Abstract
Purpose
We investigated if automated TLI selection may be a valuable strategy to identify those euploid embryos with the best chances of success.
Methods
This is a unicentric and retrospective study involving 244 patients undergoing preimplantational genetic screening (PGS) cycles with autologous oocytes or oocyte donation (OD) with single euploid embryo transferred. We examined euploid embryos selected for transfer based on morphology evaluation alone (PGS-only; control group) or by assessment using an automated TLI system (Eeva™; PGS-TLI group).
Results
In both, autologous oocytes and OD patients, significantly better implantation and clinical and ongoing pregnancy rates were obtained in the PGS-TLI group when euploid embryos with high implantation potential as predicted by the automated TLI System (Eeva™) were transferred compared with the PGS-only group. This improvement was also observed when only transfers of good morphological quality embryos were compared. TLI categories showed significant differences on blastocyst formation and euploidy rate.
Conclusions
Automated TLI combined with PGS is a useful prognostic tool to identify euploid embryos with the highest potential for implantation and pregnancy. Further, these results provide evidence that a healthy pregnancy does not only depend upon normal chromosomal status.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1265-7) contains supplementary material, which is available to authorized users.
Keywords: Preimplantation genetic screening, Automated time-lapse imaging, In vitro fertilization, Oocyte donation, Pregnancy rates
Background
The main goal of assisted reproduction techniques (ART) is to achieve a successful single healthy birth in the shortest time. However, many cases do not accomplish this partly due to the difficulty of selecting the highest quality embryo, and often leads to the transfer of more than one embryo at a time. This subsequently increases multiple birth rates, which are associated with medical risks and financial costs [1, 2]. Advances in embryo culture and cryopreservation have led to improved implantation and pregnancy rates [3, 4] and consequently, some countries have moved to a single-embryo transfer policy [5–7]. However, improved selection strategies are still needed in order to achieve higher pregnancy rates per transfer, reducing time to pregnancy while being cost-efficient.
Traditionally, embryo selection for transfer has been performed by microscopic evaluation of morphology at specific times of embryo development [8]. Morphology can be assessed at any stage of embryo development, and numerous studies have linked morphological characteristics at different developmental stages with ART outcomes [9–11]. Lately, the usefulness of this assessment is being questioned due to judgment subjectivity and low success rate [12]. In recent years, alternative methods for embryo selection have been developed [13] such as preimplantational genetic screening (PGS), which provides chromosomal analysis to improve clinical outcomes when transferring a single embryo. The presence of chromosomal abnormalities (i.e., aneuploidy) in human embryos is one of the main causes of implantation failure and miscarriage [14–17].
Thus far, PGS has shown positive results, improving pregnancy rates per transfer in poor and good prognosis patients, and egg donation cycles [18]. Results from four randomized, controlled trials (RCTs) performed with good prognosis patients undergoing in vitro fertilization (IVF) confirmed that aneuploidy analysis in human blastocysts and selection of euploid embryos for transfer significantly improves clinical outcomes, compared with selection by morphology only. These findings were valid regardless of the genetic platform used [19–25].
Although these results are encouraging, there are still cases of euploid embryos with good morphology that fail to implant [3]. Moreover, selection of euploid embryos by morphology can translate into the same implantation rates independently of the blastocyst grade [26]. Therefore, additional markers of embryo viability are needed to identify embryos with the highest potential to produce a healthy pregnancy, and several have been proposed [27, 28]. Some such as mitochondrial DNA quantification has been suggested as a complement existing method of identifying embryos with the highest chances of implanting [29, 30], while others such as proteomics findings were initially suggested as independent markers of embryo quality. However, the clinical value of these techniques are still under review and not routinely used in IVF laboratories [27, 31–33].
Among these newly developed techniques of embryo selection, one of the most widely applied in recent years is time-lapse imaging (TLI). This tool provides a non-invasive alternative to static morphological assessment of embryos, allowing the observation of cellular events that would remain undetected by conventional methods. Such technology also provides the opportunity to identify and record timing, and other kinetic parameters potentially valuable for embryo development and implantation competence assessment [34].
One of the first relevant studies revealed the correlation between early time-lapse parameters and gene expression profiling, suggesting that blastocyst formation can be predicted systematically and reliably by four-cell stage. These findings also showed that these timings are previous to the early genome activation, meaning that the fate of the embryo is decided partly by the inheritance of maternal transcripts [34]. Subsequently, these parameters were found to be associated with euploidy, where all euploid embryos were comprised within normal timings but only 30% of the aneuploid embryos [35].
Based on these previous studies, an automatic TLI system, Eeva™, was developed and commercialized for human embryo evaluation, classifying embryos into categories depending on early cleavage timings P2 (duration of 2 cell stage) and P3 (duration of 3 cell stage), being the first time-lapse system FDA cleared to aid embryo selection. Numerous studies have demonstrated that these parameters are correlated to the prediction of blastocyst formation [36], implantation rates [37, 38], and ongoing pregnancy rates [39, 40]. Unlike other time-lapse systems, Eeva has demonstrated improved embryo selection among embryologist with diverse experiences when used adjunctively with morphology [36, 41].
Moreover, many studies have found correlations between diverse morphokinetic markers and embryo morphology, blastocyst formation, pregnancy, implantation, and miscarriage rates, most of which are also focused on early cleavage markers [42–45]. In fact, several RCT and meta-analysis studies highlight the benefits of TLI for embryo selection [46–49].
Additionally, TLI has also been proposed as an alternative to PGS to predict euploid embryos. A recent study found a correlation between early cleavage timing parameters and embryo chromosome status and proposed an algorithm to classify the embryos [50]. Vera-Rodriguez and colleagues similarly found a relation between some time-lapse parameters and euploidy, and also with specific gene expression patterns [51]. A further study described the relation between late time divisions, such as time to full blastocyst formation, and euploidy rate [52]. However, none of these studies demonstrated that TLI was sufficient to predict and identify euploid embryos. Moreover, studies attempting to validate both algorithms previously proposed for euploidy prediction, based on early or late cleavage timings, found no correlation [53–55]. Therefore, latest evidence points out that TLI cannot be used as a substitute for PGS.
Combining embryo selection techniques is the next logical step to producing superior results versus those obtained through the application of a single technique. A study detailing the combination of PGS and time-lapse imaging systems, which is more available and commonly used in IVF laboratories, investigated the effect of selecting competent embryos among euploid ones by an algorithm based on morphokinetic markers previously described by Meseguer et al. [43]. The authors concluded that this combination achieves better pregnancy and implantation rates in IVF patients than selection by morphology alone [56]. However, it is clear that further research is necessary to understand the relationship between time-lapse parameters and the genetic status of the embryo, as well as its usefulness in clinical practice. In this study, we aim to provide information on the ability of the automated TLI system Eeva to diagnose euploidy and/or other parameters of embryo quality in order to identify the most competent embryo. We also investigated whether the combination of PGS by next-generation sequencing (NGS) with an automatic TLI might be a better embryo selection strategy to improve ART outcomes than PGS only and conventional morphology.
Methods
This study was approved by the IVF-Spain institutional review board. This was a retrospective review of records for patients treated in our center between October 2013 to February 2016.
Patient selection
The study included 244 patients undergoing ART treatment with PGS with either autologous (n = 81) or donated oocytes (n = 163). All patients had a body mass index of > 18–< 25 kg/m2 and had undergone single euploid embryo transfer. All oocyte donors were selected according to Spanish Law 14/2006, governing ART, were between the ages of 19–34 years, had normal menstrual cycles (26–34 days), showed no signs of polycystic ovary syndrome, and had received no endocrine treatment for the 3 months prior to the cycle under study. For autologous cycles, patients with follicle-stimulating hormone (FSH) < 12 mIU/ml and a minimum follicular count of six follicles were included. Patients presenting with uterine diseases and endometrial factors were excluded. All patients included in the study requested PGS due to either one or more of the following reasons: (1) unexplained recurrent miscarriage with two or more pregnancies lost; (2) repeated implantation failure after three or more embryo transfers; (3) previous aneuploid conceptions; and (4) sperm concentration < 1 M/ml from a testicular sperm sample.
Participants from both autologous and donated oocytes were divided into two groups: the PGS-only group (n = 37 autologous and 64 donated), where embryos were cultured into a conventional incubator at 6% CO2 and 5% O2 with 95% humidity, and embryos were selected for single transfer following euploidy criteria only; the PGS-TLI group (n = 44 autologous and 99 donated) where embryos were cultured into a conventional incubator at the same conditions with the addition of individual cameras connected to the automated time-lapse system Eeva™ (Auxogyn,Inc., Menlo Park, CA, USA) and were selected for transfer based on both PGS and TLI predictions. Once the requirements of embryo selection within each group were met, the best morphological quality embryo available was transferred.
Ovarian stimulation in autologous patients and oocyte donors
Gonadotropin-releasing hormone (GnRH) antagonist treatments (Ganirelix acetate injection; Orgalutran®; Merck and Co, Inc., USA) were used for controlled ovarian stimulation for autologous patients and oocyte donors. Ovarian stimulation was achieved using gonadotrophins (FSH and luteinizing hormone (LH)), and the dosage was adapted depending on age and follicular count (mean 150–225). LH was additionally given to patients over 38 years old. The patients were monitored using serial transvaginal ultrasound, assessment of the endometrial lining, and estradiol and progesterone levels. When a diameter of 19 mm was reached by at least three follicles, this triggered administration of a GnRH analog (Procrin®; Abbott Laboratories, Abbott Park, IL, USA) for final oocyte maturation. For all patients, oocyte retrieval was performed under transvaginal ultrasound guidance at 36 h after the trigger administration.
Ovum pick up and fertilization
For all cycles, follicles were aspirated and the oocytes were washed in Multipurpose Handling Medium™ (MHM™, Irvine Scientific, USA). After washing, oocytes were cultured in Continuous Single Culture™ (CSC™, Irvine Scientific, USA) and covered with light mineral oil (Irvine Scientific, USA) at 6% CO2 and 37 °C for 2 h prior to oocyte denudation if utilizing intra-cytoplasmic sperm injection (ICSI) and 4 h without denudation if IVF. Oocyte denudation was performed by mechanical pipetting of 80 IU/ml hyaluronidase (GM501 Hyaluronidase, Gynemed, Germany) in the same medium. Subsequently, 4 h after egg retrieval, fertilization was performed by ICSI or IVF, only performed when normozoospermic sample was available. All sperm samples were prepared by swim up in MHM for ICSI and CSC for IVF. ICSI was performed in MHM containing HEPES at × 400 magnification using a NIKON Eclipse TI-u. The injected oocytes were then immediately placed in CSC covered with light mineral oil at 6% CO2 and 37 °C until fertilization assessment. IVF was performed in 20 μl/ml of CSC with a final sperm concentration of 1 M/ml. Oocytes were cultured individually overnight at 6% CO2 and 37 °C until the fertilization assessment. Fertilization was assessed 16–18 h post-fertilization and confirmed by visualization of two pronuclei and two polar bodies using an inverted microscope. All zygotes were placed into a group culture dish for TLI.
Embryo culture, scoring, trophectoderm biopsy, and genetic analysis
All zygotes were cultured until blastocyst stage in the TLI dish (Eeva®, Merck, Germany) with 100 μl of CSC covered with 3 ml of light mineral oil at 37 °C, 6% CO2 and 5% O2, regardless of the group. Zygotes from the PGS-only group were placed into conventional incubators (Labotect C60, Göttingen, Germany) and evaluated under microscope. However, for the PGS-TLI group, zygotes were cultured at the same conditions into conventional incubators (Panasonic MCO-5M PE, Japan) with the TLI system incorporated inside. All embryos were evaluated morphologically on Days 2 and 3. The parameters analyzed were number of cells, symmetry, and fragmentation among others, as described by the Spanish Society of Reproductive Biology [57]. On Day 3, assisted hatching was performed to all embryos to facilitate trophectoderm extrusion for posterior biopsy.
Embryos within the TLI incubator (Eeva®, Merck, Germany) were evaluated through a screen, avoiding the need of disturbance. The TLI system consists of a camera placed inside a conventional incubator which produces dark-field images, allowing automatic cell-division tracking. TLI automatically calculates the time division of the embryos from two- to three-cell-stage (P2) and from three- to four-cell-stage (P3). Depending on these timings (P2 and P3), the system automatically generates on Day 3 a prediction categorizing each embryo as high, medium, or low. These categories are related to its developmental potential to reach to blastocyst stage, being high category with the best prognosis (Supplemental Fig. 1). Once the prediction was obtained on Day 3, embryos were moved from the TLI for assisted hatching and placed into conventional incubators (Labotect C60, Göttingen, Germany) until blastocyst stage.
None of the embryos were morphologically assessed on Day 4 of embryo development.
Once the embryos reached the blastocyst stage, they were evaluated for grade of expansion, inner cell mass, and trophectoderm quality as described by Gardner et al. [58, 59]. Blastocyst quality was assessed prior to the trophectoderm biopsy and classified into three groups: good (AA, BA, and AB), fair (BB, AC, and CA), and poor (BC, CB, and CC), independent of the grade of expansion. Blastocysts were biopsied when the grade of expansion and the number of cells were adequate, all were performed between Days 5 and 7 when three–five cells had hatched. Trophectoderm cells were aspirated into a biopsy pipette and separated from the blastocyst by applying laser pulses (ZILOS-tk® Laser, Hamilton Thorne Research, USA). The biopsied cells were washed with PBS 1× (Cell Signaling, USA) at 0.1% PVA (Sigma Aldrich, USA) and placed into sterile 0.2 ml PCR tubes (Eppendorf, Germany). All biopsy and manipulation procedures were completed under conditions controlled for temperature, gas, and humidity. The biopsied samples were sent to an external laboratory at 4 °C for genetic analysis by NGS (Ion Torrent, Thermo Fisher Scientific, USA). Following the biopsy, all blastocysts were vitrified using the Kuwayama protocol and stored in liquid nitrogen until results were available [60].
Embryo selection and transfer
Patients received substitutive hormonal treatment with 6 mg/day of oral estradiol valerate (Progynova, BAYER, Germany) for at least 14 days, or until their endometrium lining was > 7 mm as confirmed by echography. For the luteal phase, 400 mg of vaginal progesterone was administered every 12 h. For patients with ovarian function, a GnRH agonist was administered in the previous luteal phase.
Only euploid embryos were candidates for single-embryo transfer. For the PGS-only group, the highest morphological quality embryo was selected for transfer. In case of two equally graded blastocysts, best embryo development on Days 2 and 3 was prioritized. In the PGS + TLI group, selection was made based on TLI classification criteria, the highest TLI-classified embryo and best morphology available was selected for transfer. All transfers were performed in a subsequent cycle with frozen-thawed embryos under hormone replacement treatment. After 2 h post-thawing [60], survival and re-expansion were assessed. Embryo transfer was performed by vaginal ultrasound and soft catheter. The embryo was placed at 1 cm of uterine fundus in CSC medium previously drawn into the catheter.
Data collection and statistical analysis
Implantation and clinical and ongoing pregnancy rates were compared for the PGS-only and PGS + TLI groups. Implantation rate was considered when an intrauterine gestational sac was confirmed at the 6th week. Clinical pregnancy was determined by intrauterine gestational sac with fetal heartbeat, visualized by ultrasound examination at Week 8 following embryo transfer. Ongoing pregnancy was considered continuing pregnancy at > 12 weeks of gestation. Other variables such as fertilization, blastocyst presence, blastocyst quality, and euploidy were analyzed for both study groups. Euploidy and blastocyst rates per TLI category were also studied.
t test was used to compare patient characteristics between the study groups. Significant differences between clinical outcome values from the study groups were explored using X2 goodness-of-fit test.
The statistical analyses were performed using SPSS (IBM SPSS Statistics 22.0, USA). A two-tailed p value of< 0.05 was considered statistically significant.
Results
Patients’ characteristics
Clinical characteristics of the patients included in the study are detailed in Table 1. PGS-only control and TLI + PGS groups were comparable in both, patients seeking treatment with their own eggs and patients seeking ovodonation treatment. No significant differences in number of elective SET (eSET), age, MII numbers, fertilization, blastocyst, good morphological quality blastocyst, and euploidy rates were found between the groups.
Table 1.
Clinical characteristics for patients seeking treatment with autologous oocytes (IVF) and OD cycles in the study groups (PGS only and TLI + PGS)
| Parameters | IVF cycles | OD cycles | ||||
|---|---|---|---|---|---|---|
| PGS only | TLI + PGS | p value* | PGS only | TLI + PGS | p value¥ | |
| Number of transfers | 37 | 44 | 64 | 99 | ||
| Number of eSET transfers (%) | 23 (62.2%) | 28 (63.6%) | N.S. | 61 (95.3%) | 94 (94.9%) | N.S |
| Age | 38.62 ± 0.53 | 37.77 ± 0.45 | N.S. | 42.52 ± 0.73 | 43.07 ± 0.44 | N.S. |
| Number of MII | 13.76 ± 1.09 | 12.14 ± 0.90 | N.S. | – | – | – |
| Fertilization (%) | 82.85 ± 1.93 | 81.14 ± 1.68 | N.S. | 84.13 ± 1.03 | 84.43 ± 0.92 | N.S. |
| Blastocyst (%) | 48.17 ± 3.21 | 46.53 ± 3.00 | N.S. | 53.85 ± 1.85 | 56.94 ± 1.75 | N.S. |
| GQ blastocyst (%) | 32.01 ± 3.63 | 28.1 ± 3.50 | N.S. | 40.72 ± 2.58 | 40.57 ± 2.04 | N.S. |
| Euploid (%) | 46.59 ± 3.89 | 53.86 ± 3.22 | N.S. | 56.89 ± 2.62 | 58.89 ± 1.97 | N.S. |
NS, non-significant
*p value < 0.05, significant level; ¥p value < 0.05, significant level (groups were compared using a t test)
Automated TLI prediction results: classification, blastocyst, and euploidy rate
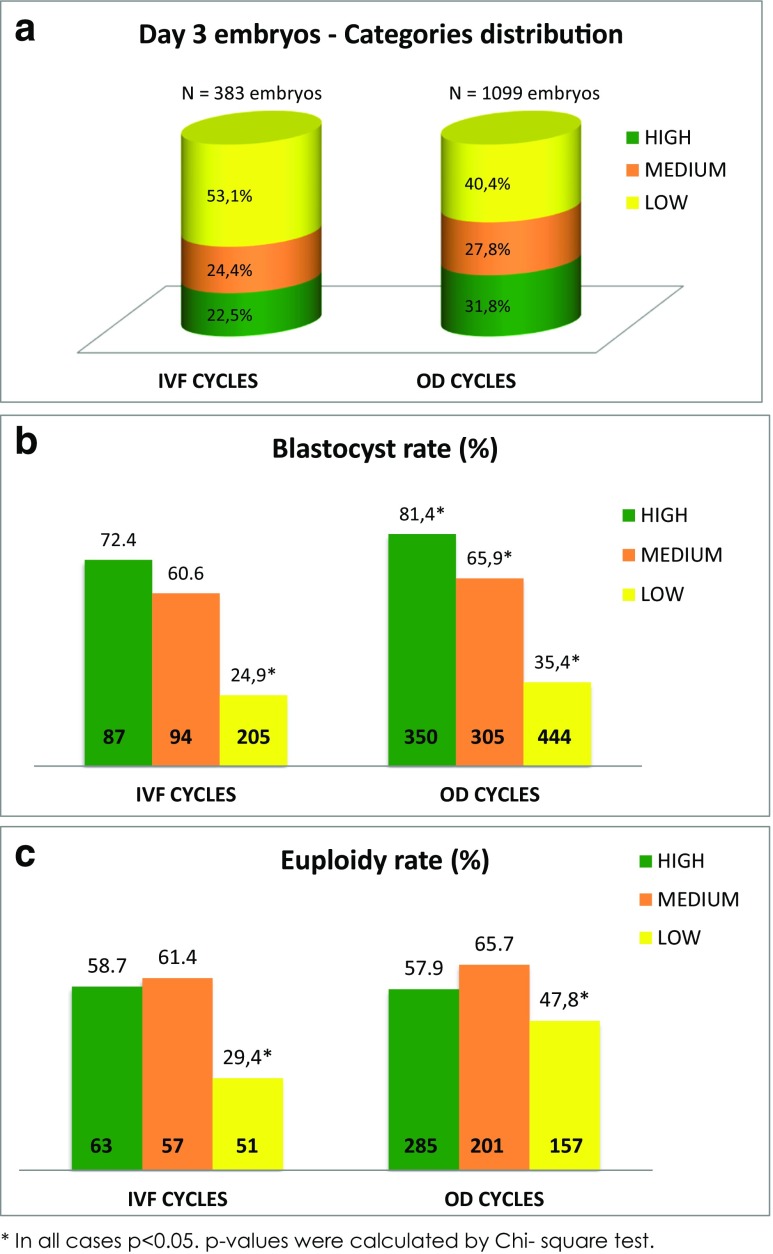
Using TLI, by Day 3, approximately half (53.1%) of the embryos from the autologous oocytes group were classified as low developmental potential, with a quarter (22.5%) classified as high developmental potential and the other quarter (24.4%) as medium (Fig. 1a). Of the OD embryos, more were designated as high potential (31.8%) with only 40.4% being classified as low developmental potential and 27.8% as medium.
Fig. 1.
Distribution of Day 3 (a), blastocyst (b), and euploid (c) rate by TLI Eeva categories for autologous oocytes (IVF) and OD embryos
The blastocyst rate was higher in both the autologous and OD for the high-classified embryo groups compared with the medium and low categories, being significantly lower for the low category in the IVF group, and significantly lower at each step down in classification in the OD group (Fig. 1b). Due to that, the percentage of embryos biopsied from each category at blastocyst stage switch, being 36.8% of the autologous and 44.3% of the OD embryos biopsied from the high Eeva category and only 29.8 and 24.4% from the low category. So, after blastocyst culture, the availability of blastocyst for biopsy from the embryos classified on Day 3 as low development potential is much lower than embryos classified as medium and high development potential (Supplemental Fig. 2).
Regarding euploidy, the percentage of genetically normal embryos was similar in both OD and own eggs groups (Table 1). When analyzing euploidy per TLI category, no significant differences were observed in euploidy rates between high and medium TLI-classified embryos, but a significantly lower euploidy rate was found in the low developmental potential category. This was the case for both autologous oocytes and OD embryos (Fig. 1c).
Clinical outcomes of PGS and PGS + TLI groups
Regarding clinical outcomes, all variables measured (implantation and clinical and ongoing pregnancy rates) were significantly improved by combining PGS and Eeva TLI system prediction for embryo selection compared with PGS alone for both autologous oocytes and OD cycles transfers (Table 2). Significantly higher implantation, clinical and ongoing pregnancy rates were observed in the PGS-TLI group cases where euploid embryos categorized as high implantation potential by the Eeva TL system were transferred as compared with the PGS-only control group where the best morphologically available euploid embryo was selected for transfer. No such differences were found when pregnancy rates from transfers of euploid embryos classified as medium or low by Eeva were compared with controls. This improvement of selecting high-Eeva-classified euploid embryos was also observed when only good morphological quality embryos were transferred (AA, AB, or BA according to Gardners criteria were analyzed) (Fig. 2). The TLI + PGS combined selection of embryos strategy rendered 2× pregnancy rates compared with the ones obtained by the selection of good morphological quality euploids.
Table 2.
Clinical results of autologous oocytes (IVF) and OD cycles comparing PGS only control group and PGS + TLI group transfers of different TLI-classified embryos
| IVF cycles | OD cycles | |||||||
|---|---|---|---|---|---|---|---|---|
| only PGS | High + PGS | Medium + PGS | Low + PGS | Only PGS | High + PGS | Medium + PGS | Low + PGS | |
| Total transfers | 37 | 20 | 18 | 6 | 64 | 64 | 28 | 7 |
| Implantation rate (%) | 35.1 | 80.0* | 55.6 | 33.3 | 60.9 | 73.4¥ | 53.6 | 14.3 |
| Clinical pregnancy (%) | 35.1 | 80.0* | 55.6 | 33.3 | 56.3 | 71.9¥ | 46.4 | 14.3 |
| Ongoing pregnancy (%) | 35.1 | 75.0* | 50.0 | 33.3 | 50 | 67.2¥ | 42.9 | 14.3 |
*p < 0.05, groups are significantly different to control group (only PGS) by X2 goodness of fit; ¥p < 0.05, significance level (groups are significantly different to control group (only PGS) by X2 goodness of fit
Fig. 2.
Implantation rates and ongoing pregnancy rates comparing all transfers to only good quality (GQ) embryo morphology transfers for autologous oocytes (IVF) (a) or OD (b) cycles
Clinical outcomes depending on blastocyst morphology and day of biopsy
Finally, all euploid transfers were compared, regardless of conventional incubation or TLI, and classified by morphology (good, fair, and poor) and day of biopsy (Day 5 or 6). Two embryos transferred (one from autologous and the other from OD group) were Day 7 and not included in this analysis. No significant differences were found between transfers of euploid embryos of good, fair, or poor morphological quality, neither in autologous oocytes or OD cycles (Supplemental Table 1). Biopsy day, however, showed an influence on autologous treatment outcomes. Day 5-biopsied embryo transfers were associated with improved implantation and clinical and ongoing pregnancy rates (Supplemental Table 1).
Discussion
Embryo selection methods are essential to increase the efficacy of ART, promote single embryo transfer, and reduce time from treatment start to live birth. Morphology evaluation remains the primary method of embryo assessment, in most IVF centers worldwide in spite of the numerous studies reporting relatively low pregnancy rates using this method [61]. Even the highest morphological quality embryo can fail to implant, and there have been several reports of cases of top-quality blastocysts being aneuploid [12, 26]. Still only one third of embryo transfers are successful. Therefore, improvements can still be implemented by the study including introduction of additional techniques such as PGS, TLI, or both combined.
Our data adds to that previously published in which the automatic algorithm applied by Eeva improves implantation rates, even allowing for varied protocol conditions [36, 37]. Researchers have agreed that clinical parameters are minimally affected by culture media type [62], oxygen concentration [63], stimulation protocol [64], insemination method [65, 66], and other confounding factors that may affect embryo kinetics. Our data also confirms previous studies which have demonstrated that Eeva categories are correlated with the probability of blastocyst formation [39, 67], as the low prediction category is highly correlated with a small blastocyst rate.
Our study indicates that TLI technology cannot substitute PGS when selecting a euploid blastocyst, since the euploidy accuracy only reaches 60–65% and is similar among high and medium categories after blastocyst culture. However, there is a better chance of selecting a euploid embryo from these categories than from the low category, as reported by previous researchers [35], which could explain previously reported better implantation results using the high and medium categories [39]. Since all embryos transferred were euploid and significant differences in implantation and clinical and ongoing pregnancy rates were found, this highlights that kinetics might be related to different embryo viability factor rather than chromosomal status. This data could support the idea of Eeva time-lapse parameters could be related to specific gene expression patterns as previously described by Wong which would determine the fate of the embryo [34] Moreover, mouse models have previously been used to show the relationship between time-lapse parameters and metabolic profiles, proposing the use of viability markers in combination with TLI to increase embryo selection efficacy [28, 68]. The objectivity afforded by PGS and TLI compared with blastocyst morphology when selecting embryos resulted in improved pregnancy rates for IVF and OD patients, than based on morphology only.
One article has previously been published combining the same methods for embryo selection by Yang et al. [56]. However, there are differences between the studies in the patient population (IVF patients age) and methods applied (type of time-lapse system (manual Embryoscope), type of morphokinetics markers [43], and the use of all fresh transfers). This prospective study using sibling oocytes, investigated only a small number of transfers and included both double and single embryo transfers. Importantly, different oxygen levels were used for the time lapse (5%) and the standard incubator (20%) for blastocyst culture. The advantages of a low oxygen concentration in blastocyst culture have previously been demonstrated [69], hence benefitting the time-lapse group in Yang’s study and blurring the effect of selection by morphokinetic parameters.
To the best of our knowledge, this is the first study combining PGS analysis by NGS with automated TLI for embryo selection. This investigation demonstrates that the combination of the aforementioned techniques is useful as a prognostic tool to identify the embryos with the highest potential of developing into a healthy baby, maximizing the chances of a successful pregnancy. In fact, there were significant differences for all the clinical results analyzed in favor of combining PGS with TLI versus PGS alone. Importantly, the two groups remained significantly different when comparing the transfer of only good morphological quality embryos transferred and also independently of the oocyte source: autologous or donor.
Thus, our research offers further insights into embryo viability, demonstrating that a successful transfer does not only depend on a normal chromosomal status of the embryo. Since ploidy was assessed by NGS in all groups, a standardized study sample was used to show the effects of embryo selection by morphology and morphokinetics parameters. Further, our study also included OD patients, who displayed a wide euploidy rate range from 16 to 100%, compared with the 60% mean (approximate).
Although many previous studies have supported TLI as an objective embryo selection method, they have been heavily criticized due to the variability in culture conditions of the groups compared. In our study, both groups were cultured in identical culture conditions (same media, dishes, CO2, O2, and humidity levels). However, one limitation of our study is the use of different conventional incubator brands from Days 1 to 3 in each group, adding a possible disparity. Another difference between groups is that embryos from the PGS-TLI group were not disturbed on Day 2 for morphology observation.
A remaining question to be answered is whether the combination of morphology grade on Days 2 and 3 plus blastocyst quality might be useful when selecting the embryo with the highest potential. Although blastocyst morphology is recommended to be assessed independently of its grade on Days 2 and 3, in the control group (PGS-only), Days 2 and 3 scores were used as second criteria in the case of similar blastocyst morphology grade in counterpart of the TLI-PGS group that uses two selection criteria in every case. Some authors already addressed this question in order to see the applicability of using Day 2 or 3 morphology scores as predictors of better outcomes with no success [70]. However, it remains unanswered whether this could be a good second criterion to select euploid blastocyst.
Our study complements previously published data [26]. We could not find a significant difference in clinical outcomes for any blastocyst morphology grade independently of the source of the eggs in contrast with recent publication that correlated blastocyst morphology especially inner cell mass with ongoing pregnancy rates [71]. However, our number of cases per group is limited and we could not consider the grade of expansion due to the performance of assisted hatching on Day 3.
On a general note, and as described in previous studies [72], differences in clinical results from Days 5 and 6 blastocysts in IVF-PGS patients were found. Although this was seen in a low number of cases, it would be advisable to transfer Day 5 embryos, if possible. Conversely in OD, the day of embryo biopsy (Day 5 or 6) did not affect clinical results, maybe due to the fact that oocyte quality is supposedly better. However, further studies are needed to confirm such hypothesis.
Conclusions
Although this investigation is limited by its retrospective nature, our research demonstrates that the correct number of chromosomes in the embryo is no guarantee for a successful pregnancy and provides further evidence that blastocyst morphology should not be solely relied upon for embryo selection. Moreover, the combination of PGS with TLI provides more information about developmental competence and embryo viability allowing the achievement of pregnancy rates close to 80–90%. Ideally, further prospective RCTs should be performed to show the benefit of PGS and automatic TLI. These trials should involve not only good prognosis patients and egg donors, but also poor prognosis patients, who provide the biggest challenge to overcome in improving fertility treatment.
Electronic supplementary material
(DOCX 73 kb)
(DOCX 83 kb)
Acknowledgments
The authors thank all the IVF-Spain team, especially the IVF Lab for their work and support. Medical writing assistance in the form of language editing and publication structure was provided by Kaedy Bryson (Zoetic Science, an Ashfield company, part of UDG Healthcare plc, Macclesfield, UK).
Authors’ contributions
E.R. is responsible for conception, design, acquisition, analysis, and interpretation of data and drafting and critical review of the article. M.E. is responsible for analysis and interpretation of data, drafting, and critical review of the article. A.L. is responsible for recruitment and acquisition of data. J.S. is responsible for analysis and interpretation of the data and critical review of the article. J.A. is responsible for the critical review of the article.
Funding
No external funding was either sought or obtained for this study.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Contributor Information
E. Rocafort, Email: eugeniarocafort@gmail.com
M. Enciso, Email: m.enciso@igls.net
A. Leza, Email: andrealezag00@gmail.com
J. Sarasa, Email: j.sarasa@iglls.net
J. Aizpurua, Email: j.aizpurua@ivf-spain.com
References
- 1.De Sutter P, Gerris J, Dhont M. A health-economic decision-analytic model comparing double with single embryo transfer in IVF/ICSI: a sensitivity analysis. Hum Reprod. 2003:1361. [DOI] [PubMed]
- 2.Bromer JG, Ata B, Seli M, Lockwood CJ, Seli E. Preterm deliveries that result from multiple pregnancies associated with assisted reproductive technologies in the USA: a cost analysis. Curr Opin Obstet Gynecol [Internet]. 2011;23:168–173. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=2011267305 [DOI] [PubMed]
- 3.Swain JE, Carrell D, Cobo A, Meseguer M, Rubio C, Smith GD. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil Steril. 2016;105:571–587. doi: 10.1016/j.fertnstert.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril [Internet]. Elsevier Inc.; 2014;102:19–26. Available from: 10.1016/j.fertnstert.2014.05.027 [DOI] [PubMed]
- 5.Vélez MP, Kadoch IJ, Phillips SJ, Bissonnette F. Rapid policy change to single-embryo transfer while maintaining pregnancy rates per initiated cycle. Reprod BioMed Online. 2013;26:506–511. doi: 10.1016/j.rbmo.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Lee AM, Connell MT, Csokmay JM, Styer AK. Elective single embryo transfer—the power of one. Contraception Reprod Med [Internet]; 2016;1:11. Available from: http://contraceptionmedicine.biomedcentral.com/articles/10.1186/s40834-016-0023-4 [DOI] [PMC free article] [PubMed]
- 7.Steinberg ML, Boulet S, Kissin D, Warner L, Jamieson DJ. Elective single embryo transfer trends and predictors of a good perinatal outcome—United States, 1999 to 2010. Fertil Steril. 2013;99:1937–1943. doi: 10.1016/j.fertnstert.2013.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaban B, Brison D, Calderón G, Catt J, Conaghan J, Cowan L, et al. Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod BioMed Online. 2011;22:632–646. doi: 10.1016/j.rbmo.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhou W, Fu L, Sha W, Chu D, Li Y. Relationship of polar bodies morphology to embryo quality and pregnancy outcome. Zygote [Internet]. 2015;1–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26198980. [DOI] [PubMed]
- 10.Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22:230–240. doi: 10.1093/humrep/del358. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJG, Klein BM, et al. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod BioMed Online. 2013;27:353–361. doi: 10.1016/j.rbmo.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–4. [DOI] [PubMed]
- 13.Obstet AG, Sigalos GA, Triantafyllidou O, Vlahos NF. Novel embryo selection techniques to increase embryo implantation in IVF attempts. Arch Gynecol Obstet. 2016;294(6):1117–1124. doi: 10.1007/s00404-016-4196-5. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs PA. Retrospective and prospective epidemiological studies of 1,500 karyotyped spontaneous human abortions. Birth Defects Res A Clin Mol Teratol. 2013:487–8. [DOI] [PubMed]
- 15.Plachot M. Chromosome analysis of spontaneous abortions after IVF. A European survey. Hum Reprod [Internet]. 1989;4:425–429. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2501337. [DOI] [PubMed]
- 16.Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod. 2002;17:446–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11821293%5Cn. http://humrep.oxfordjournals.org/content/17/2/446.full.pdf. [DOI] [PubMed]
- 17.Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27:2297–2303. doi: 10.1093/humrep/des179. [DOI] [PubMed] [Google Scholar]
- 18.Barri PN, Coroleu B, Clua E, Tur R, Boada M, Rodriguez I. Investigations into implantation failure in oocyte-donation recipients. Reprod BioMed Online. 2014;28:99–105. doi: 10.1016/j.rbmo.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril [Internet]. Elsevier Inc.; 2013;100:100–107.e1. Available from: 10.1016/j.fertnstert.2013.02.056 [DOI] [PubMed]
- 20.Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet [Internet]. 2012;5:24. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3403960&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed]
- 22.Dahdouh EM, Balayla J, García-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod BioMed Online. 2015;30:281–289. doi: 10.1016/j.rbmo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Schoolcraft WB, Surrey E, Minjarez D, Gustofson RL, Scott RT, Katz-Jaffe MG, et al. Comprehensive chromosome screening (CCS) with vitrification results in improved clinical outcome in women >35 years: a randomized control trial. Fertil Steril. Elsevier Ltd; 2012;98:S1. Available from: 10.1016/j.fertnstert.2012.07.002
- 24.Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015:473–83. [DOI] [PubMed]
- 25.Vaiarelli A, Cimadomo D, Capalbo A, Orlando G, Sapienza F, Colamaria S, Palagiano A, Bulletti C, Rienzi L, Ubaldi FM Pre-implantation genetic testing in ART: who will benefit and what is the evidence?. J Assist Reprod Genet [Internet]; 2016;33:1273–1278. Available from: 10.1007/s10815-016-0785-2 [DOI] [PMC free article] [PubMed]
- 26.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29:1173–81. [DOI] [PubMed]
- 27.Montag M, Toth B, Strowitzki T. New approaches to embryo selection. Reprod BioMed Online. 2013:539–46. [DOI] [PubMed]
- 28.Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update. 2015;21:727–747. doi: 10.1093/humupd/dmu064. [DOI] [PubMed] [Google Scholar]
- 29.Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel C-E, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet [Internet]. 2015;11:e1005241. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4454688&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed]
- 30.Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril. 2015;104:534–541.e1. doi: 10.1016/j.fertnstert.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Tejera A, Castello D, de Los Santos JM, Pellicer A, Remohi J. Meseguer M. Fertil Steril: Combination of metabolism measurement and a time-lapse system provides an embryo selection method based on oxygen uptake and chronology of cytokinesis timing; 2016. [DOI] [PubMed] [Google Scholar]
- 32.Dominguez F, Meseguer M, Aparicio-Ruiz B, Piqueras P, Quiñonero A, Simón C. New strategy for diagnosing embryo implantation potential by combining proteomics and time-lapse technologies. Fertil Steril. 2015;104:908–914. doi: 10.1016/j.fertnstert.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;32:954–62. [DOI] [PMC free article] [PubMed]
- 34.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 35.Chavez SL, Loewke KE, Han J, Moussavi F, Colls P, Munne S, et al. Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage. Nat Commun [Internet]. 2012;3:1251. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3535341&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed]
- 36.Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–419.e5. [DOI] [PubMed]
- 37.Vermilyea MD, Tan L, Anthony JT, Conaghan J, Ivani K, Gvakharia M, et al. Computer-automated time-lapse analysis results correlate with embryo implantation and clinical pregnancy: a blinded, multi-centre study. Reprod BioMed Online [Internet]. Reproductive Healthcare Ltd.; 2014;29:729–736. Available from: 10.1016/j.rbmo.2014.09.005 [DOI] [PMC free article] [PubMed]
- 38.Adamson GD, Abusief ME, Palao L, Witmer J, Palao LM, Gvakharia M. Improved implantation rates of day 3 embryo transfers with the use of an automated time-lapse-enabled test to aid in embryo selection. Fertil Steril [Internet]. Elsevier Inc.; 2016;105:369–375. Available from: 10.1016/j.fertnstert.2015.10.030 [DOI] [PubMed]
- 39.Aparicio-Ruiz B, Basile N, Pérez Albalá S, Bronet F, Remohí J, Meseguer M. Automatic time-lapse instrument is superior to single-point morphology observation for selecting viable embryos: retrospective study in oocyte donation. Fertil Steril [Internet]. 2016;106:1379–1385.e10. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0015028216626237 [DOI] [PubMed]
- 40.Kieslinger DC, De Gheselle S, Lambalk CB, De Sutter P, Kostelijk EH, Twisk JWR, et al. Embryo selection using time-lapse analysis (early embryo viability assessment) in conjunction with standard morphology: a prospective two-center pilot study. Hum Reprod. 2016;31:2450–2457. doi: 10.1093/humrep/dew207. [DOI] [PubMed] [Google Scholar]
- 41.Diamond MP, Suraj V, Behnke EJ, Yang X, Angle MJ, Lambe-Steinmiller JC, et al. Using the Eeva test™ adjunctively to traditional day 3 morphology is informative for consistent embryo assessment within a panel of embryologists with diverse experience. J Assist Reprod Genet. 2014;32:61–68. doi: 10.1007/s10815-014-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz M, Gadea B, Garrido N, Pedersen KS, Martínez M, Pérez-Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011;28:569–73. [DOI] [PMC free article] [PubMed]
- 43.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remoh J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 44.Perez S, Rubio I, Aparicio B, Beltran D, Garcia-Laez V, Meseguer M. Prospective validation of a time-lapse based algorithm for embryo selection. Fertil Steril. 2014:e322.
- 45.Chen A A., Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging: discovery, validation, and practical application. Fertil Steril [Internet]. 2013;99:1035–1043. Available from: 10.1016/j.fertnstert.2013.01.143 [DOI] [PMC free article] [PubMed]
- 46.Rubio I, Galán A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102:1287–1294. doi: 10.1016/j.fertnstert.2014.07.738. [DOI] [PubMed] [Google Scholar]
- 47.Goodman LR, Goldberg J, Falcone T, Austin C, Desai N. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril [Internet]. Elsevier Inc.; 2015; Available from: 10.1016/j.fertnstert.2015.10.013 [DOI] [PubMed]
- 48.Milewski R, Kuczyńska A, Stankiewicz B, Kuczyński W. How much information about embryo implantation potential is included in morphokinetic data? A prediction model based on artificial neural networks and principal component analysis. Adv Med Sci. 2017;62:202–206. doi: 10.1016/j.advms.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Pribenszky C, Nilselid AM, Montag M. Time-lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta-analysis. Reprod BioMed Online. 2017;35:511–520. doi: 10.1016/j.rbmo.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 50.Basile N, Nogales MDC, Bronet F, Florensa M, Riqueiros M, Rodrigo L, et al. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril [Internet]. 2014;101:699–704.e1. Available from: 10.1016/j.fertnstert.2013.12.005 [DOI] [PubMed]
- 51.Vera-Rodriguez M, Chavez SL, Rubio C, Reijo Pera RA, Simon C. Prediction model for aneuploidy in early human embryo development revealed by single-cell analysis. Nat Commun [Internet]. 2015;6:7601. Available from: http://www.nature.com/doifinder/10.1038/ncomms8601 [DOI] [PMC free article] [PubMed]
- 52.Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod BioMed Online. 2013;27:140–146. doi: 10.1016/j.rbmo.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Rienzi L, Capalbo A, Stoppa M, Romano S, Maggiulli R, Albricci L, et al. No evidence of association between blastocyst aneuploidy and morphokinetic assessment in a selected population of poor-prognosis patients: a longitudinal cohort study. Reprod BioMed Online. 2015;30:57–66. [DOI] [PubMed]
- 54.Kramer YG, Kofinas JD, Melzer K, Noyes N, McCaffrey C, Buldo-Licciardi J, et al. Assessing morphokinetic parameters via time lapse microscopy (TLM) to predict euploidy: are aneuploidy risk classification models universal? J Assist Reprod Genet. 2014;31:1231–42. [DOI] [PMC free article] [PubMed]
- 55.Reignier A, Lammers J, Barriere P, Freour T. Can time-lapse parameters predict embryo ploidy? A systematic review. Reprod BioMed Online [Internet]. Elsevier Ltd; 2018; Available from: 10.1016/j.rbmo.2018.01.001. [DOI] [PubMed]
- 56.Yang Z, Zhang J, Salem SA, Liu X, Kuang Y, Salem RD, et al. Selection of competent blastocysts for transfer by combining time-lapse monitoring and array CGH testing for patients undergoing preimplantation genetic screening: a prospective study with sibling oocytes. BMC Med Genet [Internet]. 2014;7:38. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4077552&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed]
- 57.Hurtado de Mendoza MV, Ten J. Evaluación morfológica de cada estadio de D+0 a D+3. Cuad Embriol clínica criterios ASEBIR valoración morfológica oocitos, embriones tempranos y blastocistos humanos. 2015;9–20.
- 58.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol [Internet]. 1999;11:307–311. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10369209. [DOI] [PubMed]
- 59.Gardner DK, Sakkas D. Assessment of embryo viability: the ability to select a single embryo for transfer—a review. Placenta. 2003;24:S5–S12. doi: 10.1016/S0143-4004(03)00136-X. [DOI] [PubMed] [Google Scholar]
- 60.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Maheshwari A, Papathanasiou A, Osmani B, Teoh P. Morphological assessment of embryo quality during assisted reproduction: a systematic review. Fertil Sci Res [Internet]. 2014;1:67. Available from: http://www.fertilityscienceresearch.org/text.asp?2014/1/2/67/162776
- 62.Basile N, Morbeck D, García-Velasco J, Bronet F, Meseguer M. Type of culture media does not affect embryo kinetics: a time-lapse analysis of sibling oocytes. Hum Reprod. 2013;28:634–641. doi: 10.1093/humrep/des462. [DOI] [PubMed] [Google Scholar]
- 63.Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril. 2013;99:738–744.e4. doi: 10.1016/j.fertnstert.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 64.Muñoz M, Cruz M, Humaidan P, Garrido N, Pérez-Cano I, Meseguer M. The type of GnRH analogue used during controlled ovarian stimulation influences early embryo developmental kinetics: a time-lapse study. Eur J Obstet Gynecol Reprod Biol. 2013;168:167–172. doi: 10.1016/j.ejogrb.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 65.Cruz M, Garrido N, Gadea B, Muñoz M, Pérez-Cano I, Meseguer M. Oocyte insemination techniques are related to alterations of embryo developmental timing in an oocyte donation model. Reprod BioMed Online. 2013;27:367–375. doi: 10.1016/j.rbmo.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 66.Bodri D, Sugimoto T, Serna JY, Kondo M, Kato R, Kawachiya S, et al. Influence of different oocyte insemination techniques on early and late morphokinetic parameters: retrospective analysis of 500 time-lapse monitored blastocysts. Fertil Steril. 2015;104:1175–1181.e2. doi: 10.1016/j.fertnstert.2015.07.1164. [DOI] [PubMed] [Google Scholar]
- 67.Conaghan J, Tan L, Gvakharia M, Ivani K, Shen S, Pera R, et al. Dynamic assessment of early embryo fragmentation by time-lapse analysis may improve cell cycle timing-based embryo selection. Nat Biotech Nat Comm. 2010;28:1115–211251. doi: 10.1038/nbt.1686. [DOI] [Google Scholar]
- 68.Lee YSL, Thouas GA, Gardner DK. Developmental kinetics of cleavage stage mouse embryos are related to their subsequent carbohydrate and amino acid utilization at the blastocyst stage. Hum Reprod. 2015;30:543–552. doi: 10.1093/humrep/deu334. [DOI] [PubMed] [Google Scholar]
- 69.Bontekoe S, Mantikou E, van Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane database Syst rev [Internet]. 2012;7:CD008950. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22786519. [DOI] [PMC free article] [PubMed]
- 70.Herbemont C, Sarandi S, Boujenah J, Cedrin-Durnerin I, Sermondade N, Vivot A, et al. Should we consider day-2 and day-3 embryo morphology before day-5 transfer when blastocysts reach a similar good quality? Reprod BioMed Online. 2017;35:521–8. [DOI] [PubMed]
- 71.Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril [Internet]. Elsevier Inc.; 2017;107:664–70. Available from: 10.1016/j.fertnstert.2016.11.012 [DOI] [PubMed]
- 72.Taylor TH, Patrick JL, Gitlin SA, Wilson JM, Crain JL, Griffin DK. Comparison of aneuploidy, pregnancy and live birth rates between day 5 and day 6 blastocysts. Reprod BioMed Online [Internet]. Reproductive Healthcare Ltd.; 2014;29:305–310. Available from: 10.1016/j.rbmo.2014.06.001 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 73 kb)
(DOCX 83 kb)