Abstract
Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) are organic, carcinogenic and mutagenic compounds that originate from the reaction of PAHs with NOx and OH radicals. In this study, an analytical method was developed, for the determination of seven nitro-PAHs and the method was applied to quantify the nitro-PAHs, in coffee model systems, prepared with coffee beans produced from three distinct locations and under various roasting conditions. Also, experiments were performed to study the effect of adding various amino acids on the formation of nitro-PAHs. The free radicals produced, were quantified by electron spin resonance (ESR), to assess their correlation with the formed nitro-PAHs. After extraction and cleanup, the nitro-PAHs in coffee were analyzed by gas chromatography/mass selective detection. In all heated coffee model systems, the addition of the amino acids, significantly increased the nitro-PAHs compared to the control. The ESR results were consistent with previous outcomes on the formation of nitro-PAHs.
Keywords: Coffee model system, Electron spin resonance, Nitrated polycyclic aromatic hydrocarbons, Roasting condition
Introduction
Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) are widely distributed environmental contaminants that display a broad spectrum of genotoxic, carcinogenic and mutagenic activities (Dušek et al. 2002). Nowadays, particularly, the presence of nitro-PAHs in food samples is a legitimate cause for concern because some nitro-PAHs are strong, direct-acting mutagens and carcinogens, which may be a risk to human health (IARC 1989a; Grosovsky et al. 1999). Nitropyrenes and, particularly, dinitropyrenes, are among the most potent bacterial mutagens reported in the literature (IARC 1989b; Tokiwa et al. 1981; Rosenkranz and Mermelstein 1985). Some nitro-PAHs are also carcinogenic in laboratory animals (Pitts Jr et al. 1982; Pederson and Siak 1981). Moreover, the International Agency for Research on Cancer (IARC) considered several nitro-PAHs are likely to be the basis of human carcinogens (Collins et al. 1998).
There are several reports on the occurrence of nitro-PAHs in environmental samples, like polluted air, exhaust gas, air particulates, soil, and water (Siegmund et al. 2003). Nitro-PAHs are formed either during incomplete combustion processes (pyrosynthesis) or as a result of a wide range of reactions taking place in the atmosphere, hence, their ubiquitous occurrence. For instance, the corresponding parental compounds, polycyclic aromatic hydrocarbons (PAHs) that adsorb on air particulates, react with nitrogen oxides, forming various nitro derivatives (Dušek et al. 2002). In addition, recent research showed that the formation of nitrogen oxide radical (NOx) precursors (mainly HCN and NH3) occurred through biomass and nitrogen-containing substrates (e.g. amino acids, protein) (Hansson et al. 2004; Tian et al. 2007; Becidan et al. 2007).
Some researchers have determined nitro-PAHs in foods, such as grilled and smoked meats (Becidan et al. 2007), grilled corn, pork, and grilled chicken (Miller et al. 1955). Schlemitz and Pfannhauser (1996), measured nitro-PAHs levels (2-nitronaphtalene, 2-nitrofluorene, 2-nitropyrene) in a range of foods including vegetables, fruits and spices, which could be subjected to atmospheric pollution, as well as smoked meat, coffee, tea and oils, which generate nitro-PAHs via the high temperatures used during cooking (roasting, drying and smoking).
Ziegler et al. (1999), investigated the maintenance of nitro-PAHs in foods during processing, using artificially contaminated food samples. Their results showed that nitro-PAHs remain almost quantitatively on the solid particles of the foods. Recently, Dafflon et al. (2000), investigated nitro-PAHs in smoked meat, fish, and dairy products in France. They found that in 92 investigated samples, 1- and 2-nitronaphthalene and 2-nitrofluorene, were present.
Even though nitro-PAHs have been identified in diverse foods and their carcinogenic and mutagenic potential has been proven, the literature concerning this class of contaminants in foods is limited (Larsson et al. 1988). Also, the incidence of nitro-PAHs in the food chain, has not yet been systematically investigated and a narrow range of foods have been studied. Consequently, the human dietary exposure and the related risk assessment are currently unclear and the legislation limits have not yet been established (Dennis et al. 1984; De Vos et al. 1990; Turrio-Baldassarri et al. 1996). Even though processes of incomplete combustion of organic products and NOx formation are the necessary elements to form nitro-PAHs in foods, none of the published studies have reported the influences of the heating conditions and food components, on the formation of nitro-PAHs.
In this study, an attempt has been made to elucidate the influences of the heating conditions and various amino acids, on the formation of nitro-PAHs, by electron spin resonance (ESR) using a coffee model system.
Materials and methods
Chemicals and materials
The seven nitro-PAH standards: 1-nitronaphthalene (1-NN), 2-nitronaphthalene (2-NN), 2-nitrofluorene (2-NF), 9-nitroanthracene (9-NA), 3-nitrofluoranthene (3-NF), 1-nitropyrene (1-NP), and 6-nitrochrysene (6-NC), and four internal standards: acenaphthene-d10 (Ace-d10), phenanthrene-d10 (Phen-d10), chrysene-d12 (Chrys-d12) and perylene-d12 (Pery-d12), were obtained from Supelco (Bellefonte, PA, USA). All solvents, such as dichloromethane (DCM), n-hexane, methanol, and N,N-dimethylformamide (N,N-DMFA) were HPLC grade (Burdick & Jackson, Muskegon, MI, USA). Water was purified using a Milli-Q system (Billerica, MA, USA). Sodium sulfate (Na2SO4, 99% purity) was used for liquid- extraction, and anhydrous Na2SO4 (99% purity) for dehydration (Junsei Chemical Co., Chuo-ku, Tokyo, Japan). Glutamic acid, glycine and aspartic acid, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sep-Pak silica cartridges (Waters, Milford, MA, USA) were used for solid phase extraction. PTFE test tubes (100 × 12 mm inner diameter, 1.0 mm wall thickness), used for the coffee model systems, were from Cowie Technology Group Ltd. (Middlesbrough, England, UK).
Sample preparation
Three samples of green (unroasted) coffee beans including Arabica (from Colombia and Brazil, respectively) and Robusta (Vietnam) varieties, were purchased from a local market in Seoul, Korea. The samples were heated, homogenized and stored vacuum-packed in plastic bags at − 20 °C, until analysis.
Extraction and cleanup of nitro-PAH samples
Aliquots (10 g) of the homogenized samples were placed in flat-bottomed flasks (300 mL) and then spiked with 1 mL of 20 μg/kg Ace-d10, Phen-d10, Chrys-d12 and Pery-d12, respectively. The samples were extracted with 100 mL n-hexane. The extraction efficiency of the suspension was enforced by ultrasonication in an ultrasonic bath at 50–60 Hz (Bransonic 2200, Branson Ultrasonic Corp., Danbury, CT, USA) at 28 °C (room temperature) for 60 min. The obtained extracts were filtered to remove particulate matter (110 mm filter paper; Advantec, Toyo Roshi Kaisha, Ltd., Japan). Next, the solution was sequentially extracted with 50, 25, and 25 mL N,N-DMFA: water (9:1, v/v). The extract was then diluted with 100 mL of 1% sodium sulfate solution and sequentially re-extracted with 50, 35, and 35 mL n-hexane, respectively. The combined hexane solution was washed twice with 50 mL distilled water, dried with anhydrous sodium sulfate (15 g) and concentrated on an N–N Series SB-100 rotary vacuum evaporator (Eyela, Tokyo, Japan) to 2 mL at 30 °C. The concentrated extracts were cleaned by adsorption chromatography on silica gel cartridges. The cartridges were conditioned with 6 mL cyclohexane. The extract was transferred to the cartridge quantitatively. The column was first eluted with 4 mL cyclohexane and then with 6 mL cyclohexane/DCM (1:1); the whole eluent was collected. The fraction was again evaporated and the resulting residues were re-dissolved in 1 mL. The solution was filtered through a 0.45-μm PTFE membrane filter (25 mm, 0.45 μm) from Agilent Technologies (Wilmington, DE, USA), and transferred to 2-mL amber screw-cap vials. An aliquot of this solution was analyzed by gas chromatography/mass spectrometry (GC/MS).
Analytical GC/MS conditions
An Agilent Technologies 7820A/5975 MSD GC–MS apparatus (Santa Clara, CA, USA) was used for separation, identification and quantification of individual components. The nitro-PAHs were chromatographically separated on an HP-5MS column (30 m × 0.25 mm, i.d. × 0.25 µm) from Agilent Technologies (Agilent, Palo Alto, CA, USA), by a gradient elution program. The carrier gas (helium) had a flow rate of 1.2 mL/min. A 2.0-μL aliquot of the extract was injected in the split less mode, using an Agilent 7683 Series autosampler at 280 °C. The following temperature program for the nitro-PAH analysis was used: initial 80 °C for 1 min, increased at 8 °C/min to 240 °C, and at 4 °C/min to 315 °C for 10 min. A further increase to 350 °C for 10 min. The nitro-PAHs were analyzed in the selective ion monitoring mode (SIM mode), to check for the lack of residual contamination. In the SIM mode, the mass spectrometer gathers data for masses of interest rather than looking for all masses over a wide range, thus, the SIM mode allows for detection of specific analysis with increased sensitivity relative to the full scan mode. For the molecular ions of 1-NN and 2-NN (173, 127, and 115 m/z), 2-NF (211, 165, and 253 m/z), 9-NA (223, 176, and 165 m/z), and 3-NF and 1-NP (247, 189, and 217 m/z), quantification was performed using the target molecular ions 173, 211, 223, and 247 m/z, respectively.
Method validation of GC–MS analysis for nitro-PAHs
The method developed for determining the seven nitro-PAHs was validated and calibrated by an internal standard method, using the standard mixture of the seven nitro-PAHs at 1, 2, 5, 10 and 20 μg/kg, and 20 μg/kg of Ace-d10, Phen-d10, Chrys-d12 and Pery-d12. The limit of detection (LOD) and limit of quantification (LOQ) for nitro-PAHs in heated coffee bean samples were obtained, using the signal-to-noise (S/N) ratio of 3 and 10, respectively. Recovery experiments were conducted by spiking the samples with 20 μg/kg of internal standard, for validation. The data were presented as a range, as well as mean ± standard deviation (SD). Precision (%) and accuracy (%) were acquired, by repeating the analysis, three times a day (intra-day) and on three independent days (inter-day). Precision (%) was expressed as the coefficient of variation (CV).
Influence of heating conditions on nitro-PAHs formation in coffee beans
An experiment was performed to assess the influence of roasting temperature and time on the formation of nitro-PAHs in the three coffee bean samples. The inlet air of the roaster, containing 100 g samples, was heated by using a hot air coffee roaster (Gene Cafe CBR-101, Genesis, Seoul, Korea) at 150, 180, 210, 230 and 250 °C for 5, 10 and 20 min, respectively. The roasted coffee beans were homogenized using an FM-909T(C) grinder (Hanil Co., Seoul, Korea) and then stored in a freezer at − 20 °C, in tightly sealed bottles, before extraction and analysis.
Preparation of coffee model system
The coffee model system was designed using a slightly modified method, as previously described (Arvidsson et al. 1999). Coffee beans were purchased at a local store in Ilsandong-gu, Goyang-si, Republic of Korea, homogenized (as described above) and stored at − 20 °C, until required.
Influence of various amino acids on the formation of nitro-PAHs in the coffee model system
This experiment was performed, to study the effect of various amino acids on the formation of nitro-PAHs in the coffee model system. Various amounts of glutamic acid (0–17% dry weight basis) were added to the coffee model system, respectively, and heated at 250 °C for 20 min, to establish the concentration of amino acids to be added. After determining the most effective concentration, each of the amino acids (glutamic acid, glycine and aspartic acid) was added to the coffee model system and then heated in a heating block at 250 °C for 20 min, to evaluate their effects.
Electron spin resonance (ESR) analysis
An experiment was performed to evaluate the mechanism responsible for the formation of nitro-PAHs in the coffee model system, by using ESR. The ESR signals were obtained using a Bruker ELEXSYS EPR system, at room temperature. The coffee model system samples, heated with added amino acids, were transferred to ESR tubes with a 5-mm inner diameter and analyzed in the direct spectrometry. The instrumental parameters of the ESR experiments were used as described previously (Kato et al. 1996), with slight modifications: 9.836 G microwave frequency; 1.002 mW microwave power; 2 G modulation amplitude; 100 kHz modulation frequency; 150 G sweep width; 40.96 s sweep time; 40 ms conversion time and 3510 G center field.
Statistical analysis
Statistical analysis of the concentration of the nitro-PAHs in coffee model system was performed using IBM SPSS ver. 21 (SPSS Inc., USA). Analysis of variance (ANOVA), with Duncan’s multiple range test (p < 0.05), was used to assess differences. All experiments were performed in triplicate and the data expressed as mean ± SD.
Results and discussion
Validation of nitro-PAHs in heated coffee bean products
For the calibration curves, the response factors of the seven nitro-PAHs relative to the four internal standards, were assessed at five different nitro-PAH concentration levels (1, 2, 5, 10, and 20 μg/kg). The correlation coefficient (R2), measures the chromatographic area as the concentration of the calibration curve. The correlation coefficient for the nitro-PAHs at all concentrations, was R2 > 0.99.
The LOD is defined as the lowest concentration leading to an S/N ratio of 3, whereas the LOQ is defined as the concentration leading to an S/N ratio of 10. The LOD of coffee bean was between 0.10 and 0.17 μg/kg, and the LOQ was between 0.30 and 0.50 μg/kg. The recovery values, measured using the peak area of the internal standards, varied between 94.58 and 109.64% for the coffee beans, and the relative standard deviation was between 1.84 and 4.79%.
Accuracy (%) and precision (%) were performed at five concentrations. Inter-day accuracy and precision were evaluated, by analyzing one sample on three different days. Intra-day accuracy and precision were performed, by running tree analyses on the same day under the same conditions and the results are presented in Table 1.
Table 1.
Linearity, LOD, LOQ, recovery, accuracy, and precision of each nitro- PAHs
| PAHs | R2 | LOD (μg/kg) | LOQ (μg/kg) | Recovery (%)a | Inter-day | Intra-day | ||
|---|---|---|---|---|---|---|---|---|
| Accuracy (%)b | CV (%)c | Accuracy (%)b | CV (%)c | |||||
| 1-NN | 0.9993 | 0.116 | 0.353 | 104.61 ± 1.89 | 109.66 | 2.24 | 98.62 | 1.71 |
| 2NN | 0.9994 | 0.128 | 0.387 | 109.64 ± 1.84 | 106.61 | 6.87 | 102.19 | 2.85 |
| 2-NF | 0.9978 | 0.099 | 0.300 | 96.95 ± 2.49 | 93.44 | 6.32 | 92.74 | 6.85 |
| 9-NA | 0.9987 | 0.166 | 0.502 | 107.10 ± 1.87 | 98.98 | 1.10 | 99.49 | 1.19 |
| 3-NF | 0.9979 | 0.119 | 0.361 | 109.49 ± 1.99 | 108.95 | 3.04 | 107.07 | 1.99 |
| 1-NP | 0.9970 | 0.115 | 0.349 | 98.88 ± 3.7 | 107.12 | 8.83 | 96.72 | 4.92 |
| 6-NC | 0.9986 | 0.136 | 0.411 | 94.58 ± 4.79 | 99.66 | 5.04 | 99.27 | 8.92 |
aRecovery was evaluated in samples spiked with 10 μg/kg
bAccuracy (%) = [1 − (mean concentration measured-concentration spiked)/concentration spiked] × 100
cCV (coefficient of variation, %) = (SD/mean) × 100
Influence of heating temperature and time on the nitro-PAHs formation
The levels of nitro-PAHs in the coffee beans (sourced from Colombia, Brazil and Vietnam) formed by roasting at various temperature–time combinations, are presented in Table 2. The total quantity of the nitro-PAHs formed, increased with increasing roasting temperature and time, for all coffee products.
Table 2.
Influence of roasting conditions on formation of nitro-PAHs in ground coffee bean of three different countries
| Time (min) | Temperature (°C) | Nitro-PAHs (µg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-NN | 2-NN | 2-NF | 9-NA | 3-NF | 1-NP | 6-NC | Total nitro-PAHs | ||
| Colombia | |||||||||
| 5 | 0 | 0.44 ± 0.04f | ND | ND | ND | ND | ND | ND | 0.44 ± 0.04j |
| 150 | 0.45 ± 0.04f | ND | ND | ND | ND | ND | ND | 0.45 ± 0.04j | |
| 180 | 0.64 ± 0.17ef | 0.19 ± 0.02d | ND | ND | ND | ND | ND | 0.84 ± 0.16ij | |
| 210 | 0.55 ± 0.13f | 0.41 ± 0.08c | ND | ND | ND | ND | ND | 0.95 ± 0.15i | |
| 230 | 0.89 ± 0.11de | 0.34 ± 0.07cd | 0.11 ± 0.01e | ND | 0.13 ± 0.01g | ND | ND | 1.46 ± 0.16gh | |
| 250 | 1.22 ± 0.64c | 0.58 ± 0.15b | 0.20 ± 0.02d | ND | 0.16 ± 0.01fg | 0.13 ± 0.01d | ND | 2.28 ± 0.13e | |
| 10 | 150 | 0.75 ± 0.12ef | ND | ND | ND | ND | ND | ND | 0.75 ± 0.12ij |
| 180 | 1.18 ± 0.19cd | 0.19 ± 0.02d | ND | ND | 0.16 ± 0.01fg | 0.12 ± 0.02d | ND | 1.71 ± 0.16fg | |
| 210 | 1.26 ± 0.09c | 0.19 ± 0.03d | 0.12 ± 0.01e | ND | 0.22 ± 0.01e | 0.21 ± 0.02c | ND | 1.93 ± 0.03ef | |
| 230 | 1.66 ± 0.23b | 0.38 ± 0.05c | 0.38 ± 0.02c | ND | 0.41 ± 0.01c | 0.20 ± 0.04c | ND | 3.03 ± 0.25d | |
| 250 | 1.82 ± 0.09b | 0.59 ± 0.04b | 0.42 ± 0.03c | ND | 0.63 ± 0.05b | 0.41 ± 0.05b | ND | 3.87 ± 0.24c | |
| 20 | 150 | 0.90 ± 0.19de | 0.30 ± 0.05cd | ND | ND | ND | ND | ND | 1.20 ± 0.17hi |
| 180 | 1.61 ± 0.06b | 0.32 ± 0.05cd | ND | ND | 0.2 ± 0.01ef | 0.20 ± 0.03c | ND | 2.23 ± 0.04e | |
| 210 | 1.59 ± 0.11b | 0.60 ± 0.09b | 0.20 ± 0.03d | ND | 0.28 ± 0.01d | 0.41 ± 0.02b | ND | 3.07 ± 0.18d | |
| 230 | 2.46 ± 0.33a | 0.73 ± 0.06b | 0.47 ± 0.08b | ND | 0.60 ± 0.03b | 0.43 ± 0.02b | ND | 4.69 ± 0.30b | |
| 250 | 2.66 ± 0.42a | 0.89 ± 0.21a | 0.71 ± 0.08a | ND | 0.87 ± 0.07a | 0.60 ± 0.08a | ND | 5.74 ± 0.81a | |
| Brazil | |||||||||
| 5 | 0 | 0.36 ± 0.05i | ND | ND | ND | ND | ND | ND | 0.36 ± 0.05g |
| 150 | 0.33 ± 0.08i | ND | ND | ND | ND | ND | ND | 0.33 ± 0.08g | |
| 180 | 0.41 ± 0.05gh | ND | ND | ND | ND | ND | ND | 0.41 ± 0.05g | |
| 210 | 0.71 ± 0.08fg | 0.28 ± 0.05ef | ND | ND | ND | ND | ND | 0.99 ± 0.03f | |
| 230 | 0.71 ± 0.11fg | 0.38 ± 0.07de | ND | ND | 0.14 ± 0.01g | ND | ND | 1.18 ± 0.10f | |
| 250 | 1.09 ± 0.06de | 0.49 ± 0.03cd | 0.12 ± 0.01e | ND | 0.15 ± 0.01fg | 0.13 ± 0.01f | ND | 1.98 ± 0.04d | |
| 10 | 150 | 0.50 ± 0.11gh | ND | ND | ND | ND | ND | ND | 0.50 ± 0.11g |
| 180 | 0.84 ± 0.14ef | 0.21 ± 0.07f | ND | ND | 0.13 ± 0.01g | ND | ND | 1.18 ± 0.20f | |
| 210 | 0.69 ± 0.05fg | 0.32 ± 0.11ef | 0.20 ± 0.04d | ND | 0.18 ± 0.02f | 0.18 ± 0.03e | ND | 1.59 ± 0.18e | |
| 230 | 1.22 ± 0.03cd | 0.60 ± 0.10bc | 0.30 ± 0.03c | ND | 0.36 ± 0.02d | 0.36 ± 0.03d | ND | 2.83 ± 0.05c | |
| 250 | 2.16 ± 0.25b | 0.61 ± 0.08b | 0.39 ± 0.04b | ND | 0.58 ± 0.03b | 0.41 ± 0.04c | ND | 4.15 ± 0.40b | |
| 20 | 150 | 0.70 ± 0.23fg | 0.21 ± 0.04f | ND | ND | ND | ND | ND | 0.91 ± 0.19f |
| 180 | 1.35 ± 0.08cd | 0.31 ± 0.09ef | ND | ND | 0.18 ± 0.02f | ND | ND | 1.83 ± 0.17de | |
| 210 | 1.40 ± 0.21c | 0.49 ± 0.10cd | 0.39 ± 0.01b | ND | 0.22 ± 0.02e | 0.21 ± 0.02e | ND | 2.71 ± 0.30c | |
| 230 | 2.38 ± 0.19ab | 0.52 ± 0.08bc | 0.40 ± 0.03b | ND | 0.54 ± 0.01c | 0.49 ± 0.06b | ND | 4.33 ± 0.31b | |
| 250 | 2.57 ± 0.42a | 0.80 ± 0.05a | 0.67 ± 0.06a | ND | 0.81 ± 0.02a | 0.65 ± 0.05a | ND | 5.52 ± 0.53a | |
| Vietnam | |||||||||
| 5 | 0 | 0.26 ± 0.02 h | ND | ND | ND | ND | ND | ND | 0.26 ± 0.02 h |
| 150 | 0.30 ± 0.03 h | ND | ND | ND | ND | ND | ND | 0.30 ± 0.03gh | |
| 180 | 0.47 ± 0.02g | ND | ND | ND | ND | ND | ND | 0.47 ± 0.02fgh | |
| 210 | 0.31 ± 0.01 h | 0.21 ± 0.06d | ND | ND | ND | ND | ND | 0.51 ± 0.07fg | |
| 230 | 0.65 ± 0.15de | 0.38 ± 0.13bc | ND | ND | ND | ND | ND | 1.03 ± 0.28e | |
| 250 | 0.70 ± 0.06de | 0.34 ± 0.05bc | ND | ND | ND | ND | ND | 1.07 ± 0.14e | |
| 10 | 150 | 0.50 ± 0.04fg | ND | ND | ND | ND | ND | ND | 0.50 ± 0.04fg |
| 180 | 0.60 ± 0.08ef | ND | ND | ND | ND | ND | ND | 0.65 ± 0.16f | |
| 210 | 0.80 ± 0.11cd | 0.22 ± 0.05d | ND | ND | ND | ND | ND | 1.02 ± 0.16e | |
| 230 | 0.81 ± 0.03cd | 0.29 ± 0.08cd | 0.15 ± 0.02c | ND | 0.17 ± 0.02d | 0.18 ± 0.03b | ND | 1.60 ± 0.09c | |
| 250 | 0.89 ± 0.08bc | 0.39 ± 0.08bc | 0.20 ± 0.02c | ND | 0.24 ± 0.02b | 0.15 ± 0.01c | ND | 2.01 ± 0.18b | |
| 20 | 150 | 0.51 ± 0.09fg | ND | ND | ND | ND | ND | ND | 0.51 ± 0.09fg |
| 180 | 0.70 ± 0.12de | 0.23 ± 0.07d | ND | ND | ND | ND | ND | 0.93 ± 0.15e | |
| 210 | 0.721 ± 0.04de | 0.38 ± 0.06bc | 0.19 ± 0.07c | ND | ND | ND | ND | 1.28 ± 0.01d | |
| 230 | 1.00 ± 0.15ab | 0.42 ± 0.07ab | 0.29 ± 0.08b | ND | 0.22 ± 0.04c | 0.17 ± 0.04bc | ND | 2.10 ± 0.11b | |
| 250 | 1.11 ± 0.07a | 0.49 ± 0.04a | 0.42 ± 0.04a | ND | 0.31 ± 0.03a | 0.22 ± 0.01a | ND | 2.57 ± 0.12a | |
All treatments were replicated three times and data shown are the mean ± standard deviation
Values with different superscript letters in a column indicate significant difference (p < 0.05) by ANOVA, with Duncan’s multiple range test
ND not detected, below the limit of detection
The levels of nitro-PAHs formed in the coffee beans sourced from Colombia when roasted at 150, 180, 210, 230 and 250 °C for 5, 10 and 20 min, are listed in Table 2. Five nitro-PAHs including 1-NN, 2-NN, 2-NF, 3-NF and 1-NP, were detected in the roasted Colombia coffee beans and the concentrations gradually increased as the temperature and time increased. In contrast, 9-NA and 6-NC were not detected under any of the conditions evaluated. The mean levels of total nitro-PAHs in the Colombian coffee bean samples roasted at 150, 180, 210, 230 and 250 °C for 5 min were 0.45, 0.84, 0.95, 1.46 and 2.28 μg/kg, respectively. When the samples were roasted for 10 min, the average concentrations of total nitro-PAHs were 0.75, 1.71, 1.93, 3.03 and 3.87 μg/kg, respectively. At 20 min, the total nitro-PAHs were 1.20, 2.23, 3.07, 4.69 and 5.74 μg/kg, respectively. In particular, 1-NP was only present in the Colombian coffee beans roasted at 250 °C for 5 min, whereas 1-NN was identified in all the treated samples. The highest level of 1-NP, which belongs to the IARC 2A group, was 0.60 μg/kg, when heated at 250 °C for 20 min. 1-NN was present in the highest amount at 2.66 μg/kg of nitro-PAHs, following roasting at 250 °C for 20 min, followed by 2-NN, 3-NF, 2-NF and 1-NP, which ranged from 0.60 to 0.89 μg/kg.
Table 2 shows the levels of the nitro-PAHs formed during roasting of the coffee beans sourced from Brazil. The concentrations of the nitro-PAHs gradually increased as the roasting temperature and time increased. The mean levels of total nitro-PAHs in the coffee samples roasted at 150, 180, 210, 230 and 250 °C, for 5, 10 and 20 min, ranged from 0.33 to 5.52 μg/kg. Among all of the nitro-PAHs, 1-NN was produced in the highest concentration, followed by 2-NN.
Table 2 presents the contents of nitro-PAHs formed during roasting of the coffee beans sourced from Vietnam. In these samples, the mean concentration of total nitro-PAHs was 0.30–1.07 μg/kg at 5 min, 0.50–2.01 μg/kg at 10 min, and 0.51–2.57 μg/kg at 20 min. 1-NN was formed in all the roasted coffee beans, while 9-NA and 6-NC were not detected under any of the roasting conditions investigated.
Schlemitz and Pfannhauser (1996), found the highest nitro-PAH levels in smoked food and the nitro-PAH content in olive oil derived from the oilseeds drying process with fuel gases. The high levels of nitro-PAHs found in coffee seeds should not reach toxic concentrations in the beverage because of the preparation method (Atkinson and Arey 1994). In general, an increase in the heating temperature and time enhanced the formation of nitro-PAHs in coffee bean samples, which concurs with previous study about the formation of nitro-PAHs in cooked foods (Schlemitz and Pfannhauser 1996).
Influence of various amino acids on the formation of nitro-PAHs in the coffee model system
Table 3 shows the levels of nitro-PAHs formed in the coffee model system heated at 250 °C for 20 min, in the presence of 0, 5, 9, 13 and 17% (dry weight basis, dwb) glutamic acid, which was conducted to establish the most effective concentration on the yield of the nitro-PAHs. The majority of the nitro-PAHs was increased slightly at the higher concentrations of glutamic acid. Particularly, 2-NN showed the highest concentration range among the seven nitro-PAHs; ranging from 0.71 to 1.88 μg/kg. Nitro-PAHs were most likely formed when 13% dwb (dry weight basis) glutamic acid was added and there was no significant difference found above this concentration. Therefore, further experiments were conducted using glutamic acid, glycine and aspartic acid at 13% dwb, respectively, to evaluate the influence of various amino acids on the formation of nitro-PAHs.
Table 3.
Formation of nitro-PAHs in the coffee model system with various glutamic acid concentrations and amino acid concentrations added
| Amino acid | Concentration (% dwb) | Nitro-PAHs(µg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-NN | 2-NN | 2-NF | 9-NA | 3-NF | 1-NP | 6-NC | Total nitro-PAHs | ||
| Glutamic acid | 0 | 2.34 ± 0.15c | 0.71 ± 0.05d | 0.70 ± 0.15c | ND | 0.84 ± 0.05c | 0.67 ± 0.08c | ND | 5.26 ± 0.43d |
| 5 | 2.87 ± 0.03b | 1.16 ± 0.09c | 1.07 ± 0.04b | ND | 1.34 ± 0.16b | 0.89 ± 0.05b | ND | 7.34 ± 0.13c | |
| 9 | 3.23 ± 0.32ab | 1.58 ± 0.13b | 1.41 ± 0.05a | ND | 1.60 ± 0.45b | 1.12 ± 0.07a | ND | 8.94 ± 0.33b | |
| 13 | 3.27 ± 0.42ab | 1.74 ± 0.13a | 1.42 ± 0.03a | ND | 1.92 ± 0.05a | 1.16 ± 0.03a | ND | 9.52 ± 0.61ab | |
| 17 | 3.49 ± 0.38a | 1.88 ± 0.19a | 1.45 ± 0.05a | ND | 1.93 ± 0.03a | 1.19 ± 0.04a | ND | 9.94 ± 0.56a | |
| Amino acid concentrations | |||||||||
| Control | 2.34 ± 0.15c | 0.71 ± 0.05c | 0.70 ± 0.15c | ND | 0.84 ± 0.05d | 0.67 ± 0.08c | ND | 5.26 ± 0.43c | |
| Glutamic acid | 13 | 3.36 ± 0.36a | 1.87 ± 0.15a | 1.42 ± 0.03a | ND | 1.92 ± 0.05a | 1.16 ± 0.03a | ND | 9.73 ± 0.57a |
| Aspartic acid | 13 | 2.82 ± 0.49ab | 1.76 ± 0.21a | 1.23 ± 0.13b | ND | 1.75 ± 0.02b | 0.97 ± 0.03b | ND | 8.47 ± 0.45a |
| Glycine | 13 | 2.73 ± 0.19b | 0.90 ± 0.08b | 0.80 ± 0.06c | ND | 1.02 ± 0.08c | 0.78 ± 0.10c | ND | 6.24 ± 0.46b |
All treatments were replicated three times and data shown are the mean ± standard deviation
Values with different superscript letters in a column indicate significant difference (p < 0.05) by ANOVA, with Duncan’s multiple range test
dwb dry weight basis, ND not detected, below the limit of detection
As shown in Table 3, when the correlation of the various amino acids with the formation of nitro-PAHs was evaluated, it was confirmed that the levels of most of the nitro-PAHs in all heated coffee model systems with added amino acids (ranging from 0.78 to 3.36 μg/kg) were higher than the control (ranging from 0.67 to 2.34 μg/kg), which showed a significant difference (p < 0.05). Among the amino acids, the lowest content of total nitro-PAHs was generated when glycine was added. On the contrary, adding glutamic acid and aspartic acid showed similar contents of total nitro-PAHs. Ren et al. (2011), studied the formation of NOx and N2O precursors (NH3 and HCN) from pyrolysis of biomass including co-pyrolysis of amino acids with cellulose, hemicellulose and lignin. They reported that the total conversion of nitrogen into HCN and NH3, which are NOx precursors, decreased in the order of leucine, phenylalanine, proline, glycine, glutamic acid and aspartic acid, during pyrolysis. In particular, there was only about 3.5% nitrogen in aspartic acid released as HCN and NH3 from pyrolysis, while in glutamic acid and glycine, there was, respectively, about 15.3 and 21.5% nitrogen released as HCN and NH3. However, in the current study, as above-mentioned, among the amino acids investigated, the lowest concentration of total nitro-PAHs was generated in the coffee model system when glycine was added. Therefore, further research about the effect of glycine in this type of system should be conducted. Furthermore, during the coffee roasting process, undesirable compounds, such as furan, acrylamide and PAHs may also be formed (Arisseto et al. 2008). The formation of these compounds may be associated with the coffee composition, which, as reported in previous studies (Mendes et al. 2001; Vignoli et al. 2014; Dias and Benassi 2015), varies according to species and cultivar. For instance, differences in amino acids, caffeine and chlorogenic acid levels, were described for different coffee species, cultivars and roasting degrees (Campa et al. 2005). Nitro-PAHs originate via reactions between PAHs with NOx and, thus, amino acids that affected the formation of NOx precursors had a critical impact on the formation of nitro-PAHs in the coffee model system, during heating. The results of amino acids effect on nitro-PAHs formation in this study provide valuable information to control the risk of nitro-PAHs formation in coffee for coffee processors.
Electron spin resonance (ESR) studies of the coffee system on the mechanism of formation of nitro-PAHs
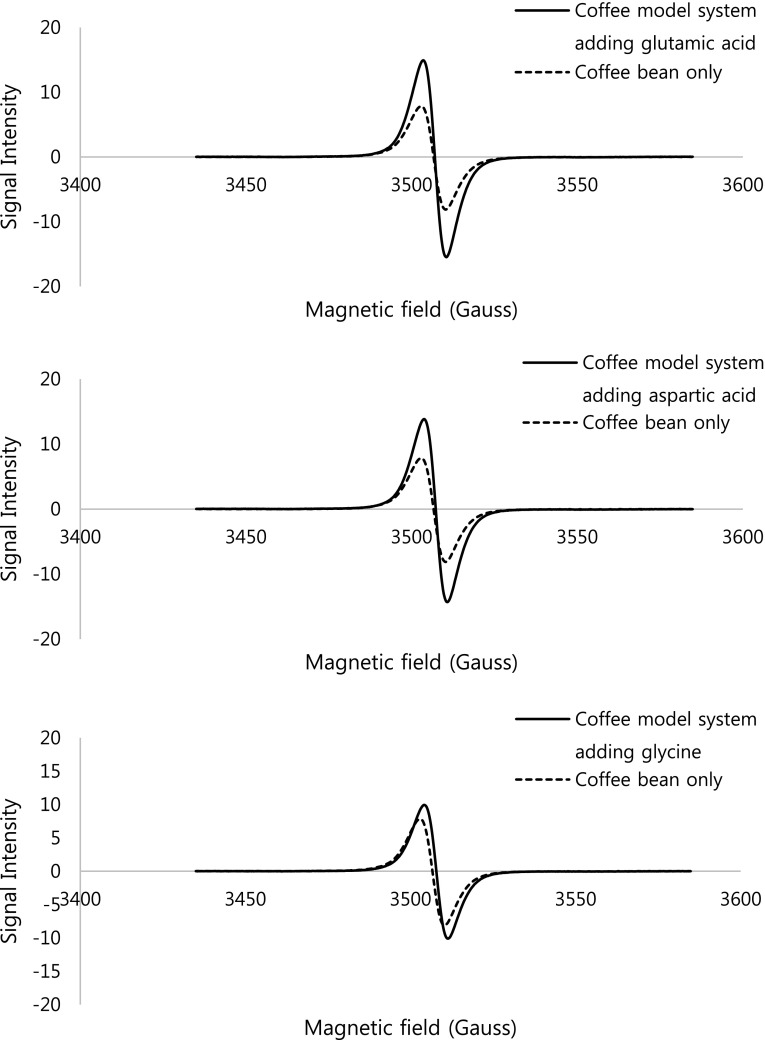
To evaluate the mechanism associated with free radicals, on the formation of nitro-PAHs in the coffee model system, an ESR experiment was performed. ESR spectroscopy is primarily used for studying materials with unpaired electrons (Elschner 1983). Particularly, it is useful for studying metal complexes or organic radicals, to identify irradiated foods or to investigate the mechanism of formation of mutagenic compounds involving free radicals. Figure 1 shows the ESR spectra of the coffee model system, as influenced by the addition of glycine, aspartic acid and glutamic acid.
Fig. 1.
Electron spin resonance spectra of the coffee model system with added glutamic acid, aspartic acid and glycine, heated at 250 °C for 20 min
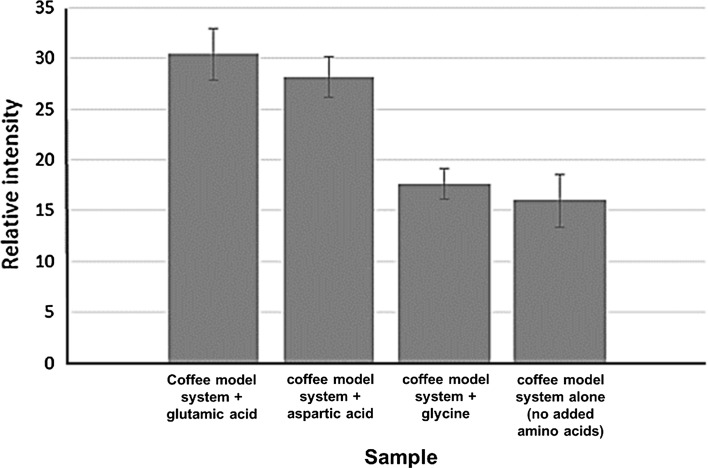
A broad signal of stable free radicals in the coffee model systems was observed. Each peak in the spectra was increased in the model system with added amino acid compared to the control. The signal intensity is measured as the distance between the maximum value and the minimum value of the spectral curve, as a percentage of the base peak and reported in arbitrary units (a.u.) (European Standard EN, 1997). In Fig. 2, the signal intensity of the control coffee model system heated at 250 °C for 20 min, without added amino acids, was 15.97 ± 2.57 a.u. and those of the coffee model system with added glutamic acid, aspartic acid and glycine were 17.62 ± 1.5, 28.13 ± 1.99 and 30.39 ± 2.54 a.u., respectively. These results correspond to the previous outcome on the formation of nitro-PAHs in the heated coffee model system. Namely, both the amount of nitro-PAHs formed and the ESR signal intensity, were increased in the coffee model system when amino acids were added.
Fig. 2.
Plot displaying the intensities of the electron spin resonance spectra of the coffee model system with added amino acids, heated at 250 °C for 20 min. Coffee model system + glutamic acid, coffee model system + aspartic acid, coffee model system + glycine, and coffee model system alone (no added amino acids)
This result was similar to other studies on mutagens. The mechanism of gas-phase OH radical-initiated reaction with PAHs to generate nitro-PAHs, has been previously described (Atkinson and Heath 1990). In the gas phase, the initial addition of OH radical to an aromatic ring leads to an OH-PAH adduct. This radical may react with NO2 to yield a nitro cyclohexadienyl radical intermediate, followed by water elimination to form the nitro-PAH. Alternatively, in ambient atmospheres, the OH-PAH adduct can also react with O2, to produce other products (Zimmermann et al. 2012). It is commonly known that PAHs are generated during fossil fuel combustion. These PAHs can react with the OH radical and nitrogen oxides, produced by combustion, forming nitro-PAHs in the presence of sunlight. After sunset, the reaction is initiated by NO3 instead of the OH radical (Atkinson and Heath 1990).
Atkinson and Arey (1994), conducted an ESR study on the formation and inhibition of the imidazoquinoxaline-type heterocyclic amines and reported that mutagens are formed through the unstable free radical Maillard intermediates. According to the study, the intermediary pyrazine cation radical that is produced throughout the reaction, has an essential role in the mutagen formation in the early stages of the Maillard reaction.
The definite mechanism of nitro-PAH formation remains unknown. However, the findings of our research regarding the correlation between the levels of the nitro-PAHs and the ESR data, might suggest another hypothesis that the mechanism of formation of nitro-PAHs, can be estimated by the occurrence of free radicals.
Conclusion
In this study, the formation of nitro-PAHs was affected by the roasting temperature–time conditions of the coffee beans and exogenous amino acids added to the coffee model system. The ESR data correlated with the level of formation of the nitro-PAHs in the coffee model system. This correlation might be explained by the participation of free radicals in the formation of nitro-PAHs.
Acknowledgements
This work was supported by the Dongguk University Research Fund of 2017 (S-2017-G0001-0001) and funded by Korea Environmental Industry & Technology Institute (A117-00197-0703-0).
References
- Arisseto AP, de Figueiredo Toledo MC, Govaert Y, van Loco J, Fraselle S, Degroodt J-M. A modified sample preparation for acrylamide determination in cocoa and coffee products. Food Anal Methods. 2008;1:49–55. doi: 10.1007/s12161-007-9001-4. [DOI] [Google Scholar]
- Arvidsson P, Boekel M, Skog K, Solyakov A, Jägerstad M. Formation of heterocyclic amines in a meat juice model system. J Food Sci. 1999;64:216–221. doi: 10.1111/j.1365-2621.1999.tb15868.x. [DOI] [Google Scholar]
- Atkinson R, Arey J. Atmospheric chemistry of gas-phase polycyclic aromatic hydrocarbons: formation of atmospheric mutagens. Environ Health Perspect. 1994;102:117. doi: 10.1289/ehp.94102s4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson BJ, Heath AW. The limits of explanation and evaluation. Fam Process. 1990;29:164–167. doi: 10.1111/j.1545-5300.1990.00164.x. [DOI] [Google Scholar]
- Becidan M, Skreiberg Ø, Hustad JE. NOx and N2O precursors (NH3 and HCN) in pyrolysis of biomass residues. Energy Fuels. 2007;21:1173–1180. doi: 10.1021/ef060426k. [DOI] [Google Scholar]
- Campa C, Doulbeau S, Dussert S, Hamon S, Noirot M. Qualitative relationship between caffeine and chlorogenic acid contents among wild Coffea species. Food Chem. 2005;93:135–139. doi: 10.1016/j.foodchem.2004.10.015. [DOI] [Google Scholar]
- Collins J, Brown J, Alexeeff G, Salmon A. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol. 1998;28:45–54. doi: 10.1006/rtph.1998.1235. [DOI] [PubMed] [Google Scholar]
- Dafflon O, Scheurer L, Koch H, Bosset JO. Le dosage des hydrocarbures aromatiques polycycliques nitrés dans le poisson, les produits carnés et le fromage par chromatographie liquide à haute performance. Mitteilungen aus Lebensmitteluntersuchung und Hygiene. 2000;91:158–171. [Google Scholar]
- De Vos R, Van Dokkum W, Schouten A, de Jong-Berkhout P. Polycyclic aromatic hydrocarbons in Dutch total diet samples (1984–1986) Food Chem Toxicol. 1990;28:263–268. doi: 10.1016/0278-6915(90)90038-O. [DOI] [PubMed] [Google Scholar]
- Dennis M, Massey R, McWeeny D, Knowles M. Estimation of nitropolycyclic aromatic hydrocarbons in foods. Food Addit Contam. 1984;1:29–37. doi: 10.1080/02652038409385820. [DOI] [PubMed] [Google Scholar]
- Dias RC, Benassi MdT. Discrimination between arabica and robusta coffees using hydrosoluble compounds: is the efficiency of the parameters dependent on the roast degree? Beverages. 2015;1:127–139. doi: 10.3390/beverages1030127. [DOI] [Google Scholar]
- Dušek B, Hajšlová J, Kocourek V. Determination of nitrated polycyclic aromatic hydrocarbons and their precursors in biotic matrices. J Chromatogr A. 2002;982:127–143. doi: 10.1016/S0021-9673(02)01340-7. [DOI] [PubMed] [Google Scholar]
- Elschner B. Ch. P. Poole, jr.: Electron-Spin-Resonance, A Comprehensive Treatise on Experimental Techniques, John Wiley and Sons, New York, Chichester, Brisbane, Toronto, Singapore 1983. 780 Seiten, Preis:£ 61. Berichte der Bunsengesellschaft für physikalische Chemie. 1983;87:1230. doi: 10.1002/bbpc.19830871245. [DOI] [Google Scholar]
- Grosovsky AJ, Sasaki JC, Arey J, Eastmond D, Parks K, Atkinson R (1999) Evaluation of the potential health effects of the atmospheric reaction products of polycyclic aromatic hydrocarbons research report. Health Effects Institute. https://www.healtheffects.org/publication/evaluation-potential-health-effects-atmospheric-reaction-products-polycyclic-aromatic [PubMed]
- Hansson K-M, Samuelsson J, Tullin C, Åmand L-E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds. Combust Flame. 2004;137:265–277. doi: 10.1016/j.combustflame.2004.01.005. [DOI] [Google Scholar]
- IARC M . IARC monographs on the evaluation of carcinogenic risk to humans. Lyon: International Agency for Research on Cancer; 1989. [Google Scholar]
- IARC M . IARC monographs on the evaluation of carcinogenic risk to humans. Geneva: World Health Organization, International Agency for Research on Cancer; 1989. [Google Scholar]
- Kato T, Harashima T, Moriya N, Kikugawa K, Hiramoto K. Formation of the mutagenic/carcinogenic imidazoquinoxaline-type heterocyclic amines through the unstable free radical Maillard intermediates and its inhibition by phenolic antioxidants. Carcinogenesis. 1996;17:2469–2476. doi: 10.1093/carcin/17.11.2469. [DOI] [PubMed] [Google Scholar]
- Larsson BK, Pyysalo H, Sauri M. Class separation of mutagenic polycyclic organic material in grilled and smoked foodsAbtrennung mutagenen polycyclischen organischen Materials in gegrillten und geräucherten Lebensmitteln Zeitschrift für Lebensmittel-Untersuchung und. Forschung. 1988;187:546–551. doi: 10.1007/BF01042387. [DOI] [PubMed] [Google Scholar]
- Mendes LC, de Menezes HC, Aparecida M, Da Silva A. Optimization of the roasting of robusta coffee (C. canephora conillon) using acceptability tests and RSM. Food Qual Prefer. 2001;12:153–162. doi: 10.1016/S0950-3293(00)00042-2. [DOI] [Google Scholar]
- Miller J, Sandin R, Miller E, Rusch H. The carcinogenicity of compounds related to 2-acetylaminofluorene: II. Variations in the bridges and the 2-substituent. Can Res. 1955;15:188–198. [PubMed] [Google Scholar]
- Pederson T, Siak JS. The role of nitroaromatic compounds in the direct-acting mutagenicity of diesel particle extracts. J Appl Toxicol. 1981;1:54–60. doi: 10.1002/jat.2550010203. [DOI] [PubMed] [Google Scholar]
- Pitts JN, Jr, Harger W, Lokensgard DM, Fitz DR, Scorziell GM, Mejia V. Diurnal variations in the mutagenicity of airborne particulate organic matter in California’s south coast air basin. Mutat Res Lett. 1982;104:35–41. doi: 10.1016/0165-7992(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Ren Q, Zhao C, Chen X, Duan L, Li Y, Ma C. NOx and N2O precursors (NH3 and HCN) from biomass pyrolysis: Co-pyrolysis of amino acids and cellulose, hemicellulose and lignin. Proc Combust Inst. 2011;33:1715–1722. doi: 10.1016/j.proci.2010.06.033. [DOI] [Google Scholar]
- Rosenkranz HS, Mermelstein R. The genotoxicity, metabolism and carcinogenicity of nitrated polycyclic aromatic hydrocarbons. J Environ Sci Health C. 1985;3:221–272. [Google Scholar]
- Schlemitz S, Pfannhauser W. Monitoring of nitropolycyclic aromatic hydrocarbons in food using gas chromatography Zeitschrift für Lebensmittel-Untersuchung und. Forschung. 1996;203:61–64. doi: 10.1007/BF01267771. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Weiss R, Pfannhauser W. Sensitive method for the determination of nitrated polycyclic aromatic hydrocarbons in the human diet. Anal Bioanal Chem. 2003;375:175–181. doi: 10.1007/s00216-002-1653-8. [DOI] [PubMed] [Google Scholar]
- Tian F-J, Yu J, McKenzie LJ, J-i Hayashi, Li C-Z. Conversion of fuel-N into HCN and NH3 during the pyrolysis and gasification in steam: a comparative study of coal and biomass. Energy Fuels. 2007;21:517–521. doi: 10.1021/ef060415r. [DOI] [Google Scholar]
- Tokiwa H, Nakagawa R, Ohnishi Y. Mutagenic assay of aromatic nitro compounds with Salmonella typhimurium. Mutat Res Lett. 1981;91:321–325. doi: 10.1016/0165-7992(81)90008-7. [DOI] [PubMed] [Google Scholar]
- Turrio-Baldassarri L, Didomenico A, Rocca CL, Iacovella N, Rodriguez F. Polycyclic aromatic hydrocarbons in Italian national and regional diets. Polycycl Aromat Compd. 1996;10:343–349. doi: 10.1080/10406639608034715. [DOI] [Google Scholar]
- Vignoli JA, Viegas MC, Bassoli DG, de Toledo Benassi M. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res Int. 2014;61:279–285. doi: 10.1016/j.foodres.2013.06.006. [DOI] [Google Scholar]
- Ziegler W, Garcia Penalver L, Preiss U, Wallnoefer P. Fate of nitropolycyclic aromatic hydrocarbons in artificially contaminated fruits and vegetables during food processing. Adv Food Sci. 1999;21:54–57. [Google Scholar]
- Zimmermann K, Atkinson R, Arey J. Effect of NO2 concentration on dimethylnitronaphthalene yields and isomer distribution patterns from the gas-phase OH radical-initiated reactions of selected dimethylnaphthalenes. Environ Sci Technol. 2012;46:7535–7542. doi: 10.1021/es3009826. [DOI] [PubMed] [Google Scholar]