Abstract
Objective
Study was planned to know vitamin D status in mothers and their newborns and effect of vitamin D deficiency on pregnancy outcome. Study design: Two hundred consecutive pregnant women with singleton pregnancy admitted to the labor ward of a tertiary care center were recruited for the study. Maternal and cord blood samples were taken and analyzed for 25(OH) D level. Maternal and fetal outcomes were studied.
Results
High prevalence of hypovitaminosis D was found among pregnant women. Eighty-six percentage had vitamin D deficiency, 9.5% had insufficiency, and only 4.5% had sufficient vitamin D level. Women with preeclampsia had statistically significant vitamin D deficiency and insufficiency as compared to patients who had normal blood pressure levels (p = 0.04). Cesarean section rate was significantly higher in patients with vitamin D deficiency and insufficiency compared to sufficient group (p = 0.004). Cord blood 25(OH) D levels strongly correlated with maternal serum 25 (OH) D levels (p = 0.001, correlation coefficient r = 0.84).
Conclusions
This study showed a very high prevalence of hypovitaminosis D among pregnant women and excellent correlation between maternal and fetal 25(OH) D levels. Hypovitaminosis D was associated with preeclampsia, increased Cesarean rate, and low birth weight babies.
Keywords: Hypovitaminosis D, Pregnancy outcomes, Newborn
Introduction
Vitamin D is a complex hormone system known to be involved in bone metabolism. Recently, vitamin D has been implicated in physiologic processes as diverse as vascular health, metabolism, immune function, and placental function. Vitamin D can be synthesized by our skin under the effect of sunlight. Despite the fact that Indian subcontinent is situated between 8.4°N and 37.6°N latitude and has adequate sunshine throughout the year, studies have shown widespread prevalence of hypovitaminosis D in India [1–3]. The reasons cited for high prevalence of VDD in our country despite favorable natural conditions are increased skin melanin pigment, less outdoor activity, vegetarian diet, wearing of more covered clothing, and no policy of fortification of food items with vitamin D [1].
There is no consensus in defining hypovitaminosis D in pregnancy and cutoff serum value range from 10 to 32 ng/ml. According to American College of Obstetrics and Gynaecology (ACOG), serum 25(OH) D concentration of at least 20 ng/mL is needed to avoid bone problems and vitamin D deficiency should be defined as circulating 25(OH) D levels less than 32 ng/ml [4]. According to Canadian Paediatric Society, serum 25(OH) D concentration below 10 ng/ml is used as a cutoff for deficiency and between 10 and 30 ng/ml as a cutoff for insufficiency [5]. Institute of Medicine (IOM) recommended circulating level of 25(OH) D to be more than 20 ng/ml [6].
The topic of maternal vitamin D requirements during pregnancy with regard to maternal and fetal outcomes has been poorly studied, and no conclusive evidence links lower levels of 25-hydroxyvitamin D with adverse pregnancy outcomes. Clinical studies establishing an association between vitamin D levels and adverse pregnancy outcomes such as preeclampsia, gestational diabetes, low birth weight, preterm labor, Cesarean delivery, and infectious diseases have conflicting results.
Maternal vitamin D deficiency is a major risk factor for vitamin D deficiency in infancy as the fetus and newborn are entirely dependent on the mother for their supply of vitamin D. Studies have shown that maternal serum 25(OH)D levels correlate positively with cord blood 25(OH)D levels [7].
As the studies regarding the prevalence of hypovitaminosis D in pregnancy and correlation of maternal and cord blood serum vitamin D levels are very sparse from India and none of these studies have correlated the pregnancy outcome with hypovitaminosis D, this study has been planned to know the vitamin D status in mothers and their newborns and effect of vitamin D deficiency on pregnancy outcome.
Methods
A total of 200 pregnant women with singleton pregnancy admitted to the labor ward for delivery and their newborns were consecutively recruited for the study. Exclusion criteria were pregnant patient with known history or evidence of overt thyroid, parathyroid or adrenal diseases, hepatic or renal failure, metabolic bone disease, type 1 diabetes mellitus and malabsorption, and not willing to participate in the study. Detailed history was recorded from the recruited pregnant women including complete demographic details, dietary history, past medical history, previous obstetric history, antenatal history including details of any antenatal complications. Intake of vitamin D tablets during pregnancy, its dose, and duration were also recorded. Labor and delivery details including induction of labor, mode of delivery, newborn birth weight, and APGAR scores were recorded. Maternal and cord blood samples (5 ml each) were drawn at the time of delivery for estimation of 25(OH) D to diagnose hypovitaminosis D and correlation between maternal and cord blood serum concentrations of vitamin D. Serum was separated from collected samples, and they were stored at − 20°C till analysis. Same sample was also tested for serum calcium. Vitamin D was analyzed by chemiluminescence method on Advia Centaur XP. Vitamin D deficiency was defined as 25(OH) D levels in blood < 20 ng/ml, and insufficiency of vitamin D was defined as 25(OH) D levels < 32 ng/ml [5]. After the estimation of vitamin D levels in the mothers and their newborns, recruited for the study, vitamin D status in mothers and their newborns was known. Outcome of pregnancy–antenatal complications (preeclampsia, gestational hypertension, eclampsia, gestational diabetes, preterm labor, PROM), rate of induction, rate of Cesarean sections, low birth weight babies (weight < 2500 g), preterm birth, and small for gestational age were studied in women with hypovitaminosis D, and it was statistically compared with the outcome in pregnant women having sufficient level of vitamin D.
Results
A total of 200 consecutive pregnant women, admitted in labor room for delivery, with singleton pregnancy willing to participate in the study were recruited. Subjects underwent biochemical investigations, including vitamin D levels and calcium levels. Maternal and fetal outcomes in relation to vitamin D levels were recorded. The descriptive data and further analyzed data are presented as follows. For detailed statistical analysis, subjects (mothers) were divided into three groups depending upon their vitamin D levels: deficient group [25(OH) D levels < 20 ng/ml], insufficiency group [25(OH) D levels 20–32 ng/ml], and sufficient group [25(OH) D levels > 32 ng/ml].
Out of the 200 cases enrolled, 172 (86%) of the subjects were vitamin D deficient and 19 (9.5%) were vitamin D insufficient. Only nine (4.5%) pregnant women had sufficient vitamin D levels. Mean value of the vitamin D level in mothers was 12.6 (ng/ml) ± 6.8 ng/ml.
Vitamin D levels were not found to be affected by difference in age, socioeconomic status, religion, rural versus urban population, BMI, and parity of pregnant women. No significant difference was seen in hypovitaminosis D prevalence in relation to dietary factors, sun exposure, and calcium and vitamin D supplements intake. This finding explained that the dose of vitamin D (500 IU/day) taken during pregnancy was not sufficient to normalize their vitamin D levels in deficient state. And also most of the subjects had sun exposure over face and hands only and that to for very less time.
Mean calcium level in deficient group was 8.6 (SD 1.2) mg/dl, in insufficient group was 9.3 (SD 1.1) mg/dl, and in sufficient group was 10.0 (SD 2.7) mg/dl. Despite high prevalence of hypovitaminosis D, mean calcium level in three groups was within normal range. (Normal serum calcium level in pregnant women in third trimester is 8.2–9.7 mg/dl.)
Fifty-two (26%) patients were found to have preeclampsia out of 200 cases enrolled. Statistically significant number of preeclampsia patients had vitamin D deficiency and insufficiency as compared to patients who had normal blood pressure levels (p = 0.04). However, statistically no correlation was seen, of vitamin D deficiency and insufficiency with gestational hypertension (p = 0.407). There was no association found between vitamin D deficiency or insufficiency with GDM, preterm labor, PROM, gestational hypertension, cholestasis, IUGR, abruption, and anemia.
Of the 200 patients, 48% had spontaneous onset of labor, 43% underwent induction of labor for various reasons, and 9% underwent elective Cesarean section. Of the patients who underwent Cesarean section, 92% had vitamin D deficiency and 6% had insufficiency as compared to patients who underwent normal vaginal delivery, 85.6% had vitamin D deficiency, and 10.8% had insufficiency, and this difference was statistically significant (p = 0.004).
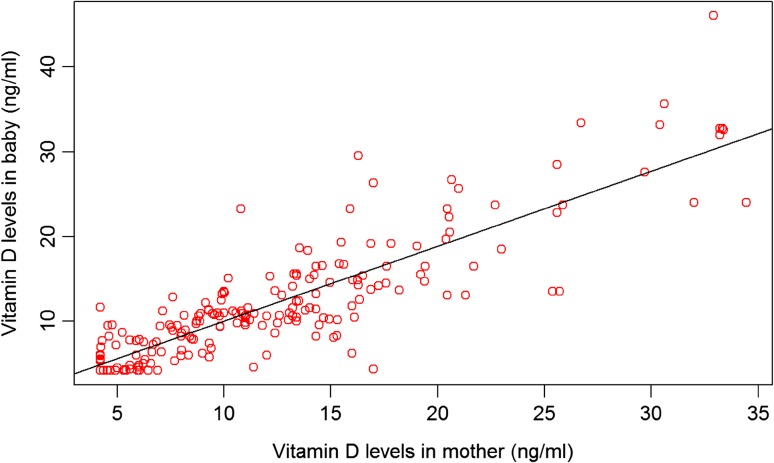
Significant correlation was seen between vitamin D levels in mothers and vitamin D levels in cord blood of their newborns (p = 0.001, correlation coefficient r = 0.84). In cord blood, deficiency was seen in 170 (85%) samples, insufficiency was seen in 23 (11.5%), and sufficiency was seen only in seven (3.5%) samples. In mothers with vitamin D deficiency, cord blood vitamin D deficiency was seen in 97.7% and insufficiency was seen in 2.3% (Table 1, Fig. 1). No vitamin D cord blood level was sufficient in mothers having vitamin D deficiency. Multivariate analysis was done to evaluate determinants of cord blood vitamin D levels. Variables presented in Table 1 were included in the model. Of these, maternal vitamin D levels and gestational age at birth significantly influenced the cord blood vitamin D levels. The variables included in the multivariate regression model were responsible for 74% of variability observed in vitamin D levels in cord blood (adjusted R square value = 0.74).
Table 1.
Correlation between maternal and cord blood vitamin D levels (n = number)
| Cord blood vitamin D | Maternal vitamin D | p value | ||
|---|---|---|---|---|
| Deficient | Insufficient | Sufficient | ||
| Deficient (n = 170) | 168 (97.7%) | 2 (10.5%) | 0 | < 0.001 |
| Insufficient (n = 23) | 4 (2.3%) | 17 (89.5%) | 2 (22.2%) | |
| Sufficient (n = 7) | 0 | 0 | 7 (77.8%) | |
Fig. 1.
Scatter plot depicting correlation between maternal and cord blood vitamin D levels (r = 0.84)
Mean birth weight of babies born to mothers having vitamin D deficiency was 2.7 (0.5) kg, those having insufficiency was 2.9 (0.5) kg, while mean birth weight of babies born to mothers having sufficient vitamin D levels was 3.0 (0.3) kg. Of the babies having birth weight < 2.5 kg, 94.7% of their mothers had vitamin D deficiency, 5.3% had insufficiency, and none had sufficient vitamin D levels (Table 2). However, of babies who had birth weight > 2.5 kg, 80.5% of their mothers had vitamin D deficiency, 12.2% had insufficiency, and 7.3% had sufficient vitamin D levels. So there was significant association of hypovitaminosis D with low birth weight babies (p = 0.01). No significant association was found between vitamin D levels with IUGR, fetal distress, and APGAR of babies. No baby in our study had congenital malformation.
Table 2.
Fetal birth weight and vitamin D levels in mother (n = number)
| Fetal birth Wt. (kg) | Deficient | Insufficient | Sufficient | p value |
|---|---|---|---|---|
| < 2.5 (n = 77) | 73 (94.7%) | 4 (5.3%) | 0 | 0.01 |
| > 2.5 (n = 123) | 99 (80.5%) | 15 (12.2%) | 9 (7.3%) |
Discussion
Our study where 200 consecutive pregnant women with singleton pregnancy were recruited showed a high prevalence of vitamin D deficiency in pregnant women. Vitamin D deficiency was seen in 86% of the mothers, insufficiency was seen in 9.5%, and only 4.5% mothers had sufficient vitamin D levels [25 (OH) D > 32 ng/ml]. Although the literature is sparse from India, few studies which have been conducted in India have also shown high prevalence of hypovitaminosis D despite India being a tropical country with abundant sunshine for most or all of the year [1, 3, 8].
Clinical studies establishing an association between vitamin D levels and adverse pregnancy outcomes such as preeclampsia, gestational diabetes, low birth weight, preterm labor, Cesarean delivery, and infectious diseases have conflicting results. Overall, most of the studies were conducted in the developed countries with very sparse data from the developing countries like India. Our study revealed that maternal hypovitaminosis D was significantly associated with preeclampsia (p = 0.04). Among the pregnant women in our study who had preeclampsia, 96.2% had vitamin D deficiency, 3.8% had vitamin D insufficiency, and none had sufficient vitamin D levels. This is in accordance with various studies whereby lower maternal serum vitamin D concentrations were found in women with preeclampsia when compared to normotensive uncomplicated women [9–11]. However, recent study done by Fernandez-Alonso et al. found no difference in mean early pregnancy maternal 25(OH) vitamin D in those who developed preeclampsia compared to those with normal pregnancies, but the number of cases enrolled was too less [12].
No statistically significant association was found in our study, of vitamin D levels with other adverse antenatal complications like gestational hypertension, gestational diabetes mellitus, preterm labor, PROM, cholestasis, and abruption.
Hypovitaminosis D was not found to be significantly associated with higher rate of induction of labor; however, Cesarean rate was higher in this group in our study. Of the patients who underwent Cesarean section, 92% had vitamin D deficiency and 6% had insufficiency as compared to patients who underwent normal vaginal delivery, 85.6% had vitamin D deficiency, and 10.8% had insufficiency, and this difference was statistically significant (p = 0.004); similar result was seen in a study by Merewood et al. [13].
In our study, cord blood 25(OH) D strongly correlated with maternal serum values of 25(OH) D. Mean maternal serum 25(OH) D level was 12.5 (SD 6.8) ng/ml, and mean cord blood 25(OH) D level was 12.3 (SD 7.1) ng/ml. Eighty-six percentage of mothers had vitamin D deficiency, and 9.5% had vitamin D insufficiency, while 85 and 11.5% of cord blood samples had vitamin D deficiency and insufficiency, respectively. In mothers with vitamin D deficiency, cord blood vitamin D deficiency was seen in 97.7% and insufficiency was seen in 2.3%. None of the cord blood sample showed sufficient vitamin D level, in mothers having vitamin D deficiency. However, in a study by Agarwal et al. [14], no significant difference (p = 0.82) in 25(OH) D3 levels between paired maternal and umbilical cord blood samples was found, but the number of subjects recruited in study was too less (n = 20).
Vitamin D deficiency and insufficiency in mothers of babies weighing < 2.5 kg were seen in 94.7 and 5.3%, respectively, and none had sufficient vitamin D levels. However, of babies who had birth weight > 2.5 kg, 80.5% of their mothers had vitamin D deficiency, 12.2% had insufficiency, and 7.3% had sufficient vitamin D levels. Hence, we observed that offspring with low birth weight had significantly higher prevalence of vitamin D deficiency and insufficiency among their mothers (p = 0.011), and this association was present even after adjusting for confounding factors like preeclampsia, gestational age, prepregnancy BMI, and anemia. Various studies have demonstrated lower birth weight in infants born to mothers with low circulating 25(OH) D concentrations [15–17], while other studies have shown no differences [18–21]. No significant association was found in terms of vitamin D levels with IUGR, fetal distress, or APGAR score of baby.
Conclusion
This study showed a very high prevalence of hypovitaminosis D (86% had vitamin D deficiency, and 9.5% had vitamin D insufficiency) among pregnant women in a tertiary care hospital of northern India and excellent correlation between maternal and fetal 25(OH) D levels. Hypovitaminosis D was associated with preeclampsia, increased Cesarean rate, and low birth weight babies. Our study showed no association of hypovitaminosis D with GDM, preterm labor, PROM, gestational hypertension, cholestasis, IUGR, abruption, anemia, fetal distress, and APGAR score. As the number of subjects (mother–infant pair) recruited was only 200, more large-scale studies are required to study the exact prevalence and effect of hypovitaminosis D on pregnancy outcomes.
Saloni Arora
has completed her MBBS from GMCH, Chandigarh, in 2011 and her post-graduation (MD) in obstetrics and gynecology from Govt. Medical College and Hospital, Chandigarh, in 2015. Presently, she is working as senior resident in Department of Obstetrics and Gynecology in Govt. Medical College and Hospital, Chandigarh.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Institutional Ethics Committee of Government Medical College and Hospital, Chandigarh, India.
Footnotes
Dr. Saloni Arora (MD) is Senior Resident at Department of Obstetrics and Gynaecology, D Block Level IV GMCH Sector 32, Chandigarh. Dr. Poonam Goel (MD) is Professor at Department of Obstetrics and Gynaecology, D Block Level IV GMCH Sector 32, Chandigarh, India. Dr. Deepak Chawla (MD, DM-Neonatology) is an Associate Professor at Department of Pediatrics, D Block Level IV GMCH Sector 32, Chandigarh, India. Dr. Anju Huria (MD) is Professor at Department of Obstetrics and Gynaecology, D Block Level IV GMCH Sector 32, Chandigarh, India. Dr. Adhi Arya is a Senior Resident at Department of Pediatrics, D Block Level IV GMCH Sector 32, Chandigarh, India.
Contributor Information
Saloni Arora, Email: saloni.arora09@gmail.com.
Poonam Goel, Email: poonam1302@yahoo.com.
Deepak Chawla, Email: drdeepak.chawla@gmail.com.
Anju Huria, Email: anjuhuria1114@gmail.com.
Adhi Arya, Email: adhiarya32@gmail.com.
References
- 1.Sachan A, Gupta R, Das V, et al. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 2.Jain V, Gupta N, Kalaivani M, et al. Vitamin D deficiency in healthy breastfed term infants at 3 months & their mothers in India: Seasonal variation & determinants. Indian J Med Res. 2011;3:267–273. [PMC free article] [PubMed] [Google Scholar]
- 3.Marwaha RK, Tandon N, Chopra S, et al. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011;106:1383–1389. doi: 10.1017/S000711451100170X. [DOI] [PubMed] [Google Scholar]
- 4.Committee ACOG. Vitamin D: screening and supplementation during pregnancy. Obstet Gynecol. 2011;118:197–198. doi: 10.1097/AOG.0b013e318227f06b. [DOI] [PubMed] [Google Scholar]
- 5.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine . Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 7.Zhila M, Arash HN, Ali RS, et al. Vitamin D status in mothers and their newborns in Iran. BMC Pregnancy Childbirth. 2007;7:1–7. doi: 10.1186/1471-2393-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahu M, Bhatia V, Agarwal A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol. 2009;70:680. doi: 10.1111/j.1365-2265.2008.03360.x. [DOI] [PubMed] [Google Scholar]
- 9.Bodnar LM, Catov JM, Simhan HN. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker AM, Haeri S, Camargo CA., Jr A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–5109. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson CJ, Alanis MC, Wagner CL. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203:361–366. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez AM, Dionis SEC, Chedraui P, et al. First-trimester maternal serum 25-hydroxyvitamin D(3) status and pregnancy outcome. Int J Gynaecol Obstet. 2012;116:6–9. doi: 10.1016/j.ijgo.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Merewood A, Mehta SD, Chen TC. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94:940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal N, Arya SC. Vitamin D3 levels in pregnant women and newborns at a private tertiary care hospital in Delhi, India. Int J Gynaecol Obstet. 2011;113:240–241. doi: 10.1016/j.ijgo.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Amirlak I, Ezimokhai M, Dawodu A, et al. Current maternal infant micronutrient status and the effects on birth weight in the United Arab Emirates. East Mediterr Health J. 2009;15:1399–1406. [PubMed] [Google Scholar]
- 16.Bowyer L, Catling PC, Diamond T, et al. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol (Oxf) 2009;70:372–377. doi: 10.1111/j.1365-2265.2008.03316.x. [DOI] [PubMed] [Google Scholar]
- 17.Leffelaar ER, Vrijkotte TG, Van EM. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth. Br J Nutr. 2010;104:108–117. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 18.Farrant HJW, Krishnaveni GV, Hill JC, et al. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63:646–652. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardawi M, Nasra HA, Ba’aqueel HS, et al. A cross-sectional study showing Vitamin D status and calcium-regulating hormones in Saudi pregnant females and their babies. Saudi Med J. 1997;18:15–24. [Google Scholar]
- 20.Sabour H, Hossein NA, Maghbooli Z, et al. Relationship between pregnancy outcomes and maternal vitamin D and calcium intake. Gynecol Endocrinol. 2006;22:585–589. doi: 10.1080/09513590601005409. [DOI] [PubMed] [Google Scholar]
- 21.Maghbooli Z, Hossein NA, Shafaei AR, et al. Vitamin D status in mothers and their newborns in Iran. BMC Pregnancy Childbirth. 2007;7(1):1. doi: 10.1186/1471-2393-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]