Abstract
Purpose
The objective of our study is to assess the relationship of embryo ploidy status in relation to embryo sex, morphological characteristics, and transfer parameters.
Methods
This is a retrospective cohort study at an academic medical center of patients who underwent in vitro fertilization with preimplantation genetic screening (PGS) from 2010 to 2015. Embryos were screened with 24-chromosome preimplantation genetic screening with day 5/6 trophectoderm biopsy. We investigated embryo euploidy in relation to morphology (expansion, inner cell mass, trophectoderm), embryo sex, biopsy day, and blastocyst cohort size. We used multivariate logistic regression to calculate odds ratios of euploidy in relation to these parameters.
Results
A total of 1559 embryos from 316 cycles and 233 patients (mean maternal age = 37.8 ± 4.2 years) were included in the analysis. Six hundred and twenty-eight blastocysts (40.3%) were found to be euploid. Expansion (p < 0.001), inner cell mass (ICM) (p < 0.01), and trophectoderm grade (p < 0.001) were significantly associated with embryo ploidy in bivariate models controlling for maternal age, while embryo sex, biopsy day, and blastocyst cohort size were not associated with embryo ploidy. In a multivariate model, we found that maternal age (p < 0.001), higher grade of expansion (p < 0.01), and better quality trophectoderm (p < 0.001 for A compared to C grade) remained significantly associated with increased embryo euploidy, but ICM grade was no longer significant. Embryo sex was not associated with ploidy status, though male embryos were found to be associated with higher trophectoderm scores (p < 0.02).
Conclusions
This is the largest study to date to investigate PGS-tested embryo sex and ploidy status. While maternal age and some morphological parameters (expansion, trophectoderm grade) are associated with euploidy in our cohort, other parameters such as embryo sex, biopsy day, and cohort size are not. Though embryo sex was not associated with euploidy, male embryos were found to be associated with higher trophectoderm grades. Additional investigation in larger studies is warranted.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1262-x) contains supplementary material, which is available to authorized users.
Keywords: Morphology, Embryo sex, Euploidy, Inner cell mass, Trophectoderm, Preimplantation genetic screening
Introduction
For patients undergoing in vitro fertilization (IVF), morphological parameters [including expansion, inner cell mass (ICM), and trophectoderm] are commonly used to select embryos for transfer. Prior studies have suggested that better morphological parameters are associated with increased likelihood of euploidy in embryos; however, it has been reported that substantial numbers of good morphology embryos are aneuploid [1–3]. Morphology may be used alone or in conjunction with preimplantation genetic screening (PGS), which gives information on ploidy of embryos prior to transfer. By selecting euploid embryos, PGS helps facilitate the transfer of fewer embryos while maintaining success rates [4, 5]. PGS may be particularly useful for patients with advanced maternal age, as increased maternal age is the most significant contributor to embryo aneuploidy [6–8].
Several studies have reported a linkage with better morphological parameters and euploidy. Alfarawati et al. [1] reported that a higher proportion of blastocysts with expansion 5/6 were euploid, and that ICM and trophectoderm were both significantly associated with ploidy status. Two other studies on blastocysts also reported that morphological parameters in addition to embryo quality were associated with euploidy [3, 9]. However, studies on the subject are limited by small sample sizes, and many have not included multivariate analyses with all morphological parameters.
PGS can also provide information on embryo sex, though not all IVF clinics disclose this information to patients. There is very limited data on PGS-tested embryo sex in relation to morphological parameters or euploidy. Alfarawati et al. reported that in their cohort, euploidy rates were similar for both sexes; however, 72% of grades 5 and 6 blastocysts were found to be male [1]. Another study also reported similar euploidy rates for males and females, but found that there was a trend towards females developing faster during the cleavage-stage embryos and males developing faster as blastocysts, although these differences were not significant [2]. A study of cleavage-stage embryos also reported no ploidy differences by embryo sex, though not all chromosomes were tested [10].
The objective of this study is use a large retrospective cohort to assess the relationship of blastocyst morphology and embryo sex and ploidy. To our best knowledge, this is the largest study to date to examine the relationship between PGS-tested embryo sex and euploidy, as well as the first to investigate this relationship in a multivariate analysis. We also aimed to investigate if embryo sex was associated with morphological parameters. This study aims to provide information that may be helpful to patients undergoing IVF and choosing embryos to transfer based on available morphological data.
Methods
This is a retrospective cohort study of patients at an academic IVF clinic who underwent 24-chromosome IVF-PGS on day 5/6 with trophectoderm biopsy between 2010 and 2015. PGS was chosen by patients for a variety of reasons, including advanced maternal age, male factor, prior IVF failure, recurrent pregnancy loss, or sex selection. We excluded all cleavage-stage embryos and embryos with incomplete information on any of the parameters studied (expansion, trophectoderm, inner cell mass (ICM), sex, biopsy day (5/6), or blastocyst cohort size). In order to study the effect of embryo sex on euploidy, we also excluded embryos with embryo sex abnormalities. IRB approval for this study was obtained through the university affiliated with the IVF clinic.
Patients underwent controlled ovarian stimulation using antagonist, long GnRH agonist (leuprolide acetate), or microdose flare protocols (Table 1). Embryos were cultured according to methods previously described [11]. Briefly, embryos were group cultured in SAGE sequential media in low oxygen conditions. Laser-assisted hatching was performed on day 3 after retrieval, followed by trophectoderm biopsy on day 5 or day 6 (post-fertilization) depending on morphological parameters. Laser was also used for trophectoderm biopsy, with removal of 3 to 5 cells. Samples were then analyzed by one of four PGS companies (Natera, Reprogenetics, Ivigen, and Blastogen) for 24-chromosome screening.
Table 1.
Baseline characteristics
| Number of unique patients | 233 |
| Number of unique cycles | 316 |
| ICSI cycles | 192 (60.8%) |
| Maternal age in years (mean, St. dev.) | 37.8 ± 4.2 |
| SART indication* | |
| Male factor | 214 (67.7%) |
| DOR | 100 (31.6%) |
| PCOS | 32 (10.1%) |
| Endometriosis | 17 (5.4%) |
| Tubal | 15 (4.7%) |
| Uterine | 8 (2.5%) |
| Unexplained | 16 (5.1%) |
| Other | 44 (13.9%) |
| Protocol | |
| Antagonist | 229 (72.5%) |
| Agonist | 50 (15.8%) |
| Flare | 34 (10.8%) |
| Other/unknown | 3 (1.0%) |
| BMI (mean, St. dev.) kg/m2** | 23.5 ± 4.5 |
| FSH (mean, St. dev.) mIU/mL** | 7.5 ± 3.3 |
| AMH (mean, St. dev.) ng/mL** | 2.5 ± 2.3 |
| Number of blastocysts for biopsy | 5.5 ± 4.0 |
*Multiple indications recorded for some patients—total percentage exceeds 100%
**Data not available for all patients
Morphological assessments were conducted on day 5 or day 6 (day of biopsy) according to the Gardner grading system to assess expansion, ICM, and trophectoderm [12]. One of six senior embryologists provided modified Gardner grades to blastocysts. While grading may have some inherent subjectivity, the embryologists all had > 6 years of experience and adhered to standardized laboratory grading protocols. For expansion, embryos with cavitation were considered blastocysts, while embryos with compaction but no cavitation were considered morulae. Blastocysts were given expansion grades from 1 to 6 and were given ICM and trophectoderm scores A, B, or C. Embryos with expansion ≤ 2 did not have sufficiently developed ICM and trophectoderm to receive grades for those two parameters. A retrospective chart review was conducted to record morphological parameters, ploidy status, embryo sex, biopsy day (5/6), and blastocyst cohort size.
Statistical analysis
Blastocyst euploidy was studied in relation to morphological parameters, embryo sex, biopsy day (5/6), and blastocyst cohort size, first in bivariate logistic regression models which controlled for maternal age for each cycle included. We then performed multivariate analysis using a model incorporating all parameters which were found to be significant in bivariate analysis. In a separate multivariate logistic regression model, we also studied morphological parameters and maternal age in relation to embryo sex. In our two multivariate models, we focused on blastocysts with expansion of 3 or greater and excluded embryos with expansion grade 2 or lower due to the fact that nearly all of these embryos were not given an ICM or trophectoderm score, which made the multivariate model collinear between expansion, ICM, and trophectoderm for these embryos.
All tests were two-sided with significance at the alpha = 0.05 level. Data analysis was performed in R Foundation for Statistical Computing version (Vienna, Austria) and Microsoft Excel.
Results
In our cohort, a total of 1559 blastocysts from 316 cycles and 233 patients (mean maternal age = 37.8 ± 4.2 years) were included in the analysis. The average number of cycles per patient = 1.4 (range 1–8 cycles), and 25% of patients had more than one cycle. Table 1 displays the baseline characteristics of the cohort. ICSI was used for 60.8% of cycles. Male factor was the leading SART indication, followed by diminished ovarian reserve (DOR), polycystic ovarian syndrome (PCOS), and endometriosis. A high percentage of male factor cases may have been due to the presence of an urologist specializing in male factor infertility associated with our infertility clinic. The majority of cycles (72.5%) used an antagonist protocol. In terms of ovarian reserve measures, the mean FSH was 7.5 mIU/mL [standard deviation (SD) 3.3] and mean AMH was 2.5 ng/mL (SD 2.3). The average blastocyst cohort size available for biopsy was 5.5 (SD 4.0).
Out of 1559 blastocysts in the cohort, 628 (40.3%) were found to be euploid (Table 2). In bivariate models controlling for maternal age, we found that expansion, (ICM), and trophectoderm grade were significantly associated with embryo ploidy. In particular, compared to the reference group of expansion grade 5/6, the ORs (95% CI) for euploidy were 0.54 (0.38–0.77), 0.54 (0.40–0.72), and 0.28 (0.19–0.42) for expansion 3/4, 1/2, and morulae, respectively, all p < 0.001. For ICM, compared to the reference group of grade A, both B grade [OR 0.67 (0.52–0.87)] and no ICM grade [OR 0.37 (0.27–0.49)] had significantly lower rates of euploidy (p < 0.01), while C grade approached significance [OR 0.59 (0.35–1.00), p = 0.05]. For trophectoderm, compared to the reference group of grade A, odds of euploidy were significantly lower for grade B [0.70 (0.52–0.94), p < 0.02] grade C [OR 0.34 (0.22–0.53), p < 0.001], and no trophectoderm grade [OR 0.33 (0.24–0.46), p < 0.001]. Embryo sex (p = 0.11), biopsy day (p = 0.15), and blastocyst cohort size (p > 0.10) were not associated with embryo ploidy when controlling for maternal age.
Table 2.
Euploidy by morphology and embryo sex—bivariate models (age adjusted)
| Total | Euploid | % Euploid | OR (95% CI) Age-adjusted | p value (age-adjusted) | |
|---|---|---|---|---|---|
| Total | 1559 | 628 | 40.3% | ||
| Expansion | |||||
| 5 or 6 | 920 | 449 | 48.8% | Reference | |
| 3 or 4 | 174 | 56 | 32.2% | 0.54 (0.38–0.77) | < 0.001 |
| 1 or 2 | 285 | 89 | 31.2% | 0.54 (0.40–0.72) | < 0.001 |
| Morulae | 180 | 34 | 18.9% | 0.28 (0.19–0.42) | < 0.001 |
| ICM | Total | ||||
| A | 399 | 212 | 53.1% | Reference | |
| B | 626 | 264 | 42.2% | 0.67 (0.52–0.87) | < 0.01 |
| C | 73 | 29 | 39.7% | 0.59 (0.35–1.00) | 0.05 |
| No ICM grade | 461 | 123 | 26.7% | 0.37 (0.27–0.49) | < 0.001 |
| Trophectoderm | Total | ||||
| A | 269 | 153 | 56.9% | Reference | |
| B | 665 | 305 | 45.9% | 0.70 (0.52–0.94) | 0.02 |
| C | 164 | 47 | 28.7% | 0.34 (0.22–0.53) | < 0.001 |
| No troph grade | 461 | 123 | 26.7% | 0.33 (0.24–0.46) | < 0.001 |
| Sex | Total | ||||
| Female | 798 | 307 | 38.5% | Reference | |
| Male | 761 | 321 | 42.2% | 1.19 (0.96–1.47) | 0.11 |
| Blastocyst biopsy day | Total | ||||
| 5 | 1222 | 492 | 40.3% | Reference | |
| 6 | 337 | 136 | 40.4% | 1.21 (0.93–1.56) | 0.15 |
| Cohort size | Total | ||||
| < 5 | 392 | 119 | 30.4% | Reference | |
| 5 to 9 | 674 | 288 | 42.7% | 1.26 (0.95–1.66) | 0.10 |
| 10 or greater | 493 | 221 | 44.8% | 0.95 (0.69–1.31) | 0.76 |
Note: Statistically significant findings denoted by italics
In a multivariate model incorporating all the significant parameters, maternal age, expansion, and trophectoderm remained significantly associated with embryo euploidy, but ICM grade was no longer significant (Table 3). Each year increase in maternal age was associated with decreased euploidy, OR 0.88 (0.85–0.91), p < 0.001. Compared to expansion grade 5/6, expansion grade 3/4 blastocysts were significantly less likely to be euploid [OR 0.59 (0.41–0.85), p < 0.01]. For trophectoderm, compared to grade A, grade B was not significantly different [OR 0.77 (0.57–1.07), p = 0.12], but grade C was associated with significantly decreased euploidy [OR 0.40 (0.25–0.64), p < 0.001].
Table 3.
Multivariate model of age and morphology in relation to euploidy
| Parameter | Category | OR (95% CI) | p value |
|---|---|---|---|
| Maternal age* | 0.88 (0.85–0.91) | < 0.001 | |
| Expansion | 5 or 6 | Reference | |
| 3 or 4 | 0.59 (0.41–0.85) | < 0.01 | |
| ICM | A | Reference | |
| B | 0.85 (0.64–1.13) | 0.26 | |
| C | 0.94 (0.52–1.68) | 0.83 | |
| Trophectoderm | A | Reference | |
| B | 0.77 (0.57–1.07) | 0.12 | |
| C | 0.40 (0.25–0.64) | < 0.001 |
Notes: Embryos with no trophoectoderm/ICM grade excluded from multivariate model due to collinearity. Statistically significant findings denoted by italics
*OR per year increase
In a separate multivariate model investigating the relationship of morphology and embryo sex, we found that embryo sex was only significantly associated with the trophectoderm, with male embryos more likely to have higher trophectoderm grades, p < 0.02 (Table 4, Supplementary Table 1). Maternal age, expansion, and ICM were not found to be associated with embryo sex.
Table 4.
Multivariate model of embryo sex (likelihood male) in relation to age and morphology
| Parameter | Category | OR (95% CI) | p value |
|---|---|---|---|
| Maternal age* | 0.99 (0.97–1.02) | 0.63 | |
| Expansion | 5 or 6 | Reference | |
| 3 or 4 | 0.79 (0.56–1.10) | 0.16 | |
| ICM | A | Reference | |
| B | 1.24 (0.94–1.64) | 0.12 | |
| C | 0.73 (0.42–1.28) | 0.28 | |
| Trophectoderm | A | Reference | |
| B | 0.63 (0.47–0.86) | < 0.01 | |
| C | 0.60 (0.39–0.94) | 0.02 |
Notes: Embryos with no trophoectoderm/ICM grade excluded from multivariate model due to collinearity. Statistically significant findings denoted by italics
*OR refers to per year increase
We also studied the type of aneuploidy (monosomy, trisomy, or complex) in relation to expansion, ICM, trophectoderm, and embryo sex (Fig. 1). Complex aneuploidy was defined as greater than two chromosomal abnormalities. Overall, percentage of embryos with at least one trisomy (37.5%) was similar to embroyos with at least one monosomy (34.7%). Among embryos with favorable morphological parameters, trisomies were the most common type of aneuploidy, while monosomies were more common among blastocysts with poor morphological parameters. Male and female embryos had a similar distribution of types of aneuploidy.
Fig. 1.
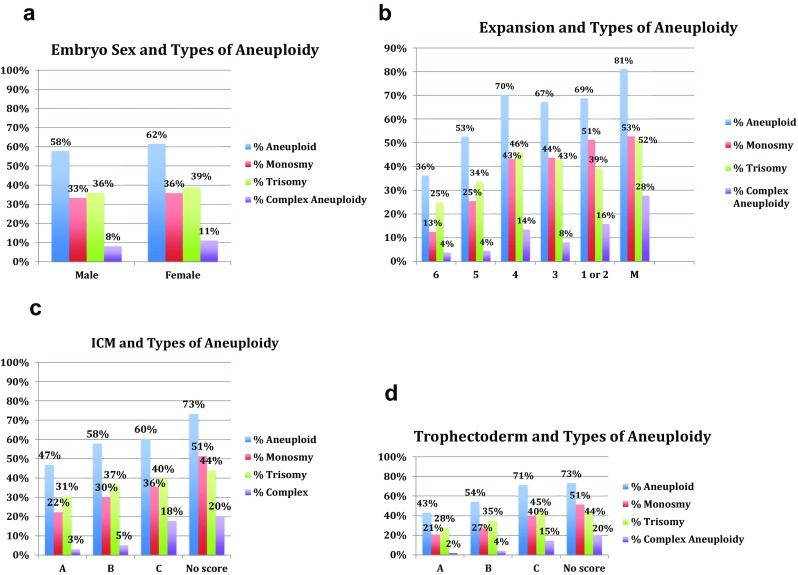
a–d Types of aneuploidy by morphology and embryo sex
Discussion
In this analysis, we observed that maternal age, expansion, and trophectoderm were significantly associated with embryo euploidy in embryos undergoing day 5/6 PGS. ICM was not a significant predictor of euploidy when all morphological parameters were analyzed in a multivariate model controlling for other confounders. Ploidy status was not found to be related to embryo sex, biopsy day, or cohort size. We also found that though embryo sex was not associated with ploidy status, male embryo sex was significantly associated with higher trophectoderm grade. These findings may be useful for patients undergoing IVF who are choosing embryos to transfer based on morphological data.
Comparison with prior literature
Embryo sex
Embryo sex has been rarely studied in relation to euploidy with PGS data. Our study is the largest to date to investigate this question of embryo sex and euploidy, and the first to investigate this outcome in a multivariate analysis. Our analysis found that embryo sex was not significantly associated with ploidy status. Previous studies have reported similar unadjusted findings. Alfarawati et al. [1] reported that in a cohort of 500 blastocysts undergoing comprehensive chromosome screening using comparative genomic hybridization, there was a similar euploidy rate for both male and female embryos. Fragouli et al. studied a cohort of 1213 mixed cleavage-stage and blastocyst embryos and reported no correlation between euploidy and embryo sex. Eaton et al. reported that in a study of 758 cleavage-stage embryos which were tested for sex chromosomes and a subset of autosomal chromosomes, male and female embryos were equally likely to be euploid (for tested chromosomes) and achieve blastocyst stage [10].
There have been very few studies on the relationship of PGS-tested embryo sex to morphological parameters. Alfarawati et al. did not study embryo sex in relation to morphological parameters in a multivariate analysis, but reported that 72% of embryos with expansion grade 5 or 6 were male embryos. In our cohort, we did not find expansion grade 5/6 embryos to be significantly different than expansion grade 3/4 embryos with relation to embryo sex, which may be due to the fact that our study had a larger sample size and used multivariate analysis to control for additional morphological parameters. Additionally, only 59/500 (11.8%) blastocysts in the Alfarawati et al. cohort had an expansion grade of 5 or 6, while in our cohort 920/1559 (59.0%) blastocysts were expansion grade 5 or 6. This suggests that the cohorts may have had underlying differences in the patient population that make a direct comparison of grade 5 or 6 embryos difficult.
Our study did observe that higher trophectoderm grades were significantly associated with male embryos. Alfarawati et al. reported that male embryos were more likely to achieve an A trophectoderm than female embryos (57 vs. 42%), though the difference was not significant. Fragouli et al. also reported that male embryos were more likely to have higher expansion grades at the blastocyst stage, while the opposite was true at the cleavage stage; however, these differences were non-significant [2]. Additionally, another study of 421 cleavage-stage embryos found that male embryos were associated with some faster morphokinetic parameters, though both sexes were equally likely to be euploid [13]. The significant findings in our cohort may have been due to larger sample size as well as multivariate analysis.
Several studies have suggested that non-PGS blastocyst transfers are slightly more likely to result in male offspring, particularly when conventional fertilization is used [14–16] although this has not been consistent in other studies [17]. The literature on this subject is extremely limited, though our analysis and some existing studies suggest that male embryos may be associated with higher morphological parameters. Further research on this subject in a larger cohort is warranted.
Morphological parameters
Though increasing maternal age is a well-established risk factor for aneuploidy [6, 18], few studies have investigated the relationship between morphological parameters and ploidy status in blastocysts. Alfarawati et al. [1] reported that a higher proportion of blastocysts with expansion 5/6 were euploid (49.2%, compared to 37.5% for grades 1–2 embryos), and that ICM and trophectoderm were both significantly associated with ploidy status on unadjusted analyses. In another study investigating the association of morphological parameters with euploidy in 956 blastocysts, Capalbo et al. [3] reported that morphological parameters were associated with euploidy in a logistic regression analysis controlling for maternal age and IVF center. This study did not look at expansion directly and instead used a measure of embryo quality (excellent, good, average, and poor) which included expansion, ICM, and trophectoderm scores in one parameter (in addition to including ICM and trophectoderm directly).
Another study of 1730 blastocysts investigating both morphological and morphokinetic parameters found that top quality ICM, TE, and grade 5–6 expansion were all correlated with significantly increased odds of euploidy, using mixed logistic regression models with adjustment for maternal age [9]. A study of 306 embryos found that “good morphology” blastocysts were significant more likely to be euploid than “poor morphology” blastocysts, though this association was not found for cleavage-stage embryos [19]. However, another study of both cleavage-stage and blastocyst-stage embryos found that there was no correlation of morphology and euploidy at the cleavage stage, and only subtle correlation at the blastocyst stage [2]. Other studies have also reported a relationship with morphology and ploidy status in cleavage-stage embryos, though some of these studies did not use 24-chromosome screening and instead examined only a subset of chromosomes with fluorescence in situ hybridization (FISH) [8, 18, 20–23].
Our study is unique in investigating euploidy in relation to maternal age, morphological parameters, and embryo sex in a single multivariate analysis. While prior studies have mostly used a composite measure of embryo quality combining multiple morphological parameters, or univariate/bivariate models with individual morphological parameters, a multivariate analysis is particularly important to investigate the contribution of each morphological parameter individually. For example, higher expansion may be more common among embryos that also have higher ICM/trophectoderm grades. Therefore, a univariate or bivariate analysis may suggest that multiple morphological parameters are predictive of euploidy even if only a subset of parameters is driving the actual association. In particular, expansion has been rarely included directly in prior studies on morphology and euploidy, and is often part of a composite embryo quality measure. Our study also features a more diverse set of indications for PGS (including male factor, PCOS, and sex selection) whereas prior studies included mostly patients who were undergoing PGS for advanced maternal age and repeated IVF failure, which makes our cohort potentially more generalizable.
Our overall results show similarities to prior studies, in finding that higher morphological parameters are associated with increased rates of euploidy. In particular, on multivariate analysis we found that a trophectoderm score C (but not B) was significantly less likely to be euploid compared to trophectoderm score A, and that expansion 3/4 was significantly less likely to be euploid compared to expansion score 5/6. However, when compared to the Capalbo et al. and Minasi et al. studies, we did not find an effect of ICM. This may be due to several factors, most importantly having the ability to study all parameters together in a multivariate analysis, as well studying expansion directly.
Prior literature has also suggested the importance of trophectoderm quality in blastocyst transfer success. An analysis of 3151 non-PGS elective single embryo transfers (eSETs) found that blastocyst stage and trophectoderm (along with maternal age) were predictive of clinical pregnancy, but ICM and embryo grade were not [24]. Another previous study on transfer of 1117 untested blastocysts found that trophectoderm but not ICM was a significant predictor of live birth outcome [25]. Though the authors theorized that this was due to the importance of trophectoderm in hatching and implantation, another possible contribution to these findings is that trophectoderm is a morphological parameter strongly related to euploidy, as observed in our analysis. Similarly, a study of 694 single-blastocyst transfers found that trophectoderm grading but not ICM grading was associated with implantation and live birth [26]. Another study of 263 blastocysts also reported that trophectoderm (but not ICM or expansion) was significantly associated with clinical pregnancy and live birth [27]. Additional study of this subject in a multivariate analysis with a larger cohort is warranted, though some existing literature and our study support a relationship between trophectoderm and euploidy.
Similar to the Alfarawati et al. study, we found that trisomies were common in embryos with higher morphological parameters, while monosomies were more common in embryos with lower morphological parameters. We also found that biopsy day (5 vs. 6) and blastocyst cohort size were not associated with euploidy, which is consistent with some prior studies on the subject. Several studies have reported that aneuploidy rates are not related to the rate of blastulation, and that slower-growing blastocysts in their cohorts had similar rates of euploidy as faster-growing blastocysts [3, 28]. A study using array CGH on 7753 embryos also found that cohort size was not significantly associated with euploidy for day 3 and day 5 embryos [29]. Our study confirms all of these independent findings in one cohort.
Strengths and limitations
Strengths of our study include the large sample size, setting in a single institution which allows for more consistent grading of embryos, and availability of detailed morphology and sex information for embryos. Our study is novel due to investigation of multiple parameters (including age, morphology, embryo sex, and characteristics of the embryo cohort) in a single cohort. In particular, literature on embryo sex and ploidy status is extremely limited. Limitations of our study include the retrospective cohort format, lack of data on transfer outcomes, the fact that PGS may carry a small rate of inaccuracy, and that multiple testing companies were used for PGS. However, in our cohort, in a separate analysis, we found no significant difference in euploidy rates by testing companies after controlling for the other factors in our multivariate model. Previous studies have found conflicting results on whether or not morphological parameters are related to birth outcomes for euploid embryos [3, 30], which warrants further investigation. In addition, our cohort may have included better prognosis patients as evidenced by mean blastocyst cohort size of 5.5 and mean AMH of 2.5 ng/mL, which should be taken into consideration for interpretation of the results. Lastly, our data spans from 2010 to 2015, and changes in laboratory techniques since then may warrant additional investigation in a contemporary cohort (however, culture conditions of our laboratory have remained relatively stable over that time).
Conclusions
This is the largest study to date on PGS-tested embryo sex and ploidy status. Our findings suggest that while maternal age and some morphological parameters (particularly expansion and trophectoderm grade) are associated with embryo euploidy, other parameters such as embryo sex, transfer day, and cohort size are not associated with ploidy status. Embryo sex was not associated with ploidy status, but male embryos were associated with higher trophectoderm grades. These findings may be useful for counseling IVF patients who are choosing embryos to transfer based on morphological data. Further study in a prospective format with larger sample size is warranted to validate our findings, as well as to investigate birth outcomes in relation to morphological parameters of euploid embryos.
Electronic supplementary material
(DOCX 38 kb)
Contributors
AW and LW participated in study conception and design. AW performed the data analysis and wrote the initial draft of the manuscript. All authors contributed to data interpretation and revisions and approval of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
IRB approval was obtained through Stanford University.
References
- 1.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20:117–126. doi: 10.1093/molehr/gat073. [DOI] [PubMed] [Google Scholar]
- 3.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–1181. doi: 10.1093/humrep/deu033. [DOI] [PubMed] [Google Scholar]
- 4.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, Treff NR, Scott Jr RT In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril 2013;100:100–7 e1, 107.e1. [DOI] [PubMed]
- 5.Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills E, Salem RD. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5:24. doi: 10.1186/1755-8166-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT., Jr The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–63 e1. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–391. doi: 10.1016/S0015-0282(16)57739-5. [DOI] [PubMed] [Google Scholar]
- 9.Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, Spinella F, Fiorentino F, Varricchio MT, Greco E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31:2245–2254. doi: 10.1093/humrep/dew183. [DOI] [PubMed] [Google Scholar]
- 10.Eaton JL, Hacker MR, Barrett CB, Thornton KL, Penzias AS. Influence of embryo sex on development to the blastocyst stage and euploidy. Fertil Steril. 2011;95:936–939. doi: 10.1016/j.fertnstert.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 11.Kort JD, Lathi RB, Brookfield K, Baker VL, Zhao Q, Behr BR. Aneuploidy rates and blastocyst formation after biopsy of morulae and early blastocysts on day 5. J Assist Reprod Genet. 2015;32:925–930. doi: 10.1007/s10815-015-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner D SWIvcohbI, Jansen R MD, editors. Toward reproductive certainty (fertility, Publishing; agbCP, 378–88. p.
- 13.Bronet F, Nogales MC, Martinez E, Ariza M, Rubio C, Garcia-Velasco JA, et al. Is there a relationship between time-lapse parameters and embryo sex? Fertil Steril. 2015;103:396–401 e2. doi: 10.1016/j.fertnstert.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 14.Chang HJ, Lee JR, Jee BC, Suh CS, Kim SH. Impact of blastocyst transfer on offspring sex ratio and the monozygotic twinning rate: a systematic review and meta-analysis. Fertil Steril. 2009;91:2381–2390. doi: 10.1016/j.fertnstert.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 15.Dean JH, Chapman MG, Sullivan EA. The effect on human sex ratio at birth by assisted reproductive technology (ART) procedures—an assessment of babies born following single embryo transfers, Australia and New Zealand, 2002-2006. BJOG: an Int J Obstet Gynaecol. 2010;117:1628–1634. doi: 10.1111/j.1471-0528.2010.02731.x. [DOI] [PubMed] [Google Scholar]
- 16.Luna M, Duke M, Copperman A, Grunfeld L, Sandler B, Barritt J. Blastocyst embryo transfer is associated with a sex-ratio imbalance in favor of male offspring. Fertil Steril. 2007;87:519–523. doi: 10.1016/j.fertnstert.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 17.Kausche A, Jones GM, Trounson AO, Figueiredo F, MacLachlan V, Lolatgis N. Sex ratio and birth weights of infants born as a result of blastocyst transfers compared with early cleavage stage embryo transfers. Fertil Steril. 2001;76:688–693. doi: 10.1016/S0015-0282(01)02010-6. [DOI] [PubMed] [Google Scholar]
- 18.Munne S, Chen S, Colls P, Garrisi J, Zheng X, Cekleniak N, et al. Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod BioMed Online. 2007;14:628–634. doi: 10.1016/S1472-6483(10)61057-7. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar G, Majumdar A, Verma IC, Upadhyaya KC. Relationship between morphology, euploidy and implantation potential of cleavage and blastocyst stage embryos. J Hum Reprod Sci. 2017;10:49–57. doi: 10.4103/0974-1208.204013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquez C, Sandalinas M, Bahce M, Alikani M, Munne S. Chromosome abnormalities in 1255 cleavage-stage human embryos. Reprod BioMed Online. 2000;1:17–26. doi: 10.1016/S1472-6483(10)61988-8. [DOI] [PubMed] [Google Scholar]
- 21.Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertil Steril. 2007;87:534–541. doi: 10.1016/j.fertnstert.2006.07.1512. [DOI] [PubMed] [Google Scholar]
- 22.Munne S. Chromosome abnormalities and their relationship to morphology and development of human embryos. Reprod BioMed Online. 2006;12:234–253. doi: 10.1016/S1472-6483(10)60866-8. [DOI] [PubMed] [Google Scholar]
- 23.Moayeri SE, Allen RB, Brewster WR, Kim MH, Porto M, Werlin LB. Day-3 embryo morphology predicts euploidy among older subjects. Fertil Steril. 2008;89:118–123. doi: 10.1016/j.fertnstert.2007.01.169. [DOI] [PubMed] [Google Scholar]
- 24.Thompson SM, Onwubalili N, Brown K, Jindal SK, McGovern PG. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): a national study. J Assist Reprod Genet. 2013;30:1577–1581. doi: 10.1007/s10815-013-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–3296. doi: 10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 26.Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril 2013;99:1283–9 e1, 1289.e1. [DOI] [PubMed]
- 27.Chen X, Zhang J, Wu X, Cao S, Zhou L, Wang Y, Chen X, Lu J, Zhao C, Chen M, Ling X. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single-blastocyst transfer cycle in a Chinese population. J Assist Reprod Genet. 2014;31:1475–1481. doi: 10.1007/s10815-014-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroener L, Ambartsumyan G, Briton-Jones C, Dumesic D, Surrey M, Munne S, et al. The effect of timing of embryonic progression on chromosomal abnormality. Fertil Steril. 2012;98:876–880. doi: 10.1016/j.fertnstert.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, Munné S. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod BioMed Online. 2012;24:614–620. doi: 10.1016/j.rbmo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, Xu K, Rosenwaks Z. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107:664–670. doi: 10.1016/j.fertnstert.2016.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 38 kb)


