Abstract
Fine particulate matter (PM2.5) air pollution and environmental temperatures influence cardiovascular morbidity and mortality. Recent evidence suggests that several air pollutants can promote dyslipidemia; however, the impact of ambient PM2.5 and temperature on high-density lipoprotein (HDL) function remains unclear. We hypothesized that daily exposures to higher levels of ambient PM2.5 and colder outdoor temperatures would impair HDL functionality. Lipoproteins, serum cholesterol efflux capacity (CEC) and HDL oxidation markers were measured twice in 50 healthy adults (age: 32.1 ± 9.6 years) living in southeast Michigan and associated with ambient and personal-level exposures using mixed models. Although prior 7-day mean outdoor temperature (4.4 ± 9.8 °C) and PM2.5 levels (9.1 ± 1.8 μg/m3) were low, higher ambient PM2.5 exposures (per 10 μg/m3) were associated with significant increases in the total cholesterol-to-HDL-C ratio (rolling average lag days 1 and 2) as well as reductions in CEC by −1.93% (lag day 5, p=0.022) and −1.62% (lag day 6, p=0.032). Colder outdoor temperatures (per 10°C) were also associated with decreases in CEC from −0.62 to −0.63% (rolling average lag days 5 and 7, p=0.027 and 0.028). Prior 24-hour personal-level PM2.5 and temperature exposures did not impact outcomes, nor were any exposures associated with changes in HDL-oxidation metrics. In conclusion, we provide the first evidence that ambient PM2.5 (even at low levels) and outdoor temperatures may influence serum CEC, a critical anti-atherosclerotic HDL function.
Keywords: Cholesterol Efflux Capacity, 3-Chlorotyrosine, 3-Nitrotyrosine, Myeloperoxidase
Introduction
Fine particulate matter (<2.5 μm diameter, PM2.5) air pollution is a leading cause of global morbidity and mortality and this is primarily attributable to the increased cardiovascular (CV) disease burden 1,2. Mounting evidence suggests that the long-term PM2.5 exposure may play a crucial role in the development and progression of atherosclerosis 3–5. Extreme swings in outdoor temperatures are also linked to CV morbidity and mortality 6–8. Epidemiologic studies suggest that PM2.5-mediated increases in low-density lipoproteins (LDL) and reductions in high-density lipoproteins (HDL) cause the CV burden 5,9–13. While low HDL levels are linked to CV risk, specific anti-atherosclerotic functions of HDL, namely the ability of HDL to off-load cholesterol from macrophages (measured as cholesterol efflux capacity, CEC) predict both prevalent and incident CV events 14–19. Myeloperoxidase (MPO), a heme-enzyme, together with a host of other systemic inflammatory responses are elevated with PM2.5 exposure 3,20,21. One pathway whereby PM2.5 might thus impair HDL function is via heightened MPO-induced oxidation of HDL22. In this context, we evaluated the effects of ambient PM2.5, along with environmental temperatures on CEC, a critical facet of anti-atherosclerotic HDL function and the role of MPO-induced HDL-oxidation on HDL function.
Methods
The study was approved by the Institutional Review Board of the University of Michigan, and all participants signed a written informed consent. Potential participants were recruited from the local community in the southeast Michigan area by advertisements. Inclusion criteria were healthy nonsmoking adults living in nonsmoking households (by self-report) aged 18–50 years old, without a history of CV disease or risk factors (screening blood pressure <140/90 mm Hg and fasting glucose <126 mg/dL). Subjects were excluded if they reported taking any antihypertensive, cholesterol, diabetes medication or any drug that could alter HDL function metrics (e.g., antioxidants, fish oil, folate). Qualifying participants (n=50) were enrolled into a repeated measures panel study conducted from August 2014 to February 2016 in Michigan (Figure S1). For a 24-hour period before the blood draws (fasting ≥8 hours), participants wore a personal environmental monitor while following usual daily activities (no travel or exercise). Participants were then discharged home and underwent a 6-day (minimum) to 3-week (maximum) washout period before repeating the same process. Full details for protocols and statistical methods can be found in the online supplement.
Each patient had their own unique seven distinct 24-hour ambient outdoor temperature and PM2.5 data exposure (24-hour average) calculated starting retrospectively from the time in the morning of each blood draw. In addition, the rolling average of exposures from 1 to 7 days in duration was calculated. Personal-level exposures and temperatures were recorded as averages for the 24-hour period before the blood draw visit in each study block 23.
Total Cholesterol, triglycerides, and HDL were measured by the enzymatic, GPO-PAP and a 2-step direct method on a Randox RX Daytona Chemistry analyzer (West Virginia, USA). HDL was prepared from plasma by sequential ultracentrifugation. HDL proteins were precipitated and delipidated, and oxidized amino acids were isolated by solid-phase extraction from an acid hydrolysate of HDL proteins. Oxidized amino acids were quantified using isotopically labeled internal standards- 13C6 tyrosine, 13C6 3-chlorotyrosine, and 13C6 3-nitrotyrosine by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) with multiple reaction monitoring (MRM) and positive ion acquisition mode22.
CEC was assessed was using J774 murine macrophages that were labeled with 2 μCi/mL 3H cholesterol (Perkin Elmer, Waltham, MA) for 24 hours in the presence of an acyl-CoA: cholesterol acyltransferase (ACAT) inhibitor (Sandoz 58–035, Santa Cruz Biotechnology, CA, USA) and equilibrated overnight with 0.3mM 8-(4-chlorophenylthio)-cyclic AMP to induce ATP-binding cassette A1 (ABCA1) expression. ApoB-depleted serum was obtained from polyethylene glycol (PEG) precipitation and used as an efflux acceptor (2.8% v/v) for 4 hours. Efflux was quantified by liquid scintillation and expressed as a percentage of total cell 3H-cholesterol content. All assays were performed in duplicate, and average values were used in calculations15.
Summary statistics were computed for continuous measures as the mean ± standard deviation (SD), as well as median (interquartile range, IQR), and for categorical variables as frequency and proportion (%). We analyzed the associations of repetitive measurements with 24-hour average outdoor ambient and personal-level PM2.5 and temperature using mixed-effect models. All analyses were performed using the statistical software package R (version 3.2.5).
Results
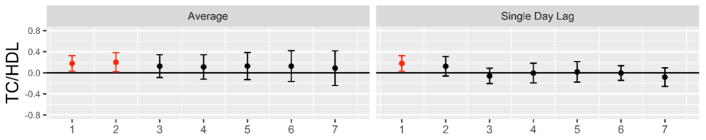
The 50 study participants (34 women) were young and overall healthy (Table 1). Prior 24-hour personal-level and 7-day outdoor-ambient exposures for temperatures and PM2.5 concentrations are provided in Table 2. PM2.5 levels were low and on-average within annual National Ambient Air Quality Standards < 12 μg/m3. There were few correlations between HDL function metrics and participant demographics (Table S1). Moreover, PM2.5 and temperatures were weakly or not-at-all correlated supporting their potential as independent factors on study endpoints (Table S2). There were trends toward higher concentrations of ambient PM2.5 over the previous week being related to increases in the total cholesterol-to-HDL ratio (Figure 1). An increase of 10 μg/m3 was associated with a mean 0.18 (p=0.022) and 0.20 (p=0.032) elevation on rolling average lag days 1 and 2, respectively. This change was not due to a lowering of HDL but rather driven by a tendency for increases in LDL-C (Figure S2). Lipoprotein changes had no significant relationships with prior 24-hour personal-level PM2.5 or any temperature exposures.
Table 1.
Clinical Characteristics of the Study Participants (n=50, 34 women)
| Variable | Obs | Mean ± SD | Min | 25th percentile | Median | 75th percentile | Max |
|---|---|---|---|---|---|---|---|
| Age (years) | 50 | 32.1 ± 9.6 | 19 | 24.0 | 28.5 | 41.0 | 50 |
| BMI (kg/m2) | 50 | 26.1 ± 5.7 | 19.0 | 21.7 | 24.9 | 29.1 | 43.5 |
| Systolic BP (mm Hg) | 200 | 107.8 ± 13.3 | 86 | 99 | 105 | 112 | 161 |
| Diastolic BP (mm Hg) | 200 | 70.2 ± 9.4 | 50 | 65 | 69 | 75 | 107 |
| Heart rate (beats/min) | 200 | 70.6 ± 11.3 | 44 | 63 | 71 | 78 | 111 |
| Total cholesterol (mg/dL) | 100 | 149.4 ± 32.3 | 72 | 129.5 | 149 | 168 | 229 |
| Triglycerides (mg/dL) | 100 | 75.3 ± 52.7 | 21 | 44 | 57 | 95 | 345 |
| LDL cholesterol (mg/dL) | 100 | 86.8 ± 25.3 | 30 | 69.2 | 82.2 | 100.4 | 158 |
| HDL cholesterol (mg/dL) | 100 | 47.6 ± 13.5 | 23 | 38 | 46 | 54.5 | 100 |
| CEC (%) | 100 | 16.16 ± 3.57 | 9.29 | 12.94 | 15.93 | 18.90 | 26.32 |
| 3-chlorotyrosine* | 100 | 7.84 ± 17.22 | <0.01 | 0.04 | 1.16 | 4.51 | 87.82 |
| 3-nitrotyrosine* | 100 | 4.68 ± 8.1 | <0.01 | 0.78 | 2.09 | 5.36 | 67.39 |
| o,o′-dityrosine* | 100 | 0.65 ± 3.34 | <0.01 | <0.01 | <0.01 | 0.18 | 31.97 |
BP, blood pressure; BMI, body mass index; CEC, cholesterol efflux capacity; HDL, high density lipoprotein; LDL, low-density lipoprotein; max, maximal value; min, minimal value; Obs, number of observations; SD, standard deviation;
mmol/mol tyrosine.
Table 2.
Ambient and Personal-Level Environmental Exposures
| Variable | Obs | Mean ± SD | Min | 25th percentile | Median | 75th percentile | Max | |
|---|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | Personal Outdoor | 95 | 12.2 ± 16.9 | 0.2 | 4.0 | 6.9 | 13.3 | 94.0 |
| 7-day average | 94 | 9.1 ± 1.8 | 5.5 | 7.7 | 9.1 | 10.7 | 12.4 | |
| Temp (°C) | Personal Outdoor | 98 | 22.0 ± 3.0 | 13.8 | 20.4 | 22.2 | 24.2 | 28.7 |
| 7-day average | 97 | 4.4 ± 9.8 | −13.4 | −3.9 | 2.9 | 12.7 | 23.2 |
Personal PM2.5 and temperature levels were averaged from all available 24-hour long period measurements performed on lag day 1 prior to blood draw during both study blocks. Outdoor PM2.5 and temperature levels were averaged over 7-day long lag periods prior to all study visits. max, maximal value; min, minimal value; Obs, number of observations; SD, standard deviation.
Figure 1. Associations of higher ambient PM2.5 concentrations with changes in total cholesterol to high density lipoprotein cholesterol ratio.
Results provided per 10 μg/m3 increase in ambient PM2.5. Average, rolling mean ambient PM2.5 concentrations averaged over all exposure days up through lag day(s) 1–7; Single day lag, individual lag day ambient PM2.5 concentrations; Linear mixed effect model adjusted for same-day ambient temperature, sex and study block. Significant associations highlighted in red. PM2.5, Fine particulate matter; TC/HDL, total cholesterol to high density lipoprotein cholesterol ratio.
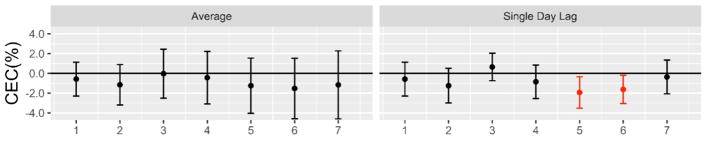
Higher ambient PM2.5 levels over the preceding week tended to be associated with impairments in serum CEC (Figure 2). The associations reached statistical significance in a delayed fashion with reductions ranging from −1.93% (p= 0.022) to −1.62% (p=0.032) for a single day increase in ambient PM2.5 of 10 μg/m3 on lag days 5 and 6, respectively. However, personal-level PM2.5 exposures during the previous 24-hours were not associated with CEC. Neither ambient nor personal-level PM2.5 exposures were associated with MPO-induced or other HDL oxidation markers (Figure S3).
Figure 2. Associations of higher ambient PM2.5 concentrations with serum cholesterol efflux capacity.
Results provided per 10 μg/m3 increase in ambient PM2.5. Average, rolling mean ambient PM2.5 concentrations averaged over all exposure days up through lag day(s) 1–7; Single day lag, individual lag day ambient PM2.5 concentrations. Linear mixed effect model adjusted for same-day ambient temperature, sex and study block. Significant associations highlighted in red. CEC, cholesterol efflux capacity (% change); PM2.5, Fine particulate matter.
Exposures to colder outdoor temperatures over the previous week were also associated with impairments in serum CEC (Figure 3). Significant decreases ranged from −0.62% (p= 0.027) to −0.63% (p=0.028) for rolling average colder temperatures (per −10°C) over lag days 6 and 7. However, personal-level temperature exposure during the previous 24-hours was not associated with CEC. Neither outdoor nor personal-level temperature exposures were significantly associated with levels of MPO-induced or other HDL oxidation markers (Figure S4).
Figure 3. Association of colder ambient temperatures with serum cholesterol efflux capacity.
Results provided per 10°C decrease in ambient temperature. Average, rolling mean temperature averaged over all exposure days up through lag day(s) 1–7; Single day lag, individual lag day temperature. Linear mixed models adjusted for same-day ambient PM2.5 level, sex, study block. Significant correlations highlighted in red. CEC, cholesterol efflux capacity (% change).
Finally, there was no effect modification (no significant interaction in the statistical models) between temperature levels and PM2.5 exposures for CEC. Therefore, we evaluated the combined effect of a colder temperature (−10°C rolling average level) and higher PM2.5 (10 μg/m3 single day level) on lag day 5. In this potential real-life situation, the adverse impact of both co-exposures led to a more substantial decrease in CEC by −2.17% (p=0.011).
Discussion
We herein demonstrated that ambient PM2.5 and outdoor temperatures each influence CEC. PM2.5 also acutely shifted lipoproteins toward a more pro-atherogenic pattern. While both environmental factors are known to affect lipids 5,9–13,24,25, this is the first report providing evidence that ambient levels of PM2.5 and colder outdoor temperatures might each independently impair HDL function. Given the critical role that CEC plays in mitigating the atherosclerotic process 14,15, our results elucidated a novel mechanistic linkage between PM2.5 and heightened CV risk 1–5. These findings may also help to explain the increase in CV events during colder seasons 6,7.
Prior studies have shown that air pollutants can promote dyslipidemia 5,9–13. Long-term PM2.5 exposures were linked to lower HDL particle numbers in Multi-Ethnic Study of Atherosclerosis-MESA 13. We also observed that PM2.5 prompted an acute increase in the total cholesterol-to-HDL ratio, primarily due to increasing trends in LDL. While important, we posited that a PM2.5-mediated impairment in HDL function would be a stronger mechanistic explanation for pro-atherosclerotic actions. Converging epidemiological and experimental evidence highlights that CEC, rather than HDL levels per se, is the critical HDL-facilitated process responsible for protecting against CV events 14,15. However, few studies have evaluated the impact of air pollutants on HDL function 16–19. Animals exposed to high PM levels developed a blunted HDL anti-oxidant capacity 16,17. In our prior study, acute exposure to concentrated PM2.5 prompted a similar response in a subset of adults 18. As far as we are aware, the lack of impact on CEC following a 2-hour exposure to coarse PM is the only previous study to evaluate the effect of any air pollutant on this endpoint 19. This makes our current findings - that ambient PM2.5 levels and not 24-hour personal PM2.5 were independently associated with a reduction in CEC over subacute time periods - both novel and clinically relevant. Our results could help further explain epidemiological studies linking PM2.5 with heightened atherosclerosis and increased CV events 3–5. Billions of people (particularly across Asia) continue to face markedly-poor air quality, with daily PM2.5 levels commonly 2 to 50-fold above those in Michigan1. The fact that even low levels of PM2.5 are linked to an impaired facet of anti-atherosclerotic HDL function supports the imperative to reduce this ubiquitous air pollutant as much as possible to protect global CV health 2.
The mechanisms responsible for our findings must remain speculative. Animal models suggest that enhanced atherosclerosis progression may have risen from HDL dysfunction due to the heightened generation of reactive oxygen and nitrative species 5,18. We posited that PM2.5 might increase MPO-induced oxidation of HDL and thereby blunt CEC 22. However, we found no evidence for increased MPO-specific (3-chlorotyrosine) or other oxidative changes (3-nitrotyrosine and o,o′-dityrosine) on HDL protein despite using a highly-sensitive assay (LC-ESI-MS/MS). It is possible that HDL oxidation/modification could have arisen from other sources that went undetected – free metal ions and radicals, various enzymes, glucose, and acute phase reactants 26–29. In the current study, blunted CEC occurred in a delayed fashion – 5 to 6 days following higher ambient PM2.5 levels but not acutely to 24-hour personal PM2.5 levels. This suggests that the underlying mechanism is a relatively slower (subacute) process, or that any acute PM2.5-mediated damage to HDL takes several days to become manifest through reduced CEC. However, the personal exposures were monitored only for the 24-hour period leading up to the blood sampling and hence failed to capture this sub-acute process. It is interesting to note that in a recent study from Shanghai, subacute (9-days) of ambient PM2.5 exposure (albeit at much higher levels) induced a wide array of adverse metabolic-stress responses (e.g., altered corticosteroids, catecholamines, amino acids, lipids, and fatty acids) 30. We, therefore, plan to evaluate if any of these mediators, or other HDL factors such as an altered proteome, are responsible for the reduction in CEC observed in our current study. It will also be important in future analyses to explore the impact on other potentially-important facets of HDL function (e.g., anti-oxidant and anti-inflammatory indices).
We have previously shown that increasing levels of PM2.5 and outdoor temperatures are associated with opposing effects on blood pressure 8. Given that people are often concomitantly exposed to multiple environmental factors, we felt it necessary to test whether there were individual effects of PM2.5 and temperatures on HDL function. We show here for the first time that colder outdoor temperatures were also independently associated with reduced CEC. The response appeared in a delayed subacute time-period (lag days 5–7), similar to that induced by PM2.5. This suggests that shared or overlapping mechanisms may be responsible; however, future studies are required in this regard. We speculate that cold-induced HDL dysfunction may help further explain the increase in CV events related to winter 6,7.
Our study was relatively small and conducted at a single location with low air pollution levels. The CEC changes observed were modest and of uncertain clinical relevance for a single individual; however, it is possible that much higher exposures to PM2.5 (such as across Asia) may be associated with more robust alterations. Moreover, PM2.5 causes serious public health problems not because of the small adverse health effects at the individual-level but because of the enormous number of people impacted worldwide on a continual involuntary basis1. We cannot exclude the possibility that other unmeasured pollutants also affected CEC. However, our prior studies demonstrate that while other co-pollutants (e.g., ozone) may have had an independent effect, they are unlikely to explain or confound the associations with PM2.5. The influence of other pollutants, the specific sources of exposure (e.g., traffic-related pollution), and particle components (e.g., metals) responsible for impairing HDL function warrant future investigation. Finally, as with all observational studies, any causation cannot be directly inferred from our findings. Nevertheless, our trial was a priori designed with the aim of evaluating the impact of PM2.5 on CEC and HDL function metrics, and there are biologically plausible explanations for our results. Follow-up larger trials in several locations with more varied PM2.5 levels would be essential to corroborate our findings as well as enhance our understanding of these novel observations. In conclusion, our study shows for the first time that subacute exposures to low levels of ambient PM2.5 as well as colder outdoor temperatures are each independently associated with near-term reductions in CEC – a vital aspect of HDL function. Future studies are needed to test whether interventions to reduce exposures to both environmental risk factors can improve HDL function and decrease CV events.
Supplementary Material
Acknowledgments
Sources of funding: National Institutes of Health (UL1RR024986; R01-ES015146; R01-HL1297778; K08-HL130944), Environmental Protection Agency (RD83479701), American Heart Association (15SDG2447015), and the University of Michigan-Peking University Joint Institute for Translational and Clinical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA, 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, Schwartz JD. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Kunzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S, Storey RF Esc Working Group on Thrombosis EAfCP, Rehabilitation Association ESCHF. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93b. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Diez Roux AV, Gassett AJ, Jacobs DR, Jr, Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA, Watson KE. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388:696–704. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y, Sun Q. Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. Biochim Biophys Acta. 2016;1860:2863–2868. doi: 10.1016/j.bbagen.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient temperature and morbidity: a review of epidemiological evidence. Environ Health Perspect. 2012;120:19–28. doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Effects of ambient temperature on the incidence of myocardial infarction. Heart (British Cardiac Society) 2009;95:1760–1769. doi: 10.1136/hrt.2009.175000. [DOI] [PubMed] [Google Scholar]
- 8.Giorgini P, Rubenfire M, Das R, Gracik T, Wang L, Morishita M, Bard RL, Jackson EA, Fitzner CA, Ferri C, Brook RD. Particulate matter air pollution and ambient temperature: opposing effects on blood pressure in high-risk cardiac patients. J Hypertens. 2015;33:2032–2038. doi: 10.1097/HJH.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 9.Kunzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, Trigo E, Gilliland FD. Ambient Air Pollutants Have Adverse Effects on Insulin and Glucose Homeostasis in Mexican Americans. Diabetes Care. 2016;39:547–554. doi: 10.2337/dc15-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang KJ, Yan YH, Chiu SY, Cheng TJ. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med. 2011;68:64–68. doi: 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- 12.Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J, Kupper L, Williams R, Neas L, Cascio W, Devlin RB, Peden DB. Coarse particulate matter (PM2. 5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Perspect. 2007;115:709–714. doi: 10.1289/ehp.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell G, Mora S, Greenland P, Tsai M, Gill E, Kaufman JD. Association of Air Pollution Exposures With High-Density Lipoprotein Cholesterol and Particle Number: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:976–982. doi: 10.1161/ATVBAHA.116.308193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Navab M, Pakbin P, Ning Z, Navab K, Hough G, Morgan TE, Finch CE, Araujo JA, Fogelman AM, Sioutas C, Hsiai T. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res. 2013;54:1608–1615. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient Particulate Pollutants in the Ultrafine Range Promote Early Atherosclerosis and Systemic Oxidative Stress. Circulation research. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan G, Yin F, Speck M, Tseng CH, Brook JR, Silverman F, Urch B, Brook RD, Araujo JA. Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol. 2016;13:26. doi: 10.1186/s12989-016-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiseyeu A, Yang H-y, Ramanathan G, Yin F, Bard RL, Morishita M, Dvonch JT, Wang L, Spino C, Mukherjee B, Badgeley MA, Barajas-Espinosa A, Sun Q, Harkema J, Rajagopalan S, Araujo JA, Brook RD. No Effect of Acute Exposure to Coarse Particulate Matter Air Pollution in a Rural Location on High Density Lipoprotein Function. Inhalation toxicology. 2014;26:23–29. doi: 10.3109/08958378.2013.850761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF, Jr, Lin H, Vasan RS, Benjamin EJ, Mittleman MA. Short-Term Exposure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruckerl R, Hampel R, Breitner S, Cyrys J, Kraus U, Carter J, Dailey L, Devlin RB, Diaz-Sanchez D, Koenig W, Phipps R, Silbajoris R, Soentgen J, Soukup J, Peters A, Schneider A. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ Int. 2014;70:32–49. doi: 10.1016/j.envint.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejike C, Wang L, Liu M, Wang W, Morishita M, Bard RL, Huang W, Harkema J, Rajagopalan S, Brook RD. Personal-level exposure to environmental temperature is a superior predictor of endothelial-dependent vasodilatation than outdoor-ambient level. Journal of the American Society of Hypertension: JASH. 2017 doi: 10.1016/j.jash.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu R, May Wu X, Malig BJ, Broadwin R, Gold EB, Qi L, Derby C, Jackson EA, Green RS. Estimating the associations of apparent temperature and inflammatory, hemostatic, and lipid markers in a cohort of midlife women. Environ Res. 2017;152:322–327. doi: 10.1016/j.envres.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartini C, Barry SJ, Whincup PH, Wannamethee SG, Lowe GD, Jefferis BJ, Lennon L, Welsh P, Ford I, Sattar N, Morris RW. Relationship between outdoor temperature and cardiovascular disease risk factors in older people. Eur J Prev Cardiol. 2017;24:349–356. doi: 10.1177/2047487316682119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagano Y, Arai H, Kita T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Natl Acad Sci U S A. 1991;88:6457–6461. doi: 10.1073/pnas.88.15.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauster M, Oskolkova OV, Innerlohinger J, Glatter O, Knipping G, Frank S. Endothelial lipase-modified high-density lipoprotein exhibits diminished ability to mediate SR-BI (scavenger receptor B type I)-dependent free-cholesterol efflux. Biochem J. 2004;382:75–82. doi: 10.1042/BJ20031882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferretti G, Bacchetti T, Marchionni C, Caldarelli L, Curatola G. Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta Diabetol. 2001;38:163–169. doi: 10.1007/s592-001-8074-z. [DOI] [PubMed] [Google Scholar]
- 29.Li XA, Yutani C, Shimokado K. Serum amyloid P component associates with high density lipoprotein as well as very low density lipoprotein but not with low density lipoprotein. Biochem Biophys Res Commun. 1998;244:249–252. doi: 10.1006/bbrc.1998.8248. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H, Kan H. Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation. 2017;136:618–627. doi: 10.1161/CIRCULATIONAHA.116.026796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.