 4% (1/25) of normal tissues (Figure 1). In cancerous tissues, Ct-OATP1B3 mRNA has a significantly higher expression frequency than Lt-OATP1B3 mRNA (p=0.0027). However, there was no significant difference in expression frequency between Ct- and Lt-OATP1B3 mRNA in normal tissues (p=0.56). Both Lt- and Ct-OATP1B3 mRNA had significantly higher expression frequency in cancerous than in normal tissues (29.2 vs. 4%, p=0.0232 for Lt-OATP1B3 and 66.7 vs. 8%, p<0.0001 for Ct-OATP1B3).
Figure 1. Expression profiles of Ct- and Lt-OATP1B3 mRNA in patients with ovarian cancer.
Ct- and Lt-OATP1B3 mRNA levels in normal and cancerous ovary tissues. The number of samples with positive expression over the total number of clinical samples in each group is shown in parentheses. The median value in mRNA expression level for each group is shown as a solid horizontal line.
Expression profile of Lt- and Ct-OATP1B3 mRNA in human prostate tissues
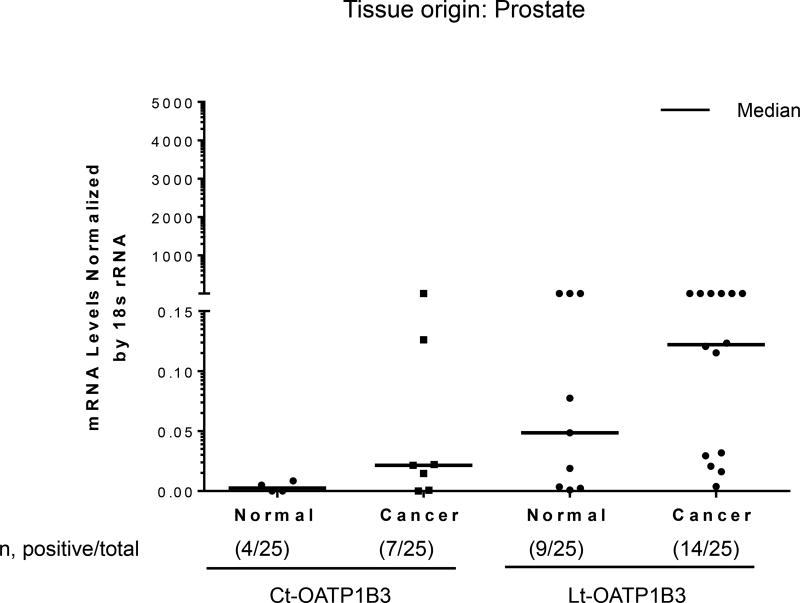
Twenty-five samples of normal prostate tissue and 25 samples of prostate cancer tissue were analyzed for the specific expression of Lt- and Ct-OATP1B3 mRNA. Expression of Ct-OATP1B3 mRNA was detected in 28% (7/25) of cancerous tissues and 16% (4/25) of normal tissues (Figure 2). Expression of Lt-OATP1B3 mRNA was detected in 56% (14/25) of cancerous tissues and 36% (9/25) of normal tissues (Figure 2). In cancerous tissues, Lt-OATP1B3 mRNA had a significantly higher expression frequency than Ct-OATP1B3 mRNA (p=0.0348). However, in normal tissues, there was no statistically significant difference in Ct- and Lt-OATP1B3 mRNA expression (p=0.096).
Figure 2. Expression profiles of Ct- and Lt-OATP1B3 mRNA in patients with prostate cancer.
Ct- and Lt-OATP1B3 mRNA levels in normal and cancerous prostate tissues. The number of samples with positive expression over the total number of clinical samples in each group is shown in parentheses. The median value in mRNA expression level for each group is shown as a solid horizontal line.
Expression profile of Lt- and Ct-OATP1B3 mRNA in human bladder tissues
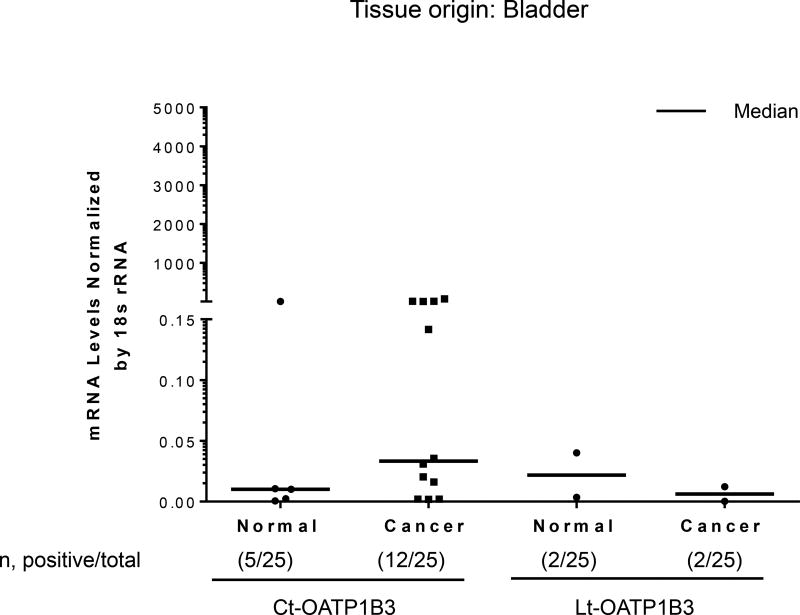
Twenty-five samples of bladder cancer tissues and 25 samples of normal bladder tissues were analyzed for the specific expression of Lt- and Ct-OATP1B3 mRNA. Expression of Ct-OATP1B3 mRNA was detected in 48% (12/25) of cancerous tissues and 20% (5/25) of normal tissues (Figure 3). Expression of Lt-OATP1B3 mRNA was detected in 8% (2/25) of both cancerous and normal tissues (Figure 3). In cancerous tissues, Ct-OATP1B3 mRNA had a significantly higher expression frequency than Lt-OATP1B3 mRNA (p=0.0075). In normal tissues, there was no statistically significant difference in the expression frequency of Ct- and Lt-OATP1B3 mRNA (p=0.26).
Figure 3. Expression profiles of Ct- and Lt-OATP1B3 mRNA in patients with bladder cancer.
Ct- and Lt-OATP1B3 mRNA levels in normal and cancerous bladder tissues. The number of samples with positive expression over the total number of clinical samples in each group is shown in parentheses. The median value in mRNA expression level for each group is shown as a solid horizontal line.
Expression profile of Lt- and Ct-OATP1B3 mRNA in human breast tissues
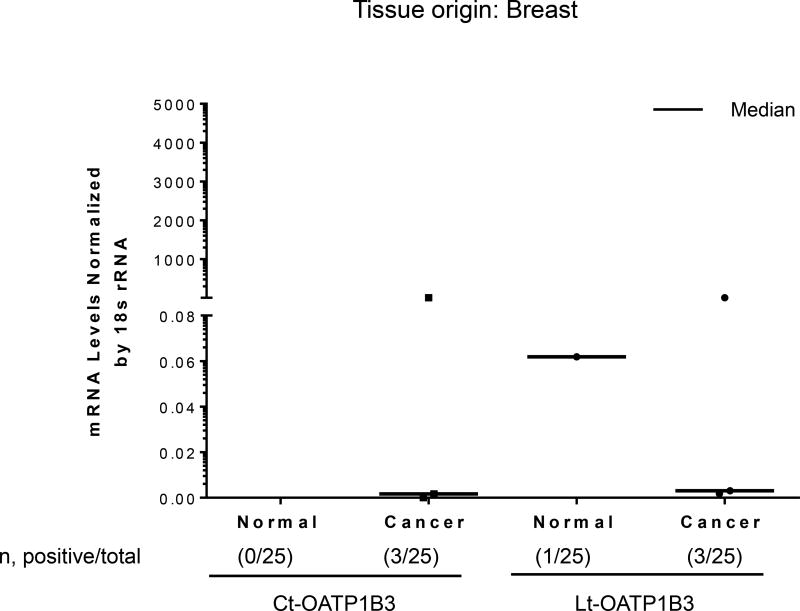
Twenty-five samples of breast cancer tissues and 25 normal breast tissues were analyzed for the specific expression of Lt- and Ct-OATP1B3 mRNA. Expression of Ct-OATP1B3 mRNA was detected in 12% (3/25) of cancerous tissues, while no Ct-OATP1B3 was detected in normal tissue (Figure 4). Expression of Lt-OATP1B3 mRNA was detected in 12% (3/25) of cancerous tissues and 4% (1/25) of normal tissues (Figure 4). In cancerous tissues, there was no statistically significant difference in the expression frequency of Lt- and Ct-OATP1B3 mRNA (p=1.00). Ct-OATP1B3 was not detected in normal breast tissues.
Figure 4. Expression profiles of Ct- and Lt-OATP1B3 mRNA in patients with breast cancer.
Ct- and Lt-OATP1B3 mRNA levels in normal and cancerous breast tissues. The number of samples with positive expression over the total number of clinical samples in each group is shown in parentheses. The median value in mRNA expression level for each group is shown as a solid horizontal line.
Expression profile of Lt- and Ct-OATP1B3 mRNA in human lung tissues
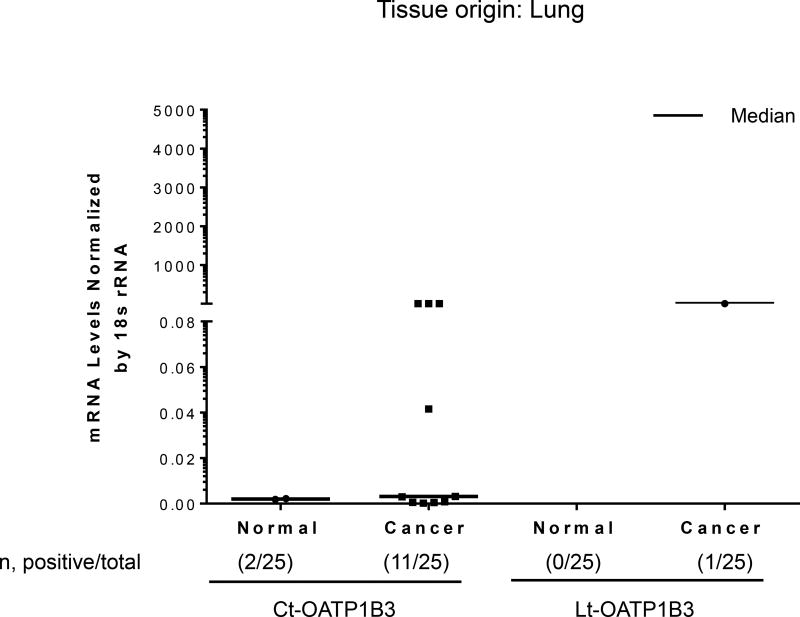
Twenty-five samples of lung cancer tissues and 25 samples of normal lung tissues were analyzed for the specific expression of Lt- and Ct-OATP1B3 mRNA. Expression of Ct-OATP1B3 mRNA was detected in 44% (11/25) of cancerous tissues and 8% (2/25) of normal tissues (Figure 5). Lt-OATP1B3 mRNA was detected in 4% (1/25) of cancerous tissue. None of normal tissues expressed Lt-OATP1B3 mRNA (Figure 5). In cancerous tissues, Ct-OATP1B3 mRNA had a significantly higher expression frequency than Lt-OATP1B3 mRNA (p=0.0016).
Figure 5. Expression profiles of Ct- and Lt-OATP1B3 mRNA in patients with lung cancer.
Ct- and Lt-OATP1B3 mRNA levels in normal and cancerous lung tissues. The number of samples with positive expression over the total number of clinical samples in each group is shown in parentheses. The median value in mRNA expression level for each group is shown as a solid horizontal line.
Plasma membrane localization of Lt-OATP1B3 expressed in ovarian cancer cell line SKOV3
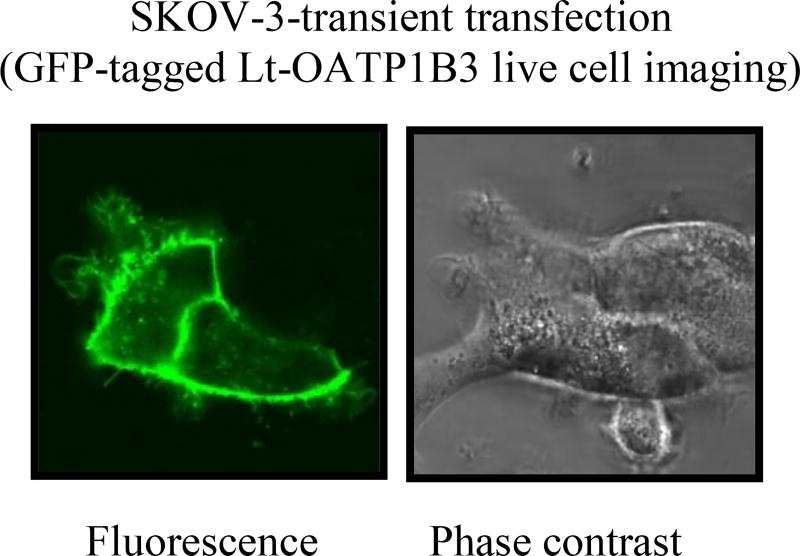
In SKOV3 cell transiently transfected with the construct expressing GFP-tagged Lt-OATP1B3, plasma membrane localization of the GFP-Lt-OATP1B3 was detected by confocal live cell imaging (Fig. 6).
Figure 6. Plasma membrane localization of GFP-Lt-OATP1B3 in SKOV3 ovarian cancer cells.
SKOV3 cells were seeded into 4-compartment 35 × 10mm CELLVIEW cell culture dishes with integrated glass bottoms (Greiner Bio One, Monroe, NC) at a density of 1.5 ×105 cells per compartment. Twenty-four hours after seeding, the cells were transfected with GFP-Lt-OATP1B3 (0.5 µg plasmid DNA per quarter well) using GenJet™ Transfecting agent per the manufacturer’s instructions. Forty-eight hours post-transfection, the culture dish was kept in an Olympus FluoView FV10i-LIV confocal laser scanning microscope (Olympus, Tokyo, Japan) supplemented with 95% O2 and 5% CO2, at a temperature of 37°C. An Olympus UPLSAPO 60× water immersion objective (N/A 1.2) with 473 nm laser excitation and an emission bandpass of 490–590 nm was used to image the cells. Confocal images were acquired using a 0.5 µm Z-step size with 2× Kalman line averaging and 1.0 airy disk confocal aperture (representative images are shown from 3 independent experiments).
4. Discussion
Expression and role of Lt-OATP1B3 in drug disposition in the liver has been well appreciated in hepatic drug disposition [31, 32]. Characterization of the extra-hepatic expression of Lt-OATP1B3 in cancers extends our understanding of the potential role of OATP1B3 in mediating the influx of anti-cancer drugs that are OATP1B3 substrates into cancer cells.
Lt-OATP1B3 mediates the uptake of many clinically important anti-cancer drugs [9–14]. Thus, understanding the specific Lt-OATP1B3 expression in cancers has potential clinical relevance for cancer therapy. The current published OATP1B3 antibodies recognize the common amino acids region of Lt- and Ct-OATP1B3; therefore, these antibodies are not anticipated to distinguish between Ct- and Lt-OATP1B3 at the protein level [8, 17, 22]. Due to the potential for simultaneous expression of Lt- and Ct-OATP1B3 in cancers, it is important to characterize whether Lt-OATP1B3 indeed transcribes in cancers, using Lt- and Ct-OATP1B3-specific PCR primers. The current study for the first time reports the expression of specific Lt-mRNA in prostate, ovarian, breast, and bladder cancer tissues. Two laboratories have reported independently that Lt-OATP1B3 protein retains its transport function when exogenously expressed in colon and ovarian cancer cells in vitro [16, 17]. In the HCT8 colon cancer cell line, which does not express OATP1B3, exogenously expressed Lt-OATP1B3 that was localized primarily on the plasma membrane [17] after transient transfection. In the current study, we observed the plasma membrane localization of transiently transfected GFP-Lt-OATP1B3 in the SKOV3 ovarian cancer cell line (Figure 6).
Many of the anti-cancer drugs that are OATP1B3 substrates are widely used for treatment of the cancer types studied here. For example, paclitaxel, docetaxel, methotrexate, cisplatin, and carboplatin are all OATP1B3 substrates [9–11, 13]. These drugs have been approved for treatment of ovarian cancer [33–35], certain prostate cancers [36], breast cancer [37], bladder cancer [35], and lung cancers [33]. In endometrial cancer, high expression levels of OATP1B3, as determined by immunohistochemical staining, were significantly correlated with type I tumors [24], and were associated with disease-free survival and/or longer overall survival after paclitaxel and carboplatin chemotherapy [24]. Muto et al. reported that OATP1B3 protein expression is correlated with disease-free and overall survival in patients with breast cancer [38]. In the present study, the expression frequency of Lt-OATP1B3 mRNA in prostate cancer tissues (56%) appeared to be higher than that in ovary (29%), bladder (8%), breast (12%), and lung (4%) cancer tissues. These findings are of great interest since expression of Lt-OATP1B3 in cancer could be associated with better therapeutic outcomes due to the ability of Lt-OATP1B3 protein to retain its transport function. The significance of these findings warrant further characterization using a larger sample size.
Due to the negligible transport function of Ct-OATP1B3 observed when expressed in cancer cells in vitro [16, 17], the clinical significance of Ct-OATP1B3 expression in cancers is unlikely to be related to its substrate transport function; however, a transport protein might have additional biological functions beyond its transport function. For example, the efflux transporter P-glycoprotein possesses anti-apoptotic properties that are independent of its transport activity [39]. Expression of Ct-OATP1B3 mRNA has been identified previously in colon, lung, and pancreatic cancers [17]. Here, we extended these findings to four additional cancer types, ovarian, prostate, bladder and breast cancer, and reproducibly detected Ct-OATP1B3 mRNA expression in lung cancers. We also present the novel finding that Ct-OATP1B3 mRNA expression frequency in ovarian, bladder, and lung cancer was significantly higher than that in the respective normal tissues. It would be interesting to further explore whether Ct-OATP1B3 expression is associated with cancer stage and whether it can be used as a diagnostic biomarker.
Our assay was not designed to compare the copy numbers of Lt- and Ct-OATP1B3 mRNA. Therefore, the relative expression of Lt- vs. Ct-OATP1B3 mRNA in these tissues remains unknown, and the expression levels of Lt- or Ct-OATP1B3 was not compared among different tissues. Future studies are warranted to compare the amounts of Lt- and Ct-OATP1B3 in relation to the expression in the liver tissues, similar to previous work [16].
5. Conclusion
The current study characterized the specific expression of Ct- and Lt-OATP1B3 mRNA expression in ovarian, prostate, bladder, breast, and lung cancers and demonstrated that Ct-OATP1B3 has significantly higher expression frequency in ovarian, bladder, and lung cancers than in respective normal tissues. Our findings are supportive for potential roles of Lt-OATP1B3 in cancer therapy.
Supplementary Material
Acknowledgments
This research was supported by NIH R01 GM094268 [W. Y]. The Olympus FV10i confocal microscope is supported by equipment grants from the NIH to Dr. Wei Yue (R01GM094268-06S1) and from the Presbyterian Health Foundation to Dr. Kelly Standifer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the University of North Carolina Animal Clinical Chemistry and Gene Expression Laboratories for their technical support in conducting RNA extraction and real-time RT-PCR. We thank Dr. Dietrich Keppler for providing the HEK293-OATP1B3 cell line.
Nonstandard Abbreviations
- HEK293
Human embryonic kidney 293
- OATP
organic anion transporting polypeptide
- Lt
Liver-type
- Ct
Cancer-type
Footnotes
The published manuscript is available at EurekaSelect via http://www.eurekaselect.com/ 10.2174/1872312812666180326110146
Conflict of Interest:
The author(s) declare no competing financial interests.
References
- 1.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14(8):543–60. doi: 10.1038/nrd4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447(5):653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 4.Bossuyt X, Muller M, Meier PJ. Multispecific amphipathic substrate transport by an organic anion transporter of human liver. J Hepatol. 1996;25(5):733–8. doi: 10.1016/s0168-8278(96)80246-7. [DOI] [PubMed] [Google Scholar]
- 5.Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120(2):525–33. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, Sugiyama Y. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33(10):1477–81. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- 7.Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Mol Aspects Med. 2013;34(2–3):396–412. doi: 10.1016/j.mam.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.König J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J. Biol. Chem. 2000;275(30):23161–23168. doi: 10.1074/jbc.M001448200. [DOI] [PubMed] [Google Scholar]
- 9.Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4(8):815–8. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- 10.Lee HH, Leake BF, Teft W, Tirona RG, Kim RB, Ho RH. Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance. Mol Cancer Ther. 2015;14(4):994–1003. doi: 10.1158/1535-7163.MCT-14-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, Nunoki K, Sato E, Kakyo M, Nishio T, Sugita J, Asano N, Tanemoto M, Seki M, Date F, Ono K, Kondo Y, Shiiba K, Suzuki M, Ohtani H, Shimosegawa T, Iinuma K, Nagura H, Ito S, Matsuno S. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120(7):1689–99. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, Goto J, Hishinuma T, Mano N. Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett. 2008;260(1–2):163–9. doi: 10.1016/j.canlet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Lancaster CS, Sprowl JA, Walker AL, Hu S, Gibson AA, Sparreboom A. Modulation of OATP1B-type transporter function alters cellular uptake and disposition of platinum chemotherapeutics. Mol Cancer Ther. 2013;12(8):1537–44. doi: 10.1158/1535-7163.MCT-12-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman EI, Hu S, Roberts JL, Gibson AA, Orwick SJ, Li L, Sparreboom A, Baker SD. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013;19(6):1458–66. doi: 10.1158/1078-0432.CCR-12-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai M, Furihata T, Matsumoto S, Ishii S, Motohashi S, Yoshino I, Ugajin M, Miyajima A, Matsumoto S, Chiba K. Identification of a new organic anion transporting polypeptide 1B3 mRNA isoform primarily expressed in human cancerous tissues and cells. Biochem Biophys Res Commun. 2012;418(4):818–23. doi: 10.1016/j.bbrc.2012.01.115. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Furihata T, Ishii S, Nagai M, Harada M, Shimozato O, Kamijo T, Motohashi S, Yoshino I, Kamiichi A, Kobayashi K, Chiba K. Unique expression features of cancer-type organic anion transporting polypeptide 1B3 mRNA expression in human colon and lung cancers. Clin Transl Med. 2014;3:37. doi: 10.1186/s40169-014-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakkar N, Kim K, Jang ER, Han S, Kim K, Kim D, Merchant N, Lockhart AC, Lee W. A cancer-specific variant of the SLCO1B3 gene encodes a novel human organic anion transporting polypeptide 1B3 (OATP1B3) localized mainly in the cytoplasm of colon and pancreatic cancer cells. Mol Pharm. 2013;10(1):406–16. doi: 10.1021/mp3005353. [DOI] [PubMed] [Google Scholar]
- 18.Imai S, Kikuchi R, Tsuruya Y, Naoi S, Nishida S, Kusuhara H, Sugiyama Y. Epigenetic regulation of organic anion transporting polypeptide 1B3 in cancer cell lines. Pharmaceutical research. 2013;30(11):2880–90. doi: 10.1007/s11095-013-1117-1. [DOI] [PubMed] [Google Scholar]
- 19.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. British journal of pharmacology. 2009;158(3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svoboda M, Wlcek K, Taferner B, Hering S, Stieger B, Tong D, Zeillinger R, Thalhammer T, Jager W. Expression of organic anion-transporting polypeptides 1B1 and 1B3 in ovarian cancer cells: relevance for paclitaxel transport. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2011;65(6):417–26. doi: 10.1016/j.biopha.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J. Pharmacol. Exp. Ther. 2004;311(1):139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- 22.Powell J, Farasyn T, Kock K, Meng X, Pahwa S, Brouwer KL, Yue W. Novel mechanism of impaired function of organic anion-transporting polypeptide 1B3 in human hepatocytes: post-translational regulation of OATP1B3 by protein kinase C activation. Drug Metab. Dispos. 2014;42(11):1964–1970. doi: 10.1124/dmd.114.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun SE, Thakkar N, Oh Y, Park JE, Han S, Ryoo G, Hahn H, Maeng SH, Lim YR, Han BW, Lee W. The N-terminal region of organic anion transporting polypeptide 1B3 (OATP1B3) plays an essential role in regulating its plasma membrane trafficking. Biochem Pharmacol. 2017;131:98–105. doi: 10.1016/j.bcp.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Ogane N, Yasuda M, Kameda Y, Yokose T, Kato H, Itoh A, Nishino S, Hashimoto Y, Kamoshida S. Prognostic value of organic anion transporting polypeptide 1B3 and copper transporter 1 expression in endometrial cancer patients treated with paclitaxel and carboplatin. Biomed Res. 2013;34(3):143–51. doi: 10.2220/biomedres.34.143. [DOI] [PubMed] [Google Scholar]
- 25.Pressler H, Sissung TM, Venzon D, Price DK, Figg WD. Expression of OATP family members in hormone-related cancers: potential markers of progression. PloS one. 2011;6(5):e20372. doi: 10.1371/journal.pone.0020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahwa S, Alam K, Crowe A, Farasyn T, Neuhoff S, Hatley O, Ding K, Yue W. Pretreatment with Rifampicin and Tyrosine Kinase Inhibitor Dasatinib Potentiates the Inhibitory Effects toward OATP1B1- and OATP1B3-Mediated Transport without Affecting Plasma Membrane Localization of the Transporters. J Pharm Sci. 2017 doi: 10.1016/j.xphs.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci U S A. 2002;99(7):4602–7. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue W, Lee J, Abe K, Sugiyama Y, Brouwer KLR. Decreased breast cancer resistance protein (Bcrp) expression and function in livers and sandwich-cultured hepatocytes from Mrp2-deficient TR- rats. Drug Metab Dispos. 2011;39:441–447. doi: 10.1124/dmd.110.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Ichihara S, Kikuchi R, Kusuhara H, Imai S, Maeda K, Sugiyama Y. DNA methylation profiles of organic anion transporting polypeptide 1B3 in cancer cell lines. Pharmaceutical research. 2010;27(3):510–6. doi: 10.1007/s11095-010-0064-3. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration, C. f. D. E. a. R. C. Office of Clinical Pharmacology, Guidance and Policy Draft guidance: In Vitro Metabolism- and Transporter- Mediated Drug-Drug Interaction Studies Guidance for Industry. 2017 [Google Scholar]
- 32.Tweedie D, Polli JW, Berglund EG, Huang SM, Zhang L, Poirier A, Chu X, Feng B International Transporter, C. Transporter studies in drug development: experience to date and follow-up on decision trees from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94(1):113–25. doi: 10.1038/clpt.2013.77. [DOI] [PubMed] [Google Scholar]
- 33.Company B-MS. TAXOL Injection package insert. 2011 Apr; [Google Scholar]
- 34.Company B-MS. PARAPLATIN Package insert. 2010 Jul; [Google Scholar]
- 35.Squibb B-M. Cisplatin package insert [Google Scholar]
- 36.Sandoz Docetaxel package insert. 2012 Mar; [Google Scholar]
- 37.DAVA Pharmaceuticals, I. Methotrexate package insert [Google Scholar]
- 38.Muto M, Onogawa T, Suzuki T, Ishida T, Rikiyama T, Katayose Y, Ohuchi N, Sasano H, Abe T, Unno M. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer science. 2007;98(10):1570–6. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tainton KM, Smyth MJ, Jackson JT, Tanner JE, Cerruti L, Jane SM, Darcy PK, Johnstone RW. Mutational analysis of P-glycoprotein: suppression of caspase activation in the absence of ATP-dependent drug efflux. Cell Death Differ. 2004;11(9):1028–37. doi: 10.1038/sj.cdd.4401440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.