Abstract
Due to the strong bitter taste, sacha inchi seeds are usually consumed after roasting, which also contributes to the elimination of antinutrients. Sacha inchi plants fully adapted to cultivation under sub-tropical climate conditions were produced in southeastern Brazil. Our main goal was to evaluate the effect of dry heating (roasting) on the antinutrient content of these seeds. We also investigated the effects of the applied roasting treatments on the antioxidant activity, proximate composition and oxidative stability of the seeds. To the best of our knowledge, this is the first report on antinutrients of sacha inchi seeds cultivated under sub-tropical conditions, outside their native tropical environment. Except for saponins, which are not heat-labile compounds, the contents of all assessed antinutrients continually reduced with the increase in roasting temperature. Roasting improved antioxidant activity and phenolic content in the seeds at the highest temperature. Oxidation changes occurred in the seed oil, and they increased with temperature. However, maximum peroxide value was within the acceptable consumption limits. As a conclusion, roasting treatments can be applied to minimize the antinutrient potential in sacha inchi seeds. Knowledge on the composition and proper processing of sacha inchi cultivated under sub-tropical conditions may support future efforts focused on the development of new production areas.
Keywords: Saponin, Phytate, Trypsin, Hemagglutination, Plukenetia volubilis, Antioxidants
Introduction
Plukenetia volubilis Linneo is an oil seed naturally found in regions of tropical rainforest climate of northern South America, such as Venezuela, Peru, Bolivia and Brazil. The crop is well-known by the Peruvian population as sacha inchi, meaning “forest peanut” (Bussmann et al. 2009). With the current trend in finding “super-foods”, great attention has been focused on sacha inchi as a novel source of omega-3 fatty acids. Although marine oils are considered the primary source of long-chain polyunsaturated fatty acids (LC-PUFAs), sacha inchi oil has been directly consumed as a supplement or incorporated into other foods and cosmetics for its high polyunsaturated fatty acid (PUFA) content, which includes 45–55% linolenic acid.
Sacha inchi belongs to Euphorbiacea, a family of plants that includes many species known to produce metabolites of toxicity to humans and livestock (Seigler 1994). Thermal treatments have been largely used to inactivate antinutrients and improve the general flavor of seeds (Nidhina and Muthukumar 2015). Indigenous peoples have traditionally consumed sacha inchi after roasting the seeds, to eliminate its beany taste and digestive impairments. On the other hand, roasting may contribute to accelerate seed lipids oxidation with some effects on shelf-life. Although the effects of roasting on the sacha inchi oil have been studied (Cisneros et al. 2014), there is little data regarding its antinutrients.
Growing interest in sacha inchi’s products lead to the investigation of its cultivation performance outside the Amazonian environment. Recent propagation studies, conducted in a subtropical climate region of Brazil, demonstrated the feasibility of producing this crop under milder conditions (Rodrigues et al. 2014). In light of these findings, we aimed to investigate the presence of antinutrients in the seeds produced by these experiments and how they are affected by roasting. Additionally, we have evaluated the effects of the applied dry-heat on the proximate composition, antioxidant activity and oil stability.
Materials and methods
Cultivation of the plant material
Around 2010, sacha inchi seeds originated from Amazonian Colombia were sown in southern Brazil, where climate and soil characteristics are quite different from conditions found in the Amazon region. This endeavor generated strong vigorous plants. From one of these plants, an experiment was set with the goal of comparing horticultural features between plants obtained via seminal and in vitro (Rodrigues et al. 2014). The experimental field was installed at the University of São Paulo in the city of Piracicaba, Brazil (22°42′30″S; 47°38′00″W), at 546 m altitude. Eucalyptus stakes and steel wires were used in the guidance of 36 plants (30 originated from seminal propagation). Cultivation was performed in clay acidic soil in a zone of humid subtropical climate (Cwa in the Köppen–Geiger classification), characterized by cool and dry winters and hot summers, with average annual temperature of 21.1 °C. Both tissue culture and seed-propagated seedlings of sacha inchi exhibited good agronomic field performance. Offspring seeds of the plants propagated via seminal in this experiment were harvested during the year of 2015 and used in the present study.
Materials and reagents
HPLC grade isoctane, ethanol and methanol were obtained from J. T. Baker (Phillipsburg, NJ, USA). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), AAPH (2,2′-Azobis(2-methylpropionamidine) dihydrochloride), DPPH (2,2-Diphenyl-1-picrylhydrazyl), ABTS (2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), vanillin, catechin, L-BAPNA (N-Benzoyl-l-arginine 4-nitroanilide hydrochloride), fluorescein, p-anisidine, trypsin, diosgenin, 5-sulfosalicylic acid hydrate, phytic acid, resin Dowex 1 × 4 chloride form, gallic acid and sodium carbonate were Sigma-Aldrich (St Louis, MO, USA). Microplates were provided by Greiner Bio-one (Frickenhauser, Germany). All analytical grade reagents were purchased from Synth (Diadema, SP, Brazil).
Seed collection and roasting
Sacha inchi is a dehiscent plant. When mature, seeds are self-dispersed (autochory), a common trait of the Euphorbiaceae family. As the plant’s capsule (fruit) becomes dry, its lobes open up releasing the seeds onto the ground. Almost year-round, fruits at a wide range of maturity can be found in sacha inchi plants. Mature seeds remain inside the capsules of the plant for a period of approximately 2 weeks before being released. However, harvest was carried out after a brief period of rain and, for that reason, lobes containing mature seeds remained closed for longer than usual. For that reason, after harvest, capsules were placed in a ventilated oven at 35 °C for 3 h to allow dryness and partial opening of the lobes. Seeds were fully unshelled and uncoated carefully to maintain integrity, and further divided into 4 groups comprising the three treatments of roasting temperatures and raw seeds, as the control. Roasting was performed for 15 min in a non-ventilated laboratory oven at 80 ± 1 °C, 120 ± 1 °C and 160 ± 1 °C, namely T80, T120 and T160, respectively. After cooling to room temperature, seeds were finely ground and stored at − 4 °C until analysis.
Proximate composition
Analyses of proximate chemical composition were carried out according to the Association of Official Analytical Chemists (1990). Crude protein was determined by micro-Kjeldahl analysis (6.25 ×). Fiber content was analyzed according to Asp et al. 1983. Carbohydrates were determined by percentage difference. For each of the four samples, analyses were carried out in triplicates.
Antinutritional factors
Tannins
Tannins were determined by the vanillin/HCl spectroscopic method described by Price et al. 1980. 0.5 g of the ground uncoated seeds were extracted with 10 mL methanol. The mixture was shaken for 20 min, centrifuged for 20 min at 4000 rpm and the supernatant was collected, completing the volume to 10 mL. To 1 mL of extract were added 5 mL of 1% vanillin in 8% HCl/methanol. The test tube was covered in tin foil and kept under agitation for 20 min at 30 °C. Readings were performed at 500 nm. Catechin dilutions were used as standard to compose a calibration curve. Results were expressed as catechin equivalents (CE mg/100 g sample).
Phytic acid
Quantification was based on Latta and Eskin (1980) using the Wade reagent (a mixture of 0.3 g FeCl3 · 6H2O in 10 mL H2O and 0.03 g sulfosalicylic acid in 10 mL H2O). Samples (0.5 g) were digested in 10 mL aqueous 0.65 N HCl for 1 h under agitation and centrifuged for 10 min at 3000 rpm. Fifteen milliliters of the supernatant, previously diluted ten times in distilled water, were eluted in an ion exchange resin column with 0.7 M sodium chloride. A fraction of 5 mL was collected and added to 1 mL of Wade reagent in stoppered test tubes. The tube was mixed and readings were performed at 500 nm after a 15-min incubation period. A curve was built using 5 mL of phytic acid dilutions as standard (0.005–0.05 mg/mL) with the addition of 1 mL Wade reagent. Water was used instead of the sample as the blank.
Saponins
Total saponins were extracted with 50% aqueous ethanol (see preparation of antioxidant extracts) and determined according to Hiai et al. (1976). Sample extract (500 µL) was added to a glass tube containing 5 mL 72% sulfuric acid. After adding 500 µL of 8% vanillin/ethanol solution, tubes were closed with glass stoppers and mixed in vortex. Incubation took place in a 60 °C water bath for 10 min. A stock solution of diosgenin 1 mg/mL was prepared in 50% ethanol and diluted to generate solutions ranging from 0.05 to 0.3 mg/mL. Readings were performed at 540 nm and saponins were expressed as diosgenin equivalents.
Hemagglutinating activity
Hemagglutinating activity test was executed according to Nowak et al. (1976) with modifications described by Mendonça-Franqueiro et al. (2011). Approximately 1 g of ground seeds were resuspended in 10 mL PBS. The mixture was kept under stirring at room temperature for 2 h. The material was centrifuged at 10.000 rpm for 10 min and the supernatant collected. The assay was performed in 96-well round-bottom plates, where 100 µL of the phosphate-buffered saline (PBS) solution containing human red blood cells at 4% were added to 50 µL of samples at different dilutions (1:1–1:105) and incubated at room temperature for 2 h. Formation of a net of red blood cells characterized hemagglutination, whereas a settling button of erythrocytes was observed at the bottom of the well for samples with no agglutination activity. Results are the minimal concentration in which the seed extract presents hemagglutinating activity.
Trypsin inhibitor activity
Trypsin is capable of cleaving L-BAPNA releasing the colorimetric agent p-nitroaniline. Thus, the ability of the samples to reduce this activity was determined according to Kakade et al. (1974), using 1 mM L-BAPNA substrate in Tris buffer (pH 8.2; 20 mM CaCl2). One hundred milligrams of each sample were resuspended in 1 mL of a 10 mM NaOH solution, adjusting the pH to 9.5. Samples were vortexed for 5 min and kept under stirring at room temperature for 2 h. After centrifugation at 10.000 rpm for 10 min, the supernatant was collected for analysis. A calibration curve was drawn from a seriated dilution of a 20 µg/mL trypsin solution in 1 mM HCl. Reaction was carried out in a 96-well flat-bottom plate, in which 45 μL Tris buffer, 45 μL of trypsin solution and 110 μL of L-BAPNA were added. Reaction was monitored using a Spectramax 190 reader (Molecular Devices, California, USA) for 10 min, at 410 nm.
Antioxidant capacity
Preparation of extracts
The antioxidant capacity of foods can be better assessed by means of solvent extracts, with the goal of concentrating and isolating the compounds of interest from the sample’s matrix. It is known that antioxidant compounds of polar nature can be highly effective in protecting lipids by acting on oil interface (Laguerre et al. 2015). Roasting has been reportedly responsible for the development of Maillard-derived compounds of polar nature that may present antioxidant activity (Pellegrini et al. 2003). Therefore, we chose to perform antioxidant capacity in vitro assays using polar extracts obtained from the 4 groups of seeds of this study. The choice of solvent for ethanol was based on preliminary tests comparing the extractability of aqueous solutions of ethanol, acetone and methanol (data not shown).
Approximately 20 mL of 50% aqueous ethanol were added to 2 g of sample in an Erlenmeyer flask kept at room temperature under agitation for 24 h. Extracts were centrifuged and the collected supernatant was evaporated to dryness at 45 °C by rotary evaporation. Residues were suspended in 5 mL methanol and stored in freezer until analysis.
Total phenolic content (TPC)
TPC was determined using the Folin–Ciocalteau reagent according to de Camargo et al. (2015). To 20 µL of the prepared extract, 100 µL of the Folin–Ciocalteau reagent were added. After 5 min, aqueous sodium carbonate 7.5% was added to complete a final volume of 195 µL in each well. Readings at 740 nm were performed after incubation for 40 min. Dilutions of gallic acid were used as the standard phenolic to build a calibration curve. Results were calculated using the equation from the standard curve, and expressed as gallic acid equivalents (mg GAE/100 g seed). All antioxidant assays were performed using a microplate reader spectrophotometer SpectraMax M5 (Molecular Devices, California, USA) and carried out in triplicates (three readings per sample).
DPPH
DPPH method (1,1-diphenyl-2-picrylhydrazyl) was assessed based on Al-Duais et al. (2009). Trolox was used as standard to generate a calibration curve. DPPH 150 mM solution was mixed with 66 µL of the sample extract to reach a final volume of 200 µL in each well. Readings were performed at 517 nm after a 45-min incubation period.
TEAC/ABTS+
The TEAC/ABTS assay was based on Al-Duais et al. (2009). To liberate the ABTS radical cation, 88 µL of aqueous potassium persulfate was added to 5 mL of 7 mM aqueous ABTS. Trolox was used as standard to generate a calibration curve. Either 20 µL of sample, potassium phosphate buffer (as blank) or standard were added to 220 µL of the ABTS solution. Readings were made at 734 nm after a 5-min incubation period.
Oxidative stability
Oil extraction
Sacha inchi oil was obtained from all samples through solvent extraction at room temperature. Approximately, 5 g of ground seeds were transferred to stoppered Erlenmeyer flasks and extracted with 30 mL n-hexane under agitation for 4 h. The content was then filtered and concentrated using rotary evaporator at 30 °C to yield approximately 1.9 g of oil. Oil aliquots were stored at − 4 °C until analysis. Three oil-extractions were performed per sample and the following determinations were carried out in triplicates.
Absorptivity in the UV spectrum
Oil samples were weighed (0.100 g) into 10 mL volumetric flasks and dissolved in isooctane. Determinations of conjugated dienes and trienes were performed at 232 and 270 nm, respectively, using a UV–VIS spectrometer (model UV-1203, Shimazu, Japan), with 1 mm path-length quartz cuvette.
Peroxide value and anisidine value
Peroxide value (PV) was determined by the iodometric AOCS method Cd 8b-90 (2003). Anisidine value (AnV) was carried out according to method Cd 18-90 (AOCS, 2003).
Statistical analysis
Significance among means was established by one-way ANOVA, at the level of p < 0.05, using Minitab 17.0 statistical software (Minitab Inc., State College, PA, USA) and Tukey’s test for multiple comparisons.
Results and discussion
Proximate composition
Results of the proximate composition of raw and roasted sacha inchi seeds are presented in Table 1. From control to T160, samples displayed gradual increase in browning, occurring more intensively in T160. As expected, the higher the temperature applied, the greater was moisture loss. Roasting did not affect fiber, ash, protein and fat contents significantly (p < 0.05). Gutiérrez et al. (2011) found similar results for protein (24.7%) and ash (4.0%) content, but considerably different for fat content (42.0%) in sacha inchi seeds of Colombian origin. Fat content was comparable to those found by Martínez-Herrera et al. (2006) (57.2–55.3%) for Jatropha curcas of different agroclimatic regions of Mexico. The high fat content in sacha inchi seeds reveal its potential as a source of oil that could be used for many nutritional, industrial and pharmaceutical applications.
Table 1.
Chemical proximate composition of raw and roasted sacha inchi seeds (g/100 g dry matter basis)
| Component | Control | T80 | T120 | T160 |
|---|---|---|---|---|
| Moisture | 5.54 ± 0.3c | 5.05 ± 0.2c | 3.52 ± 0.3b | 0.80 ± 0.2a |
| Ash | 2.90 ± 0.1a | 2.97 ± 0.2ª | 3.00 ± 0.1a | 3.02 ± 0.3a |
| Fat | 54.7 ± 0.3a | 54.8 ± 0.3ª | 54.6 ± 0.2a | 54.3 ± 0.4a |
| Protein | 29.2 ± 0.2a | 29.0 ± 0.1ª | 28.9 ± 0.2a | 28.8 ± 0.5a |
| Total fiber | 6.61 | 6.23 | 6.58 | 6.47 |
| Insoluble | 0.51 ± 0.7a | 0.44 ± 0.3ª | 0.45 ± 0.4a | 0.48 ± 0.8a |
| Soluble | 6.10 ± 0.9a | 5.79 ± 0.7ª | 6.13 ± 0.7a | 5.99 ± 0.4a |
| Carbohydrate (by difference) | 6.59 | 7.00 | 6.92 | 7.43 |
Results are mean ± SD. Letters (a, b, c) represent significant difference between samples. Determinations were carried out in triplicates
Antinutrients
The many ways in which food is processed can influence the content of its nutrients, antinutrients and other bioactive compounds. Levels of all the assessed antinutrients in sacha inchi seeds (Table 2) were affected by roasting treatments. Tannins were only detected in the control group, indicating the effectiveness of treatments for tannin decomposition. The detected content was low, compared to those found in legumes, such as common beans (6.9–32.4 mg/g) (Guzmán-Maldonado et al. 1996). Tannin content was, however, comparable to other species of Euphorbiaceae: Jatropha curcas (0.04 mg/g) (Makkar et al. 1998) and Ricinus communis (0.35 mg/g) (Akande et al. 2012). Despite presenting antioxidant, antibacterial and aflatoxin inhibitory activities (Mahoney and Molyneux 2004), many tannins have been associated to negative effects on both taste and digestibility of foods.
Table 2.
Antinutritional factors in sacha inchi seeds
| Tannins (mg/g seed) | Phytic acid (mg/g seed) | Saponins (mg/g seed) | Hemagglutination (µg seed/mL) | Trypsin inhibitor activity (µg/g seed) | |
|---|---|---|---|---|---|
| Control | 0.19 ± 0.0 | 59.4 ± 1.0a | 7.02 ± 0.2a | 0.68 | 32.2 ± 1.1a |
| T80 | nd | 45.7 ± 1.0b | 7.83 ± 0.5b | 0.97 | 23.8 ± 0.7b |
| T120 | nd | 39.1 ± 0.6c | 8.49 ± 0.3c | 3.36 | 12.6 ± 1.2c |
| T160 | nd | 5.43 ± 0.4d | 10.85 ± 0.4d | 62.5 | 1.93 ± 0.9d |
Results are mean ± SD. Letters (a, b, c, d) represent significant difference between samples. Determination of tannins, phytic acid and saponins was carried out in triplicates. Hemagglutination and trypsin inhibitor activity assays were performed in duplicates. nd not detected
Phytic acid in its salt form, known as phytates, bind with key enzymes involved in the breakdown of nutrients, negatively affecting digestibility and bioavailability of proteins and micronutrients. Roasting significantly reduced phytic acid levels at all temperatures, showing over 11-fold reduction from control to T160. Although the activity of existing phytases can be reduced with heat (Blaabjerg et al. 2010), phytates were decomposed by thermal treatments. Heat-induced physical changes within the seed may have facilitated the exposure of phytates to enzymatic degradation.
Saponins are a class of compounds comprising glucoside units linked to either a triterpene or steroid. In the past, saponins were regarded as antinutrients due to hemolytic properties and toxicity to fish (Lacaille-Dubois and Wagner 1996). Nowadays, these natural surfactants are desired by the food and beverage industry. Saponin content increased significantly with roasting temperature, which agrees with the work of Reddy and Pierson (1994) stating that saponins are not destroyed by heat. As proteins and cell walls constituents endure chemical changes during roasting, saponins may have been released from their sites in the seed matrix and become more available for detection in the analysis (Chen et al. 2007).
Agglutinating activity of red blood cells is a direct indicative of the presence of lectins. This class of proteins is largely found in plants of Leguminosae, but has also been detected in many species of Euphorbiacea (Nsimba-Lubaki et al. 1986). The control sample presented high agglutinating activity on erythrocytes. After heat treatments, the minimal concentration of sample necessary to generate hemagglutination increased with the temperature. Hemagglutinating activity dropped 92-fold from control to T160. Similar results were found by Aregheore et al. (2003), demonstrating heat-induced inactivation of lectins in Jatropha curcas meal. The undesired effects derived from lectin ingestion by humans and other non-ruminants are mainly related to impairment of the digestive system on several levels. As observed, such effects could be minimized after heating the whole seeds for a short period.
Similarly to lectins, trypsin inhibitors are protein-based agents with special importance to the animal feeding industry due to their digestive adverse effects, which include high pancreatic toxicity, growth inhibition and overall reduction of digestive efficiency (Reddy and Pierson 1994). Since most trypsin inhibitors are thermolabile, heat-treatments have been used in meals as feed, as in soybeans (Vollmann et al. 2003). Trypsin inhibitor activity was reduced in 26.3% from control (32.2 µg/g) to T80 (23.7 µg/g). This reduction continued with the increase in temperature, first in T120 (57.7%), and finally in T160 (92.4%). Similarly, Armour et al. (1998) managed to reduce lectin and trypsin inhibitor activities to minimal levels in soybean seeds, using moist heat treatments at 100 °C for 10 min of exposure. Kadam and Smithard (1987) also indicated higher efficiency of temperatures above 100 °C in the minimization of the inhibitory activity of trypsin in winged bean.
Antioxidant capacity
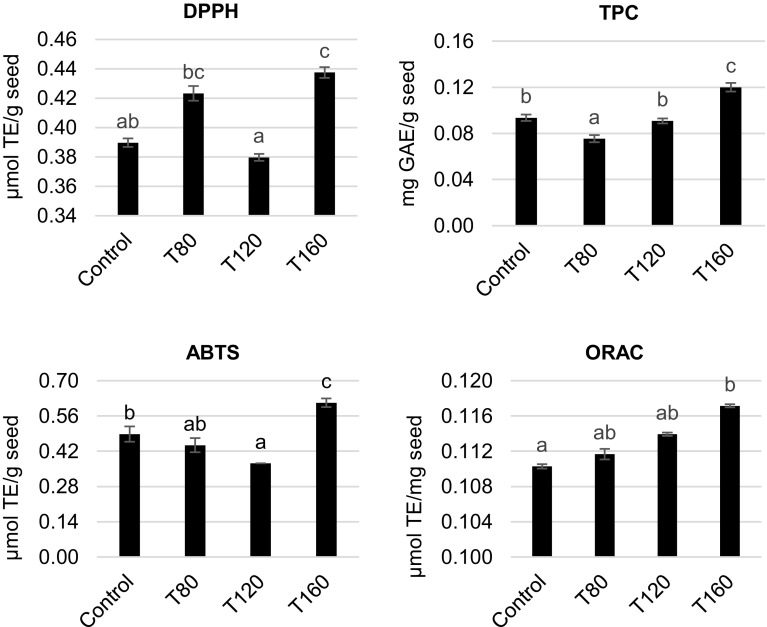
Reduction of the radical cation ABTS⋅+ by sacha inchi extracts is shown in Fig. 1. Extracts presented decreasing activity from control (0.49 µmol TE/g seed) to T120 (0.37 µmol TE/g seed), later increasing in T160 (0.61 µmol TE/g seed). The extract with higher scavenging effect on DPPH radicals (Fig. 1) was T160 (0.44 µmol TE/g seed), showing up to 13% increase as compared to its counterparts. TPC analysis (Fig. 1) revealed that antioxidant activity decreased from control (0.09 mg GAE/g seed) to T80 (0.07 mg GAE/g seed), increasing in T120 (0.09 mg GAE/g seed) and again in T160 (0.12 mg GAE/g seed), which was 22% higher than control. The ORAC assay showed that the sample’s capacity of scavenging peroxyl radicals significantly increased with roasting at 160 °C (0.12 µmol TE/mg seed) in comparison to control (0.11 µmol TE/mg seed). Overall, T160 displayed the highest antioxidant activity between samples. Similarly, Cisneros et al. (2014) found that the highest roasting temperature (102 °C for 10 min) resulted in greater antioxidant activity, assessed in sacha inchi oil. This heat-induced increase in TPC and antioxidant activity has been reported for other foods (Xu et al. 2007). In nature, phenolic compounds are found in their free form or covalently associated to other groups or molecules (Andjelkovic et al. 2006). Processing of foods, including roasting, can break up this linkage, thus increasing antioxidant activity. Also, in the roasting process, compounds of antioxidant activity derived from Maillard reactions are generated, which can also help explaining the increased antioxidant activity in samples roasted at temperatures (Pellegrini et al. 2003).
Fig. 1.
Antioxidant activity of raw and roasted sacha inchi seeds. Results are means of triplicates along with standard deviation (error bars). Letters a, b, c above bars represent significant difference between samples within the same assay. TE trolox equivalents, GAE gallic acid equivalents, TPC total phenolic content
Oxidative stability
The oxidative status of sacha inchi oil was assessed by three methodologies to identify occurring changes on different stages of oxidation (Table 3). Absorptivity in the UV region is used in the evaluation of primary oxidation products, such as conjugated dienes and trienes. As expected, the control presented the lowest oxidation levels. Thermally treated samples, however, showed no significant difference in diene measurements among them, but the presence of trienes increased with temperature. In oils with high content of polyunsaturated fatty acids, such as sacha inchi oil, it is essential to perform measurements at the region of max absorption for trienes (270 nm) to ensure a correct assessment of its oxidative status. Peroxide Value (PV) also increased with temperature, starting from 3.46 meq O2/kg oil (control) to 6.89 meq O2/kg oil (T160). PV of the control can be considered high for a fresh cold-pressed oil sample. Seeds were harvested after a long period they maturated, and had not fallen off because of the rainy period. It is possible that this could have negatively affected the sample’s oxidative status, considering the susceptibility of sacha inchi oil to oxidation reactions due to high linolenic acid content. Similar results have been found before by Anjum et al. (2006) for crude sunflower seed oil obtained by cold pressing (3.77 meq O2/kg). Herchi et al. (2012) have found that PV increases according to flaxseed maturation in the field, reaching a maximum of 3.22 meq O2/kg 42 days after flowering (DAF). The overall PV obtained for any of the samples is considered within acceptable quality limits (< 10 meq O2/kg oil) (Codex Alimentarius 2015). Anisidine Value (AnV) correlates to the presence of volatile compounds produced in the final stages of oxidation reactions. AnV also followed a trend along with temperature, increasing in over 4.2-fold from control to T160. These findings indicate there was significant formation of secondary oxidation products throughout the roasting process. Although there is no official standardized limit for AnV in vegetable oils, the generally accepted limit is 10 (Mathäus 2010). For fish oils and high omega-3 supplements the stablished limit is 20, which places all oil samples used in this study within acceptable quality limits (Ismail et al. 2016). It is important to notice that the increase in PV and AnV from 120 to 160 °C was the smallest in comparison to the oxidation-related changes found in the lower temperatures. This may be due to the higher antioxidant activity found at T160, as previously explained.
Table 3.
Oxidative stability analyses of sacha inchi seeds
| Samples | UV absorptivity | Peroxide value (meq O2/kg oil) | p-anisidine value | |
|---|---|---|---|---|
| 232 nm | 270 nm | |||
| Control | 0.65 ± 0.0a | 0.06 ± 0.0a | 3.46 ± 0.0a | 1.26 ± 0.7a |
| T80 | 1.54 ± 0.0b | 0.17 ± 0.0b | 5.59 ± 0.0b | 2.69 ± 0.4b |
| T120 | 1.56 ± 0.0b | 0.22 ± 0.0b | 6.57 ± 0.0c | 4.84 ± 0.2c |
| T160 | 1.58 ± 0.0b | 0.31 ± 0.0c | 6.89 ± 0.0d | 5.39 ± 0.0d |
Results are mean ± SD. Letters (a, b, c, d) represent significant difference between samples. Determinations were carried out in triplicates
Conclusion
Apart from saponins, roasting reduced the contents of all assessed antinutrients. Oven-roasting sacha inchi seeds at 160 °C for 15 min significantly reduced overall antinutrient content and improved antioxidant activity. Oxidation-related changes that occurred as consequence of roasting were within generally accepted oil quality limits. Roasting demonstrated to be a simple and effective procedure that can reduce antinutritional potential in sacha inchi seeds. Knowledge on the composition and processing of seeds of Plukenetia volubilis cultivated under sub-tropical conditions may endorse forthcoming actions involving novel production regions.
Acknowledgements
The first author would like to thank CAPES for the granted doctoral scholarship, and Josi for her continuous support throughout this research. Marisa Aparecida Bismara Regitano-d’Arce acknowledges the Financial Support of FAPESP (São Paulo Research Foundation, Process Nos. 2013/03650-6 and 2017/02819–8).
References
- Akande TO, Odunsi AA, Olabode OS, Ojediran TK. Physical and nutrient characterisation of raw and processed castor (Ricinus communis L.) seeds in Nigeria. World J Agric Sci. 2012;8(1):89–95. [Google Scholar]
- Al-Duais M, Müller L, Böhm V, Jetschke G. Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: use of different assays. Eur Food Res Technol. 2009;228(5):813–821. doi: 10.1007/s00217-008-0994-8. [DOI] [Google Scholar]
- Alimentarius Codex. Standard for edible fats and oils not covered by individual standards, Codex Stan 19-1981. Rome: Joint FAO/WHO; 2015. [Google Scholar]
- Andjelkovic M, Van Camp J, De Meulenaer B, Depaemelaere G, Socaciu C, Verloo M, Verhe R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006;98(1):23–31. doi: 10.1016/j.foodchem.2005.05.044. [DOI] [Google Scholar]
- Anjum F, Anwar F, Jamil A, Iqbal M. Microwave roasting effects on the physico-chemical composition and oxidative stability of sunflower seed oil. J Am Oil Chem Soc. 2006;83(9):777–784. doi: 10.1007/s11746-006-5014-1. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15. Arlington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- AOCS . Official methods and recommended practices of the American Oil Chemists’ Society. Champaign: AOCS Press; 2003. [Google Scholar]
- Aregheore EM, Becker K, Makkar HPS. Detoxification of a toxic variety of Jatropha curcas using heat and chemical treatments, and preliminary nutritional evaluation with rats. S Pac J Nat Sci. 2003;21:50–56. [Google Scholar]
- Armour JC, Chanaka Perera RL, Buchan WC, Grant G. Protease inhibitors and lectins in soya beans and effects of aqueous heat-treatment. J Sci Food Agric. 1998;78(2):225–231. doi: 10.1002/(SICI)1097-0010(199810)78:2<225::AID-JSFA109>3.0.CO;2-1. [DOI] [Google Scholar]
- Asp NG, Claes GJ, Hallmer H, Siljestron M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem. 1983;31(3):476–482. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- Blaabjerg K, Carlsson NG, Hansen-Møller J, Poulsen HD. Effect of heat-treatment, phytase, xylanase and soaking time on inositol phosphate degradation in vitro in wheat, soybean meal and rapeseed cake. Anim Feed Sci Technol. 2010;162(3–4):123–134. doi: 10.1016/j.anifeedsci.2010.09.005. [DOI] [Google Scholar]
- Bussmann RW, Téllez C, Glenn A. Plukenetia huayllabambana sp. nov. (Euphorbiaceae) from the upper Amazon of Peru. Nordic J Bot. 2009;27(4):313–315. doi: 10.1111/j.1756-1051.2009.00460.x. [DOI] [Google Scholar]
- Chen Y, Xie MY, Gong XF. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J Food Eng. 2007;81(1):162–170. doi: 10.1016/j.jfoodeng.2006.10.018. [DOI] [Google Scholar]
- Cisneros FH, Paredes D, Arana A, Cisneros-Zevallos L. Chemical composition, oxidative stability and antioxidant capacity of oil extracted from roasted seeds of sacha-inchi (Plukenetia volubilis L.) J Agric Food Chem. 2014;61:5191–5197. doi: 10.1021/jf500936j. [DOI] [PubMed] [Google Scholar]
- de Camargo AC, Regitano-d’Arce MAB, Gallo CR, Shahidi F. Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin. J Funct Foods. 2015;12:129–143. doi: 10.1016/j.jff.2014.10.034. [DOI] [Google Scholar]
- Gutiérrez LF, Rosada LM, Jiménez Á. Chemical composition of Sacha Inchi (Plukenetia volubilis L.) seeds and characteristics of their lipid fraction. Grasas Aceites. 2011;62(1):76–83. doi: 10.3989/gya044510. [DOI] [Google Scholar]
- Guzmán-Maldonado H, Castellanos J, de Mejía EG. Relationship between theoretical and experimentally detected tannin content of common beans (Phaseolus vulgaris L.) Food Chem. 1996;55(4):333–335. doi: 10.1016/0308-8146(95)00106-9. [DOI] [Google Scholar]
- Herchi W, Bouali I, Bahashwan S, Rochut S, Boukhchina S, Kallel H, Pepe C. Changes in phospholipid composition, protein content and chemical properties of flaxseed oil during development. Plant Physiol Biochem. 2012;54:1–5. doi: 10.1016/j.plaphy.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Hiai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976;29(2):116–122. doi: 10.1055/s-0028-1097639. [DOI] [PubMed] [Google Scholar]
- Ismail A, Bannenberg G, Rice HB, Schutt E, Mackay D. Oxidation in EPA- and DHA-rich oils: an overview. Lipid Technol. 2016;28(3–4):55–59. doi: 10.1002/lite.201600013. [DOI] [Google Scholar]
- Kadam SS, Smithard RR. Effects of heat treatments on trypsin inhibitor and hemagglutinating activities in winged bean. Plant Foods Hum Nutr. 1987;37:151–159. doi: 10.1007/BF01092051. [DOI] [Google Scholar]
- Kakade ML, Rackis JJ, McGhee JE, Puski G. Determination of trypsin inhibitor activity of soy products: a collaborative analysis of an improved procedure. Cereal Chem. 1974;51(3):376–382. [Google Scholar]
- Lacaille-Dubois MA, Wagner H. A review of the biological and pharmacological activities of saponins. Phytomed. 1996;2(4):363–386. doi: 10.1016/S0944-7113(96)80081-X. [DOI] [PubMed] [Google Scholar]
- Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, Decker EA, Villeneuve P. what makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit Rev Food Sci Nutr. 2015;55:183–201. doi: 10.1080/10408398.2011.650335. [DOI] [PubMed] [Google Scholar]
- Latta M, Eskin M. A simple and rapid colorimetric method for phytate determination. J Agric Food Chem. 1980;28:1313–1315. doi: 10.1021/jf60232a049. [DOI] [Google Scholar]
- Mahoney N, Molyneux RJ. Phytochemical inhibition of aflatoxigenicity in Aspergillus flavus by constituents of walnut (Juglans regia) J Agric Food Chem. 2004;52:1882–1889. doi: 10.1021/jf030812p. [DOI] [PubMed] [Google Scholar]
- Makkar HPS, Aderibigbe AO, Becker K. Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chem. 1998;62(2):207–215. doi: 10.1016/S0308-8146(97)00183-0. [DOI] [Google Scholar]
- Martínez-Herrera J, Siddhuraju P, Francis G, Dávila-Ortíz G, Becker K. Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chem. 2006;96(1):80–89. doi: 10.1016/j.foodchem.2005.01.059. [DOI] [Google Scholar]
- Matthäus B. Oxidation of edible oils. In: Decker E, Elias R, McClements DJ, editors. Oxidation in foods and beverages and antioxidant applications. 1. Cambridge: Woodhead; 2010. pp. 183–238. [Google Scholar]
- Mendonça-Franqueiro EDP, Alves-Paiva RDM, Sartim MA, Callejon DR, Paiva HH, Antonucci GA, Rosa JC, Cintra ACO, Franco JJ, Arantes EC, Dias-Baruffi M, Sampaio SV. Isolation, functional, and partial biochemical characterization of galatrox, an acidic lectin from Bothrops atrox snake venom. Acta Biochim Biophys Sin. 2011;43(3):181–192. doi: 10.1093/abbs/gmr003. [DOI] [PubMed] [Google Scholar]
- Nidhina N, Muthukumar SP. Antinutritional factors and functionality of protein-rich fractions of industrial guar meal as affected by heat processing. Food Chem. 2015;173:920–926. doi: 10.1016/j.foodchem.2014.10.071. [DOI] [PubMed] [Google Scholar]
- Nowak TP, Haywood PL, Barondes SH. Developmentally regulated lectin in embryonic chick muscle and a myogenic cell line. Biochem Biophys Res Commun. 1976;68(3):650–657. doi: 10.1016/0006-291X(76)91195-5. [DOI] [PubMed] [Google Scholar]
- Nsimba-Lubaki M, Allen AK, Peumans WJ. Isolation and partial characterization of latex lectins from three species of the genus Euphorbia (Euphorbiaceae) Physiol Plant. 1986;67:193–198. doi: 10.1111/j.1399-3054.1986.tb02442.x. [DOI] [Google Scholar]
- Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr. 2003;133(9):2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- Price ML, Hagerman AE, Butler LG. Tannin content of cowpeas, chickpeas, pigeon peas, and mung beans. J Agric Food Chem. 1980;28(2):459–461. doi: 10.1021/jf60228a047. [DOI] [PubMed] [Google Scholar]
- Reddy NR, Pierson MD. Reduction in antinutritional and toxic components in plant foods by fermentation. Food Res Int. 1994;27(3):281–290. doi: 10.1016/0963-9969(94)90096-5. [DOI] [Google Scholar]
- Rodrigues PHV, Bordignon RS, Ambrosano GMB. Horticultural performance of in vitro propagated plants of Sacha inchi. Cienc Rural. 2014;44(6):1050–1053. doi: 10.1590/S0103-84782014000600016. [DOI] [Google Scholar]
- Seigler DS. Phytochemistry and systematics of the Euphorbiaceae. Ann Mo Bot Gard. 1994;81(2):380–401. doi: 10.2307/2992104. [DOI] [Google Scholar]
- Vollmann J, Grausgruber H, Wagentristl H, Wohleser H, Michele P. Trypsin inhibitor activity of soybean as affected by genotype and fertilisation. J Sci Food Agric. 2003;83(15):1581–1586. doi: 10.1002/jsfa.1582. [DOI] [Google Scholar]
- Xu G, Ye X, Chen J, Liu D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J Agric Food Chem. 2007;55:330–335. doi: 10.1021/jf062517l. [DOI] [PubMed] [Google Scholar]