Abstract
Studies have identified strong associations between D2 receptor binding potential and neural responses to rewarding stimuli and substance use. Thus, D2 receptor perturbations are central to theoretical models of the pathophysiology of substance dependence, and epigenetic changes may represent one of the fundamental molecular mechanisms impacting the effect of alcohol exposure on the brain. We hypothesized that epigenetic alterations in the promoter region of the dopamine D2 receptor (DRD2) gene would be associated with cue-elicited activation of neural reward regions, as well as severity of alcohol use behavior. The current study leveraged functional neuroimaging (fMRI) during an alcohol reward paradigm (n=383) to test associations among DRD2 promoter methylation in peripheral tissue, signal change in the striatum during the presentation of alcohol cues, and severity of alcohol use disorder (AUD). Controlling for age, DRD2 promoter methylation was positively associated with responses to alcohol cues in the right accumbens (partial r=0.144, p=0.005), left putamen (partial r=0.133, p=0.009), right putamen (partial r=0.106, p=0.039), left caudate (partial r=0.117, p= 0.022), and right caudate (partial r=0.133, p=0.009), suggesting that DRD2 methylation was positively associated with robust activation in the striatum in response to reward cues. DRD2 methylation was also positively associated with clinical metrics of AUD severity. Specifically, controlling for age, DRD2 methylation was associated with AUDIT total (partial r=0.140, p=0.002); ICS total (partial r=0.097, p=0.044) and ADS total (partial r=0.152, p=0.001). Thus, DRD2 methylation may be a critical mechanism linking D2 receptors with functional striatal brain changes and clinical severity among alcohol users.
Keywords: alcohol dependence, DNA methylation, DRD2, epigenetics, fMRI, striatum
Introduction
Dopamine and its D2 receptor play a critical neuromodulatory role in the firing patterns of corticostriatal circuits that are central to cognition and behavior. The D2 receptor is involved in reward processing, attribution of incentive salience to sensory cues, avoidance learning, motivation, affect regulation, and decision making (see Collins and Frank, 2014; Everitt and Robbins, 2013), which are all critical processes involved in substance use initiation, escalation, potential problem use, and addiction. Consequently, D2 receptor perturbations are critical in the pathophysiology of a number of substance use disorders (Volkow et al., 2013) and other neuropsychiatric disease states such as mood disorders (Nestler and Carlezon, 2006), cognitive sequelae of schizophrenia (Simpson et al., 2010), and attention deficit hyperactivity disorder (Volkow et al., 2011). Thus, understanding the role of D2 receptor regulation in substance use disorders (SUDs) is a high priority for cognitive and clinical neuroscientists.
There has been growing interest in epigenetics of gene expression and gene splicing (Luco et al., 2011), and how epigenetic regulation may influence the etiology of disease states as diverse as cancer (Jones et al., 2016) and psychiatric disorders (Tsankova et al., 2007). Briefly, epigenetics refers to reversible changes to the genetic code that regulate gene expression without changing the DNA sequence. Rodent studies suggest that epigenetic mechanisms, including histone de-acetylation and DNA methylation, may underlie neural adaptations produced by chronic substance exposure (Nestler, 2013). DNA methylation is a type of epigenetic modification that occurs when a methyl group is added at a cytosine guanine (GpC) dinucleotide, thereby altering transcription at that genomic location and ultimately influencing gene expression (Goldberg et al., 2007). Previous research has suggested that alcohol use disorders (AUDs) are associated with methylation in numerous genes, including dopamine genes (e.g., Hagerty et al., 2016; Hillemacher et al., 2009). Although findings are mixed, most studies have demonstrated hypermethylation (i.e., increased methylation) of dopamine genes in AUD phenotypes (Hagerty et al., 2016; Hillemacher et al., 2009). Consistent with this prior work, substance use disorders represent an excellent model for the study of epigenetic regulation of D2 receptors. Substance dependence is closely associated with the mesolimbic and corticostriatal networks involved in the dopaminergic reward system (Barker et al., 2015; Engel and Jerlhag, 2014). Animal studies have shown that ethanol stimulates dopaminergic neurons, while chronic drinking leads to long-term downregulation of D2 receptors (Imperato and Di Chiara, 1986; Volkow et al., 1996). Further, positron emission tomography (PET) studies have identified strong associations between reduced D2 receptor binding potential and substance abuse (Volkow et al., 2009; Volkow et al., 2011) and functional brain responses to rewarding stimuli (Heinz et al., 2004; Volkow et al., 2013). D2 receptor availability is also associated with subjective responses to alcohol (Yoder et al., 2005). Relatedly, lower D2 methylation has been associated with abstinence and treatment seeking in individuals with a history of pathologic gambling (Hillemacher et al., 2015), which shares common neural mechanisms with substance use disorders (Potenza, 2008).
Less is known about the molecular mechanisms underlying changes in the D2 receptor. Further, DRD2 methylation has never been directly examined in relation to functional imaging response in brain regions related to reward processing and AUD. The present study aimed to determine whether epigenetic regulation of the DRD2 gene is one of the molecular mechanisms underlying changes in reward-processing regions of the brain. We hypothesized that peripheral DRD2 hypermethylation would be associated with increases in BOLD response in reward processing regions—specifically the striatum, amygdala and insula—during the presentation of alcohol versus control cues; and that DRD2 promoter hypermethylation would be related to clinical measures of AUD severity. As an exploratory aim, we also conducted whole brain analyses examining correlations between DRD2 methylation and BOLD responses during the alcohol cue task.
Materials and Methods
Sample
Heavy drinking participants were recruited from the Albuquerque metropolitan area of the United States. Subjects were included if they endorsed drinking at least 5 or more drinks per drinking occasion for men (4 or more for women) at least five times in the past month. Exclusionary criteria included previous brain injury or loss of consciousness for more than 5 minutes, a history of treatment for alcohol withdrawal, or a positive pregnancy test. Subjects were instructed to abstain from smoking cigarettes for 2 hours and from consuming alcohol for 24 hours before scanning, and had to pass a breathalyzer (i.e., obtain a reading of 0.00) immediately prior to study participation. Written informed consent, approved by the University of New Mexico Human Research Committee, was obtained from all participants.
At the study session, the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993) was used to assess symptoms of alcohol abuse and dependence over the last 6 months. The AUDIT consists of 10 items on the quantity of alcohol use and incidence of alcohol-related problems. On the AUDIT, a score of 8 or greater is associated with hazardous drinking. Participants also completed the Impaired Control Scale (ICS; Heather et al., 1993), which consists of 25 items that measure attempts and success in controlling drinking over the last 6 months, as well as belief in the ability to control drinking if attempted. Participants also completed the Alcohol Dependence Scale (ADS; Kivlahan et al, 1989), which is used to assess severity of alcohol use symptoms. On the ADS, a score of 9 or greater is associated with a diagnosis of an AUD. Finally, participants completed the Timeline Follow-Back (Sobell et al., 1979), which obtains estimates of daily alcohol, cigarette, and marijuana use. This instrument requires subjects to recall the number of drinks consumed each day over the prior 30 days. Our primary clinical measures of AUD were the AUDIT, ADS, and ICS because they cover a range of behaviors across a continuum of AUD severity.
To characterize comorbidity with co-occurring mood symptoms, we also collected measures of depression and anxiety. Subjects completed the Beck Depression Inventory-II (BDI-II; (Beck et al., 1996), a measure of depression symptom severity over the past two weeks. Scores range between 0 and 63, with categorical depression ratings of “minimal” (0–13), “mild” (14–19), “moderate” (20–28), and “severe” (29–63). Participants also completed the Beck Anxiety Inventory (BAI; (Beck and Steer, 1991) to assess for anxiety symptom severity over the past week. Items are summed to obtain a total score from 0 to 63, with categorical cutoffs of “low” (0–21), “moderate” (22–35) and “severe” (36–63).
DNA preparation and methylation assay
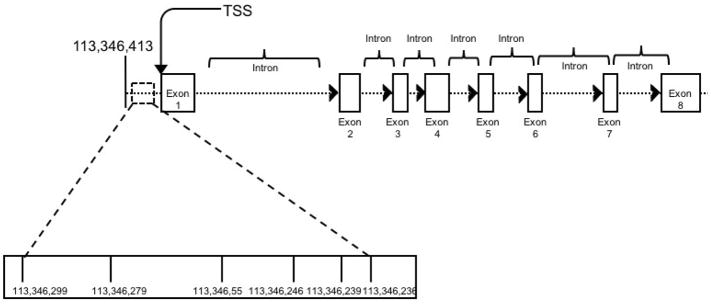
Saliva samples from all participants were obtained and stored until the time of DNA extraction. The DNA was extracted per manufacturer instructions using DNA Genotek’s (Ottowa, Ontario, Canada; http://www.dnagenotek.com) prepIt DNA extraction kit (Cat. No. PT-L2P-45). DNA was quantified using Invitrogen’s Qubit™ dsDNA BR Assay Kit (Cat. No. Q-32853) and cryogenically stored at −80°C. The DRD2 methylation custom assay covered 6 DRD2 CpG dinucleotides (113346236, 113346239, 113346246, 113346255, 113346279, 113346299) in the promoter region of the gene, ranging from −126 to −189 in reference to the transcription start site (Human Genome Assembly GRCh37.p13). The amplicon covered by the primers and subsequent analyses can be viewed in Figure 1.
Figure 1.
Assayed CpG sites fell within the promoter region of the DRD2 gene, ranging from −126 to −189 in reference to the transcription start site (Human Genome Assembly GRCh37.p13). Selected CpG sites were chosen on the basis of their location within an important regulatory region of the gene.
Given that pyrosequencing is widely used to assay CpGs and most appropriate in situations when the target CpGs have been previously identified (Nikolova et al., 2014) we used pyrosequencing to assay these six CpGs. To determine the methylation status of CpG sites near of the DRD2 gene, pyrosequencing was performed at EpigenDX (Worcester, MA, USA). Pyrosequencing quantitatively monitors the real-time incorporation of nucleotides through the enzymatic conversion of released pyrophosphate into a proportional light signal (Tost and Gut, 2007). Assays were designed by EpigenDx. Site analysis was based on the ability to generate primers located around CpG islands and that meet the requirements required for pyrosequencing of each site accurately. All primer sequences are owned by EpigenDx. Because methylation values across the six sites of interest were highly correlated, we created an average methylation variable (e.g. average methylation across the six CpG sites assayed), which was used in all analyses outlined below. Specifically, across all 472 individuals included in the full sample, the correlations across these 6 CpG dinucleotides ranged from r=.049 to r=.329, with most correlations significant at p<.01. There were three methylation outliers, (defined as individuals whose averaged methylation values were greater than 3 standard deviations above the mean), and those individuals were dropped in all methylation analyses. The pyrosequencing methods are described in detail in Supplemental Materials and Methods.
BOLD fMRI Imaging and Task
Cue-elicited response to alcohol (Alcohol cue-related reward response) and juice (Non-alcohol cue-related reward response) were measured with a taste task previously used in our laboratory (Claus et al., 2011; Filbey et al., 2008; Hutchison et al., 2008). Briefly, while subjects were in the scanner, they received small amounts (e.g. 1ml) of two different beverages presented in a pseudorandom order through Teflon tubing using a computer-controlled gustometer. They received either their preferred alcoholic beverage (e.g., wine, mixed drink) or litchi juice during a 24 second taste-delivery period, during which participants were instructed to Taste Alcohol (or Juice; seconds 1–10 and 12–22) and Swallow (seconds 10–12, 22–24). The taste cue presentation was followed by a 16 second washout period, during which the word “REST” appeared on the screen and no taste stimuli were presented. Each trial began with a “Ready” prompt for 2 seconds, designating the start of the trial. Participants completed six trials of each taste cue in each of two 9-minute runs. This task measures neural activation during receipt of an alcohol taste cue compared to an appetitive, non-alcohol taste cue. The critical contrast (Alcohol reward vs. non-Alcohol reward) reflects alcohol-induced neural activation over and above activation in response to normal (non-alcoholic Juice) appetitive cues.
BOLD fMRI data acquisition
fMRI data was collected on a 3-T Siemens Trio scanner with a 12-channel radio frequency coil using a gradient-echo echo-planar imaging (EPI) sequence: TE=29 ms, TR=2 s, flip angle=75°, 33 slices with slice thickness=3.5 mm, slice gap=1mm, 64×64 matrix, 3.75mm×3.75mm. Only subjects with motion less than 2mm translation or 0.035 radians rotational were included in the primary analysis for this study (Weiland et al., 2013) resulting in 86 individuals being excluded for motion and a sample of 383 individuals with imaging data. Alternative motion criteria consistent with Siegel et al. (2014) were applied post-hoc in order to further validate the results.
BOLD fMRI data processing
Functional images were preprocessed using an automated pipeline based around SPM 8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) including realignment, slice-timing correction, spatial normalization to Montreal Neurological Institute (MNI) space, reslicing, and smoothing with a 6 mm full-width half-max Gaussian kernel (Scott et al., 2011). Regressors of interest were created for the following conditions: alcohol cue, alcohol baseline, juice cue and juice baseline according to the timing scheme used in previous studies (Claus et al., 2011; Filbey et al., 2008).
BOLD fMRI data analysis and ROI selection
Statistical analyses of fMRI data were performed using a general linear model in SPM8 using the canonical hemodynamic response function. Although the primary contrast of interest was Alcohol versus Juice, contrast maps were computed for each participant for each of three possible contrasts: Juice versus Baseline, Alcohol versus Baseline, and Alcohol versus Juice. Based on our a priori interest in the striatum, region of interest (ROI) analysis was performed. BOLD activation for bilateral caudate, nucleus accumbens, putamen, insula, and amygdala were extracted from each individual’s contrast maps for correlation with mean methylation, controlling for age (Claus et al., 2011). Percent signal change values were extracted for all ROIs using the MarsBar toolbox (Brett et al., 2002). ROIs were defined based on the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002).
Exploratory Whole Brain Data Analysis
In order to be inclusive, we also conducted an exploratory second-level whole-brain analysis. Using a primary voxel-level threshold of p < .01, we examined correlations between mean DRD2 methylation and the primary contrast of interest, Alcohol vs. Juice, as well as Alcohol vs. Baseline and Juice vs. Baseline.
Primary statistical analysis
In order to avoid Type I error inflation that would result from testing each CpG individually, our analyses focused on the average methylation across the 6 a priori CpGs (mean DRD2 methylation). Further, we report corrected p-value correct for False discovery via Benjamini-Hochberg (Benjamini & Hochberg, 1995). Partial Pearson product moment correlations were used to investigate the associations among average DRD2 methylation and 1) signal change in reward regions during exposure to alcohol cues over and above a normally rewarding beverage cue and baseline, and 2) clinical measures of AUD severity (i.e., total scores on alcohol dependence scales). Given the impact of age on methylation patterns, for all correlation analyses between methylation and all other variables, we used partial correlations covarying for age. Note that we examined partial correlations between methylation and clinical measures in the full sample (n=472), rather than the 383, given that imaging data were not included in this set of analyses.
Results
Demographics and drinking behavior
The sample included in the imaging analyses was comprised of 383 participants ages 21 to 56 years (mean age=30.13, SD=8.92 years), 238 men and 145 women. The sample was 52.6% White, 16.2% Hispanic, 7.8% Native American, 2.1% Black, 1% Asian, 13.7% mixed race and 6.6% unknown or declined to state. Participants’ scores on the (AUDIT; Saunders et al., 1993) ranged from 3 to 39 (mean=14.81, SD=7.01). The sample largely consisted of hazardous drinkers (86% of the sample endorsed an AUDIT score of 8 or greater). Scores on the Impaired Control Scale (ICS-FC; Heather et al., 1993) ranged from 1 to 43 (mean=18.60, SD=9.55), and scores on the Alcohol Dependence Scale (ADS; Kivlahan et al., 1989) ranged from 0 to 43 (mean=10.29, SD=6.97). Over half of the sample had symptoms consistent with AUD (56% of the sample endorsed an ADS score of 9 or greater). TLFB days of marijuana use over the past 30 days ranged from 0 days to 30 days (mean=4.35, SD=8.66) and cigarette smoking days over the past 30 days ranged from 0 to 30 (mean=9.41, SD=12.67). In terms of concurrent mood symptoms, BDI total score ranged from 0 to 47 (mean=9.69, SD=8.46) and BAI total score ranged from 0 to 53 (mean=8.52, SD=8.89).
Peripheral DRD2 Methylation and BOLD Response in the Brain
Given the role of striatal dopamine in reward response, the primary analyses focused on associations among mean DRD2 methylation and signal change in striatal regions of interest (ROIs) in the contrast between the alcohol cue and the normal appetitive cue (i.e., juice). After FDR correction, results indicated significant positive correlations between mean DRD2 methylation and signal change for Alcohol versus Juice (controlling for age) in the following regions: right NAcc: partial r=0.144, p=0.013 (95% confidence interval [CI]=.046–.242; all CIs for partial correlations were determined using 1000 bootstraps); left putamen: partial r=0.133, p=0.014 (95% CI=.037–.231); right putamen: partial r=0.106, p=0.044 (95% CI=.016–.187); right caudate: partial r=0.14, p=0.014 (95% CI=.044–.222); and left caudate: partial r=0.117, p=0.029 (95% CI=.02–.215). No other contrasts or regions showed significant correlations with mean DRD2 methylation. Importantly, we also ran post-hoc models excluding participants for framewise displacement (n=453, FD > 0.9) consistent with Siegel et al. (2014), and significant [right accumbens (partial r=0.114, p=0.02; 95% CI=.022–.204) and left putamen (partial r=0.095, p=0.04, 95% CI=.029–.185)] or trend level results [right putamen (partial r=0.098, p=0.06, 95% CI=.006–.188), left caudate (partial r=0.079, p= 0.09, 95% CI=.013–.17), and right caudate (partial r=0.089, p=0.06, 95% CI=.032–.1800] held for all of our previously associated ROIs; further, effect sizes did not significantly differ across the two sampling methods. Given that dopaminergic genetic methylation has been influenced by depressive symptoms (Wiers et al., 2015), we also ran secondary models that controlled for co-occurring mood symptoms by additionally covarying BDI and BAI score and each of the above correlations remained significant (p < .05). Figure 2 presents scatter plots of striatal regions showing significant activation against mean DRD2 methylation. Across these regions, individuals with higher methylation demonstrated greater activation during the alcohol versus litchi contrast; thus, that DRD2 methylation was associated with increase responsiveness to alcohol-specific reward (over and above appetitive reward responses).
Figure 2.
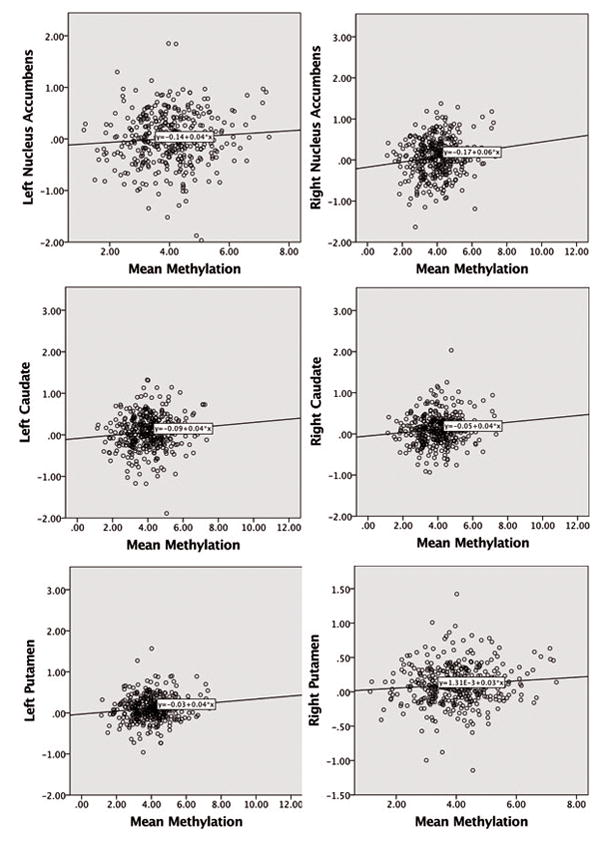
Scatter plots showing correlations between mean DRD2 methylation and Alcohol vs. Juice activation in the bilateral NAcc, caudate, and putamen (n=383).
Exploratory Whole Brain Results
No significant activation was found for the Juice vs. Baseline contrast, and no clusters passed error correction for the Alcohol vs. Baseline or Juice vs. Baseline contrasts. However, as hypothesized, at a significance threshold of p <.01, we found positive correlations between mean DRD2 methylation and the Alcohol vs. Juice reward contrasts in regions associated with reward processing, similar to those found for main effect of the taste task in our previous study (Claus et al, 2011), see Table 1 for peak cluster coordinates and statistics. Figure 3 presents statistical brain maps of the correlations with BOLD response for Alcohol vs. Juice and Alcohol vs. Baseline, and mean DRD2 methylation.
Table 1.
Activation during fMRI taste task that are correlated with average DRD2 methylation across 6 CpG sites
| CONTRAST /DIRECTION | MNI Coordinates | Cluster Size (voxels) | Peak T | Cluster Level p (FWE-corr) | ||
|---|---|---|---|---|---|---|
| Brain Region | x | y | z | |||
| Alcohol vs. Juice/POSITIVE CORRELATION | ||||||
|
|
||||||
| L superior temporal/middle temporal/insula/postcentral/supramarginal/putamen | −57 | −6 | 3 | 455 | 5.17 | .010 |
Figure 3.
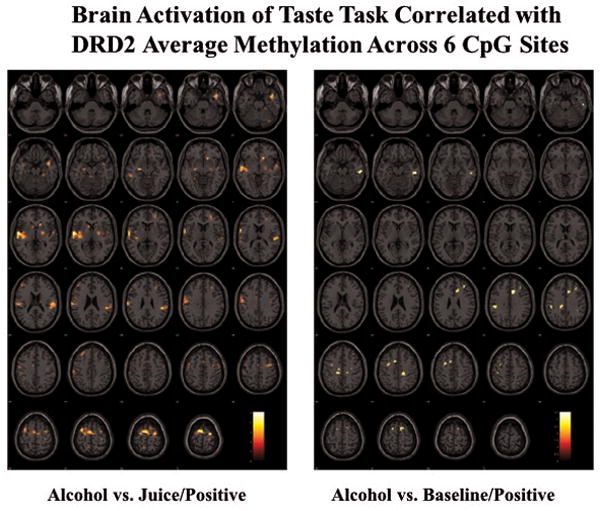
Statistical brain maps of correlations between mean DRD2 methylation and BOLD response for whole brain reward contrasts.
Peripheral DRD2 Methylation and Clinical Severity
Consistent with the brain findings, after FDR correction, each of our clinical measures of AUD severity were positively correlated with mean DRD2 methylation, after controlling for age (n=472; AUDIT: partial r=0.140, p=0.008 [95% CI=.043–.230]; ICS total: partial r=0.097, p=0.044 [95% CI=−.005–.196]; ADS total: partial r=0.152, p=0.008 [95% CI=.056–.245]). The direction of effects are as expected, given our findings that higher methylation is associated with greater alcohol-specific striatal reward responding, and the research linking alcohol-specific reward responsivity to increased AUD severity (Claus et al., 2011). In secondary models that controlled for co-occurring mood symptoms by additionally covarying BDI and BAI score, each of the above correlations remained significant (p < .05) with the exception of ICS total score.
Discussion
Based on the premise that mid-brain dopaminergic neurons are critical for reward learning (Collins and Frank, 2014), and their mediating role in the development of AUDs (Volkow et al., 2013), our study examined the extent to which methylation of the DRD2 promoter is related to functional brain responses to rewarding stimuli. We further examined the extent to which this regulation may be associated with clinical variability in AUD, which critically involves dysfunctional reward learning. This was tested in a heavy drinking sample that varied on measures of clinical severity, and the results indicated that greater methylation in the DRD2 promoter was associated with robust activation of the striatum in response to alcohol versus appetitive reward cues. Previous research has made a direct link from preferential activation of the striatum to alcohol over and above a normally appetitive cue like juice with AUD severity (see Claus et al., 2011). Thus, these findings contribute to the understanding of this pathway and highlight a relationship between DRD2 methylation, functional brain responses to reward cues, and AUD severity.
The results suggest a moderately sized positive relationship between DRD2 methylation and measures of AUD severity, such that higher levels of methylation were associated with greater alcohol dependence. In addition, the fMRI analysis in heavy drinkers demonstrated a positive relationship between DRD2 methylation and the extent to which alcohol cues activate reward structures over and above a normally rewarding appetitive stimulus (i.e., the juice cue), such that greater methylation was associated with a significantly greater BOLD activation in response to alcohol cues as compared to juice cues. This effect was localized to the striatum (e.g., accumbens, putamen, and caudate), which is consistent with previous studies demonstrating that the response to drug cues involves the ventral and dorsal striatum (Heinz et al., 2004; Volkow et al., 2006). These observations are also consistent with numerous prior studies in animals and humans linking lower levels of D2 receptors with greater levels of substance use (see Volkow et al., 2006), and greater number of D2 receptors with lower levels of alcohol use (Thanos et al., 2001; Volkow et al., 2006). In addition, our work is consistent with studies reporting associations between higher levels of methylation in the dopamine transporter gene (DAT1) and increased measures of both alcohol craving and cue reactivity (Hillemacher et al., 2009; Wiers et al., 2015). Our findings suggest that epigenetic mechanisms may regulate D2 receptor availability and bias the reward system in favor of drug-related cues. Although it is not clear from the present study, a logical extension of these findings is that DRD2 methylation may also represent a specific treatment target for medication or behavioral treatments for AUDs. Future studies that pharmacologically manipulate epigenetic regulation (particularly DNA methylation), as well as studies that correlate DRD2 methylation with receptor binding and those that examine DRD2 methylation before and after treatment, will help establish the utility of epigenetic marks as potential treatment targets.
Although no prior study has directly examined DRD2 methylation in association with AUD and related phenotypes, our findings are consistent with a recent review reporting that several candidate gene studies have found hypermethylation of promoter regions across specific genes (AVP, DNMT3B, HERP, HTR3A, OPRM1, and SNCA) in AUD (Zhang & Gelernter, 2017). Further, a number of recent papers have reported associations between fMRI measures and methylation in candidate genes, including SLC6A4 (Nikolova et al., 2014) OXTR (Puglia et al., 2015), and NR3C1 (Vukojevic et al., 2014). While our results are focused on downstream neural and behavior effects, they map onto a number of studies that suggest that methylation changes may impact neural function via changes in receptor availability and transcription (Moore et al., 2013). Our results suggest that future studies should directly test a hypothesized mediating relationship between DRD2 methylation/epigenetic regulation and AUD severity, via striatal function and D2 expression. There is a complex interplay between transcription factor binding and methylation in promoter regions, and the functional significance of promoter methylation may depend on the activity of transcription factors (Smith and Meissner, 2013). Because epigenetics and transcription factors play an important role in CNS development more broadly, it is possible that exposure to environmental triggers (e.g., stress, toxins, etc.) during development may result in differential levels of transcriptional activity and/or methylation, thereby altering the expression of D2 receptors, which in turn represents a risk factor for a number of psychiatric disorders, including substance abuse disorders.
Alternatively, alcohol abuse itself, either during a critical developmental period or at any time, may be a trigger that modifies the DRD2 epigenetic landscape and changes D2 expression. Future longitudinal studies will be necessary to determine whether and how alterations in DRD2 promoter methylation specifically impact neurodevelopment and transcription, whether they are a cause or consequence of alcohol abuse, and whether these changes contribute more broadly to variability in neurocognitive processes that have downstream implications for behavior. Likewise, animal studies (e.g. Rodenas-Ruano et al., 2012) could be used to experimentally determine whether alcohol exposure triggers changes in DNA methylation and specific transcription factors, leading to downstream effects on D2 receptors. Evaluating the developmental role of epigenetics on the regulation of D2 receptors and neurocognition throughout the lifespan will require longitudinal studies with DNA collection at multiple time points. Given the importance of the D2 receptor for neurocognition more broadly (Collins and Frank, 2014), and the implications for a number of specific neuropsychiatric disorders (Volkow et al., 2013), this should be a high priority area of research.
An important limitation of the present study is the reliance on measurement of DRD2 methylation in peripheral tissue. Given that the measurement of methylation levels in human brain tissue is not viable in vivo, the majority of previous epigenetic studies focused on the human brain have been conducted using post-mortem samples (Hagerty et al., 2016), which may not reflect the dynamic and neurodevelopmental processes underlying AUD. Thus, our findings are limited by the assumption that peripheral DRD2 methylation is a proxy for central nervous system epigenetic regulation. However, a number of studies have suggested that, for some genes, DNA methylation is correlated across tissue types (for review see Tylee et al., 2013), including two recent studies that examined the correlation between peripheral methylation and protein expression in the brain in vivo using PET, suggesting that peripheral DNA methylation may represent a useful proxy for methylation and protein expression in the brain (Shumay et al., 2012; Wang et al., 2012). Alternatively, it is possible that DRD2 methylation could at least serve as a biomarker for alcohol dependence, given the increasing evidence that methylation in peripheral cells could serve as a biomarker for various neuropsychiatric disorders (Ai et al., 2012).
As noted in a previous review, epigenetic studies should ideally provide a foundation for advancing more fine-grained mechanistic hypotheses about how DNA methylation may alter transcription and down-stream changes in neural function and behavior, which can subsequently be tested in future animal models or human studies (Nikolova and Hariri, 2015). The present study has attempted to address this objective by covering a targeted portion of the DRD2 promoter region and by advancing hypotheses regarding the role of methylation and risk for substance use disorder. Notably, our use of a targeted amplicon based on a putatively functional set of CpGs, strengthens the ability to form mechanistic hypotheses that can be tested in future studies. However, the current study is also limited in that our analysis only covered an 800 bp region of the DRD2 promoter. Methylation in other areas of the gene may be important. In addition, to simultaneously examine the role of transcription factors and DNA methylation in D2 receptors and function, future animal studies will be needed. Ultimately, these findings should be leveraged to design more specific mechanistic hypotheses for future research.
Our findings provide novel evidence of the role of DRD2 promoter methylation in the neurophysiology of AUD and build on a strong theoretical model implicating D2 receptor function in the development of AUD. Continuing investigations on the function of DRD2 and the role of DRD2 methylation in its regulation are a priority research area, and studies in this area will undoubtedly contribute to further elucidating the neurobiology of AUD and myriad other pathologies that involve D2 reception function.
Supplementary Material
Acknowledgments
This research is supported by NIH/NIAAA R01AA014886 and R01AA012238 to KH. LCB is supported by NIH/NIDA K23DA033302.
Footnotes
Authors contribution
LCB, HCK, ABD, and KEH were responsible for the study concept and design. LCB and HCK conceptualized and performed the primary data analysis. RET, EDC, and BJW contributed to the acquisition and analysis of imaging data. All authors assisted with the interpretation of findings and drafting of the manuscript. All authors critically reviewed content and approved final version for publication.
References
- Ai S, Shen L, Guo J, Feng X, Tang B. DNA methylation as a biomarker for neuropsychiatric diseases. International Journal of Neuroscience. 2012;122:165–176. doi: 10.3109/00207454.2011.637654. [DOI] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ. Corticostriatal circuitry and habitual ethanol seeking. Alcohol. 2015;49:817–824. doi: 10.1016/j.alcohol.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beck AT, Steer RA. Relationship between the Beck anxiety inventory and the Hamilton anxiety rating scale with anxious outpatients. Journal of Anxiety Disorders. 1991;5:213–223. [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16:S497. [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AG, Frank MJ. Opponent actor learning (OpAL): modeling interactive effects of striatal dopamine on reinforcement learning and choice incentive. Psychological review. 2014;121:337–366. doi: 10.1037/a0037015. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E. Alcohol: mechanisms along the mesolimbic dopamine system. Progress in brain research. 2014;211:201–233. doi: 10.1016/B978-0-444-63425-2.00009-X. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neuroscience and biobehavioral reviews. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcoholism, clinical and experimental research. 2008;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hagerty SL, Bidwell LC, Harlaar N, Hutchison KE. An Exploratory Association Study of Alcohol Use Disorder and DNA Methylation. Alcoholism, clinical and experimental research. 2016;40:1633–1640. doi: 10.1111/acer.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Tebbutt JS, Mattick RP, Zamir R. Development of a scale for measuring impaired control over alcohol consumption: a preliminary report. Journal of studies on alcohol. 1993;54:700–709. doi: 10.15288/jsa.1993.54.700. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. The American journal of psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Buchholz V, Hussein R, Bleich S, Meyer C, John U, Bischof A, Rumpf H-J. Alterations in DNA-methylation of the dopamine-receptor 2 gene are associated with abstinence and health care utilization in individuals with a lifetime history of pathologic gambling. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015;63:30–34. doi: 10.1016/j.pnpbp.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Hartl T, Wilhelm J, Kornhuber J, Bleich S. Promoter specific methylation of the dopamine transporter gene is altered in alcohol dependence and associated with craving. Journal of psychiatric research. 2009;43:388–392. doi: 10.1016/j.jpsychires.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, Horton WJ, Filbey F. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Archives of general psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. The Journal of pharmacology and experimental therapeutics. 1986;239:219–228. [PubMed] [Google Scholar]
- Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nature reviews Genetics. 2016;17:630–641. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- Kivlahan DR, Sher KJ, Donovan DM. The Alcohol Dependence Scale: a validation study among inpatient alcoholics. Journal of studies on alcohol. 1989;50:170–175. doi: 10.15288/jsa.1989.50.170. [DOI] [PubMed] [Google Scholar]
- Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell. 2011;144:16–26. doi: 10.1016/j.cell.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular basis of memory for addiction. Dialogues in clinical neuroscience. 2013;15:431–443. doi: 10.31887/DCNS.2013.15.4/enestler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Hariri AR. Can we observe epigenetic effects on human brain function? Trends in cognitive sciences. 2015;19:366–373. doi: 10.1016/j.tics.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Koenen KC, Galea S, Wang C-M, Seney ML, Sibille E, Williamson DE, Hariri AR. Beyond genotype: serotonin transporter epigenetic modification predicts human brain function. Nature neuroscience. 2014;17:1153–1155. doi: 10.1038/nn.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas-Ruano A, Chavez AE, Cossio MJ, Castillo PE, Zukin RS. REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nature neuroscience. 2012;15:1382–1390. doi: 10.1038/nn.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Scott A, Courtney W, Wood D, de la Garza R, Lane S, King M, Wang R, Roberts J, Turner JA, Calhoun VD. COINS: An Innovative Informatics and Neuroimaging Tool Suite Built for Large Heterogeneous Datasets. Frontiers in neuroinformatics. 2011;5:33. doi: 10.3389/fninf.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumay E, Logan J, Volkow ND, Fowler JS. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics. 2012;7:1151–1160. doi: 10.4161/epi.21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human brain mapping. 2014;35:1981–1996. doi: 10.1002/hbm.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nature reviews Genetics. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- Sobell M, Sobell L, Maisto S. Alcoholism treatment assessment research instruments(NIAA treatment handbook series 2) Rockville, MD: National Institute on Alcohol Abuse; 1979. Time line follow back assessment method [TLFB] pp. 167–188. [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R. Overexpression of dopamine D2 receptors reduces alcohol self-administration. Journal of neurochemistry. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Tost J, Gut IG. DNA methylation analysis by pyrosequencing. Nature protocols. 2007;2:2265–2275. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nature reviews Neuroscience. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Tylee DS, Kawaguchi DM, Glatt SJ. On the outside, looking in: a review and evaluation of the comparability of blood and brain “-omes”. Am J Med Genet B Neuropsychiatr Genet. 2013;162b:595–603. doi: 10.1002/ajmg.b.32150. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Maynard LJ, Wong CT. Predominance of D2 receptors in mediating dopamine’s effects in brain metabolism: effects of alcoholism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4527–4535. doi: 10.1523/JNEUROSCI.5261-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism: Clinical and Experimental Research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Fowler JS, Goldstein RZ, Klein N, Logan J, Wong C, Swanson JM. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular psychiatry. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukojevic V, Kolassa IT, Fastenrath M, Gschwind L, Spalek K, Milnik A, Heck A, Vogler C, Wilker S. Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. 2014;34:10274–10284. doi: 10.1523/JNEUROSCI.1526-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Szyf M, Benkelfat C, Provencal N, Turecki G, Caramaschi D, Cote SM, Vitaro F, Tremblay RE, Booij L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PloS one. 2012;7:e39501. doi: 10.1371/journal.pone.0039501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Shumay E, Volkow ND, Frieling H, Kotsiari A, Lindenmeyer J, Walter H, Bermpohl F. Effects of depressive symptoms and peripheral DAT methylation on neural reactivity to alcohol cues in alcoholism. Translational psychiatry. 2015;5:e648. doi: 10.1038/tp.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O’connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D2 receptor availability is associated with subjective responses to alcohol. Alcoholism: Clinical and Experimental Research. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gelernter J. DNA methylation and alcohol use disorders: Progress and challenges. The American journal on addictions. 2017;26(5):502–515. doi: 10.1111/ajad.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.