Abstract
Purpose
To determine the expected out-of-pocket costs of IVF with preimplantation genetic testing for aneuploidy (PGT-A) to attain a 50%, 75%, or 90% likelihood of a euploid blastocyst based on individual age and AMH, and develop a personalized counseling tool.
Methods
A cost analysis was performed and a counseling tool was developed using retrospective data from IVF cycles intended for PGT or blastocyst freeze-all between January 1, 2014 and August 31, 2017 (n = 330) and aggregate statistics on euploidy rates of > 149,000 embryos from CooperGenomics. Poisson regression was used to determine the number of biopsiable blastocysts obtained per cycle, based on age and AMH. The expected costs of attaining a 50%, 75%, and 90% likelihood of a euploid blastocyst were determined via 10,000 Monte Carlo simulations for each age and AMH combination, incorporating age-based euploidy rates and IVF/PGT-A cost assumptions.
Results
The cost to attain a 50% likelihood of a euploid blastocyst ranges from approximately $15,000 U.S. dollars (USD) for younger women with higher AMH values (≥ 2 ng/mL) to > $150,000 for the oldest women (44 years) with the lowest AMH values (< 0.1 ng/mL) in this cohort. The cost to attain a 75% versus 90% likelihood of a euploid blastocyst is similar (~ $16,000) for younger women with higher AMH values, but varies for the oldest women with low AMH values (~ $280,000 and > $450,000, respectively). A typical patient (36–37 years, AMH 2.5 ng/mL) should expect to spend ~ $30,000 for a 90% likelihood of attaining a euploid embryo.
Conclusions
This tool can serve as a counseling adjunct by providing individualized cost information for patients regarding PGT-A.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1275-5) contains supplementary material, which is available to authorized users.
Keywords: Preimplantation genetic screening, PGT, Aneuploidy, Counseling tool, Cost analysis
Introduction
Many patients undergoing in vitro fertilization (IVF) elect to use preimplantation genetic testing for aneuploidy (PGT-A), hoping to optimize their chances of having a live birth and reduce their risk of miscarriage by selecting a euploid embryo for transfer [1–7]. While the utility of PGT-A for different patient populations has been widely debated in the literature, the use of this technology continues be a large part of many reproductive endocrinology practices [5, 8–14]. Oocyte aneuploidy increases with age, while the embryo yield per cycle decreases with age [15, 16]. Hoping to minimize the overall cost of PGT-A and maximize the likelihood of success, some patients undergo multiple stimulation cycles and bank blastocysts prior to having PGT-A [17, 18]. The optimal number of blastocysts, the likely number of cycles needed to obtain them, and the predicted cost of achieving a high likelihood of having a euploid embryo for transfer are important factors when counseling patients considering this approach.
Anti-müllerian hormone (AMH) level, while not a marker for IVF pregnancy, is a useful predictor of the response to gonadotropin medication during a stimulation cycle [19, 20]. Women who present to fertility clinics with very low AMH levels, and particularly older patients, often have few eggs retrieved and a low number of embryos created despite maximal stimulation with gonadotropins over multiple cycles [19, 21, 22]. For these patients, obtaining a blastocyst suitable for biopsy may be achievable only at very high cost or may never be attainable. In such cases, clinics may consider recommending a fresh embryo transfer of an untested embryo instead of PGT-A. However, deciding whether to pursue PGT-A is complex and is likely to be influenced by many factors and patient-specific characteristics.
Given the increasing utilization of PGT-A and the need for individualized counseling tools, the purposes of this study were as follows: 1) to determine the expected age-related cost of IVF with PGT-A to attain a 50%, 75%, and 90% likelihood of having at least one euploid blastocyst, 2) to determine whether there is an age and AMH value at which PGT-A should perhaps be discouraged due to the low chance of success and excessive cost, and 3) to develop a personalized, evidence-based counseling tool based on age, AMH, and estimated cost that helps guide patients and clinicians considering PGT-A.
Materials and methods
IVF cycles and laboratory protocols
We conducted a retrospective analysis of 330 IVF or IVF/ICSI cycles started from January 1, 2014 to August 31, 2017 for which PGT or a blastocyst freeze-all was planned at the Center for Infertility and Reproductive Surgery at Brigham and Women’s Hospital. Patients’ first cycles for which PGT (n = 304) or a freeze-all (n = 26) was intended were included. Cycles were excluded if 1) a donor egg or a gestational carrier was used, 2) embryos were frozen at the two pronuclear (2pn) or cleavage stage, or 3) the cycle was intended for long-term fertility preservation for a cancer or medical diagnosis. Approval for this study was obtained from the Partners HealthCare Institutional Review Board.
All gametes and embryos were cultured at 37 °C in a humidified incubator under an atmosphere of CO2 (5–6%), O2 (5%), and N2 (89–90%). IVF or ICSI was performed 4–6 or 3–5 h after oocyte retrieval, respectively, followed by a fertilization check 16–18 h after fertilization. A single step medium (25 μL microdrops, Global Total, IVFOnLine, Guelph, Ontario, Canada) was used to culture 2pn zygotes under mineral oil. Embryos were evaluated on day 3 between 66 and 69 h post-insemination and then moved to a fresh drop of equilibrated Global Total medium for culture to day 5. Blastocyst morphology was then evaluated on day 5 between 112 and 115 h and scored according to the stage of development and quality of the inner cell mass (ICM) and trophectoderm (TE). Embryos that were ineligible for biopsy or freeze on day 5 were left in culture and evaluated on day 6. Good-quality embryos were defined as hatching/hatched blastocysts with “A” or “B” grades for both the ICM and TE. Fair-quality embryos were defined as expanding, full, or expanded blastocysts with “A” or “B” grades for both the ICM and TE, or hatching/hatched blastocysts with at least one “C” grade for the ICM or TE. Poor-quality embryos were considered as: 1) any morula or blastocyst stage with a “D” grade for the ICM or TE, 2) any early blastocyst, or 3) any full, expanding, or expanded blastocyst with at least one “C” grade for the ICM or TE. In our center, all full, expanding, expanded, or hatched blastocysts with an “A” or “B” grade for the ICM or TE or a “C” grade for either the ICM or TE are eligible for TE biopsy (biopsiable embryos); early blastocysts and any blastocyst with a “C” grade for both the ICM and TE, or a “D” for either the ICM or TE are ineligible.
Methodological assumptions
Determination of number of “biopsiable” blastocysts
The number of biopsiable blastocysts was identified directly for those cycles undergoing PGT. When PGT was not performed, the number of biopsiable blastocysts was defined as the number of embryos fulfilling the “biopsiable” criteria, as described above. In cases of a cycle order change resulting in a fresh day 3 transfer instead of PGT or a freeze-all on day 5, the presumed number of biopsiable blastocysts was determined as follows: if a pregnancy was achieved, the number was derived from the number of supernumerary blastocysts that fulfilled the biopsy criteria, plus the number of documented gestational sacs (e.g., if a twin pregnancy was achieved following a day 3 transfer, the number of presumed biopsiable blastocysts was increased by two). Most blastocyst freeze-alls were intended for the prevention of ovarian hyperstimulation syndrome, embryo banking in advance of a future surgical procedure needed prior to transfer (e.g., a myomectomy), for use with a gestational carrier, or for patients’ personal reasons/requests.
Determination of the probability of a euploid blastocyst
To determine the probability of blastocyst euploidy, aggregate age data by individual year from CooperGenomics was utilized. This included PGT-A results of 149,831 embryos obtained using array comparative genomic hybridization (aCGH) or next-generation sequencing (NGS) from January 1, 2013 to December 31, 2017 (unpublished data). The percentage of euploid blastocysts was independent of embryo cohort size and was inversely related to the female partner’s age.
Cost analysis
Poisson regression was used to model the average number of biopsiable blastocysts obtained per cycle based on age and AMH from the 330 cycles for which PGT or a freeze-all on day 5 were initially intended. AMH values were logarithmically transformed prior to the regression because of the magnitude of AMH variation among patients. From the regression, the projected probability distributions of biopsiable blastocysts per cycle were derived.
The cost analysis was performed using the following assumptions:
The baseline cycle cost for self-pay patients was $8000 + $2000 for ICSI.
The average cost per vial (containing 75 IU of FSH and 75 IU of LH) of human menopausal gonadotropin (HMG) or 75 IU of follicle stimulation hormone (FSH) was $75. This was approximated by comparing medication costs at multiple pharmacies using the Fertility Drug Calculator1 application and using the lowest prices available. Total dose requirements and medication costs were calculated based on different AMH values using a linear regression with log transformations.
The PGT-A cost was derived from an approximate $1500 cost for the biopsy procedure, regardless of the number of embryos biopsied, plus $150/embryo analyzed for the PGT-A testing.
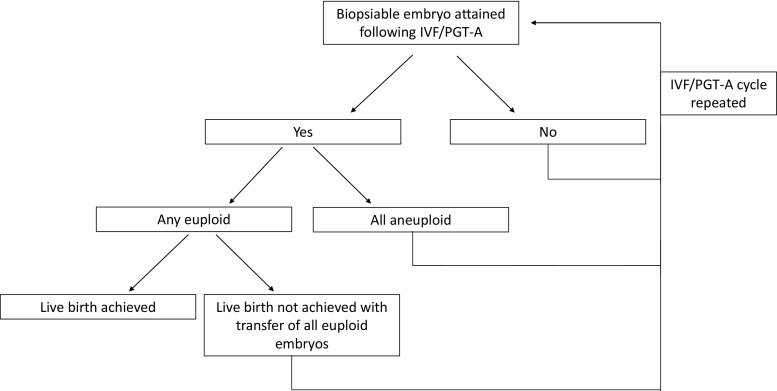
Monte Carlo simulations were used to determine the expected cost of having at least one euploid blastocyst, using patient age and AMH as inputs. Additional parameters included the probability that any biopsiable blastocyst will be euploid as a function of age, and the cost assumptions noted above. Each simulation consisted of multiple cycles, with each cycle containing a number of biopsiable blastocysts randomly chosen from the Poisson probability distribution according to the regression for that particular age and AMH, until a euploid blastocyst was achieved. For each age and AMH combination, 10,000 simulations were performed for predicting the cost of a euploid blastocyst. The cost at which there is a 50%, 75%, or 90% likelihood of having at least one euploid embryo was determined by identifying the 50th, 75th, or 90th percentile in cost, respectively, within each set of 10,000 simulations. An additional 10,000 simulations per age and AMH were performed to estimate the cost of a 50%, 75%, and 90% likelihood of live birth, which assumed a 60% likelihood of birth following transfer of a euploid blastocyst [23, 24]. The decision tree model would proceed as depicted in Fig. 1, and the accumulative cost determined using Monte Carlo simulations. Statistical analyses were performed using Matlab (version R2015a, MathWorks, Natick, MA). Demographic data are presented as mean ± standard deviation or median (range).
Fig. 1.
This figure depicts the decision tree model. The first node occurs with the determination of whether a biopsiable embryo is attained following an IVF/PGT-A cycle and is continued until a euploid embryo is achieved. The accumulative cost is determined using Monte Carlo simulations
Results
Cycle outcomes
Of the 330 cycles included, 304 (92%) and 26 (8%) cycles were initially intended for PGT or a blastocyst freeze-all, respectively. Patient demographic and cycle information for the study population are shown in Table 1. The average patient age was 36.7 years old. Most cycles employed a GnRH antagonist protocol (75%) and the majority utilized ICSI (67%). Of the 304 patients who started their cycle planning to have PGT, 277 (91%) ultimately had embryos biopsied. The median number of embryos biopsied was 3. Twenty-six (8.6%) patients ultimately opted against PGT entirely, instead electing to undergo a fresh embryo transfer. These patients were older (39.0) and had a lower AMH (mean 1.9, median 1.3, interquartile range (IQR) 0.6–2.5). The average number of biopsiable blastocysts was 0.9 in this group, 11 of whom had no embryo suitable for biopsy. Sixteen (5.3%) patients underwent a fresh transfer with their remaining eligible embryos biopsied. Six patients who initially intended on having a blastocyst freeze-all ultimately opted for a fresh transfer instead due to low numbers of embryos. The majority (32/48; 67%) of fresh transfers occurred on day 3. Of patients who had a fresh transfer, 22 (46%) achieved a pregnancy, of which 13 (59%) were biochemical pregnancies and 9 (41%) were clinical pregnancies. Six patients (12.5%) had live births and there is one additional ongoing pregnancy.
Table 1.
Population demographics and cycle characteristics among patients undergoing cycles for which PGT or a freeze-all was initially intended
| Parameter | N% or median (IQR) |
|---|---|
| Age | 36.7 ± 4.5 |
| BMI | 25.0 ± 5.6 |
| AMH | 2.5 (1.2–4.8) |
| Day 3 FSH | 7.4 (6.3–8.9) |
| Gravidity | 1 (0–3) |
| Parity | 0 (0–1) |
| Diagnosis | |
| Unexplained | 25.8% |
| Male factor | 20.6% |
| Tubal factor | 5.2% |
| Ovulatory dysfunction/PCOS | 7.0% |
| Endometriosis | 4.5% |
| DOR | 12.7% |
| Othera | 51.8% |
| Stimulation protocolb | |
| Poor responder | 20.0% |
| Good responder | 80.0% |
| ICSI (% cycles) | 67.0% |
| Number of eggs retrieved | 14 (9–21) |
| 16.0 ± 9.7 | |
| Number of mature oocytes | 10 (6–16) |
| 11.8 ± 7.8 | |
| Number of 2PNs | 8 (4–14) |
| 9.8 ± 6.6 | |
| Number of blastocysts | 6 (2–10) |
| 6.7 ± 5.4 | |
| Number of blastocysts biopsied (among patients who had PGT; N = 277) |
4 (1–7) |
| 4.6 ± 3.8 | |
Values represent n(%) or median (interquartile range)
aThe most common diagnoses that comprise the “Other” category include uterine factor infertility and PGT for non-long-term banking indications. PGT solely for family balancing is not currently offered at our institution
bPoor responder protocols include microflare, patch, letrozole, and ultra-low dose Lupron (ULDL). Good responder protocols include antagonist and luteal Lupron stimulations other than ULDL
Biopsiable blastocysts
The expected number of biopsiable blastocysts obtained per cycle based on age and AMH is shown in Table 2. The predicted number of biopsiable blastocysts was positively correlated with AMH levels and negatively correlated with age (p-trend < 10−10 for both). In our study population, women ≥ 42 years with AMH ≤ 0.1 are expected to have < 1 biopsiable blastocyst obtained/cycle, and so are predicted to require more than one cycle to obtain a suitable blastocyst for biopsy, on average. All other age and AMH combinations are expected to produce at least 1 biopsiable blastocyst per cycle.
Table 2.
Expected number of biopsiable blastocysts/cycle
| Age (years) | AMH (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 1 | 2 | 3 | 5 | 10 | |
| 30 | 1.6 | 2.4 | 2.9 | 3.8 | 4.9 | 5.8 | 7.0 | 9.1 |
| 35 | 1.3 | 1.9 | 2.3 | 3.1 | 4.0 | 4.6 | 5.6 | 7.3 |
| 36 | 1.2 | 1.9 | 2.2 | 2.9 | 3.8 | 4.4 | 5.4 | 7.0 |
| 37 | 1.2 | 1.8 | 2.2 | 2.8 | 3.6 | 4.3 | 5.2 | 6.7 |
| 38 | 1.1 | 1.7 | 2.1 | 2.7 | 3.5 | 4.1 | 5.0 | 6.4 |
| 39 | 1.1 | 1.6 | 2.0 | 2.6 | 3.3 | 3.9 | 4.7 | 6.2 |
| 40 | 1.0 | 1.6 | 1.9 | 2.5 | 3.2 | 3.7 | 4.5 | 5.9 |
| 41 | 1.0 | 1.5 | 1.8 | 2.4 | 3.1 | 3.6 | 4.4 | 5.7 |
| 42 | 0.9 | 1.4 | 1.7 | 2.3 | 2.9 | 3.4 | 4.2 | 5.4 |
| 43 | 0.9 | 1.4 | 1.7 | 2.2 | 2.8 | 3.3 | 4.0 | 5.2 |
| 44 | 0.9 | 1.3 | 1.6 | 2.1 | 2.7 | 3.1 | 3.8 | 5.0 |
Poisson regression was used to predict the number of biopsiable blastocysts obtained per cycle. Results are rounded to the nearest tenth
Cost of a euploid embryo
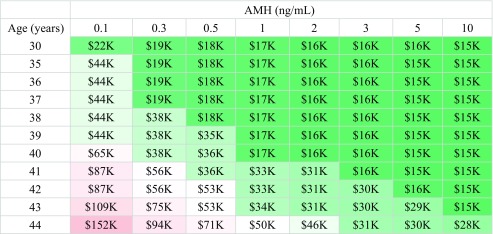
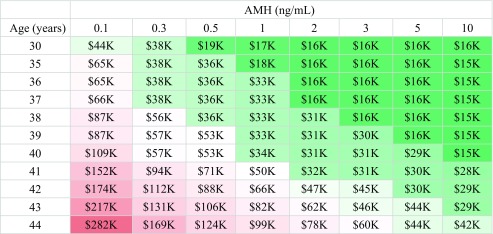
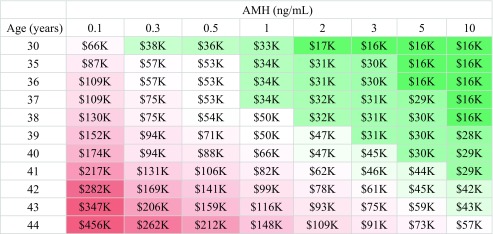
The probability of a blastocyst being euploid as a function of age based on CooperGenomics data, as well as the corresponding number of blastocysts needed to obtain a 50%, 75%, or 90% likelihood of having a euploid embryo, are shown in Supplemental Table 1. Our analyses show that the expected total treatment cost for a 50% likelihood of attaining at least one euploid embryo ranged from approximately $15,000 for younger women with higher AMH values (≥ 2 ng/mL) to > $150,000 for the oldest women in our study population (44 years) with AMH ≤ 0.1 ng/mL (Table 3). There is little difference in cost for a 75% versus 90% likelihood of attaining a euploid embryo for the youngest women with the highest AMH values in this cohort (Tables 4 and 5). The difference in cost to attain a 75% versus 90% likelihood of a euploid embryo becomes more pronounced with increasing age and decreasing AMH. Women with the highest AMH values (≥ 10 ng/mL) can expect to pay $16,000 up to age 39 for a 90% likelihood of attaining a euploid embryo. The “average” patient in our study population was 36–37 years old, with an AMH of 2.5 and, according to our analyses, would be expected to spend approximately $16,000 for a 50% likelihood of attaining a euploid embryo and $30,000 for a 90% likelihood of attaining a euploid embryo. There is less of a difference in cost to attain a euploid embryo across AMH values for women 30–36 years of age. The cost differential across AMH values increases with age, with the oldest patients’ projected cost increasing more as AMH decreases compared with younger women. For example, to attain a 90% likelihood of a euploid embryo, a woman 30 years old with an AMH of ≤ 0.1 versus ≥ 10 ng/mL should expect to pay $66,000 versus $16,000, respectively—a difference of approximately $50,000. Women 44 years old with an AMH of ≤ 0.1 versus ≥ 10 ng/mL should expect to pay $456,000 versus $57,000, a difference of approximately $400,000.
Table 3.
Cost in thousands to attain a 50% likelihood of a euploid embryo
Table 4.
Cost in thousands to attain a 75% likelihood of a euploid embryo
Table 5.
Cost in thousands to attain a 90% likelihood of a euploid embryo
Tables 3, 4, and 5. Ten thousand Monte Carlo simulations per cell were used to estimate the treatment cost to obtain a 50%, 75%, or 90% likelihood of having at least one euploid embryo. Results are rounded to the nearest $1000
Redder cells indicate higher cost estimates, while greener cells indicate lower cost estimates
Figure 2 depicts the probability of attaining a euploid blastocyst over an increasing number of cycles for different patient ages and AMH values. The “average” 36–37-year-old with an AMH of 2–3 ng/mL would have approximately an 80–85% chance of obtaining a euploid blastocyst after a single cycle; after 2 cycles, the likelihood increases to > 90%. A 38-year-old patient with an AMH of 0.1 ng/mL would have < 40% chance of obtaining a euploid blastocyst after a single cycle; after 3 cycles, that likelihood increases to approximately 70%. A 44-year-old with an AMH of 0.1 ng/mL would have < 40% chance of obtaining a euploid blastocyst even after 5 cycles. At a higher AMH value of 5 ng/mL, a 44-year-old would have about a 90% chance of having a euploid blastocyst; notably, only one 44-year-old in our database had an AMH value that high.
Fig. 2.
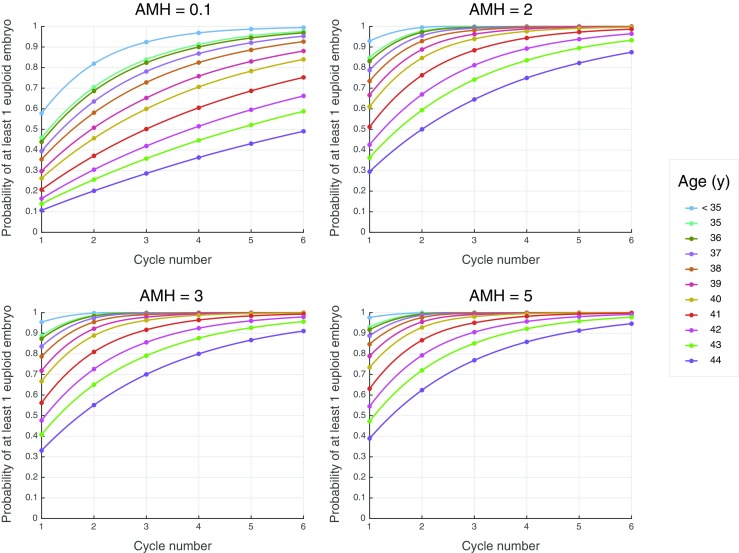
This figure depicts the probability of obtaining a euploid blastocyst over an increasing number of cycles for different patient ages and AMH values. Each graph was created using 10,000 Monte Carlo simulations
Discussion
Preimplantation genetic testing for aneuploidy is an attractive technology that has the potential to increase IVF success rates through the selection of a euploid embryo for transfer. However, deciding whether to undergo PGT-A is a complex decision based on a multitude of factors for different patients (Genoff Garzon et al.)[25]. Patients may have varying thresholds at which they feel comfortable transferring a tested versus untested embryo. Likewise, patients come from different financial means. Ideally, the decision to undergo PGT-A will be made by a fully informed patient, with physician guidance. It is important to counsel patients regarding the likelihood of obtaining a euploid embryo and what their expected costs may be, based on their age and AMH. This present study was undertaken to fill a gap in the literature regarding the anticipated costs of PGT-A on an individual patient basis and to allow for more comprehensive and personalized patient counseling.
Studies regarding both the cost-effectiveness and utility of PGS for different patient populations have been mixed (Mersereau et al., [26]; Twisk et al.[8]; Scott et al.[14]; Dahdouh et al.[15]; Murugappan et al.[6]; Kang et al.[27]; Collins et al.[28]). A recent study found IVF with PGT-A to be cost-effective for women over the age of 37 years [28], based on an analysis of previously published cost estimates of pregnancy-related events from a public health care lens. While that study helps to evaluate how PGS impacts the overall cost to society of pregnancy among women who undergo PGT-A, it is limited in its generalizability and cannot be used as an individualized counseling tool. The study assumed the same cost estimates for all women > 37 years old and presumed that they will have obtained a blastocyst from a single IVF cycle, thus eliminating the poorest prognosis patients. It did not consider ovarian reserve parameters, nor did their cost estimates include IVF cycle costs. Their study was therefore not designed to be a personalized counseling tool. Cost considerations are important factors for individuals to consider when deciding whether to pursue PGT-A, and personalized counseling tools are needed. To our knowledge, the current study is the first to evaluate the cost of PGT-A by individual year of age and over a range of AMH values, and our results have been built into a user-friendly instrument for patient counseling.
In the current study, the median cost estimate to attain a 75% or 90% likelihood of a euploid blastocyst (i.e., for women with a median age and AMH of 37 years and 2.5 ng/mL, respectively) would be approximately $16,000 or $30,000, respectively (Tables 4 and 5). Women in the highest age categories with the lowest AMH values were projected to need to spend upward of $200,000 over multiple IVF cycles to achieve this same likelihood. As there is typically some embryo attrition from days 3 to 5, and not all euploid embryos necessarily progress to biopsiable blastocysts in the laboratory, these patients may ultimately be better served by undergoing fresh embryo transfers of one or more embryos on day 3. One could argue that patients 41–42 years of age with an AMH < 0.5 ng/mL or > 42 with an AMH < 1 ng/mL be discouraged from undergoing PGT-A, unless they are prepared to spend significant time and approximately $100,000 to undergo multiple cycles of IVF for a 75% likelihood of attaining a euploid embryo. The cost to attain a 50% likelihood of a euploid embryo for these women is less, at approximately $55,000–90,000 USD (Table 3). For many, but not all, this would prove cost-prohibitive. It is important to counsel patients who opt for PGT-A regarding the risk of not having any embryos available for biopsy or for transfer, a risk that increases with age [15]. However, it is equally important to counsel patients about the increased risk of miscarriage with transfer of an untested embryo at higher ages. It is impossible to put a price, in terms of both dollars and time spent, on an aneuploid miscarriage that may have otherwise been avoided by transferring a euploid embryo. While euploidy does not eliminate the chance of a miscarriage, it does decrease this risk [27, 29]. In this present study, a small number of patients (< 10%) ultimately opted for a fresh embryo transfer instead of PGT or a freeze-all; fewer than half achieved a pregnancy, and the majority of pregnancies were biochemical, with only 15% of patients with a fresh transfer having a live birth or ongoing pregnancy.
Approximately half of the women in our study population were 37 years or older, and these patients had a median AMH of 1.8 ng/mL. Such patients should be advised that they may require multiple IVF cycles to obtain a high likelihood of a euploid blastocyst. They may therefore wish to bank embryos and have PGT-A run once to potentially decrease the overall treatment price. In Tables 3, 4, and 5, the cells shaded green represent a treatment cost of approximately $50,000 or less; for patients meeting those highlighted combinations of age and AMH, PGT-A may be more cost-friendly. Note that in this study, the higher cost differential at older ages by AMH suggests that AMH plays a larger role in the cost of treatment cycles for older women; this is largely driven by age-related aneuploidy rates.
It is important to note that these price estimates reflect those required to obtain a 50%, 75%, or 90% likelihood of obtaining a euploid embryo, but this does not necessarily mean a live birth would result following transfer. Assuming that transfer of a euploid blastocyst confers an approximate 60% likelihood of a live birth [14, 23, 24], a patient may need multiple transfers of euploid embryos in order to achieve a birth. This would translate to a higher out-of-pocket price to delivery, rather than price to transfer (Supplemental Tables 2A–C). These estimates additionally include the cost of frozen embryo transfer(s), which may increase the expected cost by approximately $2500–$3000 per transfer. As NGS begins to replace aCGH as the preferred method of PGT-A, the detection sensitivity for embryo mosaicism will likely increase and may result in more embryos deemed aneuploid [30, 31]. It is unclear how this will impact the likelihood of identifying a suitable embryo for transfer, or the likelihood of live birth following a euploid embryo transfer. Moreover, the cost of PGT-A may decrease over time as our technology advances; as an example, non-invasive PGT is inching toward reality [32, 33]. This would eliminate the embryo biopsy step and make the process for PGT overall less costly for patients.
This study has several important strengths. Notably, to our knowledge, this is the first study that presents cost projections for IVF with PGT-A taking the range of both age and AMH values into consideration. From our cost analysis, we have developed a patient counseling tool to aid in individual decision-making regarding the use of PGT-A. The use of Monte Carlo simulation models allowed for hundreds of thousands of simulations for cost approximation based on real-patient data. Patients with infertility of all diagnoses were included, allowing the results to be more generalizable. We included patients who intended on having a blastocyst embryo freeze at cycle start, as providers may stimulate more aggressively knowing that a leuprolide trigger could be used to avoid ovarian hyperstimulation syndrome. Similarly, we did not exclude patients who ultimately opted against PGT-A or a freeze-all in order to capture those patients who may be of poorer prognosis, thereby incorporating their embryo transfer data into the model.
There are limitations to this study, including the retrospective nature of chart review and the use of aggregated PGT-A data from multiple institutions. The sample size was relatively small, and we made assumptions about modeling distributions and pricing that likely differ across IVF centers. Raw counts for each age and AMH combination are shown in Supplemental Table 3, and our model may be less accurate for age and AMH combinations in which there were fewer patients. We assumed that transfer of an embryo on day 3 that resulted in a pregnancy would have likely become a biopsiable blastocyst in the laboratory. However, we cannot say for sure that an embryo resulting in a pregnancy would have survived ex-utero, and conversely, that an embryo that did not result in a pregnancy would have failed to blastulate in culture. Given the relatively few day 3 transfers and pregnancies overall, we do not feel that this significantly impacts our results. We included a $2000 cost for ICSI in our model, as most cycles in our cohort utilized ICSI. However, PGT-A does not require ICSI, so treatment costs are expected to be several thousand dollars lower for cycles using IVF without ICSI. This model relies only on age and AMH and there may be other factors that additionally contribute to the overall cost.
While the efficacy and merit of PGT-A continue to be investigated in our community, this tool can act as a counseling adjunct by providing individualized cost information that allows patients and clinicians to make better-informed choices regarding their fertility care.
Electronic supplementary material
(DOCX 32.4 kb)
Conflicts of interest
E.G. receives royalties from UpToDate, Springer BioMed Central, Advance Medical, and Sanders and Parks and receives research funding from Serono, unrelated to this work. C.R. is an ASRM board member, receives royalties from Springer and the University of Cambridge, is a lecturer at Ferring, Inc., and is an IVF program consultant, unrelated to this work. S.M. and L. R. are employed by CooperGenomics. The remaining authors report no conflicts of interest.
Ethical approval
For this type of study, formal consent is not required.
Footnotes
References
- 1.Harper J, Jackson E, Sermon K, Aitken RJ, Harbottle S, Mocanu E, Hardarson T, Mathur R, Viville S, Vail A, Lundin K. Adjuncts in the IVF laboratory: where is the evidence for “add-on” interventions? Hum Reprod. 2017;32:485–491. doi: 10.1093/humrep/dex004. [DOI] [PubMed] [Google Scholar]
- 2.Sermon K, Capalbo A, Cohen J, Coonen E, De Rycke M, De Vos A, et al. The why, the how and the when of PGS 2.0: current practices and expert opinions of fertility specialists, molecular biologists, and embryologists. Mol. Hum. Reprod. 2016;22:845–857. doi: 10.1093/molehr/gaw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capalbo A, Romanelli V, Cimadomo D, Girardi L, Stoppa M, Dovere L, Dell’Edera D, Ubaldi FM, Rienzi L. Implementing PGD/PGD-A in IVF clinics: considerations for the best laboratory approach and management. J Assist Reprod Genet. 2016;33:1279–1286. doi: 10.1007/s10815-016-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhart MB, Hines RS, Penman A, Holland AC. How do patient perceived determinants influence the decision-making process to accept or decline preimplantation genetic screening? Fertil Steril. 2016;105:188–193. doi: 10.1016/j.fertnstert.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Dahdouh EM, Balayla J, García-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104:1503–1512. doi: 10.1016/j.fertnstert.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Murugappan G, Ohno MS, Lathi RB. Cost-effectiveness analysis of preimplantation genetic screening and in vitro fertilization versus expectant management in patients with unexplained recurrent pregnancy loss. Fertil Steril. 2015;103:1215–1220. doi: 10.1016/j.fertnstert.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Wei S, Hu J, Quan S. Can comprehensive chromosome screening technology improve IVF/ICSI outcomes? A meta-analysis. PLoS One. 2015;10:e0140779. doi: 10.1371/journal.pone.0140779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twisk M, Mastenbroek S, Hoek A, Heineman M-J, van der Veen F, Bossuyt PM, Repping S, Korevaar JC. No beneficial effect of preimplantation genetic screening in women of advanced maternal age with a high risk for embryonic aneuploidy. Hum Reprod. 2008;23:2813–2817. doi: 10.1093/humrep/den231. [DOI] [PubMed] [Google Scholar]
- 9.Gleicher N, Orvieto R. Is the hypothesis of preimplantation genetic screening (PGS) still supportable? A review J Ovarian Res. 2017;10:21. doi: 10.1186/s13048-017-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ubaldi FM, Cimadomo D, Capalbo A, Vaiarelli A, Buffo L, Trabucco E, Ferrero S, Albani E, Rienzi L, Levi Setti PE. Preimplantation genetic diagnosis for aneuploidy testing in women older than 44 years: a multicenter experience. Fertil Steril. 2017;107:1173–1180. doi: 10.1016/j.fertnstert.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Weissman A, Shoham G, Shoham Z, Fishel S, Leong M, Yaron Y. Preimplantation genetic screening: results of a worldwide web-based survey. Reprod BioMed Online. 2017;35:693–700. doi: 10.1016/j.rbmo.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NEA, Arts EGJM, de Vries JWA, Bossuyt PM, Buys CHCM, Heineman MJ, Repping S, van der Veen F. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 13.Mastenbroek S, Repping S. Preimplantation genetic screening: back to the future. Hum Reprod. 2014;29:1846–1850. doi: 10.1093/humrep/deu163. [DOI] [PubMed] [Google Scholar]
- 14.Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–663.e1. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 16.La Marca A, Minasi MG, Sighinolfi G, Greco P, Argento C, Grisendi V, et al. Female age, serum antimüllerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2017;108:777–783.e2. doi: 10.1016/j.fertnstert.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Orris JJ, Taylor TH, Gilchrist JW, Hallowell SV, Glassner MJ, Wininger JD. The utility of embryo banking in order to increase the number of embryos available for preimplantation genetic screening in advanced maternal age patients. J Assist Reprod Genet. 2010;27:729–733. doi: 10.1007/s10815-010-9474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Effect of embryo banking on U.S. national assisted reproductive technology live birth rates. Yu Y, editor. PLoS One. 2016;11:e0154620. [DOI] [PMC free article] [PubMed]
- 19.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, la Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, Wallace WH, Anderson RA. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 20.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Wu C-H, Chen Y-C, Wu H-H, Yang J-G, Chang Y-J, Tsai H-D. Serum anti-Müllerian hormone predicts ovarian response and cycle outcome in IVF patients. J Assist Reprod Genet. 2009;26:383–389. doi: 10.1007/s10815-009-9332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges E, Braga DPAF, Setti A, de Figueira RC, Iaconelli Júnior A. The predictive value of serum concentrations of anti-Müllerian hormone for oocyte quality, fertilization, and implantation. JBRA Assist Reprod. 2017;21:176–182. doi: 10.5935/1518-0557.20170035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100:100–107.e1. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 24.Forman EJ, Hong KH, Franasiak JM, Scott RT. Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol. 2014;210:157.e1–157.e6. doi: 10.1016/j.ajog.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Genoff Garzon MC, Rubin LR, Lobel M, Stelling J, Pastore LM. Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin Genet 2018;1–21. [DOI] [PubMed]
- 26.Mersereau J, Plunkett B, Cedars M. Preimplantation genetic screening in older women: a cost-effectiveness analysis. Fertil Steril. 2008;90:592–598. doi: 10.1016/j.fertnstert.2007.07.1307. [DOI] [PubMed] [Google Scholar]
- 27.Kang H-J, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106:597–602. doi: 10.1016/j.fertnstert.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Collins SC, Xu X, Mak W. Cost-effectiveness of preimplantation genetic screening for women older than 37 undergoing in vitro fertilization. J Assist Reprod Genet. 2017;34:1515–1522. doi: 10.1007/s10815-017-1001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod. 2012;27:1217–1222. doi: 10.1093/humrep/des020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai H-H, Chuang T-H, Wong L-K, Lee M-J, Hsieh C-L, Wang H-L, Chen SU. Identification of mosaic and segmental aneuploidies by next-generation sequencing in preimplantation genetic screening can improve clinical outcomes compared to array-comparative genomic hybridization. Mol Cytogenet. 2017;10:14. doi: 10.1186/s13039-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maxwell SM, Colls P, Hodes-Wertz B, McCulloh DH, McCaffrey C, Wells D, et al. Why do euploid embryos miscarry? A case-control study comparing the rate of aneuploidy within presumed euploid embryos that resulted in miscarriage or live birth using next-generation sequencing. Fertil Steril. 2016;106:1414–1419.e5. doi: 10.1016/j.fertnstert.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Fang R, Chen L, Chen D, Xiao J-P, Yang W, Wang H, Song X, Ma T, Bo S, Shi C, Ren J, Huang L, Cai LY, Yao B, Xie XS, Lu S. Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci. 2016;113:11907–11912. doi: 10.1073/pnas.1613294113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feichtinger M, Vaccari E, Carli L, Wallner E, Mädel U, Figl K, Palini S, Feichtinger W. Non-invasive preimplantation genetic screening using array comparative genomic hybridization on spent culture media: a proof-of-concept pilot study. Reprod BioMed Online. 2017;34:583–589. doi: 10.1016/j.rbmo.2017.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 32.4 kb)