Abstract
A complete factorial design (23) was used to determine the influence of chia mucilage concentration (CM), locust bean gum (LBG) and base maturation time (BMT) on 14 formulations of goat milk frozen dessert. Chia mucilage was obtained by chia grain hydration (1:40) under stirring for 2 h, at 80 °C and pH of 9.0. The samples were centrifuged, as well as lyophilised to compare yields. The extraction yield of lyophilised CM was lower than 10%. The addition of LBG and CM at higher levels, influenced by BMT, increased the moisture content and the apparent viscosity of the base mixture. These formulations presented higher values for texture and lower of overrun due to the difficulty of incorporating and stabilising bubbles during whipping and freezing processes. The melting rate was also dependent on the variables analysed, and a delay in melting was observed, even in the formulations with lower fat content. CM increased the luminosity parameter proportionally to its content and provided a significant reduction of fat (up to 3.10 g 100 g−1) and energy value. The application of CM reduced the texture value, which was an interesting technological characteristic for frozen dessert.
Keywords: Ice cream, Hydrocolloids, Apparent viscosity, Melting rate, Overrun
Introduction
Ice cream and related products are classified as frozen desserts, which include ice cream, frozen custard, frozen confections, ice milk, sherbets, water ices, and mellowing products (Deosarkar et al. 2016).
Ice cream is a kind of frozen dessert characterised as a complex system, consisting of an emulsion of fat globules particles, air bubbles and ice crystals dispersed in an unfrozen phase (Goff 1997). According to Silva Junior and Lannes (2011), components such as fats influence mechanical properties, melting resistance, and palatability of ice creams.
However, Javidi et al. (2016) emphasised that consumers have been concerned about health, searching for low-energy foods and challenging the industry to formulate new alternatives to substitute fat components. Goff (2008) reported progress in the field of ice cream science, especially by using new ingredients; this can be observed in the studies of, Mahdian and Karazhian (2013), Abdel-Haleem and Awad (2015), Azari-Anpar et al. (2017a) and Zhang et al. (2018) who proposed fat substitutes in ice cream.
According to Mun et al. (2009), the greatest challenge in fat replacement is the maintenance of organoleptic characteristics and rheological properties of products, and it is often necessary to combine ingredients with different functions. For Clarke (2004), the combination of two or three hydrocolloids in the ice cream base may have synergistic effects that are able to interact with water and promote thickening, gelling and emulsifying properties, which are similar to fat particles in aqueous systems (Lim et al. 2010).
Hydrocolloids from grains have been reported in studies about frozen dessert. Campos et al. (2016), Javidi et al. (2016) and El-Aziz et al. (2015) used chia mucilage, basil gum and flaxseed gum, respectively. Chia grains (Salvia hispanica L.) have been authorized for use as ingredient in foodstuff through Commission Decision 2009/827/EC (European Communities, 2009). This grains have 5–6% of mucilage, which is rich in soluble and dietary fibre, and it is composed of high molecular weight polysaccharides (0.8–2.0 Da), essentially xylose, glucose and glucuronic acid, which are responsible for the water retention and volume increase of the grain in aqueous medium (Gômes and Colín 2008; Lin et al.1994).
According to Kuntz (2009), the locust bean gum (LBG) extracted from carob seeds (Ceratonia siliqua L.) is a source of galactomannan and is used as a thickener to reduce the growth rate of ice crystals and improve the melting characteristics in ice cream. LBG can be associated with other hydrocolloids as demonstrated by Guven et al. (2003) and Sharma and Hissaria (2009), who affirmed the synergism of LBG with carrageenan gum and guar gum.
Considering the studies to characterise technological applications of natural gums, the aim of this work was to develop formulations of goat milk frozen dessert using different concentrations of chia mucilage (CM) and locust bean gum (LBG) in different base maturation time (BMT) of mix, in order to verify the effects on apparent viscosity (mPa s−1), compression force (g), overrun (%), melting rate (g min−1) and energy value (kcal 100 g−1). Characterisation of goat milk (centesimal composition, acidity, defatted dry extract and density), chia mucilage (centesimal composition and extraction yield) and frozen dessert formulations (centesimal composition, total solids and colour and rheological parameters) was also performed.
Materials and methods
Material
The in natura goat milk (3.87 g 100 g−1 of fat) from Saanen breed was obtained from a rural producer (Itaipulandia, Parana, Brazil), according to the norms of good agricultural practices (GAPs). All formulations were standardized with the same concentration of goat’s milk fat. Chia grains were purchased from Dubai Alimentos LTDA (Ijui, Rio Grande do Sul, Brazil); locust bean gum (E410) was kindly provided by a company (Medianeira, Parana, Brazil); and the other ingredients were purchased in the local market.
Mucilage extraction from chia grains
The methodology proposed by Muñoz et al. (2012) was followed with adaptations. The seeds in a proportion of 1:40 (chia:water) were added with distilled water at 80 °C and pH 8.0, under constant agitation for 2 h. The mixture was centrifuged (model 4K 15, mark Sigma, Luton, England) at 2.600 rpm and 20 °C for 20 min. The supernatant (mucilage) was transferred to packages made of high density polyethylene (previously sanitised with sodium hypochlorite 200 mg L−1) and stored at 4 ± 1 °C until use. Since the chia mucilage was added to the frozen dessert to replace the oil base, the amount of hydrated mucilage was the same that is recommended for vegetable shortening (30 g). However, in order to compare yields, a sample of wet mucilage (in triplicate) was dehydrated in lyophiliser Free Zone 6 (model 7753522, mark Registered Labconco, Kansas City, MO, USA), with the following operating conditions: temperature of + 40 °C on heater and − 40 °C in gas, in a 36 h period.
Development of standard formulation of frozen dessert with goat milk
The goat milk (1 L) was heated to 40 °C and the following ingredients were added, respectively: powder milk (120 g), sucrose (100 g), industrial stabilisers (2.5 g) and glucose syrup (40 g), and they were mixed until the complete dissolution. The vegetable shortening (30 g) was added to goat milk at 60 °C, the mixture was pasteurised (80 °C/25 s) and cooled (5 ± 1 °C). Cocoa powder (40 g) and commercial emulsifier (2.5 g) was added and homogenised for 10 min, conducted to maturation (6 ± 1 °C/24 h) and submitted to stirring and freezing in an frozen dessert maker (model Bak 16, mark Skymsen, Paraná, Brazil) at − 12 °C for 15 min. The frozen dessert was transferred into a high-density polyethylene container (previously cleaned) and stored at − 18 ± 1 °C until analysis.
Complete factorial design (CFD, 23)
In order to develop the statistical design, some preliminary tests were performed based on the standard formulation of frozen dessert. The vegetable shortening is a low cost ingredient and it is commonly used in frozen desserts to increase creaminess and fat contents. In the current study, chia mucilage was used to replace shortening. Goat’s milk has its fat fraction standardized (3.80 g 100 g−1 ± 0.10) for all the formulations; therefore, it was not accounted for the different levels of fat replacement. The LBG was investigated as a stabilizer and emulsifier in frozen dessert processing. The base maturation time (BMT) was established in 24 h for these initial conditions. From the data obtained in the pre-tests step, it was possible to develop a CFD (23), totalling 11 tests (T) with three central points.
The substitution levels applied to variables x1 (LBG) e x2 (CM) were 50, 75 and 100% on the mass of stabilizer/emulsifier and vegetable shortening, respectively; and the base maturation time (BMT) represented the variable x3, which was studied at 12, 18 and 24 h. All the experiments performed in this study is shown in Table 1: the matrix of experimental design, the standard formulation and two external controls. The effects of apparent viscosity (mPa s−1), hardness (g), overrun (percentage), melting rate (g min−1) and energy value (kcal 100 g−1) were studied to characterize all formulations of goat milk frozen dessert.
Table 1.
Matrix of treatments of goat milk frozen dessert in CFD (23), with real and codified levels (between brackets) of variables under analysis
| Treatmentsa | x1 | x2 | x3 | Description of treatments |
|---|---|---|---|---|
| T1 | − 1 (2.5) | − 1 (15) | − 1 (12) | x1 (2.5 g L−1), x2 (15 g L−1) and x3 (12 h) |
| T2 | − 1 (2.5) | − 1 (15) | 1 (24) | x1 (2.5 g L−1), x2 (15 g L−1) and x3 (24 h) |
| T3 | 1 (5.0) | − 1 (15) | − 1 (12) | x1 (5.0 g L−1), x2 (15 g L−1) and x3 (12 h) |
| T4 | 1 (5.0) | − 1 (15) | 1 (24) | x1 (5.0 g L−1), x2 (15 g L−1) and x3 (24 h) |
| T5 | − 1 (2.5) | 1 (30) | − 1 (12) | x1 (2.5 g L−1), x2 (30 g L−1) and x3 (12 h) |
| T6 | − 1 (2.5) | 1 (30) | 1 (24) | x1 (2.5 g L−1), x2 (30 g L−1) and x3 (24 h) |
| T7 | 1 (5.0) | 1 (30) | − 1 (12) | x1 (5.0 g L−1), x2 (30 g L−1) and x3 (12 h) |
| T8 | 1 (5.0) | 1 (30) | 1 (24) | x1 (5.0 g L−1), x2 (30 g L−1) and x3 (24 h) |
| T9 | 0 (3.75) | 0 (22.5) | 0 (18) | x1 (3.75 g L−1), x2 (22.5 g L−1) and x3 (18 h) |
| T10 | 0 (3.75) | 0 (22.5) | 0 (18) | x1 (3.75 g L−1), x2 (22.5 g L−1) and x3 (18 h) |
| T11 | 0 (3.75) | 0 (22.5) | 0 (18) | x1 (3.75 g L−1), x2 (22.5 g L−1) and x3 (18 h) |
| ST (standard) | 0 | 0 | 24 | x1 (0 g L−1), x2 (0 g L−1) and x3 (24 h) |
| TC1 (control 1) | 5 | 0 | 24 | x1 (5.0 g L−1), x2 (0 g L−1) and x3 (24 h) |
| TC2 (control 2) | 0 | 30 | 24 | x1 (0 g L−1), x2 (30 g L−1) and x3 (24 h) |
ax1 locust bean gum—LBG (g L−1). x2: chia mucilage with moisture—CM (g L−1). x3: base maturation time—BMT (h)
Analytical methods
Yield of extraction of chia mucilage
The yield percentage was calculated for the wet mucilage and after drying, according to Eqs. 1 and 2.
| 1 |
| 2 |
Centesimal composition and total solids
The centesimal composition of CM and frozen dessert the formulations was determined following the method of AOAC (2005) for moisture content, ash, crude protein (conversion factor of total nitrogen: 6.25 for CM and 6.38 for frozen dessert) and total lipids. The analysis of total carbohydrates (TC) and total solids (TS) was carried out according to the Eqs. 3 and 4, respectively.
| 3 |
| 4 |
Overrun
The incorporation of air in the frozen dessert was calculated using the method described by Marshall et al. (2003). A known volume of base mix and the same volume of frozen dessert were weighed (m m−1) and the overrun was calculated by Eq. 5.
| 5 |
Melting rate
The calculation of the melting rate of the frozen dessert was performed in accordance with Soukoulis et al. (2008), with adaptations. The samples were stored at − 18 ± 1 °C until the time of analysis, and the test was performed at a temperature of 25 ± 1 °C. Each formulation of frozen dessert (100 g) was deposited on a screen (mesh 0.5 × 0.5 cm), and the melted material was weighed on an analytical balance (model BCW15, mark Welmy, Brazil) every 10 min for up to 90 min, and the quantity of drained frozen dessert was calculated as a function of time.
Rheological parameters
The rheological parameters were evaluated using a Brookfield rotational rheometer with concentric cylinder (model DV-III Ultra, mark Brookfield Engineering Laboratories, Stoughton, MA, USA) and spindle ULA-SCa34. The data were collected using the software 32 Rheocal, version 2.5 (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA). A thermostatic bath (model TE-184, mark Tecnal, Brazil) was used to keep the temperature at 5 ± 0.1 °C, and the samples of frozen dessert were analysed when the temperature reached 5.3 °C. The flow behaviour was assessed using the linear increase of the deformation rate from 5 to 30 s (ascending curve) and from 30 to 5 s (descending curve) with a total time of 5 min, with taking of 34 points for each curve. The rheological model Power Law was used to describe the flow behaviour; the index of consistency of frozen dessert samples (Eq. 6) and the apparent viscosity of the mix (mPa s) were calculated with the deformation rate of 5 s (ascending curve).
| 6 |
where σ = shear stress (Pa); K = index of consistency [Pa (sn)−1]; γ = deformation rate (s); n = rate of flow behaviour (dimensionless).
Instrumental texture
For the instrumental texture, the compression force (g) was measured, where the samples were weighed (80 g) and packed to a height of 30 mm in plastic pots with a diameter of 55 mm, at a temperature of − 18 °C. The results were obtained with the texturometer (model Stable Micro Systems, mark TA. HD plus, Surrey, England). The equipment conditions for the test were: pre-test: 20 mm s−1; test: 20 mm s−1; post-test: 10 mm s−1; distance: 15 mm: strength 5 kg. Probe. model. Delbrin, cylindrical, 0.5 inch.
Colour parameters
The colour was determined in a colourimeter equipment (model Chroma Metter CR-400 s, mark Konica Minolta, Japan) and the coordinates of the CIE/LAB were: L (brightness), a [shades of red (a +) to green (a −)] and b [shade of yellow (b +) the blue (b −)]. The numerical values of a and b were used to calculate the hue angle (h°), which indicates the colour tone, and chroma (C), that indicates the colour saturation of the sample, as shown in Eqs. 7 and 8, respectively.
| 7 |
| 8 |
The coordinates of L, a and b can be used to calculate the ΔE, which indicates the difference in the “perception” of color, including brightness, tone and saturation of the tests prepared with the mixture of CM and LBG and the standard test (PT). The value of ΔE was calculated by Eq. 9.
| 9 |
where ΔL = L (standard test) − L (test with CM + LBG); Δa = a (standard test) − a (test with CM + LBG); Δb = b (standard test) − b (test with CM + LBG). According to Cecchini et al. (2011), the greatest value of ΔE is the biggest difference between two colours, according to the description: unnoticeable (ΔE < 1); minimal (1 ≤ ΔE < 2); only noticeable (2 ≤ ΔE < 3); noticeable (3 ≤ ΔE < 5); strong difference (5 ≤ ΔE < 12); different color (ΔE ≥ 12).
Energy value
For the calculation of the energy value, a frozen dessert mass of approximately 0.5 g on dry basis was conducted to the calorimetry bomb (model C-200, mark Ika Works). The values were expressed in kcal 100 g−1.
Statistical analysis of the data
The results of the formulations of frozen dessert underwent an analysis of variance; when significant differences at the level of 5% probability were detected, Tukey’s Studentized test was applied, using Statistic version 7.0 (Statistic 2004). The same software was used to analyse the effect of the CFD (23) on the results of apparent viscosity of mix, compression force, overrun, melting rate and energy value (Fig. 1). All analyses were performed in triplicate and the final result expressed as the average of these repetitions.
Fig. 1.
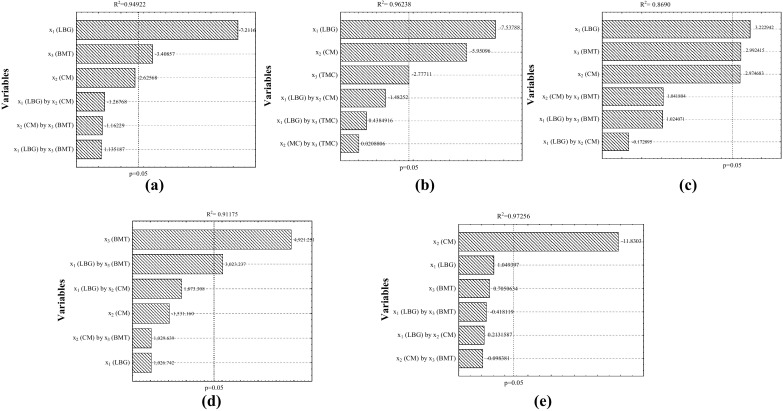
Standardized effects of the studied variables in CFD (23) on the responses: a overrun (%); b melting rate (g min−1); c apparent viscosity (mPa s−1); d hardness (g); e energy value (kcal 100 g−1) in frozen dessert
Results and discussion
Characteristics of the chia mucilage
This study was based on what was proposed by Muñoz et al. (2012) for the extraction of mucilage from chia seeds, obtaining a high moisture content in the wet mucilage (99.66 g 100 g−1 ± 0.03) and a yield of 18.25% ± 1.65 (wet mass).
Preliminary tests were performed using three different times: 2.5, 3 and 3.5 h, for chia’s hydration. All conditions did not differ (p > 0.05) and total exudative mass was the same as the obtained by Muñoz et al. (2012). Thus, the minimum time of 2 h was applied for chia hydration, followed by the extraction of mucilage.
Most available studies in the literature mention the yield of the CM after drying. The present study found 8.49% (± 0.27) of mucilage after freeze-dying, and this result was higher than previously related by Capitani et al. (2015), who found 3.70% (lyophilisation followed by friction for separation of gum from the seeds) and 3.80% of yield (vacuum filtration for separation of seeds followed by lyophilisation of the gum). There are several conditions that may affect the amount of mucilage extracted, such as the proportion chia:water, temperature of extraction, the contact time of the grains and the water with or without movement, the stirring time and, especially, the technique used to separate the mucilage. These studies emphasized the use of different methodologies, resulting in different percentages of yield (Campos et al. 2016: 4.95%; Muñoz et al. 2012: 6.97%; Segura-Campos et al. 2014: 10.90%). These indications lead us to consider new research proposals, because although there are conditions studies for the extraction of this gum, it is necessary to check the most appropriate methods to separate mucilage from the grains, since the rates of yield may increase in this step.
In wet mucilage of chia, the total carbohydrate content (0.19 g 100 g−1 ± 0.03) represented the principal fraction. The other constituents were crude protein (0.07 g 100 g−1 ± 0.01), total lipids (0.05 g 100 g−1 ± 0.01) and ash (0.03 g 100 g−1 ± 0.01). Reyes-Caudillo et al. (2008) reported the presence of polysaccharides (xylose and d-glucuronic acid) and glucose, which are propitious to form mucilaginous phase. This property is considered very important for new ingredients to replace fat in foodstuff formulation, i.e. frozen dessert.
Centesimal composition of the frozen dessert
The results of centesimal composition (Table 2) showed that for ash and crude protein, there was no significant difference (p < 0.05) among the tests. Aboulfazli, Baba and Misran (2014) also observed that the increase in water retention could promote the reduction of ice crystals, contributing to the melting of ice creams. In this way, an increase in moisture content can be observed (Table 2) in the tests with higher concentrations of LBG than the central point, the treatments were T3, T4, T7, T8, T9, T10 and T11, and they did not differ significantly (p < 0.05). In contrast, when reducing the LBG to a lower concentration (2.5 g 100 g−1), it should be noted that the moisture also decreased in T1, T2, T5 and T6.
Table 2.
Chemical and physical parameters of goat milk frozen dessert samples
| Treatments* | Moisture (g 100 g−1) | Ash (g 100 g−1) | Protein (g 100 g−1) | Fat (g 100 g−1) | Sugar total (g 100 g−1) | Total solids (g 100 g−1) | Overrun (%) | Melting rate (g min−1) |
|---|---|---|---|---|---|---|---|---|
| T1 | 66.55 ± 0.13de | 1.44 ± 0.02a | 5.12 ± 0.20a | 6.00 ± 0.20b | 20.89 ± 0.51abc | 33.45 ± 0.13ab | 35.07 ± 0.67b | 0.906 ± 0.01a |
| T2 | 67.01 ± 0.73bcde | 1.45 ± 0.03a | 5.07 ± 0.37a | 6.07 ± 0.21b | 20.40 ± 0.92abcd | 32.99 ± 0.73abcd | 29.71 ± 0.67c | 0.861 ± 0.02ab |
| T3 | 69.14 ± 0.52abc | 1.45 ± 0.01a | 5.31 ± 0.22a | 6.13 ± 0.32b | 17.97 ± 0.69de | 30.86 ± 0.52cde | 23.57 ± 0.16de | 0.822 ± 0.01c |
| T4 | 69.47 ± 1.11ab | 1.44 ± 0.04a | 5.30 ± 0.07a | 6.10 ± 0.26b | 17.69 ± 0.85e | 30.53 ± 1.11de | 21.47 ± 1.32ef | 0.800 ± 0.01c |
| T5 | 66.88 ± 0.92cde | 1.45 ± 0.02a | 5.03 ± 0.07a | 4.03 ± 0.15f | 22.61 ± 0.75a | 33.12 ± 0.92abc | 35.00 ± 1.60b | 0.846 ± 0.01bc |
| T6 | 67.23 ± 0.26bcde | 1.44 ± 0.00a | 5.06 ± 0.09a | 4.07 ± 0.35ef | 22.20 ± 0.42ab | 32.77 ± 0.26abcd | 25.27 ± 0.32d | 0.814 ± 0.01 cd |
| T7 | 69.50 ± 0.05ab | 1.45 ± 0.01a | 5.30 ± 0.06a | 4.10 ± 0.44def | 19.64 ± 0.47bcde | 30.50 ± 0.05de | 18.78 ± 0.29 h | 0.739 ± 0.00ef |
| T8 | 70.22 ± 0.53a | 1.44 ± 0.02a | 5.34 ± 0.15a | 4.17 ± 0.29cdef | 18.83 ± 0.57cde | 29.78 ± 0.53e | 13.33 ± 0.49 h | 0.705 ± 0.01f |
| T9 | 68.87 ± 0.52abcd | 1.45 ± 0.02a | 5.17 ± 0.12a | 4.83 ± 0.29cde | 19.69 ± 0.29bcde | 31.13 ± 0.52bcde | 22.35 ± 0.81ef | 0.836 ± 0.01bc |
| T10 | 68.82 ± 0.44abcd | 1.46 ± 0.01a | 5.16 ± 0.07a | 4.90 ± 0.20c | 19.66 ± 0.66bcde | 31.18 ± 0.44bcde | 21.43 ± 0.67ef | 0.833 ± 0.02bc |
| T11 | 68.76 ± 0.37abcd | 1.46 ± 0.01a | 5.16 ± 0.07a | 4.87 ± 0.15 cd | 19.75 ± 0.31bcde | 31.24 ± 0.37bcde | 22.73 ± 0.51ef | 0.832 ± 0.02bc |
| ST | 64.83 ± 2.19e | 1.44 ± 0.03a | 5.08 ± 0.02a | 7.10 ± 0.20a | 21.55 ± 2.13ab | 35.17 ± 2.19a | 42.90 ± 0.23a | 0.889 ± 0.02a |
| TC1 | 68.13 ± 0.72abcd | 1.45 ± 0.00a | 5.32 ± 0.11a | 7.07 ± 0.31a | 18.03 ± 0.84de | 31.87 ± 0.72bcde | 20.51 ± 0.84 fg | 0761 ± 0.01de |
| TC2 | 67.12 ± 0.90bcde | 1.44 ± 0.05a | 5.12 ± 0.20a | 4.00 ± 0.10f | 22.43 ± 0.96a | 32.88 ± 0.90abcd | 42.10 ± 0.73a | 0.844 ± 0.01bc |
*Means followed by the same letter in the column do not differ by Tukey’s test (p < 0.05)
The addition of CM reduced the content of lipids, with an emphasis on a reduction of up to 3.10 g 100 g−1 of this component in TC2 (addition of 100% of CM) when compared to ST (7.10 g 100 g−1 of fat) and TC1 (7.07 g 100 g−1 of fat), which differed significantly (p < 0.05) from the other tests. Therefore, reductions in lipid contents could be observed according as the vegetable shortening had been completely or partially replaced by CM. Since the goat’s milk in natura had 3.87 g 100 g−1 of lipids, and TC2 had the lowest average for this attribute (4.00 g 100 g−1 ± 0.10), this component is possibly derived from the raw material used.
The contents of carbohydrates and total solids had large variations among the tests and showed a positive linear correlation (r = 0.73), since the different proportions of CM and LBG also affect moisture content (r = − 0.24), and consequently, the amount of total carbohydrates.
Overrun
The incorporation and stabilisation of air are very important to the texture of the frozen dessert and, according to Marshall et al. (2003), the ingredients that make up the mix are fundamental to increase overrun, such as the proteins, fats and hydrocolloids. However, among the studied variables in the CFD (23) the LBG and BMT have led to a significant negative effect (p < 0.05) on the content of incorporated air (Fig. 1a). The increase in the concentration of LBG promoted the reduction of 11.82% in the air incorporation. The same was observed for the BMT, which also had a negative influence (5.66%), preventing an increase in the volume of air. Similar results were described by Abdel-Haleem and Awad (2015), who found an overrun reduction with the increase of barley flour concentration from 1 to 4%.
The apparent viscosity provided behavioural changes in the overrun because by increasing the concentration of variables x1 and x3, a thickening of the mix could be observed, causing difficulties to the air incorporation during stirring and freezing; this prevented the formation and stabilisation of the air cells. Similar results were described by El-Aziz et al. (2015) and Javidi et al. (2016); when using a combination of different hydrocolloids, they reported a reduction in the percentage of overrun due to increased apparent viscosity of mix, since the concentrations of these gums were increased.
In tests with lower values of LBG and BMT (Table 2), even in association with CM (T1 and T5), the volume increase of the frozen dessert was outstanding. Another relevant fact is related to TC2 and ST, which had the highest averages for overrun, differing from the other tests. A decrease of 56.33% of fat could be observed from TC2 (100% of CM) to ST (0% of CM); although fat is considered an important ingredient for air incorporation in frozen dessert, the replacement of vegetable shortening by chia mucilage presented similar overrun values in this kind of foodstuff.
Melting rate
The melting property of frozen dessert is critical parameter for the product. The increase of the BMT and the highest concentrations of CM and LBG reduced the melting rate in the frozen dessert formulations in 0.03, 0.07 and 0.09 g min−1, respectively (Fig. 1b). These parameters were desirable, because, according to Sworn (2004), the individual molecules of the hydrocolloids can move freely, however, in a concentrated system, they begin to have more contact with each other, limiting the movement of water and increasing ice crystals.
In all tests, the initial fusion was observed starting from 25 min, with a greater flow of melting at the end of analysis (80–90 min). According to Early (2000), the maturation of base mix provides beneficial changes to the mixture, such as complete hydration of proteins and stabilisers, contributing to an increase in the viscosity and resistance to melting.
It is believed that x1, x2 and x3, at the highest levels, influenced the melting of the frozen dessert because the thickening of the mix provided by the addition of hydrocolloids was proportional to the reduction of the fusion rate. This fact may be related to the need for greater heat transfer to melt the frozen dessert in high viscosity systems. As mentioned above, gums influenced the increase in moisture content in the formulation of the frozen dessert, which probably reduced the water available for ice crystal formation during stirring and freezing, preventing its collapse and accelerated melting in frozen dessert. These results meet the study by Kuntz (2009), which mentions the high capacity of LBG in connecting with water, making this gum useful in the preparation of frozen desserts in order to reduce the size of ice crystal, once the moisture is retained within the product.
Javidi et al. (2016) also reported that the addition of basil gum considerably reduced the fusion rate of ice cream samples with lower fat content (2.5% of fat) at all the concentration levels (0.35–0.55%), in comparison to the control formulation (10% fat). Azari-Anpar et al. (2017b) also observed that ice cream formulations containing gums of Lepidium perfoliatum and Lepidium sativum showed improvement in their melt rate, drip time, viscosity, hardness and tackiness.
The present study also showed that the substitution of vegetable shortening by CM was not crucial to raise the melting rate, since the addition of combined 50% of CM and LGB reduced the fusion rate, statistically differing (p < 0.05) from the standard test (7.10 g 100 g−1 of lipids), as specified in Table 2. This information shows that the use of CM as a substitute for the oil base can play a similar role in the structure of the frozen dessert, reducing its rate of fusion.
Rheological parameters
The Power Law model used to calculate the index of consistency (K) and the rate of flow behaviour (n) was appropriately adjusted to the data of the curve of shear stress versus the rate of deformation for all tests of frozen dessert analysed (R2 > 0.95) (Table 3).
Table 3.
Rheological parameters of the goat milk frozen dessert samples obtained using the Power Law model
| Treatments* | Linear equation adjustment | Apparent viscosity (mPa s) | K (mPa sn) | n | R 2 |
|---|---|---|---|---|---|
| T1 | σ(γ) = 8.051 + 1.041γ | 185 ± 0.60j | 0.43 | 0.64 | 0.9900 |
| T2 | σ(γ) = 12.34 + 1.368γ | 354 ± 1.15i | 0.69 | 0.59 | 0.9925 |
| T3 | σ(γ) = 25.36 + 2.584γ | 517 ± 0.96 g | 1.37 | 0.58 | 0.9900 |
| T4 | σ(γ) = 29.71 + 2.967γ | 788 ± 1.15d | 1.61 | 0.58 | 0.9829 |
| T5 | σ(γ) = 17.12 + 1.993γ | 487 ± 6.94 h | 0.94 | 0.60 | 0.9867 |
| T6 | σ(γ) = 27.32 + 2.653γ | 762 ± 3.46e | 1.54 | 0.56 | 0.9930 |
| T7 | σ(γ) = 34.70 + 2.963γ | 651 ± 0.60f | 1.82 | 0.56 | 0.9578 |
| T8 | σ(γ) = 46.37 + 4.799γ | 1.286 ± 1.92a | 2.56 | 0.58 | 0.9910 |
| T9 | σ(γ) = 40.94 + 4.148γ | 827 ± 0.29c | 2.27 | 0.57 | 0.9900 |
| T10 | σ(γ) = 43.91 + 4.427γ | 850 ± 0.96b | 2.45 | 0.57 | 0.9925 |
| T11 | σ(γ) = 43.1 + 3.865γ | 830 ± 1.67c | 2.17 | 0.59 | 0.9839 |
| ST | σ(γ) = 1.398 + 0.374γ | 56 ± 1.16 m | 0.09 | 0.77 | 0.9853 |
| TC1 | σ(γ) = 3.227 + 0.622γ | 110 ± 1.15 k | 0.19 | 0.71 | 0.9911 |
| TC2 | σ(γ) = 2.277 + 0.421γ | 70 ± 2.31 l | 0.11 | 0.75 | 0.9808 |
*Means followed by the same letter in the column do not differ by Tukey’s test (p < 0.05)
The viscosity is a very important parameter for frozen dessert, providing a desirable texture to these products. In systems with hydrocolloids addition, the viscosity of the fluid is variable, as reported by Klesment et al. (2014), who observed that increased viscosity in ice cream resulted from the association between guar gum and carrageenan.
The apparent viscosity of the mix was dependent on the concentration of LBG, CM and the BMT, promoting a positive and significant effect (p < 0.05) when these variables were analysed separately (Fig. 1c). These results demonstrated that higher concentrations of variables x1, x2 e x3 promoted an increase of apparent viscosity, as showed on Table 3. Considering this parameter, the value found for T8 (1.286 mPa s−1) was seven times higher than T1 (185 mPa s−1). The LBG was the variable that showed greater effectiveness, increasing the apparent viscosity of mix in 363.500 mPa s−1 at the maximum concentration level of hydrocolloid. The analysis of K values (Table 3) showed that the tests had greater consistency and a lower index of fluidity (n) in accordance with the addition of LBG and the increase of BMT, indicating a higher degree of resistance of the fluid.
Although the combination of the variables x1 and x2 showed no significant effect (p > 0.05), it could be observed that the addition of only one of the hydrocolloids in the tests TC1 and TC2 provided a decrease in the apparent viscosity in comparison to the other formulations that combined LBG and CM. However, these results are still higher than the ones for the standard test (without the addition of hydrocolloids). According to Pinheiro et al. (2011), the LBG presents synergic effect with other thickeners, and a special affinity with xanthan and carrageenan gums. Chia mucilage may has interacted with xylose in association with mannose and galactose chain that constitute the LBG.
The addition of gum, isolated or combined, may influence the viscosity of the mix due to a greater retention of water and hydration of proteins, and may increase the level of intermolecular attractions. These conditions have been previously noted; in the present study, it was observed a strong positive correlation (r = 0.89) between the increase of moisture content (and the concentration of LBG) and the elevation of the apparent viscosity of the mix. The ability of these gums to solubilise in an aqueous medium also seems to have an effect on the viscosity. Kuntz (2009) observed that both the CM and the LBG have greater solubility with an increasing concentration and temperature of hydration (60–90 °C), which may have occurred during the process of pasteurisation of the frozen dessert mix (80 °C/25 s).
The analysed frozen dessert was found to be a non-Newtonian fluid with pseudoplastic flow because the index of behaviour of the flow (n) was less than 1 in all tests (Table 3). The frozen dessert prepared with different concentrations of LBG and CM, in distinct BMT also behaved as thixotropic fluids because the apparent viscosity decreased with time at a constant shear rate.
According to Samavati and Skandari (2014), the pseudoplasticity is the result of an orientation effect, because the molecules are disordered when at rest, but when a tension is applied they initiate an ordering process. As the rate of shear stress is increased, the molecules of the polymers, which are randomly positioned in long chains, become increasingly aligned in the flow direction, resulting in less interaction with the chains of the adjacent polymer. Thus, the higher the tension applied, the greater the sorting, and consequently, the lower the apparent viscosity. Consequently, a greater proportion of hydrocolloids used in frozen dessert promoted a greater sorting of molecules with the increase in the applied tension, thus reducing the viscosity over time.
Instrumental texture
In the instrumental texture, BMT had a positive effect on the compression force in the frozen dessert tests, indicating that the variation from the lowest level to the top, in the range studied, resulted in an increase of 4921.251 g. The interaction time with the other variables showed a positive effect and was significant (p < 0.05) only for the LBG (Fig. 1d); however, when separately assessed, neither the LBG nor the CM were significant (p < 0.05). In addition, the BMT had an influence on LBG variable, increasing the compression force in 3023.237 g, this value was almost 3 times higher than the force used in this same variable alone (1026.742 g).
The base maturation time (BMT) can influence the composition of other ingredients of the mix, particularly the concentration of stabilisers used; according to Clarke (2004), they continue hydrating and swelling during the maturation of the mix, enhancing its characteristics; some of these components can form complexes with other ingredients in the formulation. This information is consistent with the results of the compression force in the tests (Table 4). The test T8 had the highest average for hardness (14,507.51 g) among other treatments and differed statistically (p < 0.05) from the others; the strength value necessary to compress T8 was 4.91 times higher than T7 (2953.61 g), which presented the same concentrations of LBG and CM, but a lower BMT value (12 h).
Table 4.
Instrumental analyses of goat milk frozen dessert samples
| Treatments* | Hardness (g) | Color parameters | Energy value (kcal 100 g−1) | |||||
|---|---|---|---|---|---|---|---|---|
| L | A | B | h° | C | ΔE | |||
| T1 | 7957.04 ± 711.81cde | 28.40 ± 1.38b | 2.17 ± 0.60a | 8.25 ± 0.66a | 83.78 ± 3.01a | 8.74 ± 0.78ab | 2.95 ± 1.10bc | 225.66 ± 61.25abc |
| T2 | 10,611.68 ± 1985.99bc | 28.49 ± 0.43b | 2.23 ± 0.90a | 8.42 ± 0.35a | 84.71 ± 6.48a | 8.74 ± 0.50a | 3.04 ± 0.07bc | 226.35 ± 28.61abc |
| T3 | 5409.66 ± 1071.48efg | 28.23 ± 1.00bc | 2.25 ± 0.77a | 8.35 ± 0.79a | 83.23 ± 6.05a | 8.67 ± 0.74a | 2.77 ± 0.80bc | 226.37 ± 1.22abc |
| T4 | 11,300.18 ± 522.98b | 28.36 ± 0.71b | 2.16 ± 1.07a | 8.29 ± 0.14a | 83.87 ± 7.89a | 8.61 ± 0.16a | 2.92 ± 1.01bc | 226.66 ± 20.14ab |
| T5 | 4790.11 ± 653.78 fg | 32.30 ± 2.14a | 2.33 ± 0.44a | 6.52 ± 0.68bc | 77.91 ± 6.04a | 6.95 ± 0.48 cd | 7.11 ± 2.10a | 218.32 ± 17.52 cd |
| T6 | 6303.11 ± 1085.90def | 32.28 ± 0.46a | 2.44 ± 0.59a | 6.24 ± 0.89c | 75.95 ± 6.88a | 6.73 ± 0.77d | 7.17 ± 0.45ª | 219.00 ± 24.76bcd |
| T7 | 2953.61 ± 122.76 g | 32.28 ± 1.40a | 2.36 ± 0.51a | 6.62 ± 0.20bc | 78.28 ± 4.33a | 7.03 ± 0.27 cd | 7.06 ± 1.53a | 219.40 ± 13.18bcd |
| T8 | 14,507.51 ± 1515.26a | 32.30 ± 0.41a | 2.58 ± 0.25a | 6.61 ± 0.15bc | 76.27 ± 2.40a | 7.10 ± 0.13bcd | 7.09 ± 0.43a | 219.46 ± 17.75bcd |
| T9 | 9493.10 ± 710.74bc | 29.44 ± 0.27b | 2.76 ± 0.35a | 7.44 ± 0.85abc | 77.40 ± 0.62a | 7.94 ± 0.92abcd | 4.12 ± 0.36b | 222.97 ± 35.93abcd |
| T10 | 8756.77 ± 892.29bcd | 29.32 ± 0.39b | 2.85 ± 0.35a | 7.74 ± 0.30ab | 77.46 ± 3.28a | 8.25 ± 0.17abc | 3.96 ± 0.48b | 220.90 ± 37.96abcd |
| T11 | 9485.37 ± 1003.06bc | 29.32 ± 0.49b | 2.40 ± 0.14a | 7.62 ± 0.07abc | 80.55 ± 0.92a | 7.99 ± 0.11abcd | 3.95 ± 0.72b | 222.57 ± 29.15abcd |
| ST | 5839.21 ± 109,886defg | 25.46 ± 0.49d | 2.33 ± 0.21a | 8.44 ± 0.26a | 82.81 ± 1.74a | 8.76 ± 0.24a | – | 227.93 ± 12.89a |
| TC1 | 5281.79 ± 1243.38efg | 25.55 ± 0.51 cd | 2.30 ± 0.20a | 8.47 ± 0.21a | 83.11 ± 1.29a | 8.78 ± 0.23a | 0.10 ± 0.25c | 227.73 ± 11.89a |
| TC2 | 10,187.60 ± 344.32bc | 32.26 ± 0.29a | 2.18 ± 0.11a | 6.67 ± 0.16bc | 81.19 ± 3.22a | 7.02 ± 0.12 cd | 7.03 ± 0.60a | 216.14 ± 22.32d |
*Means followed by the same letter in the column do not differ by Tukey’s test (p < 0.05)
Energy value
The energy value of CM influenced the results, showing a significant effect at the level of 95% of significance (Fig. 1e); there was a reduction of 7.21 kcal 100 g−1 from the minimum to the maximum level of concentration of hydrocolloids in the tests of frozen dessert. The reduction in lipid content due to the replacement of the vegetable shortening resulted in the reduction of the energy value in all tests. It should be noted that ST and TC1 differed significantly from T2, T5, T6, T7 and T8 (Table 4), which had 100% of vegetable shortening replaced by CM, with a maximum decrease of 11.79 kcal 100 g−1 in TC2, when compared to the standard test (227.93 kcal 100 g−1). These values also remained lower in other treatments with partial replacement of the oil base.
Colour parameters
Regarding the colour parameters evaluated (Table 4), it was observed that the frozen dessert presented toning in the region between the red (a +) and yellow (b +) with values of the hue angle situated between 77.40 and 84.71 h°. Concerning the parameter chroma (C), the values found showed oxygen saturation close to zero, indicating more neutral colours.
For brightness, it was observed that the formulations of frozen dessert were dark, since the values found were lower than 50 (L < 50). There was a trend in the increase of brightness with the increase of CM, as described in tests T5, T6, T7, T8 and TC2, which had 100% of vegetable shortening replaced by CM. The tests with partial reduction of fat (75 and 50%) showed higher values of L when compared to tests ST and TC1, with higher contents of fat. The inverse was observed for the parameter b, where the tests with the maximum level of concentration of CM (T2, T5, T6, T7 and T8) showed lower averages. This may be related to the yellowish colour of vegetable shortening and to the appearance of transparent gel formed by chia mucilage; once it presented high moisture content, this may have contributed to the dispersion of the solid particles and to an increase in the reflectance of light in tests with a higher presence of CM.
The variation of ΔE was also significant (p < 0.05); the most distinguished results detected in relation to the standard (EP) were again in tests T5, T6, T7, T8 and TC2, showing a great difference (5 ≤ ΔE < 12). In TC1, where the value of total lipids (7.07 g 100 g−1 ± 0.31) was statistically equal to the standard (7.10 g 100 g−1 ± 0.20), the variation in colour remained unnoticeable (ΔE = 0.10).
Evaluating the low luminosity, the slope of the hue angle and the chromaticity of a and b positive formations, it can be assumed that the combination of these parameters resulted in brown-coloured frozen dessert, which is characteristic of products made with cocoa.
Conclusion
CM, when lyophilised, showed a reduced yield due to the removal of water. The increase in the concentration of LBG, CM and BMT improved the moisture content, due to the greatest hydration of proteins during the maturation stage, promoting the thickening of the mix. The increase in the apparent viscosity also influenced the low overrun, hindering the incorporation and stabilisation of air bubbles and increasing the instrumental texture, especially in tests with maximum levels of LBG and BMT. The BMT and the LBG were also significant in the melting rate, reducing the fusion of frozen dessert with the increase of these variables. The CM seems to have contributed to the increase in the proportions of overrun, reducing the content of total lipids by more than 50% and, consequently, the energy value. The variables investigated in this study were important, however, it is necessary to perform sensory analysis in new studies to evaluate the responses of potential consumers of this kind of foodstuff. Considering all analyses, it was verified that sample T5 (LBG = 2.5 g L−1, CM = 30 g L−1 and BMT = 12 h) presented the best results for overrun (35.00% ± 1.60), melting rate (0.846 g min−1 ± 0.01) and apparent viscosity (487 mPa s−1 ± 6.94). Other interesting factors were lower values of crude energy, total lipids and hardness than the control test.
Acknowledgements
To Capes, CNPq, Araucária Foundation for financial support and for the scholarship offered. To the State University of Maringa and Federal Technological University of Parana for making the resources and technology available to support the development of this research.
Contributor Information
Marcia A. Chaves, Phone: +55 45 9821-3732, Email: marcia_alves_chaves@hotmail.com, http://www.uem.br
Juliane Piati, http://www.md.utfpr.edu.br.
Luana T. Malacarne, http://www.md.utfpr.edu.br
Ruana E. Gall, http://www.md.utfpr.edu.br
Eliane Colla, http://www.md.utfpr.edu.br.
Paulo R. S. Bittencourt, http://www.md.utfpr.edu.br
Aloisio H. P. de Souza, http://www.uem.br
Sandra T. M. Gomes, http://www.uem.br
Makoto Matsushita, Phone: +55 44 3011 3655, Email: mmakoto@uem.br, http://www.uem.br.
References
- Abdel-Haleem AMH, Awad RA. Some quality attributes of low fat ice cream substituted with hulless barley flour and barley β-glucan. J Food Sci Technol. 2015;52:6425–6434. doi: 10.1007/s13197-015-1755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboulfazli F, Baba AS, Misran M. Effect of vegetable milks on the physical and rheological properties of ice cream. Food Sci Technol Res. 2014;20:987–996. doi: 10.3136/fstr.20.987. [DOI] [Google Scholar]
- AOAC . AOAC official methods of analysis. 18. Washington: AOAC; 2005. [Google Scholar]
- Azari-Anpar M, Khomeiri M, Ghafouri-Oskuei H, Aghajani N. Response surface optimization of low-fat ice cream production by using resistant starch and maltodextrin as a fat replacing agent. J Food Sci Technol. 2017;54:1175–1183. doi: 10.1007/s13197-017-2492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari-Anpar M, Soltani Tehrani N, Aghajani N, Khomeiri M. Optimization of the new formulation of ice cream with native Iranian seed gums (Lepidium perfoliatum and Lepidium sativum) using response surface methodology (RSM) J Food Sci Technol. 2017;54:196–208. doi: 10.1007/s13197-016-2451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos BE, Ruivo TD, da Scapim MR, Madrona GS, de Bergamasco RC. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT Food Sci Technol. 2016;65:874–883. doi: 10.1016/j.lwt.2015.09.021. [DOI] [Google Scholar]
- Capitani MI, Corzo-Rios LJ, Chel-Guerrero LA, Betancur-Ancona DA, Nolascos SM, Tomás MC. Rheological properties of aqueous dispersions of chia (Salvia hispanica L.) mucilage. J Food Eng. 2015;149:70–77. doi: 10.1016/j.jfoodeng.2014.09.043. [DOI] [Google Scholar]
- Cecchini M, Contini M, Massantini R, Monarca D, Moscetti R. Effects of controlled atmospheres and low temperature on storability of chestnuts manually and mechanically harvested. Postharvest Biol Technol. 2011;61:131–136. doi: 10.1016/j.postharvbio.2011.03.001. [DOI] [Google Scholar]
- Clarke C. The science of ice cream. Cambridge: Royal Society of Chemistry; 2004. [Google Scholar]
- Deosarkar SS, Kalyankar SD, Pawshe RD, Khedkar CD. Ice cream: composition and health effects. Encycl Food Health. 2016;3:385–390. doi: 10.1016/B978-0-12-384947-2.00385-8. [DOI] [Google Scholar]
- Early R. Tecnologia de los produtos lácteos. Zaragoza: Acribia; 2000. [Google Scholar]
- El-Aziz MA, Haggag HF, Kaluoubi MM, Hassan LK, El-Sayed MM, Sayed AF. Physical properties of ice cream containing cress seed and flaxseed mucilages compared with commercial guar gum. Int J Dairy Sci. 2015;10:160–172. doi: 10.3923/ijds.2015.160.172. [DOI] [Google Scholar]
- European Communities (2009) Commission Decision. Regulation EC no. 258/97 of the European Parliament and of the Council. Authorising the placing on the market of Chia seed (Salvia hispanica) as novel food ingredient under. Official Journal of the European Union, 11 Jan 2009
- Goff HD. Colloidal aspects of ice cream—a review. J Dairy Sci. 1997;7:363–373. doi: 10.1016/S0958-6946(97)00040-X. [DOI] [Google Scholar]
- Goff HD. 65 years of ice cream science. Int Dairy J. 2008;18:754–758. doi: 10.1016/j.idairyj.2008.03.006. [DOI] [Google Scholar]
- Gômes JAH, Colín SM. Caracterización Morfológica de Chia (Salvia hispanica L) Rev Fitotec Mex. 2008;31:105–113. [Google Scholar]
- Guven M, Karaca OB, Kacar A. The effects of the combined use of stabilizers containing locust bean gum and of the storage time on Kahramanmaraş-type ice creams. Int J Dairy Technol. 2003;56:223–228. doi: 10.1046/j.1471-0307.2003.00108.x. [DOI] [Google Scholar]
- Javidi F, Razavi SMA, Behrouzian F, Alghooneh A. The influence of basil seed gum, guar gum and their blend on the rheological, physical and sensory properties of low fat ice cream. Food Hydrocoll. 2016;52:625–633. doi: 10.1016/j.foodhyd.2015.08.006. [DOI] [Google Scholar]
- Klesment T, Stekolstsikova J, Laos K. Influence of guar gum/furcellaran and guar gum/carrageenan stabilizer systems on the rheological and sensorial properties of ice cream during storage. Proc Estonian Acad Sci. 2014;63:193–198. doi: 10.3176/proc.2014.2.09. [DOI] [Google Scholar]
- Kuntz L. Locust bean gum: good as gold. Food Prod Des. 2009;18:1–2. [Google Scholar]
- Lim J, Inglett GE, Lee S. Response to consumer demand for reduced-fat foods: multifunctional fat replacers. Jpn J Food Eng. 2010;11:147–152. [Google Scholar]
- Lin KY, Daniel JR, Whistler RL. Structure of chia seed polysaccharide exudates. Carbohydr Polym. 1994;23:13–18. doi: 10.1016/0144-8617(94)90085-X. [DOI] [Google Scholar]
- Mahdian E, Karazhian R. Effects of fat replacers and stabilizers on rheological, physicochemical and sensory properties of reduced-fat ice cream. J Agric Sci Techol. 2013;15:1163–1174. [Google Scholar]
- Marshall R, Goff HD, Hartel RW. Ice cream. New York: Kluwer; 2003. [Google Scholar]
- Mun S, Kim YL, Kang CG, Park KH, Shim JY, Kim YR. Development of reduced-fat mayonnaise using 4aGTase-modified rice starch and xanthan gum. Int J Biol Macromol. 2009;44:400–407. doi: 10.1016/j.ijbiomac.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Muñoz LA, Cobos A, Diaz O, Aguilera JM. Chia seeds: microstructure, mucilage extraction and hydration. J Food Eng. 2012;108:216–224. doi: 10.1016/j.jfoodeng.2011.06.037. [DOI] [Google Scholar]
- Pinheiro AC, Bourbon AI, Rocha C, Ribeiro C, Maia JM, Gonçalves MP, Teixiera JA, Vicente AA. Rheological characterization of [kappa]-carrageenan/galactomannan and xanthan/galactomannan gels: comparison of galactomannans from nontraditional sources with conventional galactomannans. Carbohydr Polym. 2011;83:392–399. doi: 10.1016/j.carbpol.2010.07.058. [DOI] [Google Scholar]
- Reyes-Caudillo E, Tecante A, Valdivia-López MA. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008;107:656–673. doi: 10.1016/j.foodchem.2007.08.062. [DOI] [Google Scholar]
- Samavati V, Skandari F. Recovery, chemical and rheological characterization of gum from Assyrian pulm. Int J Biol Macromol. 2014;67:172–179. doi: 10.1016/j.ijbiomac.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Segura-Campos MR, Ciau-Solís N, Rosado-Rubio G, Chel-Guerrero L, Betancur-Ancona D. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int J Food Sci. 2014;14:1–5. doi: 10.1155/2014/241053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma BR, Hissaria PK (2009) Hydrocolloids: competent ice cream stabilizers. Chemical Weekly, pp 193–198. http://www.shplindia.com/mcp/download/1355735275Hydrocolloids-Competent%20Ice%20Cream%20Stabilizers.pdf. Accessed 26 July 2016
- Silva Junior E, Lannes SCS. Effect of different sweetener blends and fat types on ice cream properties. Food Sci Technol (Campinas) 2011;31:217–220. doi: 10.1590/S0101-20612011000100033. [DOI] [Google Scholar]
- Soukoulis C, Chandrinos I, Tzia C. Study of the functionality of selected hydrocolloids and their blends with k-carrageenan on storage quality of vanilla ice cream. LWT Food Sci Technol. 2008;41:1816–1827. doi: 10.1016/j.lwt.2007.12.009. [DOI] [Google Scholar]
- Statistic . Statistica: data analysis software system, version. Tulsa: Statsoft; 2004. [Google Scholar]
- Sworn G. Hydrocolloid thickeners and their applications. In: Philips GO, Williams PA, editors. Gums and stabilizers for the food industry. Oxford: RSC Publishing; 2004. pp. 13–22. [Google Scholar]
- Zhang H, Chen J, Li J, Wei C, Ye X, Shi J, Chen S. Pectin from citrus canning wastewater as potential fat replacer in ice cream. Molecules. 2018;23:2–11. doi: 10.3390/molecules23010002. [DOI] [PMC free article] [PubMed] [Google Scholar]


