Abstract
Background
Polycystic ovarian syndrome (PCOS), a commonly prevalent endocrinopathy among reproductive age group women, is most often associated with obesity. Increased insulin resistance appears to be the central pathophysiologic mechanism responsible for various complications of PCOS. This makes ‘weight loss’ as the first-line treatment approach in PCOS. So various trials have tried to compare metformin (an insulin-sensitizing agent) and orlistat (an anti-obesity drug) aiming to achieve weight loss and hence higher ovulation rate for the group of obese PCOS patients. Keeping an eye on all these background facts, we designed this systematic review and metaanalysis to compare the effects of metformin and orlistat on various aspects of PCOS and to pick the better among the two drugs.
Materials and Methods
This is a systemic review of randomized control trials that studied the effectiveness of orlistat versus metformin in terms of improvement in ovulation rate, weight loss, lipid profile, etc. Systematic literature search over the period January 2000–December 2016 was performed in the following electronic databases: Medline, embase, google scholar, pubmed and The Cochrane Library and only randomized controlled clinical trials were included in our study. All authors carefully went through all sources of information independently.
Results
According to this study, weight loss, testosterone level after 4 weeks of treatment, total serum cholesterol and triglyceride level showed significant fall in orlistat-treated group.
Conclusion
Our review shows that orlistat is a more effective drug than metformin and should be the preferred drug in obese PCOS in combination with weight loss.
Keywords: Polycystic ovarian syndrome, Orlistat, Metformin, Obesity
Introduction
Polycystic ovarian syndrome (PCOS) is the most commonly prevalent endocrinopathy of reproductive age group women and is characterized by chronic anovulation and androgen excess with the predominant clinical manifestation being oligomenorrhea, hirsutism, and acne. The prevalence is as high as 15% when Rotterdam criteria are used for its diagnosis [1]. Increased insulin resistance (IR) is the central pathophysiologic mechanism responsible for increased risk of developing type 2 diabetes, the adverse cardiovascular risk as well as androgen excess and infertility in PCOS patients. Obesity which has been recently designated as an epidemic is often associated with PCOS. The prevalence was 40–60% among women with PCOS [2]. This makes ‘weight loss’ as the first-line treatment approach in overweight PCOS women [2].
These pathophysiological mechanisms have led to trials involving pharmacotherapy like metformin (an insulin-sensitizing agent) and orlistat (an anti-obesity drug) aiming to achieve weight loss and hence higher ovulation rate for the group of obese PCOS patients.
This is a systemic review of randomized control trials that studied the effectiveness of orlistat versus metformin in terms of improvement in ovulation rate, weight loss, lipid profile, etc.
Materials and Methods
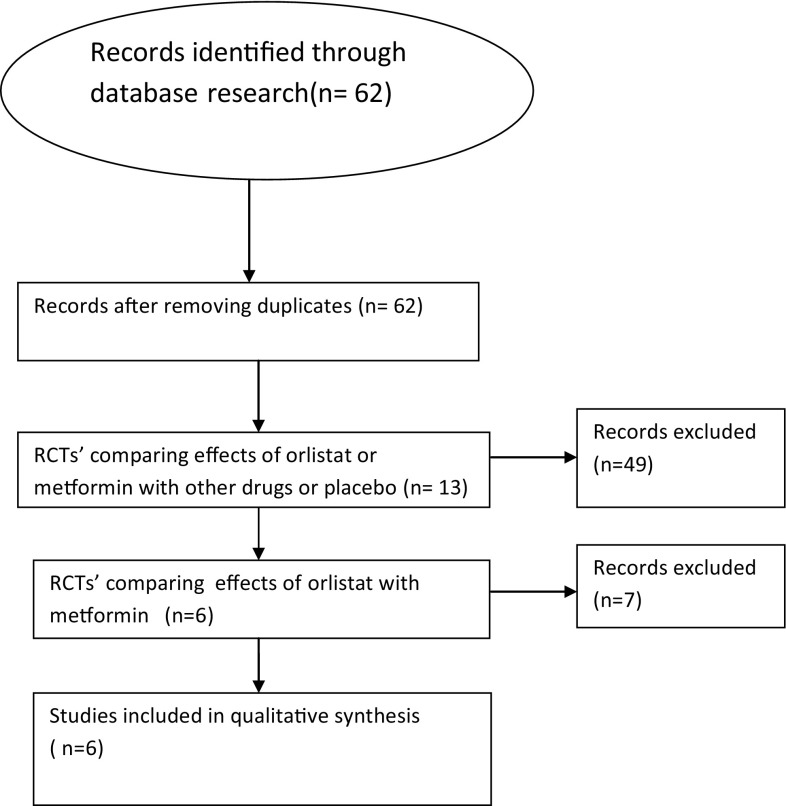
Systematic literature search was performed in the following electronic databases: Medline, embase, google scholar, pubmed and The Cochrane Library. We performed a search over the period January 2000–December 2016 and only randomized controlled clinical trials (RCT) comparing the effects of orlistat and metformin were included. Search terms were as follows: “orlistat”, “metformin”, “polycystic ovary syndrome”, “insulin resistance”, etc. Six randomized control trials were eligible for our study. All authors carefully went through all sources of information independently. One review author extracted data from the included studies and the second author checked the extracted data. Information on the characteristics of a trial was extracted from each included trial. The characteristics of each study has been described in Table 1. All the statistical analysis was done using Statistical Software SPSS Version 20. Flow chart of the study selection is shown in Fig. 1.
Table 1.
Characteristics of the included studies
| References | Country | PCOS criteria | Types of studies | Dosage | Length (weeks) | Age (PCOS-orlistat group vs. Metformin group) Mean ± SD | N (PCOSorlistat/metformin group) |
|---|---|---|---|---|---|---|---|
| Jayagopal et al. [6] | UK | NA | Prospective, randomized, open-label study | Orlistat: 120 mg three times per day; Metformin: 500 mg once daily for the first week, 500 mg twice daily for the next week and 500 mg three times daily for the remainder of the study period | 12 | 27.0 ± 4.12 (all participants) | 10/11 |
| Metwally et al. [3] | UK | Rotterdam | Randomized controlled trial | Orlistat: 120 mg twice daily Metformin: initially as 500 mg once daily after meals for one a week followed by 500 mg twice daily for 4 weeks then increased in 500 mg increments every 4 weeks to a maximum of 2000 mg per day | 12 | 30.6 (± 1.6) vs. 28.8 (± 0.9) [p value 0.34] | 20/20 |
| Cho et al. [8] | UK | Rotterdam | Randomized, open-label parallel study | Orlistat: 120 mg three times per day; Metformin: 500 mg once daily for the first week, 500 mg twice daily for the next week and 500 mg three times daily for the remainder of the study period | 12 | 26.4 ± 6.7 (all participants) | 10/10 |
| Ghandi et al. [5] | Poland | Rotterdam | Randomized, open-label parallel study | Orlistat: 120 mg three times per day; Metformin: 500 mg once daily for the first week, 500 mg twice daily for the next week and 500 mg three times daily for the remainder of the study period | 12 | 27.0 ± 44.0 (all participants) | 40/40 |
| Kumar et al. [4] | India | Rotterdam | Randomized controlled trial | Orlistat: 120 mg two times per day; Metformin was incremented stepwise to maximum 500 mg 3 times a day | 12 | NA | 30/30 |
| Kujawska-Łuczak et al. [7] | Iran | Rotterdam | Prospective randomized open-label study | Orlistat: 120 mg three times per day; Metformin: 500 mg twice daily | 12 | 31.4 ± 8.2 years (all participants) | 36/37 |
Fig. 1.
Flow chart showing the selection of literatures that are included in our study
Results
As shown in Table 2, the ovulation rate is higher for orlistat group in the studies done by Metwally et al. [3] (40 vs. 25%; p value − 0.10) and Kumar et al. [4] (33.3 vs. 23.3%; p value − 0.418). On the contrary, Ghandi et al. [5] have shown higher ovulation rate for metformin (15% for orlistat group vs. 30% for metformin group; p value − 0.108). But none of these studies have statistically significant results.
Table 2.
Effect on ovulation rate (%)
In view of Table 3, Jayagopal et al. [6] showed that the reduction in weight and after treatment with orlistat was more significant than seen in the metformin-treated group (4.69 vs. 1.02%, p value − 0.006). Similarly according to Kujawska-Łuczak et al. [7], the percentage change of weight loss and BMI was more in orlistat-treated group than that of metformin (− 3.2 ± 0.8 vs. − 1.7 ± 0.4; p value < 0.05 for weight loss and − 4.9 ± 1.3 vs. − 9.4 ± 2.3; p value < 0.05 for BMI). On comparison, the difference between the above said groups was found to be statistically significant for the concerned parameters. On the other hand, the studies by Metwally et al. [3], Kumar et al. [4], Ghandi et al. [5] and Cho et al. [8] could not found a statistically significant result between both arms as far as weight loss or BMI is concerned (p value 0.40, 1.000, > 0.05, 0.07 respectively for the above four studies).
Table 3.
Effect on BMI and weight loss
| Study group | Percentage change in BMI | Percentage change in wt loss | ||
|---|---|---|---|---|
| Orlistat | Metformine | Orlistat | Metformine | |
| Jayagopal et al. [6] | NA | NA | 4.69 ± 1.2 | 1.02 ± 0.9 |
| p value | 0.006 | |||
| Metwally et al. [3] | (−) 0.7 | (−) 2 | NA | NA |
| p value | 0.40 | |||
| Ghandi et al. [5] | (−) 4.48 ± 0.47 | (−) 4.55 ± 0.7 | NA | NA |
| p value | > 0.05 (non significant) | |||
| Kumar et al. [4] | − 8.12 ± 6.17 | − 8.40 ± 0.65 | NA | NA |
| p value | 1.000 | |||
| Kujawska-Łuczak et al. [7] | − 3.2 ± 0.8 | − 1.7 ± 0.4 | − 9.4 ± 2.3 | − 4.9 ± 1.3 |
| p value | < 0.05 | < 0.05 | ||
| Cho et al. [8] | (− 5.70) ± 0.80 | (− 3.40) ± 1.00 | NA | NA |
| p value | 0.07 | |||
Metwally et al. [3] have shown that there is a significant fall in testosterone level after 4 weeks of treatment with orlistat while for metformin this period appears to be 8 weeks, but without significant change in SHBG concentration in either group. According to Ghandi et al. [5], patients with orlistat have a significant fall in serum testosterone level after 12 week of treatment (percentage change from baseline serum testosterone level is − 19.37 ± 3.52 with p value < 0.001) while for those with metformin the fall in serum testosterone level was not statistically significant (percentage change from baseline −17.30 ± 5.30 with p value 0.053). Similarly, Cho et al. [8] have shown significant fall in free androgen index from baseline (− 22·9 ± 7.4; p value 0.017 for metformin arm vs. − 20.8 ± 5.8; p value 0.007 for orlistat arm) and significant increase in SHBG concentration from baseline (13.3 ± 3.1; p value < 0.05 for orlistat arm vs. 14.3 ± 5.0; p value < 0.05 for metformin arm) in both groups. While comparing the fall in testosterone level in orlistat-treated group with those treated with metformin, none of the concerned studies shows statistically significant result (Table 4).
Table 4.
Effect on testosterone and SHBG levels
| Study group | Change in testosterone levels (ng/dl) over 12 week | Change in SHBG levels (ng/dl) over 12 week | ||
|---|---|---|---|---|
| Orlistat | Metformine | Orlistat | Metformine | |
| Jayagopal et al. [6] | − 16.8 + 7.1 | − 14.69 + 9.6 | + 4.16 + 2.9 | + 1.46 + 4.0 |
| p value | 0.973 | 0.197 | ||
| Metwally et al. [3] | Sig fall after 4 weeks therapy | Sig fall after 8 weeks therapy | No significant change | No significant change |
| Ghandi et al. [5] | − 19.37 ± 3.52 (significant fall) | − 17.30 ± 5.30 (non significant fall) | NA | NA |
| Non significant | ||||
| Kumar et al. [4] | (−) 17.68 + 4.18 | (−) 12.89 + 3.12 | 4.67 + 7.83 | 13.2 + 33.78 |
| p value | 0.712 | 0.438 | ||
| Cho et al. [8] | NA | NA | 13.3 ± 3.1 (significant increase) | 14.3 ± 5.0 (significant increase) |
| p value | Non significant | |||
As far as lipid profile is concerned (Table 5), Ghandi et al. [5] showed that treatment with orlistat resulted in a significant decline in total serum cholesterol and triglyceride on the contrary metformin treatment caused a significant reduction in serum triglyceride only but not that of serum cholesterol. When the above result is compared between metformin and orlistat-treated groups, the more fall in total cholesterol level that was associated with orlistat treatment was found to be statistically significant (− 9.39 ± 2.43 vs. −1.54 ± 1.76 for orlistat and metformin arm, respectively; p value 0.023). Similarly, the more fall in total cholesterol level (− 9.51 + 1.56 vs. − 4.33 + 2.90; p value 0.037) and serum LDL level (− 5.66 + 2.07 vs. − 1.32 + 1.82; p value 0.04) that was associated with orlistat treatment group was also found to be statistically significant as compared with that of metformin arm in the study by Kumar et al. [4].
Table 5.
Effect on lipid profile over 12 weeks
| Study group | Change in total cholesterol | Change in triglyceride | LDL | HDL | ||||
|---|---|---|---|---|---|---|---|---|
| Orlistat | Metformine | Orlistat | Metformine | Orlistat | Metformine | Orlistat | Metformine | |
| Jayagopal et al. [6] | − 1.68 ± 4.5 | + 3.23 ± 3.8 | + 0.23 ± 9.5 | − 0.63 ± 8.4 | − 5.97 ± 4.8 | + 2.75 ± 4.1 | + 7.49 ± 4.6 | + 4.33 ± 3.9 |
| p value | 0.557 | 0.654 | 0.282 | 0.468 | ||||
| Ghandi et al. [5] | − 9.39 ± 2.43 | − 1.54 ± 1.76 | − 15.26 ± 4.93 | − 19.97 ± 3.40 | NA | NA | NA | NA |
| p value | 0.023 | Non significant | ||||||
| Kumar et al. [4] | (−) 9.51 + 1.56 | (−) 4.33 + 2.90 | (−) 5.24 + 3.14 | (−) 0.98 + 3.34 | (−) 5.66 + 2.07 | (−) 1.32 + 1.82 | 2.88 + 2.3 | 2.37 + 2.68 |
| p value | 0.037 | 0.391 | 0.004 | 0.796 | ||||
Cho et al. [8] showed a significant improvement in insulin resistance with orlistat treatment (p value 0.013) but not with metformin treatment (p value 0.17) as assessed by HOMA-IR. Similarly, the biological variability of HOMA-IR was reduced significantly only in the orlistat-treated group(p value 0.015). None of any other study groups showed significant improvement in any of the biochemical parameters for assessing insulin resistance as described in Table 6.
Table 6.
Effect on insulin resistance
| Study group | Change in FBS | Change in fasting insulin | HOMA-IR | |||
|---|---|---|---|---|---|---|
| Orlistat | Metformine | Orlistat | Metformine | Orlistat | Metformine | |
| Jayagopal et al. [6] | (−) 2.15 + 1.0 | (−) 0.99 + 1.3 mmol/l | (−) 12.5 + 5.8 mic IU/ml | (−) 7.39 + 8.2 | (−) 10.8 + 6.0 | (−) 7.19 + 8.4 |
| p value | 0.426 | 0.426 | 0.756 | |||
| Kumar et al. [4] | 0.90 + 2.29 | (−) 2.10 + 2.16 | 8.35 + 5.54 | (−) 0.86 + 4.12 | 10.56 + 7.45 | (−) 3.78 + 3.78 |
| p value | 0.178 | 0.28 | 0.301 | |||
| Kujawska-Łuczak et al. [7] | 0.31 ± 1.06 | 0.09 ± 0.69 | − 2.4 ± 3.6 | − 2.5 ± 13.8 | − 0.58 ± 0.96 | − 0.38 ± 2.56 |
| p value | Non significant | Non significant | Non significant | |||
| Cho et al. [8] | NA | NA | NA | NA | − 19.7 ± 6.4 (significant fall) | − 16.1 ± 6.8 (non significant fall) |
| p value | Significant difference | |||||
Discussion
Metformin is an oral biguanide antihyperglycemic drug which, besides being an insulin sensitizer, seems to have a significant impact on ovulation rates also. On the other hand, orlistat promotes weight loss by decreasing fat absorption from intenstine (about 30%) [9] by irreversibly inhibiting gastric and pancreatic carboxylester lipase. Because weight loss is related to improvement in ovarian function, orlistat seems to have a role in betterment in ovulation rate. This fact indicates that the association of improvement of ovulation rate with either drug seems to be indirectly through weight loss and neither drug seems to have any direct effect on ovulation induction.
As far as reduction in weight and BMI is concerned Jayagopal et al. [6], Cho et al. [8] and Kujawska-Łuczak et al. [7] have found orlistat to be more effective than metformin. Although the weight losing effects of metformin have also been described in various studies [10], the metformin therapy is not associated with a smooth course as gastrointenstinal side effects are a major barrier to patient compliance. In fact in various studies, almost all dropouts occurred only in the metformin arm. This fact suggests that orlistat may be a more preferable weight reducing agent due to better tolerability and compliance.
Obesity leading to decreased concentrations of SHBG is the prime cause for increased serum testosterone concentrations. Interestingly, we could not found any significant improvement in plasma concentrations of SHBG in any of the RCTs included in our study. So there must be other mechanisms that lead to decreased testosterone concentrations. The most logical explanation would be, moderate weight loss achieved by either metformin or orlistat, even though insufficient to improve serum SHBG concentrations, but may be sufficient enough to decrease serum testosterone levels via an attenuating effect on insulin like growth factor binding protein-I and thus inhibiting its stimulatory effect on P450c17α. (Thus decreased activity of P450c17α, leads to decreased levels of serum testosterone).
Although none of the RCTs showed any significant difference between effects of two drugs in terms of change in serum testosterone level, orlistat had its edge in preference to metformin as shown by Metwally et al. [3] and Ghandi et al. [5]. While orlistat showed an early effect (as early as 4 weeks) in the former study; metformin did not show any significant fall of serum testosterone level in the latter study. Also in another study, metformin reduced weight and waist circumference but did not affect testosterone level [11]. The delayed effect of metformin on androgen concentrations on some of the studies may indicate the need for a longer duration of therapy or may be a result of the initial low dose of metformin.
As shown by Ghandi et al. [5] and Kumar et al. [4] clearly orlistat has better effect than metformin in terms of improvement in lipid parameters.
Cho et al. [8] showed a significant improvement in insulin resistance in the orlistat-treated arm only and the difference was statistically significant as compared to metformin-treated arm. Neither of the other two RCTs included in our systematic review showed any improvement in insulin resistance by any of the drugs.
This could be explained due to the large variability in HOMA-IR values, which can be prevented in the future studies using a larger study sample.
On summarizing our systemic review results (Table 7), the list of various aspects of PCOS that are found to be improved with orlistat treatment and bearing a statistically significant difference with that of metformin are: percentage change in BMI, percentage change in weight loss, serum LDL level, serum total cholesterol level and insulin resistance in terms of HOMA-IR. Nevertheless, nausea and diarrhoea are the side effects of metformin which reduce patients compliance and suitability of its use.
Table 7.
Summary of our systematic review
| Studies that favour Orlistat with significant difference | Studies that favour Metformin with significant difference | |
|---|---|---|
| Ovulation rate | None | None |
| Percentage change in BMI | 1. Kujawska-Łuczak et al. [7] 2. Cho et al. [8] |
None |
| Percentage change in weight loss | 1. Jayagopal et al. [6] 2. Kujawska-Łuczak et al. [7] |
None |
| S. testosterone/free androgen index | Nonea | None |
| SHBG | None | None |
| LDL | 1. Kumar et al. [4] | None |
| Total Cholesterol | 1. Ghandi et al. [5] 2. Kumar et al. [4] |
None |
| Triglyceride | None | None |
| HOMA-IR | 1. Cho et al. [8] | None |
aAlthough none of the studies shows a statistically significant results while comparing between the orlistat and the metformin arm, but some of the studies like Ghandi et al. [10] and Cho et al. [13] have found a statistically significant improvement from baseline values over the course of the treatment with orlistat only and not with metformin. Other studies like Metwally et al. [8] found a delayed effect of metformin in comparison to orlistat (8 vs. 4 weeks) to find a statistically significant improvement result from baseline values. These facts also favour the use of orlistat in PCOS in comparison to metformin
On the basis of our systematic review, we can now recommend to prefer orlistat over metformin for use in cases of PCOS, especially in conjunction with exercise. However, at this stage we need to address some of the common adverse effects and drug interactions of orlistat which should be kept in mind while prescribing this drug.
Adverse Effects
Common side effects, those have been identified with the consumption of orlistat, include increased bowel movement, oily stool, oily spotting and stomach pain [12]. But these gastrointenstinal adverse events generally resolve with ongoing orlistat treatment. Again as we have already discussed, treatment with metformin is also associated with similar gastrointenstinal side effects.
Some cases of serious liver injury have been reported since 1999. The US FDA review regarding the same identified a total of 13 cases of severe liver injury, between April 1999 and August 2009 out of an estimated 40 million people worldwide who had used orlistat. The U.S. FDA advised healthcare professionals to continue prescription of orlistat in August 2009, because severe liver injury was rare. Systemic adverse effects have rarely been reported with orlistat and, furthermore, many of the systemic adverse effects that have been associated with orlistat have not been reported in clinical trials, but in reports of weaker validity, such as case reports.
Contraindications
Chronic malabsorption syndrome or cholestasis is the contraindications for orlistat therapy.
Drug Interactions
Orlistat has been reported to interact with pharmacokinetics and pharmacodynamics of some drugs like fat-soluble vitamins, warfarin, amiodarone, ciclosporin, lamotrigine, valproic acid, vigabatrin, gabapentin, thyroxine [13]. To describe each and every interaction in detail is beyond the scope of this review. But here, we are highlighting some of the important interactions.
Interaction with Fat Soluble Vitamin
Orlistat is reported to cause decreased absorption of fat soluble vitamins. There is a significant reduction in absorption of betacarotene and vitamin E, but not vitamin A, in healthy volunteers with short-term use of orlistat [13]. In most of the studies although the plasma concentrations of fat soluble vitamins (A, D, E and betacarotene) decreased among subjects taking orlistat, but it remained within the clinical reference range during the entire study period [13].
To reduce the gastrointestinal events, orlistat should be taken with a low fat diet. Patients should be strongly encouraged to take a multivitamin supplement that contains fat-soluble vitamins to ensure adequate Nutrition.
Interaction with Thyroxin
Thyroxine absorption is influenced by the content of the GI tract. Orlistat may bind to thyroxine and prevent its absorption from the small bowel. Clinicians should be aware of this potential interference of this drug with thyroxine absorption [13]. Levothyroxine and orlistat should be administered at least 4 h apart.
Interaction with Warfarin
Orlistat may reduce the absorption of fat-soluble vitamin K, thus resulting in a lowering of warfarin dose requirements. This is why patients on warfarin treatment should be closely monitored [13]. However, there is no conclusive evidence regarding the same.
At present, there is no guideline regarding the duration of use of orlistat for PCOS. However, based on the various randomized control trials that studied the various effects of orlistat, a treatment duration of minimum 3–6 month can be accepted as reasonable [3–8].
Limitations of the Study
Relatively less number of study population of the RCTs included in our systematic review constitute the only relative limitation of our study. However, after gathering a lot of fact in favour of orlistat this systematic review can safely recommend it over metformin for treatment of PCOS.
Conclusion
The analysis of these trials showed that both orlistat and metformin were associated with nonsignificant improvement, in many of the endocrine and metabolic parameters studied. However, the changes seen in terms of percentage change from baseline were more marked in the orlistat-treated group, perhaps suggesting that weight reduction had an overall stronger impact on these parameters than the insulin-sensitizing effect of metformin, the mechanism of which remains largely unknown. Based on the results of this systemic review of randomized control trials and also keeping an eye over the side effect profile of metformin, we recommend to prefer orlistat as a safe and effective therapy over metformin for treatment of polycystic ovarian syndrome preferably in combination with weight reduction. More research is recommended in this area.
Soumya Ranjan Panda
He is presently working as a senior Resident in the Department of Obstetrics and Gynecology, IMS, BHU, Varanasi, UP.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Soumya Ranjan Panda is senior Resident, Department of Obstetrics and Gynecology, IMS, BHU, Varanasi, UP. Madhu Jain is a Professor and Head, Department of Obstetrics and Gynecology, IMS, BHU, Varanasi, UP. Shuchi Jain is an Assistant Professor, Department of Obstetrics and Gynecology, IMS, BHU, Varanasi, UP. Riden Saxena is a Phd Scholar, Department of Obstetrics and Gynecology, IMS, BHU, Varanasi, UP. Smrutismita Hota is a Junior Resident, Department of Radiodiagnosis, IMS, BHU, Varanasi, UP.
References
- 1.Fauser BCJM, Tarlatzis BC, Rebar RW, et al. Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. doi: 10.1093/humrep/der396. [DOI] [PubMed] [Google Scholar]
- 2.Moran LJ, Pasquali R, Teede HJ, et al. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril. 2009;92:1966–1982. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Metwally M, Amer S, Li TC, et al. An RCT of metformin versus orlistat for the management of obese anovulatory women. Hum Reprod. 2009;24:966–975. doi: 10.1093/humrep/den454. [DOI] [PubMed] [Google Scholar]
- 4.Kumar P, Arora S. Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J Hum Reprod Sci. 2014;7(4):255–261. doi: 10.4103/0974-1208.147492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghandi S, Aflatoonian A, Tabibnejad N, et al. The effects of metformin or orlistat on obese women with polycystic ovary syndrome: a prospective randomized open-label study. J Assist Reprod Genet. 2011;28:591–596. doi: 10.1007/s10815-011-9564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayagopal V, Kilpatrick ES, Holding S, et al. Orlistat is as beneficial as metformin in the treatment of polycystic ovarian syndrome. J Clin Endocrinolmetab. 2005;90:729–733. doi: 10.1210/jc.2004-0176. [DOI] [PubMed] [Google Scholar]
- 7.Kujawska-Łuczak M, Musialik K, Szulińska M, et al. The effect of orlistat versus metformin on body composition and insulin resistance in obese premenopausal women: 3-month randomized prospective open-label study. Arch Med Sci. 2016 doi: 10.5114/aoms.2016.62014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho LW, Kilpatrick ES, Keevil BG, et al. Effect of metformin, orlistat and pioglitazone treatment on mean insulin resistance and its biological variability in polycystic ovary syndrome. Clin Endocrinol. 2009;70:233–237. doi: 10.1111/j.1365-2265.2008.03309.x. [DOI] [PubMed] [Google Scholar]
- 9.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 10.English PJ, Ashcroft A, Patterson M, et al. Metformin prolongs the postprandial fall in plasma ghrelin concentrations in type 2 diabetes. Diabetes Metab Res Rev. 2007;23:299–303. doi: 10.1002/dmrr.681. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal N, Rice SP, Bolusani H, et al. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo—controlled, crossover trial. J Clin Endocrinol Metab. 2010;95:722–730. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- 12.Orlistat: MedlinePlus Drug Information [Internet]. Medlineplus.gov. 2016 [cited: Sep 15 2016]. https://medlineplus.gov/druginfo/meds/a601244.html.
- 13.Filippatos TD, Derdemezis CS, Gazi IF, et al. Orlistat-associated adverse effects and drug interactions. Drug Saf. 2008;31(1):53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]