Abstract
Beekeeping has been widely promoted in many countries as a major contributor to rural development. Honey is a sweet and viscous liquid which has sweetness due to the presence of monosaccharides. The major constituents of honey are sugars, water, proteins, enzymes, acids and minerals, while the major causes of quality deterioration include heating at high temperatures, high moisture content, adulteration, poor packaging and poor storage conditions. Heating not only eases the processing of bottling by reducing the viscosity of honey, but also reduces the water content in honey to prevent fermentation and delays the granulation by destroying large sugar nuclei. The paper discusses about the different honey moisture reduction systems designed by research workers as well as beekeepers at farm level and the different quality parameters affected by thermal treatment of honey.
Keywords: Beekeeping, Honey, Temperature, Storage, Fermentation, Moisture reduction systems
Introduction
The activity of beekeeping provides a beneficial food and income source for rural households in developing countries. Beekeeping has been widely promoted in many countries as a major contributor to rural development (Kugonza and Nabakabya 2008). Products such as honey, beeswax, bee pollen, propolis, royal jelly, venom, queen bees and larvae are of socio-economic value (Krell 1996). Honey is a sweet and viscous liquid which has sweetness due to the presence of monosaccharides such as fructose and glucose. Its composition and chemical properties make it suitable for long-term storage. However, honey may crystallize after a period of time and this sometimes affect colour and consumer’s acceptability. The colour, flavour and aroma are the important quality characteristics of honey from consumer’s point of view. Honey is generally evaluated by physico-chemical analysis of its properties. Several of these constituents are of great importance to the honey industry as they influence the storage quality, granulation, texture, flavour and the nutritional and medicinal quality of the honey. The quality of honey is important for both local and international markets to ensure competitive prices and human health. Honey quality is an aspect generally disregarded by producers and processors especially in developing economies. The International Honey Commission (IHC) has proposed certain constituents as a measure of quality criteria for honey. These include moisture content, electrical conductivity, reducing sugars, amount of fructose and glucose, sucrose content, individual sugars, minerals, free acidity, diastase, HMF, invertase and proline (Bogdanov 1999). Proper understanding and standardisation of honey components and attributes that are most vulnerable during processing cannot be ignored.
The major constituents of honey are sugars, water, proteins, enzymes, acids and minerals, while the major causes of quality deterioration include heating at high temperatures, high moisture content, adulteration, poor packaging and poor storage conditions (Krell 1996). However, heating not only eases the bottling process by reducing the viscosity of honey (Anklam 1998), but also reduces the water content in honey to prevent fermentation (Subramanian et al. 2007). It destroys the sugar crystal nuclei to retard granulation by dissolving them during processing (Turhan et al. 2008; Escriche et al. 2009). The colour also becomes uniform throughout the sample for the preference of consumers (Abu-Jdayil et al. 2002) along with destruction of sugar tolerant osmophilic yeasts to prolong the shelf life of honey (Guo et al. 2011). The present review aims to discuss the importance of honey processing, different systems developed for moisture reduction of honey and the major quality parameters affected by the heat treatment.
Processing of honey
Heating is the utmost important processing step in honey production because it directly affects the quality of honey. Honey is generally heated indirectly due to bad effect of direct heating. Processing not only brings down the moisture content to safe limit but also delays crystallization in honey, thereby increases its shelf life.
Moisture in honey
Moisture is one of the most important parameter of honey quality. The amount of water present in honey determines its stability against fermentation and granulation (Dyce 1979). Honey having high water content ferments easily with time. So, it is necessary to process the honey by subjecting it to thermal treatment to prevent fermentation by sugar tolerant yeasts (Fallico et al. 2004). Treatment in a closed system minimizes losses of volatile aroma during heating. Processing temperature had significant effect on the moisture content. Honey processed at 60 °C had higher moisture (17.98%) as compared to 17.06 and 16.40% at 70 and 80 °C respectively while the processing time had non-significant effect on the moisture content of honey packaged in glass jars, plastic jars and poly pack pouches. The storage had significantly decreasing effect on the moisture content of honey packaged in plastic jars and poly pack pouches. The moisture content of honey decreased to 16.41 and 16.63% in plastic jars and poly pack pouches respectively from initial 18.10% after 12 months of storage (Minhas 2010). Chua et al. (2014) reduced the moisture content of honey to less than 20% after 30 min of heating at 90 °C and also concluded that higher the temperature (90 °C), higher the kinetic of water reduction. But heating of honey above 90 °C could result in caramelization of sugar (Yener et al. 1987).
Different systems have been developed at farmer as well as commercial level across the globe for reducing the moisture content of honey. These systems work either on thermal or microwave heating. The systems use rotating discs, cones, passing through holes or wire mesh for increasing the surface area of honey so as to facilitate faster and uniform moisture reduction.
Thermal heating based moisture reduction systems
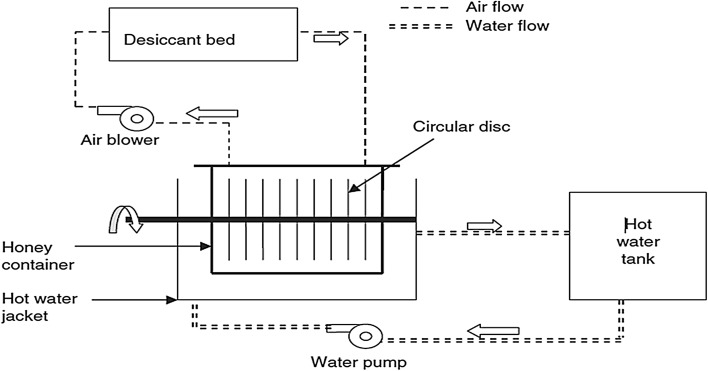
Gill et al. (2015) developed a small scale honey dehydrator (Fig. 1) to reduce moisture content of honey below 17%. Experiments were conducted for honey moisture reduction using drying air at ambient temperature, 30 and 40 °C and water at 35, 40 and 45 °C. Hot water was circulated in a water jacket around the honey container to heat honey. The heated honey was pumped through a sieve having 122 uniformly spaced holes of 0.5 cm diameter to form honey streams through which drying air passes for moisture removal. The honey streams help in increasing the exposed surface area of honey in contact with drying air. The maximum drying rate per square meter area of honey exposed to drying air at 40 °C was 197.0 g/h-m2 whereas it was minimum (74.8 g/h-m2) corresponding to the drying air at ambient temperature (8–17 °C). The energy cost of honey moisture content reduction from 25.2 to 16.4% was Rs. 6.20 to Rs. 17.36 per kilogram of honey. Singh et al. (2011) developed a desiccant honey dehydrator (Fig. 2) that heats and dehumidifies air to reduce moisture content of honey using a silica gel desiccant bed. Re-circulation was employed to prolong use-period of the desiccant bed. The moisture reduction was done with dehumidified or ambient air at 35 and 45 °C. The honey moisture was also reduced in open for reference purposes. The maximum moisture evaporation rates using dehumidified air, ambient air and open pan moisture reduction at 45 °C were 132, 78.7 and 52 g/h-m2, respectively.
Fig. 1.
A small scale honey dehydrator (Gill et al. 2015)
Fig. 2.
A desiccant honey dehydrator (Singh et al. 2011)
Semkiw et al. (2008) studied the conditions under which the excess water evaporates from unripe honey and studied the dynamics of the process. The study was conducted for 3 years in which 79 samples of unripe honey, 79 samples of dehydrated honey and 69 samples of in-hive-ripening honey were collected. Dehydration of honey samples was performed using air dehumidifier. The moisture content of honey reduced from 22.9 to 15.95% in 36 h. Wakhle et al. (1996) developed a honey moisture reduction unit which consisted of falling film evaporator (Fig. 3). In this multiple effect evaporation system, raw honey was preheated (40–45 °C) and then filtered through 80 μm polypropylene microfilter. This honey was heated up to 60–65 °C in first effect to destroy osmophilic yeast cells, held at 60 °C for evaporation of water under vacuum and then cooled in third effect before passing into settling tanks for bottling. The system had a capacity of processing 300 kg of honey per day. Samples of honey from three species viz. A. cerana, A. mellifera and A. dorsata were processed in the developed unit. The moisture content was reduced from 22.5 to 18.5% for A. cerana honey, 21.5 to 18.5% for A. mellifera honey and 24.5 to 19.5% in case of A. dorsata honey. It was observed that higher the moisture content in honey, higher was the reduction in same time. Also, more the extent of moisture reduction, there was increase in the values of reducing and non-reducing sugars, specific gravity etc. The processing cost was found to be Rs. 8 per kg of honey.
Fig. 3.

Layout of vacuum based honey processing-cum-moisture reduction system (Wakhle et al. 1996)
Maxwell (1987) used heating coils to exchange heat from re-circulating hot water to honey (Fig. 4). The honey was heated up to 49 °C and was then pumped to an evaporating table of size 1.2 m × 2.4 m which was heated up to 35 °C using air heater. The honey was spread on the table as a thin film of 0.025–0.04 m thickness where it was removed by blowing air over the table. In a trial run, about 75 kg of honey was dried up to moisture content of 17% in 1 h by using two electric fans at one end of the table. On the other hand, Ellis (1987) reduced the moisture of honey stored in barrels by heating a room up to 45 °C with the help of wood stove (Fig. 5). The heat in the room raises the temperature of honey up to 32 °C. Portable air compressor was used to generate bubbles as a stirring medium for uniform distribution of temperature inside honey by using 0.95 cm copper pipe. Warm air was also supplied into each barrel to evaporate water from honey. A fan was used to move the humid air over the barrels towards wood stove so that it can absorb or eliminate the moisture from the room. The system was run for 19 h which reduced the moisture content from 18.5 to 17.7%. Murrell and Henley (1988) suggested a method to reduce the moisture content of honey stacked in barrels. The barrels can be put in a room heated up to 27–30 °C and dehumdifier can be used to absorb excess moisture from the air. Proper ventilation and provision of ceiling fan for every 9–11 m2 area is beneficial for proper circulation of air for moisture reduction. It was suggested that electric heater can be used to provide more heat in the room or incoming air can be brought in the room through fan from passive solar collector.
Fig. 4.
A honey moisture reduction system using heating coils and table (Maxwell 1987)
Fig. 5.
Honey moisture reduction using wood stove heating and compressed air (Ellis 1987)
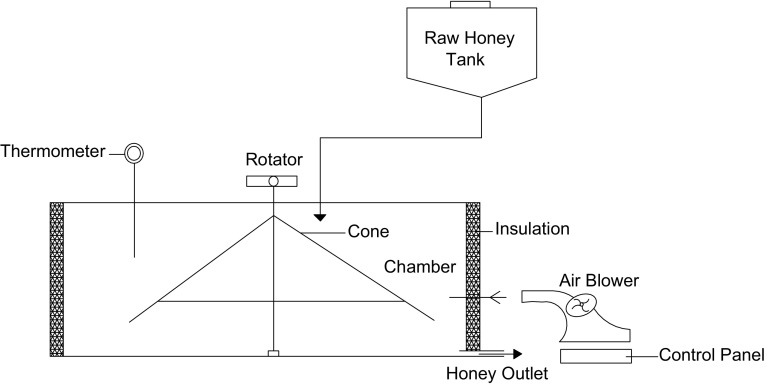
Kuehl (1988) made an apparatus that consisted of an enclosed housing with an inlet port on the upper side and outlet port at the lower edge (Fig. 6). Honey enters the inlet port and flows downward across a series of trays arranged in a zig-zag fashion up to the outlet port. Metal screens were used over each tray for spreading the honey uniformly across the trays. An evaporator coil and heater was used to dry and warm the air which was circulated over the honey layer for removal of moisture. Under operation, it can reduce the honey moisture content from 20 to 18.5% in one pass for air flow rate of about 28 m3/min and temperature of about 49 °C. While Wakhle et al. (1988) designed a small scale honey moisture reduction system consisted of a rotating stainless steel cone of 0.60 m diameter and 0.65 m height arranged in an insulated chamber (Fig. 7). A portable blower was used to supply hot and filtered air. The moisture content of honey was reduced from 25.50 to 22.50% in a single run when air at 65–67 °C was blown in the system.
Fig. 6.
An apparatus for honey moisture reduction (Kuehl 1988)
Fig. 7.
Rotating cone type honey moisture reduction system (Wakhle et al. 1988)
Platt and Ellis (1984) removed the moisture from honey as a thin contact film by using rotating discs at speed of 10 rpm in a honey moisture removal chamber (Fig. 8). Hot air was blown at temperature of 45–75 °C. In batch run, moisture of 1468 g honey with air blowing at 0.22 m/min was reduced from 26.6 to 15.2% in 2 h whereas it was reduced from 29.6 to 16.9% in 1 h when honey flows at 15.3–16.0 g/min with air flow at 50 °C and 27% relative humidity.
Fig. 8.
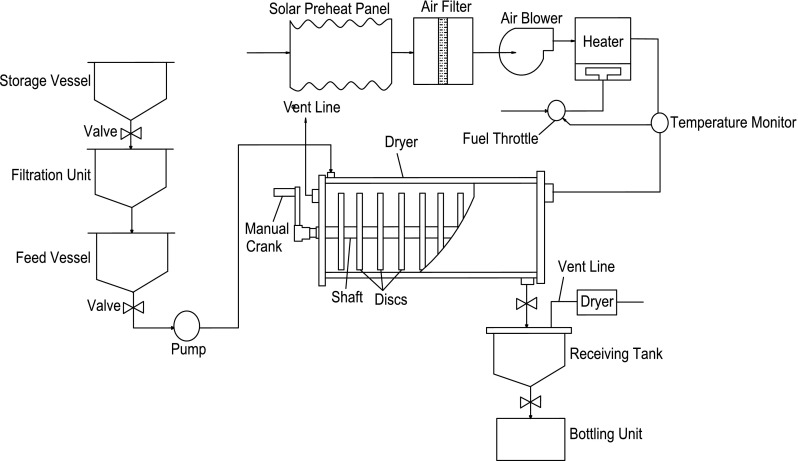
Rotating discs type honey moisture reduction system (Platt and Ellis 1984)
Solar energy based moisture reduction systems
Much work has not been done so far to develop a system using solar energy for heating the honey for moisture removal as very less literature is available on this aspect. However, a system was made by Paysen (1987) at farm level that used solar energy using green house effect. The system consisted of an oversized solar panel of size 3.66 m × 4.88 m used on the southern side of the room in a building to trap more heat. The honey was fed on the tray rack having 24 trays from top to bottom making total surface area exposed for honey to be 37 m2. The trays were inclined with one another at an angle of 30° in a zig-zag manner from top to bottom. The honey was fed at the top of trays from 5 cm honey pipe and was made to flow from top to bottom from slits provided at the end of trays and it was re-circulated till it reaches moisture content of about 18%. The shades were provided in the room to avoid direct exposure of honey to sunlight.
Microwave based honey moisture reduction
Microwave heating is a new, selective technique for short and intensive thermal processing. Its application is well known in the food industry, particularly for tempering, blanching, drying and pasteurization of food material (Ghazali et al. 1994). Microwave heating is more affected by the presence of water in foods (Tulasidas et al. 1995) as water is the major absorber of microwave energy in food, therefore, higher the moisture content, better the heating effects. In contrast to conventional heating, microwaves penetrate the material, interact with it and generate heat leading to its rapid heating. Materials containing polar molecules such as water are rapidly heated when exposed to microwave radiation due to molecular friction generated by dipolar rotation in the presence of an alternating electric field. Since honey contains a substantial amount of water (18–24%) as well as large amounts of dissolved sugars (70–80%), microwave radiation could be effectively used for heating honey. For natural honey with 18% moisture content, the dielectric loss increases with temperature for frequencies above 1 GHz. The dielectric loss of honey was found to increase with water content at low frequencies due to ionic conduction (Guo et al. 2011).
Microwave heating studies were conducted in a micro-convective oven with turntable attachment (Hebbar et al. 2003). Experiments were carried out at different power levels (PL) ranging from 10 to 100 on a microwave having maximum power of 850 W and for different heating periods of 15–90 s. The samples were mixed for 2 s at regular interval of every 15 s to provide uniform heating. The product temperature was measured at the end of the heating period. The samples were cooled to room temperature immediately after the heat treatment to preserve quality. Reduction in moisture content was above 9% at power levels of 50, 70 and 100 when the samples were heated for 60 s. Larger reduction in moisture content was not observed at lower power levels.
Microwave vacuum drying was also investigated as a potential method for obtaining high quality dried honey (Cui et al. 2008). The temperatures applied to honey being dried were very close to the water saturation temperatures corresponding to the vacuum levels applied (30 and 50 mbar) at the beginning of the drying period. It was observed that the higher the microwave power intensity, the faster the drying rate. Similarly, Kowalski et al. (2012) showed that a short microwave treatment (0–2 min) with a low power level (63 W) did not influence the honey quality.
Effect of thermal heating on honey quality
Heating of honey to prevent fermentation by sugar-tolerant yeasts and to keep it liquid as long as possible causes several desirable and undesirable changes. Diastase activity and hydroxymethylfurfural (HMF) are considered as the main parameters for its quality evaluation after heating. Other than these quality parameters, there is change in viscosity (needed for easy flow), pH, acidity and antioxidant activity of honey. Colour, an important physical parameter, is required for consumer preference also under goes change during heating which is also studied by many research workers.
Effect on viscosity
Viscosity of honeys is an important property that has been studied by various researchers (Yanniotis et al. 2006; Bhandari et al. 1999). Viscosity has an influence on the physico-chemical and sensory properties of honey (Juszczak and Fortuna 2006) and knowledge about the rheological properties of honey is useful in its processing, handling and storage (Ahmed et al. 2007). Honey has high viscosity (1.36 Ns/m2 at 25 °C and 21.5% moisture) that cause problems in handling and processing. Viscosity of honey depends on factors such as temperature, water content, chemical constitution, amount and size of crystals present in it (Juszczak and Fortuna 2006; Yoo 2004). As honey is heated, it initially undergoes a very rapid decrease in viscosity up to 30 °C but after that the change is very slow. Besides temperature and moisture content, variations in viscosity are attributed to the composition of individual sugars and non-sugar and colloidal material (Subramanian et al. 2007). Generally honey is considered to exhibit Newtonian behavior (Sopade et al. 2002; Bhandari et al. 1999) but some researchers also reported about non-Newtonian behavior of honey, which is due to the presence of protein colloids (Ahmed et al. 2007; Juszczak and Fortuna 2006). Yanniotis et al. (2006) reported the Newtonian behavior of honey after studying the effect of moisture content on its viscosity at different temperatures. Escobedo et al. (2006) also reported that honey samples at the 12th week of storage showed tendency towards non-Newtonian behaviour due to the presence of crystals which are mainly responsible for change in flow behavior.
Effect on enzymes in honey
Any type of honey possesses several kinds of enzyme that play both nutritional and analytical role in the product. One of the most important honey enzymes is diastase that is capable to break down glycosidic linkages in oligo- and polysaccharides i.e. starch into simple sugars. It is very sensitive to heat. Therefore proper heating and storage is of utmost importance to retain the market value of honey. The activity of this enzyme decreases with the time of storage and that of heating. Diastase activity is measured as the diastase number (Hooper 1983). The starch-digesting enzymes of honey are used as indicators of honey quality because of their heat sensitivity (Subramanian et al. 2007). Huidobro et al. (1995) determined the diastase activity of honey and reported values in the range of 11.3–34.5 diastase number. Sahinler and Gul (2005) in a study assessed that heating treatment at 55–65 °C did not have much effect on diastase number but was more affected by storage time. However, honey produced in warmer climates has been observed to have lower diastase activity (LaGrange and Sanders 1988).
The diastase activity first increased from 40 to 50 °C and then decreased almost regularly with the increase in temperature up to 80 °C (Khan et al. 2015a). But Hasan (2013) found that heat treatment of honey at 55, 65, 75 °C for 5, 15, 20 and 25 min did not affect diastase and invertase activity but they were more affected by storage time. Diastase can also be inactivated in short time using microwave treatment but it becomes difficult to establish rate constant (Kowalski et al. 2012). The diastase activity is also more sensitive to prolongation of heating time than to increasing the temperature. After 3 h at 50 °C, the enzymatic activity is more drastically reduced (53.71%) than in cases of heating at higher temperature but shorter time ranges such as 48.29% for 0.5 h at 100 °C or 49.55% for 1 h heating at 80 °C (Cozmuta et al. 2011). Hernandez et al. (2015) applied thermal treatments (pasteurization and tyndallization) to Apis mellifera and Tetragonisca angustula honeys, to evaluate the effect on some physicochemical and microbiological parameters associated to honey quality. The honey bottled in amber glass recipients was pasteurized to 65 °C for 15 and 21 min and was tyndallized to 80 °C during 5 and 7 min. Honey samples heat treated for pasteurization and tyndallization during 15 and 21 min showed statistically significant differences in diastase activity, in comparison to untreated honey. According to Tosi et al. (2008), diastase activity loss occurred as temperature increases. The decrease in diastase activity was more significant at higher rates of temperature increase. On the contrary, during the isothermal heating (temperature remains constant) the diastase activity decreased for short time treatments (typically for 120 s) but increased when time was increased. The effect was closely related to all temperatures under study i.e. 60–100 °C with the exception of 100 °C treatments in which the diastase activity became null.
Effect on hydroxymethyl furfural in honey
Hydroxymethyl furfural (HMF) is formed from fructose in the presence of acid. Honey is acid enough to facilitate this change. Its production is very slow in honey at normal temperatures during the handling process. Naturally occurring levels of HMF are about 10 mg/kg (Crane 1990). The amount formed, however, increases with increasing heat treatment. Heating honey above 75 °C for a few minutes or storing honey at temperatures above 27 °C for several months increases HMF levels. A maximum content of less than 40 mg/kg is allowed in honey in the international market. Amounts exceeding this maximum limit are considered a main indicator of honey deterioration (White 1979; Bogdanov and Martin 2002) either through heating or long periods of storage. Higher values of HMF also points towards the possibility of honey adulteration by invert syrup (Doner 1977). Moreover, there is always variation in the formation of HMF in different honey types which is mainly due to sugar composition and pH (Singh and Bath 1997). Heating of honey having low pH results in more HMF formation (Hase et al. 1973).
Sahinler and Gul (2005) observed that HMF content increased significantly both with storage time and heating. Similarly, Cozmuta et al. (2011) found a strong impact of heating temperature and time on formation of HMF in honey when subjected to thermal treatment at 50, 80 and 100 °C for 0.5–5 h time. Similarly, Hasan (2013) assessed the impact of heating and storage time (at 23 °C) in three types of Iraqi honey heated at 55, 65, 75 °C for 5, 15, 20 and 25 min and concluded that HMF content of honey was significantly affected by storage time and heat treatment. Khan et al. (2015a) studied the kinetics of HMF formation by applying isothermal heat treatment with different time–temperature combinations of 40–80 °C for 5–25 min. Mustard and mixed floral honey samples were taken for the study. The HMF level increased regularly from 5 to 25 min. for both the honey samples at each temperature and the increase was higher at 70 and 80 °C indicating the temperature dependence on formation of HMF. The HMF content proportionally increase with the increase of heating time up to 60 min at 63 and 90 °C and the kinetic studies showed that the HMF formation in honey followed zero order kinetic model for the first 60 min of heating at 90 °C (Chua et al. 2014). Singh and Bath (1998) computed second order polynomials for HMF formation in Eucalyptus lanceolatus, Brassica juncea and Trifolium honey types by heating them at 65, 85 and 95 °C for 5, 15 and 30 min. They found significant effect of temperature and time on HMF formation in Trifolium and Eucalyptus lanceolatus honey whereas heating time was more significant in Brassica juncea honey. It was concluded that second order polynomials can be used as a tool to study the effect of processing temperature and period on HMF formation in different honey types.
Chakraborti and Bhattacharya (2014) investigated 21 honey samples collected from different places of India. It was found that 16 samples contain higher values of HMF in respect to international standard limit (80 mg/kg) during storage (Codex 2001) and concluded that the high HMF content in most of the honey samples was might be due to their exposure in high heat stress during processing and storage. In case of microwave heating, the increase in power levels has more significant effect on the formation of HMF in different types of honey as compared to duration of heating (Bath and Singh 1999, 2001). Diab and Jarkas (2015) conducted a study to assess the influence of thermal treatment and storage conditions on the content of 5-hydroxymethylfurfural (HMF) in different types of Latakia honey using microwave as well as conventional methods at 50, 80, 100 °C for 1, 2 and 3 h intervals. It was found that microwave heating had insignificant effect on HMF content in the four crystallized samples of honey. According to Bartakova et al. (2011), HMF could be dissolved using microwave radiation which would change the role of HMF as an indicator of the damage done to honey by heating. In addition, it was found from the study of microwave heating that despite the honey was exposed to high temperature levels (80–90 °C) at the highest power levels and the longest time periods, there was no significant increase in HMF content as expected during conventional heating.
Ribeiro et al. (2012) evaluated the evolution of HMF levels in fresh extracted honeys submitted to different temperatures ranging from 30 to 100 °C during time intervals of 30, 45, 60, 180 and 720 min. The highest values were observed in samples subjected to heating over 720 min and the limit was exceeded at 70 °C. The findings indicated that the HMF content gradually increases when the honey is heated at high temperatures for long periods. Tosi et al. (2004) found that heating of honey at 80 °C, during 60 and 30 s in transient and isothermal stages, respectively, destroyed all microorganisms responsible for quality damage without spoiling honey. Besides, crystallization beginning delayed between 4 and 9 weeks on four selected honey samples stored at 4 and 20 °C respectively. Therefore, it has been suggested that temperature and duration of heating during processing must be controlled in relation to the floral source from which the honey has been extracted to avoid excess formation of HMF (Singh and Bath 1997, 1998).
Effect on pH and acidity in honey
Fallico et al. (2004) studied the effect of conditioning on four unifloral Sicilian (Orange, Eucalyptus, Sulla, Chestnut) honeys and reported that initial pH played important role in HMF formation during heat treatment especially at low temperatures i.e. different pH values could lead to different HMF levels. They found that at lower temperature (50 °C), Chestnut honey with the highest pH did not form any HMF even after 144 h (6 days) of heating as compared to other three samples. On the other hand, Samira (2016) reported that pH decreases rapidly linearly by increasing the heating temperature of honey and so there is increase in acidity of honey. The change in acidity during heating is caused by chemical reaction between sugars and amino acids.
Effect on antioxidative properties of honey
According to Aljadi and Kamaruddin (2004), the composition and antioxidant capacity of honey depend on the floral sources, seasonal and environmental factors as well as processing methods. Increased heat treatment of honey leads to development of antioxidant activity which has positive effects on human health due to formation of Maillard reaction products (Turkmen et al. 2006). It increased linearly with increasing heating time at 50 and 60 °C but logarithmically at 70 °C. Kowalski (2013) analyzed four types of honey viz. honeydew, lime, acacia, buck wheat for antioxidant activity after microwave (1.26 W/g for 6 min) and thermal heat treatment at 90 °C for 60 min. In the case of acacia honey, there were no significant changes measured by either method. In the case of lime and buckwheat honey an increase in antioxidant properties was found, under the influence of the microwave field. In case of conventionally heated lime honey, the content of polyphenols also increased in contrast to the buckwheat honey, which showed a decline in these properties. It was observed that the changes lead to increase in antioxidant potential of honey. Khan et al. (2015b) also reported an increase in antioxidant activity at 50 °C in Mustard based and mixed floral honey types but the increase was low in comparison to other temperatures viz, 60, 70 or 80 °C. Both the honey types followed first order kinetics at 50 °C.
Chaikham and Prangthip (2015) in their study on antioxidative properties of longan flower honey during thermal treatment found that samples heated at 50 and 70 °C for 5 min had the highest amounts of total phenolic compounds and total flavonoids. Antioxidative activity slightly decreases during thermal treatments with no significant difference. It was concluded that the increase of the phenolic compounds in heated samples might be due to the extraction by thermal processing. On the contrary, when heated at 100 °C, the levels of antioxidant compounds and properties diminished significantly with increasing processing time.
Effect on colour of honey
The honey colour is one of its most changing features. Heating of honey causes non-enzymatic browning due to Maillard reaction which occurs when sugars condense with free amino acids to form a variety of brown pigments. It is believed that Maillard reaction products (MRPs) act as antioxidants. Polyphenols, ascorbic acid and other carbonyl compounds even if formed during oxidative reactions participate in Maillard reaction itself (Manzocco et al. 2001). It causes darkening affect in honey. The degree of darkening is affected by the temperature and/or time of storage (Pereyra et al. 1999). But browning that occurred by heating is not desirable for consumers. Hence, a balance between positive and negative effects should be taken into account before simulating their formation during processing (Turkmen et al. 2006). Khan et al. (2015b) found that colour changes in honey at all temperatures can be depicted by first order kinetic model. There was also significant correlation between antioxidant activity and colour change in honey after thermal treatment. Colour is also one of the major quality degradation parameter during storage of honey and is dependent on moisture content along with storage temperature of honey (Bulut and Kilic 2009).
Conclusion
Apart from conventional heating, different moisture reduction systems have been designed across the globe in which either discs, cones or small screen holes have been used for increasing surface area of honey to be dried and vacuum or heated air for removal of moisture from the system. Different trials have also been done by the beekeepers for reduction of moisture directly in a room from honey storage drums. Honey is thermally treated as a part of processing which is required for easy handling, to dissolve large sugar granules, to reduce moisture for increasing shelf life, to destroy spoilage microorganisms i.e. fermentation causing yeast. This also helps in delaying granulation in honey. But honey quality is significantly affected by storage time and heating that may cause deterioration. From the study of different quality parameters as discussed, diastase activity, HMF and colour are more affected by heat treatment and are functions of both time and temperature.
References
- Abu-Jdayil B, Al-Majeed GA, Al-Malah KIM, Zaitoun S. Heat effect on rheology of light and dark-colored honey. J Food Eng. 2002;51(1):33–38. doi: 10.1016/S0260-8774(01)00034-6. [DOI] [Google Scholar]
- Ahmed J, Prabhu ST, Ragvahan GSV, Ngadi M. Physico-chemical, rheological, calorimetric and dielectric behavior of selected Indian honey. J Food Eng. 2007;79:1207–1213. doi: 10.1016/j.jfoodeng.2006.04.048. [DOI] [Google Scholar]
- Aljadi AM, Kamaruddin MY. Evaluation of the phenolic content and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518. doi: 10.1016/S0308-8146(02)00596-4. [DOI] [Google Scholar]
- Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998;63:549–562. doi: 10.1016/S0308-8146(98)00057-0. [DOI] [Google Scholar]
- Bartakova K, Drackova M, Borkovc IO, Lenka V. Impact of microwave heating on hydroxymethylfurfural content in Czech honeys. Czech J Food Sci. 2011;29(4):328–336. doi: 10.17221/110/2009-CJFS. [DOI] [Google Scholar]
- Bath PK, Singh N. A comparison between Helianthus annuus and Eucalyptus lanceolatus honey. Food Chem. 1999;67:389–397. doi: 10.1016/S0308-8146(99)00132-6. [DOI] [Google Scholar]
- Bath PK, Singh N. Effect of microwave heating on hydroxymethylfurfural formation and browning in Helianthus annuus and Eucalyptus lanceolatus honey. J Food Sci Technol. 2001;38(4):366–368. [Google Scholar]
- Bhandari B, D‘Aray B, Chow S. A research note: rheology of selected Australian honeys. J Food Eng. 1999;41:65–68. doi: 10.1016/S0260-8774(99)00078-3. [DOI] [Google Scholar]
- Bogdanov S. Honey quality and international regulatory standards: review by the International Honey Commission. Bee World. 1999;90:61–69. doi: 10.1080/0005772X.1999.11099428. [DOI] [Google Scholar]
- Bogdanov S, Martin P. Honey authenticity: a review. Mitt Lebensm Hyg. 2002;93:232–254. [Google Scholar]
- Bulut L, Kilic M. Kinetics of hydroxymethylfurfural accumulation and color change in honey during storage in relation to moisture content. J Food Process Preserv. 2009;33:22–32. doi: 10.1111/j.1745-4549.2008.00233.x. [DOI] [Google Scholar]
- Chaikham P, Prangthip P. Alteration of antioxidative properties of longan flower honey after high pressure, ultrasonic and thermal processing. Food Biosci. 2015;10:1–7. doi: 10.1016/j.fbio.2015.01.002. [DOI] [Google Scholar]
- Chakraborti T, Bhattacharya K. Quality assessment of some Indian honeys in storage through HMF content and invertase activity. Int J Pharm Pharm Sci. 2014;6(2):827–830. [Google Scholar]
- Chua LS, Adnan NA, Abdul-Rahaman NL, Sarmidi MR. Effect of thermal treatment on the biochemical composition of tropical honey samples. Int Food Res J. 2014;21(2):773–778. [Google Scholar]
- Codex Alimentarius Commission Adopting the draft revised standard for honey. Alinorm. 2001;1(25):22–24. [Google Scholar]
- Cozmuta AM, Cozmuta LM, Varga C, Marian M, Peter A. Effect of thermal processing on quality of polyfloral honey. Romanian J Food Sci. 2011;1(1):45–52. [Google Scholar]
- Crane E. Bees and beekeeping: science, practice and world resources. London, UK: Heinemann Newness; 1990. pp. 388–451. [Google Scholar]
- Cui ZW, Sun LJ, Chen W, Sun DW. Preparation of dry honey by microwave-vacuum drying. J Food Eng. 2008;84:582–590. doi: 10.1016/j.jfoodeng.2007.06.027. [DOI] [Google Scholar]
- Diab DA, Jarkas B. Effect of storage and thermal treatment on the quality of some local brands of honey from Latakia markets. J Entomol Zool Stud. 2015;3(3):328–334. [Google Scholar]
- Doner LW. The sugars of honey: a review. J Sci Food Agric. 1977;28:443–456. doi: 10.1002/jsfa.2740280508. [DOI] [PubMed] [Google Scholar]
- Dyce EJ. Producing finely granulated or creamed honey. In: Crane E, editor. Honey—a comprehensive survey. London: Heinemann; 1979. pp. 293–306. [Google Scholar]
- Ellis M. Lowering the moisture content of small lots of extracted honey. Am Bee J. 1987;127:182–183. [Google Scholar]
- Escobedo RM, Ordonez YM, Flores MEJ, Lopez GFG. The composition, rheological and thermal properties of Tajonal (Viguiera Dentata) Mexican honey. Int J Food Prop. 2006;9:299–316. doi: 10.1080/10942910600596159. [DOI] [Google Scholar]
- Escriche I, Visquert M, Juan-Borras M, Fito P. Influence of simulated industrial thermal treatments on the volatile fractions of different varieties of honey. Food Chem. 2009;112(2):329–338. doi: 10.1016/j.foodchem.2008.05.068. [DOI] [Google Scholar]
- Fallico B, Zappala M, Arena E, Verzera A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004;85:305–313. doi: 10.1016/j.foodchem.2003.07.010. [DOI] [Google Scholar]
- Ghazali HM, Ming TC, Hashim DM. Effect of microwave heating on the storage and properties of starfruit honey. ASEAN Food J. 1994;9:30–35. [Google Scholar]
- Gill RS, Hans VS, Singh S, Pal Singh P, Dhaliwal SS. A small scale honey dehydrator. J Food Sci Technol. 2015;52(10):6695–6702. doi: 10.1007/s13197-015-1760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu Y, Zhu X, Wang S. Temperature dependent dielectric properties of honey associated with dielectric heating. J Food Eng. 2011;102:209–216. doi: 10.1016/j.jfoodeng.2010.08.016. [DOI] [Google Scholar]
- Hasan SH. Effect of storage and processing temperatures on honey quality. J Babylon Univ/Pure Appl Sci. 2013;21(6):2244–2253. [Google Scholar]
- Hase S, Suzuki O, Odate M, Suzuki S. Changes in quality of honey on heating and storage-I. Changes in hydroxymethylfurfural (HMF) content of honey. J Food Sci Technol. 1973;20:248–256. doi: 10.3136/nskkk1962.20.248. [DOI] [Google Scholar]
- Hebbar HU, Nandini KE, Lakshmi MC, Subramanian R. Microwave and infrared heat processing of honey and its quality. Food Sci Technol Res. 2003;9(1):49–53. doi: 10.3136/fstr.9.49. [DOI] [Google Scholar]
- Hernandez C, Correa A, Quicazan M. Effect of the tyndallization on the quality of Colombian honeys. Chem Eng Trans. 2015;43:19–24. [Google Scholar]
- Hooper T. Guide to bees and honey. 2. New York: Sterling Pub Co Inc.; 1983. [Google Scholar]
- Huidobro JF, Santana FJ, Sanchez MP, Sancho MT, Muniategui S, Simal-Lozano J. Diastase, invertase and ß-glucosidase activities in fresh honey from north-west Spain. J Apic Res. 1995;34(1):39–44. doi: 10.1080/00218839.1995.11100884. [DOI] [Google Scholar]
- Juszczak L, Fortuna T. Rheology of selected Polish honeys. J Food Eng. 2006;75:43–49. doi: 10.1016/j.jfoodeng.2005.03.049. [DOI] [Google Scholar]
- Khan ZS, Nanda V, Bhat MS, Khan A. Kinetic studies of HMF formation and diastase activity in two different honeys of Kashmir. Int J Curr Microbiol App Sci. 2015;4(4):97–107. [Google Scholar]
- Khan ZS, Khan IA, Naik HR, Bhat MS. Kinetic studies on anti-oxidant activity vis-à-vis colour in two Kashmir honeys. Appl Biol Res. 2015;17(1):15–23. doi: 10.5958/0974-4517.2015.00003.8. [DOI] [Google Scholar]
- Kowalski S. Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. Food Chem. 2013;141:1378–1382. doi: 10.1016/j.foodchem.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Kowalski S, Lukasiewicz M, Bednarz S, Panus M. Diastase number changes during thermal and microwave processing of honey. Czech J Food Sci. 2012;30:21–26. doi: 10.17221/123/2010-CJFS. [DOI] [Google Scholar]
- Krell R. FAO agricultural services bulletin. 124. Rome: Food and Agricultural Organisation; 1996. Value added products from bee keeping; p. 371. [Google Scholar]
- Kuehl LJ (1988) Apparatus for removing moisture from honey. US Patent No. 4763572
- Kugonza DR, Nabakabya D. Honey quality as affected by handling, processing and marketing channels in Uganda. Tropicultura. 2008;26(2):113–118. [Google Scholar]
- LaGrange V, Sanders SW. Honey in cereal-based new food products. Cereal Foods World. 1988;33:833–838. [Google Scholar]
- Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol. 2001;11:340–346. doi: 10.1016/S0924-2244(01)00014-0. [DOI] [Google Scholar]
- Maxwell H. A small-scale honey drying system. Am Bee J. 1987;127:284–286. [Google Scholar]
- Minhas S (2010) Nutritional, storage and value addition studies on raw and heat processed honey. Ph.D. thesis, CSKHPKV, Palampur
- Murrell D, Henley B. Drying honey in a hot room. Am Bee J. 1988;128:347–351. [Google Scholar]
- Paysen J. A method for drying honey on a commercial scale. Am Bee J. 1987;127:273–282. [Google Scholar]
- Pereyra GA, Burin L, Pilar BM. Color changes during storage of honeys in relation to their composition and initial colour. Food Res Int. 1999;32:185–191. doi: 10.1016/S0963-9969(99)00075-7. [DOI] [Google Scholar]
- Platt JL Jr., Ellis JRB (1984) Removing water from honey at ambient pressure. US Patent No. 4472450
- Ribeiro ROR, Carneiro CS, Mársico ET, Cunha FL, Junior CAC, Mano SB. Influence of the time/temperature binomial on the Hydroxymethylfurfural content of floral honeys subjected to heat treatment. Ciênc Agrotecnol. 2012;36(2):204–209. doi: 10.1590/S1413-70542012000200009. [DOI] [Google Scholar]
- Sahinler N, Gul A. Effect of heating and storage on honey hydroxymethylfurfural and diastase activity. J Food Technol. 2005;3(2):152–157. [Google Scholar]
- Samira N. The effect of heat treatment on the quality of Algerian honey. Researcher. 2016;8(9):1–6. [Google Scholar]
- Semkiw P, Skowronek W, Skubida P. Changes in water content of honey during ripening under controlled condition. J Apic Sci. 2008;52(1):57–63. [Google Scholar]
- Singh N, Bath PK. Quality evaluation of different types of Indian honey. Food Chem. 1997;58(1–2):129–133. doi: 10.1016/S0308-8146(96)00231-2. [DOI] [Google Scholar]
- Singh N, Bath PK. Relationship between heating and hydroxymethylfurfural formation in different honey types. J Food Sci Technol. 1998;35(2):154–156. [Google Scholar]
- Singh S, Gill RS, Singh PP. Desiccant honey dehydrator. Int J Ambient Energy. 2011;32(2):62–69. doi: 10.1080/01430750.2011.584450. [DOI] [Google Scholar]
- Sopade PA, Halley P, Bhandari B, D’Arcy B, Doebler C, Caffin N. Application of the Williams Landel Ferry model to the viscosity–temperature relationship of Australian honeys. J Food Eng. 2002;56:67–75. doi: 10.1016/S0260-8774(02)00149-8. [DOI] [Google Scholar]
- Subramanian R, Hebbar HU, Rastogi NK. Processing of honey: a review. Int J Food Prop. 2007;10(1):127–143. doi: 10.1080/10942910600981708. [DOI] [Google Scholar]
- Tosi E, Re E, Lucero H, Bulacio L. Effect of honey high-temperature short-time heating on parameters related to quality, crystallisation phenomena and fungal inhibition. Lebensm Wiss Technol. 2004;37:669–678. doi: 10.1016/j.lwt.2004.02.005. [DOI] [Google Scholar]
- Tosi E, Martinet R, Ortega N, Lucero H, Re E. Honey diastase activity modified by heating. Food Chem. 2008;106:883–887. doi: 10.1016/j.foodchem.2007.04.025. [DOI] [Google Scholar]
- Tulasidas TN, Raghavan GSV, Van de Voort F, Girard R. Dielectric properties of grapes and sugar solutions at 2.45 GHz. J Microw Power Electromagn Energy. 1995;30:117–123. doi: 10.1080/08327823.1995.11688266. [DOI] [PubMed] [Google Scholar]
- Turhan I, Tetik N, Karhan M, Gurel F, Tavukcuoglu RH. Quality of honeys influenced by thermal treatment. LWT Food Sci Technol. 2008;41:1396–1399. doi: 10.1016/j.lwt.2007.09.008. [DOI] [Google Scholar]
- Turkmen N, Sari F, Poyrazoglu ES, Velioglu YS. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006;95(4):653–657. doi: 10.1016/j.foodchem.2005.02.004. [DOI] [Google Scholar]
- Wakhle DM, Nair SK, Phadke RP. Reduction of excess moisture in honey-I, a small scale unit. Indian Bee J. 1988;50:98–100. [Google Scholar]
- Wakhle DM, Phadke RP, Pais DVE, Nair SK. Design for honey processing unit—part II. Indian Bee J. 1996;58:5–9. [Google Scholar]
- White JW. Composition of honey. In: Crane E, editor. Honey—a comprehensive survey. London: Heinemann; 1979. pp. 157–158. [Google Scholar]
- Yanniotis S, Skaltsi S, Karaburnioti S. Effect of moisture content on the viscosity of honey at different temperatures. J Food Eng. 2006;72:372–377. doi: 10.1016/j.jfoodeng.2004.12.017. [DOI] [Google Scholar]
- Yener E, Ungan S, Ozilgen M. Drying behavior of honey–starch mixtures. J Food Sci. 1987;52:1054–1058. doi: 10.1111/j.1365-2621.1987.tb14274.x. [DOI] [Google Scholar]
- Yoo B. Effect of temperature on dynamic rheology of Korean honeys. J Food Eng. 2004;65(3):459–463. doi: 10.1016/j.jfoodeng.2004.02.006. [DOI] [Google Scholar]