Abstract
Changes in physico-chemical qualities (pH, total acidity, total and reducing sugar, total phenolic and vitamin C), astringency compounds (condensed and hydrolysable tannin), antioxidant activities [2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical] and flavor volatile compounds in Lactobacillus plantarum-fermented cashew-apple-juice (CAJ) and 11.4 °Bx concentrated-cashew-apple-juice (CCAJ) was investigated. Total phenolics remained unchanged throughout fermentation period, whereas condensed tannins increased and hydrolysable tannins decreased indicating reduced astringency compounds. Antioxidant activity based on both DPPH and ABTS radical scavenging activities marginally declined in some stages but overall were sustained during fermentation. Although the DPPH· radical based antioxidant activity of fermented CAJ was greater than that of fermented 11.4 °Bx CCAJ, a higher ABTS·+ radical scavenging activity was found in fermented 11.4 °Bx CCAJ, reflecting higher water soluble antioxidants. Results also indicated that DPPH· radical scavenging activity was positively correlated to vitamin-C and condensed tannins but not hydrolysable tannins. ABTS·+ radical scavenging activity was also positively correlated to condensed tannins and not hydrolysable tannins. The vitamin-C that increased during initial 12 h fermentation, decreased from 2516 to 2150 mg AAE/L at the end of 72 h fermentation. Fermented CAJ had a remarkable sweet aroma with a fruity note of two major compounds; 3-methyl-1-butanol (14.20 × 107) and 2,6-dimethyl-4-heptanol (14.76 × 107). The high phytochemicals and volatile compounds in fermented CAJ indicated that it could serve as a functional beverage with potential health benefits with reduced astringency due to lower hydrolysable tannins.
Keywords: Total phenolics, Condensed tannins, Hydrolysable tannins, Vitamin C, Sweet fruity aroma
Introduction
Cashew apple is an agricultural waste with excellent sources of bioactive compounds and nutritional components such a vitamins and it can be processed into a beverage with fine sweet flavor with specific aroma (Silveira et al. 2012). Cashew apples contain ascorbic acid four and ten times higher than orange and pineapple juice, respectively (Berry and Sargent 2011). In addition they contain phenolic compounds such as anarcardic acid, cardol and tannins as well as carotenoids which can act as potential antioxidants. However, strong radical scavenging activity proanthocyanidins (condensed tannins) are responsible for the typical astringency of some fruits (He et al. 2011). These condensed tannins in cashew apple are mainly built of (−)-epigallocatechin [and/or (+)-gallocatechin] associated with some (−)-epicatechin [and/or (+)-catechin] units (Queiroz et al. 2011). Despite the high level of tannin contents that cause astringency, cashew apples can be processed into a beverage due to its fleshy pulp, soft peel, lack of seeds, high sugar concentration and strong exotic flavor (Chapin and Lauderdale 2003; Rufino et al. 2010) from volatile constituents such as, esters (38–80% of total volatiles), alcohols (30% of total volatiles), acids (15% of total volatiles), aldehydes, ketones, and ethers (Garruti et al. 2003).
Fermentation is a traditional technology that can be used in plant foods to enhance the shelf-life, nutritional and organoleptic qualities and remove undesirable compounds (Hernandez et al. 2007). In targeting such fermentations probiotic lactic acid bacteria were used in pomegranate juice (Mousavi et al. 2013), and cashew apple juice (Pereira et al. 2011). Based on this rationale cashew apple juice is a good juice matrix for lactic acid bacterial (LAB) fermentation to produce a healthy alternative functional food and with beneficial flavor attributes (Garruti et al. 2006; Pereira et al. 2011; Salmerón et al. 2014).
Among LAB, Lactobacillus plantarum is considered as GRAS (generally recognized as safe) and has been used for centuries in food preservation and is commonly found in fermented vegetable food products (Hernandez et al. 2007; Rodríguez et al. 2009). Moreover, L. plantarum is known to degrade tannins and produces tannase after 24 h of growth on minimal media containing tannic acid (Rodríguez et al. 2009). Tannase catalyzes the hydrolysis of ester bonds in hydrolysable tannins and gallic acid ester which are used in the food industry as substrates for the synthesis of propylgallate, a potent antioxidant (Rodríguez et al. 2009). In addition, L. plantarum produces active enzymes—amylase, β-glucosidase, decarboxylase, lactate dehydrogenases, peptidase, phenolic acid decarboxylases, phenol reductase and proteinase (Hur et al. 2014). Furthermore, β-glucosidase could improve antioxidant activity (Hur et al. 2014). Based on these attributes we have targeted L. plantarum for development of non-dairy probiotic beverage products from cashew apple.
Among the important quality parameters for the consumer aroma is critical (Braga et al. 2013) as volatile constituents influence on the sensory properties of newly developed products (Garruti et al. 2003). The production of aroma compounds is highly influenced by several different factors such as fermentation conditions, production processes and microbial strains (Garruti et al. 2006). During fermentation, volatile compounds could be generated by enzymatic activity through metabolic pathways of L. plantarum (Salmerón et al. 2014). Therefore in the design of functional beverage products of fermented cashew apple using L. plantarum physico-chemical qualities and volatile compounds related to aroma can be targeted. Therefore this study focused on investigation of the physico-chemical, astringency, antioxidant activity and volatile compounds of L. plantarum-fermented fresh cashew apple juice (CAJ) and compared it with 11.4 °Bx concentrated cashew apple juice (CCAJ) to target cashew apple as a source of functional beverage.
Materials and methods
Raw material
In this study we used cashew apples and concentrated cashew apple juice from the Heritage Grower Corporation Ltd., located in Southern Thailand. Cashew apples were cleaned with filtered water and then cut into small pieces. The edible pieces were blended using a Waring blender for 5 min as fresh cashew apple juice (CAJ) with total soluble solids at 11.4 °Bx and kept at − 20 °C during the period of the study. Concentrated cashew apple juice (CCAJ) with 78.6 °Bx was adjusted to 11.4 °Bx with distilled water with the total soluble solids of CCAJ equal to CAJ total soluble solids. Fresh CAJ and 11.4 °Bx CCAJ were pasteurized for 15 min at 70 °C, prior to further study.
Probiotic lactic acid culture preparation
Lactobacillus plantarum TISTR 543 was obtained from the Thailand Institute of Scientific and Technological Research (TISTR), Pathum Thani, Thailand. Cell cultivation was carried out at 30 °C in an incubator with the cell density determined spectrophotometrically at 600 nm, and reached 1.000 which corresponds to 15 × 108 colony forming units (CFU) per milliliter (mL) (CFU/mL), as in the McFarland scale (Chapin and Lauderdale 2003).
Cashew apple fermentation
Fermentation experiments were conducted in sealed Erlenmeyer flasks, each containing 20 g of pasteurized CAJ or 11.4 °Bx CCAJ without supplementary nutrients or water. The L. plantarum TISTR 543 was cultured in MRS broth at 30 °C and equivalent of 15 × 108 CFU/mL was inoculated in pasteurized CAJ or 11.4 °Bx CCAJ. The fermentation process was performed at 30 °C for 72 h. Samples were taken at 0, 12, 24, 48 and 72 h for chemical and microbiological analyses. Pasteurized fresh cashew apple juice served as the control.
Viable cell counts
Cell viable counts of fermented CAJ or 11.4 °Bx CCAJ was obtained by serial dilution to 10−6 with 0.7% NaCl solution. Aliquots of 0.1 mL of diluted sample were plated in triplicate in MRS Agar (spread plate method). The plates containing 20–350 colonies were counted after incubation for 72 h at 37 °C and Log CFU/mL was recorded.
pH and total titratable acidity (%TTA)
The pH of fermented CAJ or 11.4 °Bx CCAJ was measured during the fermentation periods using a pH meter (Mettler Toledo, Fisher Scientific, UK) which was calibrated with buffer solutions at pH 4.0 and 7.0 (Laboratory Chemical, New Zealand). Total titratable acidity, expressed as percentage of lactic acid, was determined by titration with 0.1 M NaOH using the method of AOAC (2000).
Total sugar and reducing sugar
Reducing sugar contents were analyzed as glucose equivalents by Nelson–Somogyi method (Somogyi 1952). Total sugar contents were analyzed as glucose equivalents by the phenol sulfuric acid method of Dubois et al. (1956). Absorbance was determined using UV-Spectrophotometer (Biomate 3, Genesys 10, Thermo Electron Corporation, Germany).
Vitamin C content
Vitamin C contents were determined following the AOAC method for vitamin C (ascorbic acid) in vitamin preparations and juice (AOAC 2000). Results are expressed as milligram ascorbic acid equivalent (AAE)/L of sample.
Total phenolic contents
Total phenolics in all samples were determined by the Folin–Ciocalteu colorimetric method according to Huang et al. (2005). Sample (10 µL) was added to the test tube and mixed with 90 µL distilled water and 2 mL of 2% (w/v) Na2CO3 solution. After incubation for 3 min, 100 µL of Folin–Ciocalteu reagent was added. After standing for 30 min at room temperature the results were expressed as gram gallic acid equivalent (GAE)/L of sample using spectrophotometric method at 750 nm.
Condensed tannin contents
Total tannins or condensed tannins were measured using the modified vanillin assay described by Sun et al. (1998). Three milliliters of 4% methanol vanillin solution and 1.5 mL concentrated HCl were added to 100 μL of sample. The mixture was kept for 15 min and the amount of total condensed tannins was expressed as milligram (+)-catechin equivalent (mg CE/L) of sample using spectrophotometric method at 500 nm. The calibration curve range was 0–400 mg/mL (R2 = 0.999). Triplicate measurements were taken for all samples.
Hydrolysable tannin content
Hydrolysable tannins were determined according to the method of Saad et al. (2012). A volume of 2.5 mL of KIO3 aqueous solution (2.5% w/v) was added to the test tube and heated for 7 min at 30 °C, and then 0.5 mL of sample was added. After an additional 2 min of tempering at 30 °C, the absorbance was measured at 550 nm, using distilled water as a blank. Results were expressed as milligram tannic acid equivalent (mg TAE)/L of sample through the calibration curve using tannic acid solution (5000 mg/mL) prepared by solubilization of 0.125 g of tannic acid in 25 mL of methanol (80%). The calibration curve range was 0–4000 mg/mL (R2 = 0.999). Triplicate measurements were taken for all samples.
Determination of antioxidant capacity
DPPH radical scavenging activity assay
The DPPH radical scavenging activities of samples were measured according to the method of Bersuder et al. (1998) with a slight modification. A 10 µL aliquot of the sample was added to the test tube and mixed with 30 µL of distilled water. Then, 2 mL of 0.1 mM DPPH· dissolved in 95% ethanol was added to the sample. The mixture was shaken and left for 30 min in the dark at room temperature. Absorbance was recorded at 517 nm and percent DPPH· scavenging activity (%) was calculated using the following equation:
where Ablank is the absorbance of 40 µL distilled water mixed with 2 mL of 0.1 mM ethanolic DPPH· solution, Asample is the absorbance of 40 µL aqueous sample mixed with 2 mL of 0.1 mM ethanolic DPPH· solution, and Acontrol is the absorbance of 40 µL aqueous sample mixed with 2 mL of ethanol (all analyzed at 517 nm).
ABTS radical cation decolorization (ABTS·+) scavenging activity assay
The ABTS·+ scavenging activity of samples on ABTS method was measured according to Shan et al. (2005) where ABTS, a colorless chromogen substance, is changed into its colored monocationic radical form (ABTS·+) by an oxidizing agent. In this study, the ABTS·+ radical cation was generated by reacting 7 mM ABTS and 2.45 mM potassium persulfate in the dark for 24 h at 20 °C. Before analysis, the ABTS·+ solution was diluted with ethanol to obtain an absorbance of 0.700 ± 0.020 at 734 nm. Then, the absorbance of diluted ABTS·+ solution was recorded. An aliquot of the sample (10 µL) was added to a test tube and mixed with 70 µL distilled water. After adding 4 mL diluted ABTS·+ solution (prepared daily) to the sample, the absorbance was measured at 734 nm after exactly 6 min of initial mixing. Percentage of ABTS radical scavenging activity (%) was calculated according to the following equation:
where Ac is absorbance of 80 µL distilled water mixed with 4 mL diluted ABTS·+ solution, Am is absorbance of 80 µL sample mixed with 4 mL diluted ABTS·+ solution. Scavenging activity was expressed as percentage disappearance of ABTS·+.
Determination of volatile compounds
Analysis of volatile compounds in fermented CAJ and 11.4 °Bx CCAJ was performed in a headspace solid-phase microextraction (HS-SPME) gas chromatoghaphy mass spectrometry (GC–MS) (Model 7890A MS, Agilent Technologies, Santa Clara, CA, USA) operating with an ionization voltage of 70 eV. Each sample (3 mL) was placed in a 20 mL glass vial and heated at 40 °C for 1 min in a GC–MS heating block for headspace analysis. Volatile compounds were absorbed onto a SPME fibre (50/30 µm DVB/Carboxen™; Supelco, Bellefonte, PA) for 7 min. After equilibrium, the SPME fibre was desorbed into the injector port at 240 °C for 13 min, and the injector was operated in splitless mode. Helium was used as the carrier gas at 1.0 mL/min. Separation of volatiles was attained through a HP-5 capillary column (30 m × 0.25 mm, 0.25 µm film thickness; J&W Scientific Inc., Folsom, CA, USA). Temperature programming was set at 80 °C initially followed by an increase to 230 °C at 5 °C/min, and holding this temperature for 5 min, giving a total analysis time of approximately 35 min. Identification of volatile compounds was based on comparison of their retention time and mass spectrum with data in the Wiley 275 and NIST libraries at a percentage of quality match over 85%, and the data was compared to previously published literature. Retention index (RI) values of samples were obtained from the comparison with alkane standards (C8 − C20).
Statistical analysis
All experiments were carried out in triplicate, and the results are expressed as mean ± SD (standard deviation). The SAS Statistical Analysis System (SAS Institute, USA) was used to analyze the experimental data. Significant differences (p < 0.05) between means were separated by Duncan’s Multiple Range Test (DMRT).
Results and discussion
Physico-chemical quality changes of fermented CAJ and 11.4 °Bx CCAJ
Viable cell counts
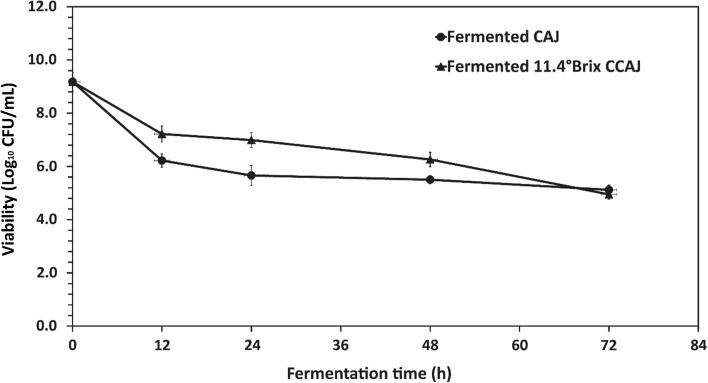
Sufficient L. plantarum could survive after some decline on pasteurized CAJ and 11.4 °Bx CCAJ under microaerophilic conditions at 37 °C for 72 h (Fig. 1). Population of L. plantarum in CAJ and 11.4 °Bx CCAJ decreased from the initial 9.18 Log10 CFU/mL to 5.12 Log10 CFU/mL and 4.95 Log10 CFU/mL, respectively. This reflected a population decline greater than 50% after 72 h fermentation but was sufficient for biotransformation activities. The decline in viable cell counts might be due to pH differences between pretreatment (MRS broth, pH 5.4) and fermentation medium, where the initial pH of fermented CAJ and 11.4 °Bx CCAJ was 4.0 and 4.5, respectively. The pH on the higher acidic side induces stress on the microorganisms and therefore potentially contributed to decreased microbial growth at the early stage of fermentation (lag phase) (Mousavi et al. 2013). However, the viable cell number was stable during fermentation time from 48 to 72 h (p ≥ 0.05) after initial the declining phase. Previously Salmerón et al. (2014) also reported that L. plantarum could survive in low pH of fermented cereal. Further the primary metabolites formed during the first growth stage of fermentation may now be biotransformed to secondary metabolites in the declining phase that may also contribute to declining growth (Salmerón et al. 2014).
Fig. 1.
Change in population of Lactobacillus plantarum in fermented cashew apple juice (CAJ) and fermented 11.4 °Bx concentrated cashew apple juice (CCAJ) at 0, 12, 24, 48 and 72 h of fermentation at 37 °C
The pH and total titratable acidity (%TTA)
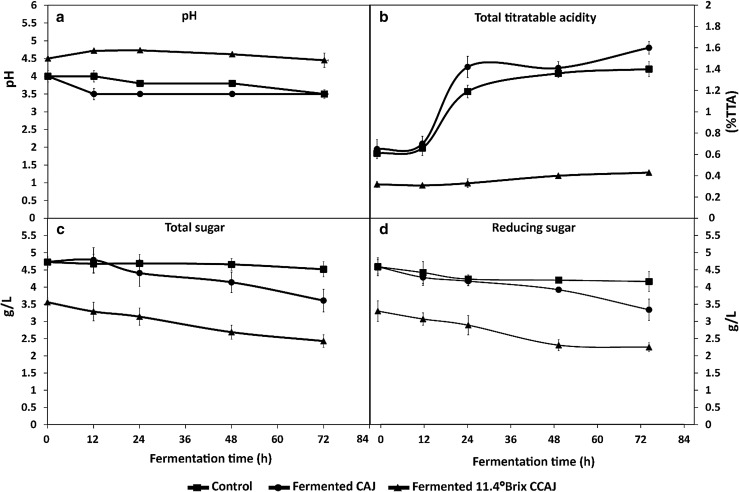
Changes in pH of CAJ and 11.4 °Bx CCAJ fermented with L. plantarum at 37 °C for 72 h are shown in Fig. 2a. During initial 24 h fermentation, pH of fermented 11.4 °Bx CCAJ increased from 4.50 to 4.73, and slightly decreased to 4.45 at the end of 72 h fermentation. Meanwhile, pH of the fermented CAJ decreased rapidly from 4.00 to 3.50 after 12 h fermentation. Thereafter, the pH remained constant for L. plantarum fermented CAJ until 72 h of fermentation (pH 3.50), similar to the pH of the control (pH 3.50) at 72 h. The pH decrease is generally associated with organic acids produced by L. plantarum, indicating pH of fermented culture had changed in response to metabolic activities (Raimbault and Tewe 2001). Results were in accordance with Nagpal et al. (2012), who reported that pH of fruit juices decreased during fermentation with L. plantarum, due to the secretion of organic acids such as citric, acetic or lactic acids.
Fig. 2.
Change in pH value (a), total titratable acidity (%TTA) (b), reducing sugar (c) and total sugar (d) of Lactobacillus plantarum in fermented cashew apple juice (CAJ) and fermented 11.4 °Bx concentrated cashew apple juice (CCAJ) at 0, 12, 24, 48 and 72 h of fermentation at 37 °C. Data is expressed as the average of three determinations ± SD
In this study, L. plantarum CAJ produced significantly more TTA than that of fermented 11.4 °Bx CCAJ. After 72 h, TTA of fermented CAJ increased from 0.65 to 1.60%, whereas TTA of fermented 11.4 °Bx CCAJ increased from 0.32 to 0.43% (Fig. 2b). It was obvious that TTA of either fermented CAJ or CCAJ was closely correlated with its pH values. This correlation of low pH and high acidity were in agreement with the findings by Ibanoglu et al. (1995) for Tarhana fermentation and Salmerón et al. (2014) for cereal fermentation. It is potentially due to lactic acid production following sucrose hydrolysis which occurs at low pH values (Costa et al. 2013).
Total sugar and reducing sugar
Sugar contents of the cashew apple have usually been assessed in terms of total sugar or reducing sugar. It is interesting to note that reducing sugar and total sugar of CAJ (4.59 and 4.74 g/L) were higher than 11.4 °Bx CCAJ (3.30 and 3.56 g/L), which might be due to the dilution to 11.4 °Bx from the concentrated cashew apple juice (CCAJ). Changes in reducing sugar and total sugar contents of fermented CAJ and fermented 11.4 °Bx CCAJ are shown in Fig. 2c, d. Results indicated that their contents decreased significantly (p < 0.05) in fermented CAJ and CCAJ compared to control. However, the decrease of reducing sugar and total sugar of fermented CAJ was greater than fermented 11.4 °Bx CCAJ. The control pasteurized CAJ without inoculation was more stable over 0–72 h and values were 4.59–4.16 g/L for reducing sugar and 4.73–4.52 g/L for total sugar, respectively. The values of reducing sugar and total sugar during 0–72 h fermentation for CCAJ were 3.30–2.25 and 3.56–2.43 g/L, and for CAJ were 4.59–3.34 and 4.74–3.61 g/L, respectively. Therefore the CAJ fermented with L. plantarum consumed sugar at a much faster rate than of fermented 11.4 °Bx CCAJ. The decrease in sugar concentrations during fermentation is largely due to not only bioconversion into lactic acid, but also the utilization during growth and metabolism of L. plantarum (Raimbault and Tewe 2001; Salmerón et al. 2014). During fermentation, hetero-fermentative lactic acid bacteria could utilize glucose via the pentose phosphate pathway (PPP), yielding lactic-acid, ethanol, and CO2, resulting in extremely low pH and high acidity (Salmerón et al. 2014). In the present investigation, results also indicated that L. plantarum could potentially utilize fruit sugars and produce lactic acid without any additional nutrient supply. The decrease in sugar contents of fermented CAJ and 11.4 °Bx CCAJ was correlated with decreasing pH and increasing TTA. In case of control, while reducing sugar and total sugar contents remain relatively stable over 0–72 h, the reduction in pH may be potentially due to pasteurization-induced non-biological acidification linked to adduct formation (Nie et al. 2013) without rapid sugar utilization as seen in L. plantarum-mediated fermentation.
Vitamin C or ascorbic acid contents
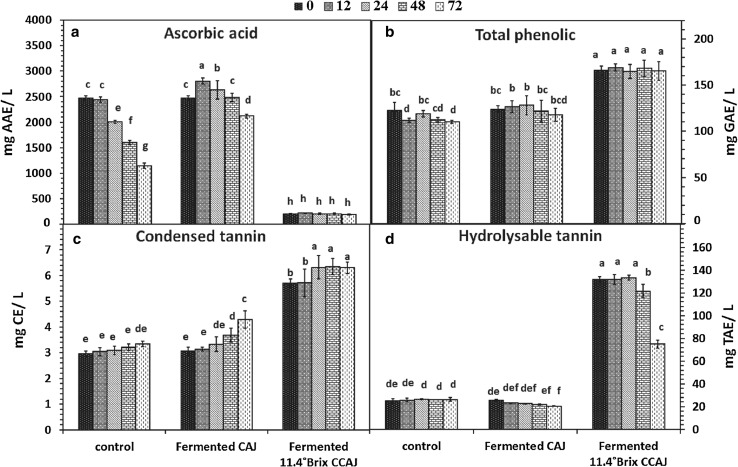
Vitamin C or L-ascorbic acid; a water-soluble vitamin that is ubiquitous in fresh fruits and vegetables, has many nutritional and clinical benefits for human health. It especially acts as a strong antioxidant through its electron donation potential (Suntornsuk et al. 2002). Results indicated that initial level vitamin C contents in fermented CAJ and control juice were higher than that found in 11.4 °Bx CCAJ (Fig. 3a). The 12X reduced content of vitamin C in 11.4 °Bx CCAJ was potentially due to both dilution effect and heat accelerated degradation (Nicoli et al. 1999). The vitamin C contents of fermented CAJ increased from 2474.88 to 2804.86 mg AAE/L from 0 to 12 h, and then decreased by the end of 72 h fermentation (Fig. 3a). The increase in vitamin C contents at 12 h of fermentation could be due to new microbial biosynthesis of vitamin C (Adetuyi and Ibrahim 2014). Moreover, the decrease in vitamin C after 12 h fermentation might be due to degradation, especially as vitamin C is very susceptible to chemical and enzymatic oxidation such as induced by ascorbate oxidase (a pH dependent enzyme that could convert ascorbic acid to dehydroascorbic acid) to non-antioxidant type substances (Adetuyi and Ibrahim 2014).
Fig. 3.
Ascorbic acid (a), total phenolic (b), condensed tannin (c), and hydrolysable tannin (d) content change in fermented cashew apple juice (CAJ) and fermented 11.4 °Bx concentrated cashew apple juice (CCAJ) at 0, 12, 24, 48 and 72 h of fermentation at 37 °C. Data is expressed as the average of three determinations ± SD. Data that does not share the same capital case letter on top of the vertical bar are significantly different at 5% according to Duncan’s multiple range test
Total phenolic contents
Phenolic acids are associated with color, sensory qualities, nutritional and antioxidant properties of foods (Hernandez et al. 2007; Shrestha et al. 2013). Total phenolic content of either of control or CAJ at 0 h were lower than total phenolics of 11.4 °Bx CCAJ (Fig. 3b).The total phenolics of the control remained relatively unchanged until 48 h and slightly reduced during 72 h of fermentation. Whereas total phenolics of fermented CAJ increased slightly from 123.97 to 128.20 mg GAE/L during 0–24 h fermentation and then decreased slightly after 24–72 h of fermentation. The highest total phenolic content was found in fermented 11.4 °Bx CCAJ with their values remaining unchanged ranging from 164.75 to 169.21 mg GAE/L. The increase of total phenolic at the beginning of fermentation time to 24 h may be potentially linked to antioxidant type bioactive compounds such as phenolic acids and flavonoids which may have increased through lactic acid bacteria-mediated fermentation (Escudero-López et al. 2013; Hernandez et al. 2007). Subsequently in later stages until 72 h phenolics of cashew juice such as gallic acid and flavonoids might bind with sugar or amino acid (Shrestha et al. 2013), resulting in lower soluble phenolic contents (Adetuyi and Ibrahim 2014; Shrestha et al. 2013). During fermentation, total soluble phenolics that increased in the fermented fruit substrate might also be mobilized and degraded, resulting in lower phenolic contents (Ankolekar et al. 2012). Some phenolic acids, especially hydroxycinnamic, can be enzymatically degraded during microbial fermentation by the action of enzyme such as polyphenol oxidase (Adetuyi and Ibrahim 2014).
Astringency compounds changes of fermented CAJ and 11.4 °Bx CCAJ
Condensed tannin contents
Biologically active compounds with antioxidant properties including condensed tannins or proanthocyanidins are responsible for the typical astringency of some fruits, but also show strong radical scavenging activity (He et al. 2011). Condensed tannin contents of CAJ (3.06 mg CE/L) were lower than that found in 11.4 °Bx CCAJ (5.71 mg CE/L). Condensed tannin contents of fermented 11.4 °Bx CCAJ at all fermentation time were higher than that of fermented CAJ and the control (Fig. 3c). However, condensed tannin contents in CAJ fermented with L. plantarum increased incrementally throughout fermentation (p < 0.05), where its content after 72 h fermentation was 4.29 mg CE/L. The highest condensed tannin content was found in fermented 11.4 °Bx CCAJ where it reached 6.32 mg CE/L by 24 h from initial level of 5.72 mg CE/L and then stabilized. The lowest content was in control juice that remained unchanged (Fig. 3c).
Hydrolysable tannin contents
Hydrolysable tannin contents of control juice (25.03 mg TAE/L) and fermented CAJ (25.58 mg TAE/L) were lower than that found in 11.4 °Bx CCAJ (131.62 mg TAE/L) at 0 h. However, hydrolysable tannin contents in fermented 11.4 °Bx CCAJ decreased throughout the fermentation process (p < 0.05), and it was lowest at 72 h (74.97 mg TAE/L) (Fig. 3d). In this study, the lowest hydrolysable tannin was found in CAJ fermented with L. plantarum for 72 h (20.63 mg TAE/L). Results indicated that hydrolysable tannins decreased with increasing condensed tannins. It is possible that L. plantarum could produce tannase during growth on media containing tannins (Rodríguez et al. 2009), which could then catalyze the hydrolysis reaction of ester bonds, resulting in decreased hydrolysable tannins (Rodríguez et al. 2009). Overall in this study, hydrolysable tannin contents of control juice and fermented CAJ were 5–10 times greater than condensed tannin contents, which were higher than the findings of Rufino et al. (2010). Although the results indicated a great astringency related compounds content of CAJ from tannins, the decreasing hydrolysable tannin contents in fermented CAJ indicated decrease in astringency related metabolites.
Antioxidant capacity changes of fermented CAJ and 11.4 °Bx CCAJ
The influence of different factors on the effectiveness of antioxidants in complex heterogeneous foods and biological systems cannot be evaluated using only a one-assay protocol. For that reason, two systems were chosen to evaluate the antioxidant capacity (ABTS·+ and DPPH·) of CAJ during fermentation where ABTS·+ is based on hydrogen atom transfer mechanism (HAT), whereas DPPH· is based on electron transfer mechanism (ET) (Badarinath et al. 2010).
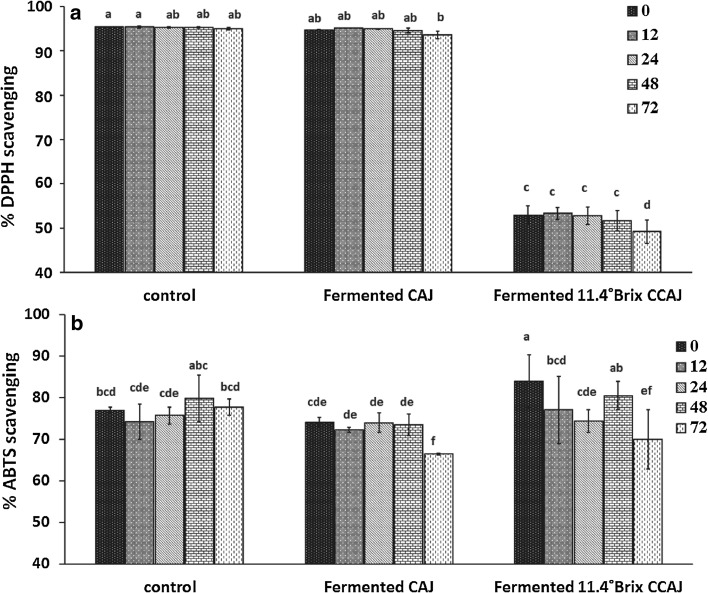
Figure 4a shows DPPH radical scavenging activity of fermented CAJ and fermented 11.4 °Bx CCAJ produced under L. plantarum fermentation. The DPPH radical scavenging of CAJ fermented with L. plantarum slightly decreased (p < 0.05) from 94.78 to 93.54% at the end of 72 h fermentation which was similar to the antioxidant values of control (95.46–95.00%). The DPPH radical scavenging activity of fermented 11.4 °Bx CCAJ was four times lower than fermented CAJ and control, decreasing from 52.97 to 49.17% at the end of fermentation.
Fig. 4.
DPPH (a) and ABTS (b) free radical scavenging of fermented cashew apple juice (CAJ) and fermented 11.4 °Bx concentrated cashew apple juice (CCAJ) at 0, 12, 24, 48 and 72 h of fermentation at 37 °C. Data is expressed as the average of three determinations ± SD. Data that does not share the same capital case letter on top of the vertical bar are significantly different at 5% according to Duncan’s multiple range test
In this study, ABTS radical scavenging activities of fermented 11.4 °Bx CCAJ were higher than fermented CAJ and control with values being 83.95, 74.07, and 77.04%, respectively at 0 h (Fig. 4b). During 72 h fermentation time, ABTS radical scavenging activity of fermented CAJ and fermented 11.4 °Bx CCAJ decreased significantly (p < 0.05), whereas it remained constant for control. The decrease of ABTS radical scavenging activity was not in agreement with DPPH radical scavenging activity. The values obtained by the DPPH method were higher than those found by the ABTS method in case of control and fermented CAJ and remained at the higher level throughout the 72 h. In contrast the fermented 11.4 °Bx CCAJ had very low DPPH radical scavenging activity than ABTS radical scavenging activity and marginally decreased over the 72 h. This could be due to the fact that the ABTS method was more sensitive to determine the antioxidant capacity of 11.4 °Bx CCAJ due its initial high concentration from CAJ and then dilution to lower Brix resulting in higher water soluble antioxidants. Further, as a result of concentration and then dilution it may have been possible to determine antioxidant capacity at lower inhibitory concentrations (Badarinath et al. 2010). It is interesting to note that radical scavenging activity of fermented CAJ remained greater than 50% after 72 h fermentation using both DPPH and ABTS assays, indicating a good potential for free radical scavenging of CAJ and good range of both water soluble and less water soluble antioxidants. This might also be due to the complex structure of cashew apple phenolics (Berry and Sargent 2011) which could be resistant to degradation or enzymatic hydrolysis during L. plantarum fermentation. At the same time in case of 11.4 °Bx CCAJ the initial concentration from CAJ and subsequent dilution potentially resulted in more water soluble antioxidants and therefore responding better to ABTS assay. Results also indicated that DPPH· radical scavenging activity was positively correlated to vitamin C and condensed tannins but not hydrolysable tannins. ABTS· + radical scavenging activity was also positively correlated to condensed tannins and not hydrolysable tannins. Overall L. plantarum mediated fermentation allowed antioxidant metabolites and related antioxidant activity to be sustained and at the same time astringency metabolite like hydrolysable tannin reduced.
Volatile compositions of fermented CAJ and 11.4 °Bx CCAJ
One of the most important characteristics of food products for consumer palatability is volatile flavor. Volatile compounds of CAJ and 11.4 °Bx CCAJ fermented with L. plantarum were identified by comparison of their mass spectra by SPME–GC–MS on HP-5 column with libraries and published data (Table 1). In total, 17 flavor volatile compounds were identified in control at 0 h incubation. Volatile compounds of control were predominantly alcohol, followed by ester, and then acid. Major compounds such as 2,6-dimethyl-4-heptanol, ethyl-3-methyl-butanoate, and 3-methyl-1-butanol indicated that the odor descriptions were fruity, sweet, pineapple, whiskey, and malt type. These findings are consistent with other research reported previously for CAJ and cashew apple-based alcoholic beverages by the Osme gas chromatography/olfactometry technique (Garruti et al. 2006). As the fermentation time increased, 3-methyl butanoic acid, 3-hydroxy-2-butanone, 3-methyl-1-butanol and 3-methylbutyl-3-methyl butanoate increased, whereas ethyl-3-methyl butanoate, 1-hexanol, ethyl-(2E)-2-methyl-butenoate, and ethyl-2-hydroxy caproate decreased (Table 1). Results also indicated that the fruity odor decreased, but the whiskey and acid odor increased. There were 15 volatile compounds identified for L. plantarum fermented CAJ, which were predominantly ester, followed by alcohol, and acid. Major compounds of fermented CAJ were ethyl-3-methyl-butanoate, 2,6-dimethyl-4-heptanol and 3-methyl-1-butanol. According to Garruti et al. (2003, 2006), among the detected compounds, the presence of ethyl-3-methyl butanoate, ethyl hexanoate, and ethyl-2-methyl-2-butenoate contributed to the fruity and cashew-like aroma of cashew apple-based alcoholic beverage. Fermentation time increased acid volatile compounds (3-methyl butanoic acid and 2-methyl butanoic acid) and as 3-methyl-1-butanol also increased (Table 1). Moreover, the increase of unpleasant odor of fermented CAJ was due to 2-methyl butanoic acid (sweaty and cheese), ethyl isovalerate (ethyl 3-methyl butanoate) (sickly sweet and fruit flavors), and isovaleric acid (3-methyl butanoic acid) (pungent/sour odor). Moreover, 3-methyl-1-butanol contributing to the overripe cashew apple odor (Garruti et al. 2003) is derived from leucine and is considered the most quantitatively important alcohol in Chinese ciders (Fan et al. 2011). Ethyl hexanoate (imparts fruity notes) and ethyl octanoate, reported as the odor-active compounds in CAJ (Garruti et al. 2003), were detected in control and in low quantities in fermented CAJ (Table 1). One important identified volatile is 1-hexanol which is considered to be a characteristic volatile compound of fruits and is produced during the enzymatic oxidation process of linoleic acid (Fan et al. 2011). Moreover, volatile compounds of fermented CAJ were quite compatible with those reported for fermented apple juice (Braga et al. 2013). It was found that inoculation with lactic acid bacteria generated a change in flavor profiles of fermented CAJ. For fermented 11.4 °Bx CCAJ, only 2 flavor volatile compounds were identified; furfural and 3-methyl-1-butanol, and as the fermentation time increased, 3-methyl-1-butanol increased, but furfural decreased (data not shown). Odor description of volatile flavor compounds of 11.4 °Bx CCAJ was bread, almond, sweet, creamy, malt, dried cashew, and cocoa which might be a result of caramelization, and condensation of amino groups and reducing compounds by the Maillard reaction due to heating of CAJ.
Table 1.
Volatile compounds of cashew apple juice (CAJ) fermented for 0, 12, 24, 48 and 72 h, at 37 °C
| Volatile compoundsa | Odor descriptionb | RIc | Peak area (× 107) (% relative peak area*) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Fermented CAJ | |||||||||
| 0 h | 12 h | 24 h | 48 h | 0 h | 12 h | 24 h | 48 h | |||
| 3-Hydroxy-2-butanone (acetoin) |
Butter, cream | 718 | 3.50 (3.69) |
3.66 (4.57) |
5.47 (5.67) |
3.99 (4.70) |
3.30 (4.95) |
4.77 (5.43) |
5.87 (7.68) |
4.88 (6.54) |
| 3-Methyl-1-butanol | Whiskey, malt, burnt | 736 | 11.80 (12.43) |
9.06 (11.32) |
15.01 (15.55) |
9.64 (11.37) |
9.23 (13.85) |
13.30 (15.15) |
14.80 (19.38) |
14.20 (19.02) |
| 3-Methyl butanoic acid (isovaleric acid) |
Sweat, acid, rancid | 851 | 1.68 (1.77) |
1.75 (2.18) |
2.43 (2.51) |
1.81 (2.14) |
– | 1.93 (2.20) |
2.90 (3.80) |
2.48 (3.32) |
| 2-Methyl butanoic acid | Cheese, sweat | 854 | 7.56 (7.96) |
6.92 (8.65) |
8.01 (8.30) |
7.18 (8.47) |
3.13 (4.70) |
6.33 (7.21) |
6.51 (8.53) |
7.37 (9.87) |
| Ethyl-3-methyl-butanoate (ethyl isovalerate) |
Fruit | 873 | 15.48 (16.30) |
1.14 (1.43) |
14.27 (14.79) |
10.78 (12.71) |
16.68 (25.01) |
17.96 (20.47) |
12.71 (16.64) |
12.01 (16.07) |
| 1-Hexanol | Resin, flower, green | 877 | 2.43 (2.56) |
1.89 (2.36) |
2.27 (2.35) |
1.85 (2.18) |
1.84 (2.76) |
2.79 (3.18) |
2.60 (3.40) |
2.47 (3.31) |
| Ethyl-(2E)-2-methyl-2-butenoate ((E)-ethyl tiglate) | Fruity type odor | 940 | 2.69 (2.83) |
2.43 (3.03) |
2.58 (2.68) |
2.04 (2.41) |
4.15 (6.23) |
4.31 (4.91) |
2.50 (3.27) |
2.25 (3.01) |
| Benzaldehyde | Almond, burnt sugar | 962 | 6.28 (6.61) |
7.57 (9.46) |
5.34 (5.53) |
4.95 (5.84) |
3.63 (5.44) |
4.48 (5.11) |
4.13 (5.41) |
3.94 (5.28) |
| Ethyl hexanoate | Apple peel, fruit | 1002 | 2.32 (2.44) |
2.50 (3.13) |
2.52 (2.62) |
2.16 (2.55) |
2.31 (3.47) |
2.93 (3.34) |
1.46 (1.91) |
1.43 (1.92) |
| 1-Methyl-2-phenyl-1H-Indole | – | 1047 | – | – | 0.96 (0.99) |
1.22 (1.44) |
1.15 (1.73) |
1.43 (1.63) |
1.16 (1.51) |
1.20 (1.61) |
| 2,6-Dimethyl-4-heptanol | Fruity and sweet odor | 1059 | 27.17 (28.61) |
28.16 (35.19) |
24.41 (25.29) |
25.33 (29.87) |
14.13 (21.19) |
18.57 (21.16) |
14.73 (19.28) |
14.76 (19.76) |
| 4-Methyl-4-heptanol | Piney, citrus | 1068 | 11.04 (11.62) |
11.60 (14.49) |
9.88 (10.23) |
10.37 (12.23) |
5.94 (8.91) |
7.95 (9.06) |
6.40 (8.38) |
6.49 (8.69) |
| Ethyl dl-2-hydroxy caproate (ethyl 2-hydroxyhexanoate) |
Fruity type odor | 1099 | 0.56 (0.59) |
0.64 (0.80) |
0.33 (0.34) |
0.35 (0.41) |
– | – | – | – |
| 3-Methylbutyl-3-methyl-butanoate (isoamyl isovalerate) |
Fruity odor | 1108 | 0.75 (0.79) |
1.08 (1.35) |
1.26 (1.30) |
1.34 (1.57) |
0.98 (1.47) |
0.83 (0.94) |
– | 0.66 (0.89) |
| 2-Phenylethanol | Floral type odor | 1124 | 1.12 (1.18) |
1.14 (1.43) |
1.11 (1.15) |
1.09 (1.29) |
– | – | – | – |
| Ethyl octanoate (ethyl caprylate) |
Fruit, fat | 1196 | – | – | – | 0.17 (0.20) |
0.20 (0.30) |
0.19 (0.22) |
– | – |
| Benzenepropanol (3-phenyl-1-propanol) |
Balsamic type odor | 1201 | 0.60 (0.63) |
0.49 (0.61) |
0.66 (0.68) |
0.52 (0.61) |
– | – | 0.63 (0.82) |
0.54 (0.72) |
aAll compounds were identified by comparison with mass spectra and retention index database
bOdour descriptions were cited from www.flavornet.org and recent reports
cRI (retention index) calculated with a HP-5 stationary phase using a series of alkanes between C8 and C20
*% Relative peak area of volatile compounds is expressed as (compoundpeak area/total compoundspeak area) × 100
–Not detectable
The major other volatile compounds identified in L. plantarum fermented CAJ were ethanol, whereas ethyl acetate and acetic acid were detected in lower amounts (data not shown). In this study L. plantarum might have had the ability to synthesize ethanol during fermentation due to the alcohol dehydrogenase activity, an enzyme able to convert acetaldehyde to ethanol. Ethyl acetate (odor note of pineapple) was detected in fermented CAJ which was higher than control over all fermentation times. These findings are consistent with research reported previously for cereal-based probiotic beverages with L. plantarum (Salmerón et al. 2014). It was also found that acetic acid was the most abundant among short chain free fatty acids in fermented CAJ, but it was not found in control and fermented 11.4 °Bx CCAJ. Moreover, the volatile production depends on the substrate in the fermentation process. This increase in volatile acidity in fermented CAJ may be due to the synthesis by L. plantarum.
Conclusion
Cashew apple juice, a good source of antioxidant compounds (total phenolic, condensed tannin, hydrolysable tannin, and ascorbic acid) and antioxidant capacity, causes unacceptable flavor and taste. This study found that condensed tannins of CAJ fermented with L. plantarum significantly increased but hydrolysable tannins decreased, resulting in reduction of astringent taste related metabolites over the 72 h fermentation period. Results also suggested that though lactic acid fermentation resulted in marginal decrease of antioxidant compounds in some stages of fermentation their overall antioxidant activity was sustained from initial levels. At the same time astringency related metabolite like hydrolysable tannins was reduced and volatile compounds linked with desirable flavor that had marked characteristic of cashew apple flavor increased. Fermented CAJ with sustainable high levels of antioxidant compounds and other enhanced beneficial flavor phytochemicals is therefore a valuable target for designing bioactive probiotic functional beverage. This study provides sound foundation for future optimization of value added functional food and ingredient products from cashew apple juice through beneficial lactic acid fermentation.
Acknowledgements
This work was supported by the Royal Golden Jubilee Ph.D. Program (PHD/0186/2553), and National Research University Project of Thailand. The cashew apple was kindly provided by Heritage Grower Corporation Ltd.
References
- Adetuyi FO, Ibrahim TA. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger Food J. 2014;32:128–137. doi: 10.1016/S0189-7241(15)30128-4. [DOI] [Google Scholar]
- Ankolekar C, Johnson K, Pinto M, Johnson D, Labbe RG, Greene D, Shetty K. Fermentation of whole apple juice using Lactobacillus acidophilus for potential dietary management of hyperglycemia, hypertension, and modulation of beneficial bacterial responses. J Food Biochem. 2012;36:718–738. doi: 10.1111/j.1745-4514.2011.00596.x. [DOI] [Google Scholar]
- AOAC . Association of official analytical chemists. 14. Washington, D.C.: AOAC; 2000. [Google Scholar]
- Badarinath A, Rao KM, Chetty CMS, Ramkanth S, Rajan T, Gnanaprakash K. A review on in vitro antioxidant methods: comparisions, correlations and considerations. Int J PharmTech Res. 2010;2:1276–1285. [Google Scholar]
- Berry AD, Sargent SA. Cashew apple and nut (Anacardium occidentale L.) Cambridge: Woodhead Publishing Limited; 2011. [Google Scholar]
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine-glucose model system I: investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Braga CM, et al. Classification of juices and fermented beverages made from unripe, ripe and senescent apples based on the aromatic profile using chemometrics. Food Chem. 2013;141:967–974. doi: 10.1016/j.foodchem.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Chapin KC, Lauderdale T. Reagents, stains, and media: bacteriology. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of clinical microbiology. 8. Washington, D.C.: ASM Press; 2003. p. 358. [Google Scholar]
- Costa MGM, Fonteles TV, De Jesus ALT, Rodrigues S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: process optimisation and product stability. Food Chem. 2013;139:261–266. doi: 10.1016/j.foodchem.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Escudero-López B, et al. Fermented orange juice: source of higher carotenoid and flavanone contents. J Agric Food Chem. 2013;61:8773–8782. doi: 10.1021/jf401240p. [DOI] [PubMed] [Google Scholar]
- Fan W, Xu Y, Han Y. Quantification of volatile compounds in chinese ciders by stir bar sorptive extraction (SBSE) and gas chromatography-mass spectrometry (GC-MS) J Inst Brew. 2011;117:61–66. doi: 10.1002/j.2050-0416.2011.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Garruti DS, Franco MRB, da Silva MAAP, Janzantti NS, Alves GL. Evaluation of volatile flavour compounds from cashew apple (Anacardium occidentale L.) juice by the Osme gas chromatography/olfactometry technique. J Sci Food Agric. 2003;83:1455–1462. doi: 10.1002/jsfa.1560. [DOI] [Google Scholar]
- Garruti DS, Franco MRB, da Silva MAAP, Janzantti NS, Alves GL. Assessment of aroma impact compounds in a cashew apple-based alcoholic beverage by GC-MS and GC-olfactometry. LWT Food Sci Technol. 2006;39:372–377. doi: 10.1016/j.lwt.2005.02.006. [DOI] [Google Scholar]
- He L, Xu H, Liu X, He W, Yuan F, Hou Z, Gao Y. Identification of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant capacities by HPLC–ABTS + assay. Food Res Int. 2011;44:1161–1167. doi: 10.1016/j.foodres.2010.05.023. [DOI] [Google Scholar]
- Hernandez T, Estrella I, Perez-Gordo M, Alegria EG, Tenorio C, Ruiz-Larrrea F, Moreno-Arribas MV. Contribution of malolactic fermentation by Oenococcus oeni and Lactobacillus plantarum to the changes in the nonanthocyanin polyphenolic composition of red wine. J Agric Food Chem. 2007;55:5260–5266. doi: 10.1021/jf063638o. [DOI] [PubMed] [Google Scholar]
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Hur SJ, Lee SY, Kim Y-C, Choi I, Kim G-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Ibanoglu S, Ainsworth P, Wilson G, Hayes GD. The effect of fermentation conditions on the nutrients and acceptability of tarhana. Food Chem. 1995;53:143–147. doi: 10.1016/0308-8146(95)90779-7. [DOI] [Google Scholar]
- Mousavi ZE, Mousavi SM, Razavi SH, Hadinejad M, Emam-Djomeh Z, Mirzapour M. Effect of fermentation of pomegranate juice by Lactobacillus plantarum and Lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids and phenolic compounds. Food Biotechnol. 2013;27:1–13. doi: 10.1080/08905436.2012.724037. [DOI] [Google Scholar]
- Nagpal R, Kumar A, Kumar M. Fortification and fermentation of fruit juices with probiotic lactobacilli. Ann Microbiol. 2012;62:1573–1578. doi: 10.1007/s13213-011-0412-5. [DOI] [Google Scholar]
- Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol. 1999;10:94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- Nie S, Huang J, Hu J, Zhang Y, Wang S, Li C, Marcone M, Xie M. Effect of pH, tempterature and heating time on the formation of furan in sugar–glycine model systems. Food Sci Hum Wellness. 2013;2:87–92. doi: 10.1016/j.fshw.2013.05.001. [DOI] [Google Scholar]
- Pereira ALF, Maciel TC, Rodrigues S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res Int. 2011;44:1276–1283. doi: 10.1016/j.foodres.2010.11.035. [DOI] [Google Scholar]
- Queiroz C, da Silva AJR, Lopes MLM, Fialho E, Valente-Mesquita VL. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale L.) after processing. Food Chem. 2011;125:128–132. doi: 10.1016/j.foodchem.2010.08.048. [DOI] [Google Scholar]
- Raimbault OA, Tewe OO. Protein enrichment of sweet potato by solid substrate fermentation using four monoculture fungi Nigerian. J Biotechnol. 2001;9:1–4. [Google Scholar]
- Rodríguez H, et al. Food phenolics and lactic acid bacteria. Int J Food Microbiol. 2009;132:79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Rufino MDSM, Pérez-Jiménez J, Tabernero M, Alves RE, De Brito ES, Saura-Calixto F. Acerola and cashew apple as sources of antioxidants and dietary fibre. Int J Food Sci Technol. 2010;45:2227–2233. doi: 10.1111/j.1365-2621.2010.02394.x. [DOI] [Google Scholar]
- Saad H, Charrier-El Bouhtoury F, Pizzi A, Rode K, Charrier B, Ayed N. Characterization of pomegranate peels tannin extractives. Ind Crops Prod. 2012;40:239–246. doi: 10.1016/j.indcrop.2012.02.038. [DOI] [Google Scholar]
- Salmerón I, Thomas K, Pandiella SS. Effect of substrate composition and inoculum on the fermentation kinetics and flavour compound profiles of potentially non-dairy probiotic formulations. LWT Food Sci Technol. 2014;55:240–247. doi: 10.1016/j.lwt.2013.07.008. [DOI] [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and charactrization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Shrestha AK, Dahal NR, Ndungutse V. Bacillus fermentation of soybean: a review. J Food Sci Technol Nepal. 2013;6:1–9. [Google Scholar]
- Silveira MS, Fontes CP, Guilherme AA, Fernandes FA, Rodrigues S. Cashew apple juice as substrate for lactic acid production. Food Bioprocess Technol. 2012;5:947–953. doi: 10.1007/s11947-010-0382-9. [DOI] [Google Scholar]
- Somogyi M. Determination of reducing sugars by Nelson–Somogyi method. J Biol Chem. 1952;200:245. [Google Scholar]
- Sun B, Ricardo-da-Silva JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998;46:4267–4274. doi: 10.1021/jf980366j. [DOI] [Google Scholar]
- Suntornsuk L, Gritsanapun W, Nilkamhank S, Paochom A. Quantitation of vitamin C content in herbal juice using direct titration. J Pharm Biomed Anal. 2002;28:849–855. doi: 10.1016/S0731-7085(01)00661-6. [DOI] [PubMed] [Google Scholar]