Abstract
Yeast strain plays a central role in the formation of aroma and flavour of fruit wine. The effect of four commercial Saccharomyces cerevisiae strains (D254, VIC, BV818 and CECA) on volatile compounds of fermented pineapple (Ananas comosus L. Merr.) juice was investigated. Alcohols and esters were the most abundant groups in terms of the amounts of identified volatiles in four pineapple wines, followed by acids and sulphur compounds. Different S. cerevisiae strains possess various capacities to release or synthesize volatiles during pineapple wine fermentation. For global aroma, strain D254 yielded the highest total number and concentration of volatiles and could be used as a starter culture for the making of intense pineapple wine. Strain BV818 produced wine with the highest amounts of volatiles with OAVs > 1 and scored the highest in global aroma. Thus, BV818 might be the appropriate strain that could impart characteristic aromas and enhance wine complexity. The relative content of esters formed by strain VIC was higher than that yielded by the other strains. Strain CECA produced the highest relative contents of alcohols, aldehydes and ketones. However, VIC and CECA were not ideal starter cultures because of their low sense scores. This study provided a foundation for the production of pineapple wine with the desired flavour profile.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3338-0) contains supplementary material, which is available to authorized users.
Keywords: Saccharomyces cerevisiae, Volatile compound, Pineapple, Fruit wine
Introduction
Pineapple (Ananas comosus L. Merr.), which belongs to the Bromeliaceae family, is one of the most popular tropical fruits widely planted in southern China. Pineapple is normally consumed fresh, but its availability is restricted by its seasonal fruiting character and limited shelf-life. Developing pineapple products can overcome seasonal problems and reduce post-harvest losses of excess fruits, thereby promoting agricultural diversification and increasing economic value (Roda et al. 2017). With the popularity and diversity of fruit wine, pineapple wine has been considered as an appealing product of pineapple. Pineapple contains high amounts of nutrients and exudes an inviting fruity aroma. Fresh pineapple has a water content of up to 80%, and its juice can be easily extracted and filtered, suggesting that pineapple is suitable for wine making (Bamidele and Fasogbon 2017; Chanprasartsuk et al. 2010).
Aroma is an important factor that determines the flavour and quality of the final fruit wine product (Jiang and Zhang 2010). Volatile compounds that originate from fruits and are produced during alcoholic fermentation and aging process are responsible for the aroma of fruit wines (Duarte et al. 2010). Fermentative volatile constituents synthesized by yeast considerably vary during wine making, and they are essential for wine flavour. Yeast strain plays a central role in the formation of aroma and flavour of fruit wine. Volatile components released from the flavour precursors of fruits by yeast and yeast-derived volatiles synthesized during fermentation can influence the flavour characteristic of fruit wine. The distinguishing flavour profiles remarkably affect the sensory properties of wines fermented with different yeast strains despite the same fermentation. The selection of a suitable yeast strain is critical for producing the desired fruit wine. Nowadays, various wine yeast strains are commercially available. Appropriate yeasts contribute to an efficient and smooth fermentation process and largely provide a consistent and predictable quality of wines (Rodríguez et al. 2010; Sun et al. 2011). Ndip et al. (2001) investigated the effects of various yeasts isolated from fruits on the sugar and ethanol tolerance and acceptable score of pineapple wine. Pino and Queris (2010) identified thirteen types of volatile compounds in pineapple wine. Dellacassa et al. (2017) also evaluated the characteristics of volatile aroma compounds in pineapple wine in Angola. However, to the best of our knowledge, limited studies have investigated the effect of yeast strains on the profile of volatile compounds in pineapple wine.
This study aimed to evaluate the influence of four commercial S. cerevisiae strains (D254, VIC, BV818 and CECA) on the production of volatile compounds in pineapple wine.
Materials and methods
Yeast strains
Four commercial S. cerevisiae strains were used in this research. Strain D254 was purchased from LALVIN (Denmark). Strains VIC, BV818 (var. bayanus), and CECA were purchased from Angel (China).
Fruit juice preparation and fermentation conditions
Pineapple (Ananas comosus L. Merr.) planted in Hainan of China was purchased from the local fruit market and the peeled pineapple was pressed using a juice extractor.
The pineapple juice had a soluble solid level of 15°Brix, a pH value of 3.9 and total acidity 4.86 g/L (expressed as g/L of tartaric acid). The fruit juice was adjusted to a total soluble solid of 22°Brix and a final pH of 3.8 using sucrose and citric acid, respectively, prior to fermentation. Subsequently, 80 mg/L sodium metabisulphite was added to the juice. After statically placing for 4 h, 200 mg/L of activated (hydration in 5% of glucose solution at 37 °C for 30 min) commercial yeast strains were added to the juice, and the main fermentation was conducted at 22 °C without stirring. When the residual sugar was lower than 2 g/L, the wines were centrifuged at 1500×g for 10 min, and the supernatants were sampled. All fermentations were performed in triplicates.
Physicochemical properties
The general physicochemical properties of the wines were analyzed according to Zhu et al. (2014). Total soluble solid was assessed using a hand refractometer (WYT, HaoChuang, ChengDu, China). Residual sugar and acidity were determined by titration, and the results were expressed as glucose and tartaric acid, respectively. Volatile acidity was obtained by distillation combined with titration, and the results were expressed as acetic acid. Alcoholic content was obtained by distillation combined with a portable densimeter (DA-130N, KEM, Tokyo, Japan). Similarity, total acid of pineapple juice was determined by titration, and the result was expressed as tartaric acid. pH of pineapple juice was determined by using a pH meter (SevenEasy S20, METTLER TOLEDO, Zurich, Switzerland). Each measurement was conducted three times.
Volatile compounds
The volatile compounds of the pineapple wines (kept at 18 °C for 20 days) were extracted by headspace-solid phase microextraction (HS-SPME) and analysed by gas chromatography-mass spectrometry (GC–MS). Each sample (5 g) was placed in a 20 mL vial sealed with silicone septa (Sigma Chemical Co.). Exactly 50 μL of 1,3-dichlorobenzene (100 mg/L) was used as the internal standard. Before extraction, the SPME fibre (75 μm CAR/PDMS, Supelco, Bellefonte, PA, USA) was inserted into the GC injector at 250 °C for 30 min. Then, the fibre was exposed to the headspace over the sample, where extraction was allowed to occur in a thermostatted bath adjusted to 60 °C with continued agitation by a magnetic stirrer for 30 min. Finally, the fibre was desorbed in the GC injector for 3 min.
Compound analysis was performed using an Agilent 7890A GC (Agilent, Santa Clara, CA, USA) coupled with an Agilent 5975C mass spectrometer system equipped with a HP-5x capillary column (60 m × 0.25 mm inner diameter, 0.25 μm film thickness, Agilent). Helium (purity 99.999%) was used as the carrier gas at 1.0 mL/min. Desorption of analytes from the SPME fibre occurred at 250 °C for 3 min under a splitless mode. The oven was held at an initial temperature of 50 °C, and subsequently raised to 300 °C at a rate of 3 °C/min and held for 5 min. The mass spectra were obtained in an electron impact mode of 70 eV, and the range of mass (m/z) was scanned from 30 to 450 atomic mass units. The volatile compounds were identified by comparing retention indices and retention times with those obtained for authentic standards, or those of literature data, or with mass spectra in the Wiley7n Database (Agilent Technologies Inc.).
Odour activity values (OAVs)
The contribution of each aromatic compound to the overall flavour of the wines was evaluated by the odour activity value, which was calculated by the ratio of the concentration of a compound to its odour threshold value (Velázquez et al. 2015; Moyano et al. 2009).
Quantitative descriptive analysis (QDA)
The fermented pineapple wines were evaluated by a well-trained panel of 10 members (4 males and 6 females, among 20–50 years of age). 20 mL of wine sample was presented to panelists in plastic cups at room temperature of 22 ± 1 °C. Five important sensory terms including fruity, sweet, green, fatty and global aroma were selected by the panelists during discussion to describe and differentiate the samples, and a scale from 0 to 5 was used to score each attribute’s intensity, where 0 indicated that the descriptor was not perceived, and the intensity of values 1–5 was gradually enhanced.
qRT-PCR
Cultures were sampled at 16 h and the expression levels of BAT1, BAT2, ATF1, ATF2, EHT1, EEB1 and IAH1 were assessed. The total cellular RNA was extracted using a yeast RNA kit (Omega, Madison, EI, USA). By utilizing mRNA as a template, cDNA was synthesized using a Reverse Transcription System Kit (Takara, China). The expression levels of seven genes were assessed through real-time quantitative PCR (qRT-PCR) using an Ultra SYBR Two-Step qRT-PCR kit with ROX (reference dye for real-time PCR, TIANGEN, China). Actin was used as the loading control. The primers used for amplifying the target genes and the reference gene ACT1 are listed in Table S1 in supplementary material. The expression level of the target genes in the strain D254 was normalized to the reference gene. Experiments were conducted thrice.
Statistical analysis
Statistical analyses were carried out using the SPSS version 18.0 software. ANOVA and Duncan’s multiple range tests were applied to the data to determine significant differences. Differences at P < 0.05 were considered statistically significant. Principal component analysis (PCA) was applied to establish the relation between wine samples and volatile compounds (García-Carpintero et al. 2011) using PLS_TOOLBOX 5.02Version under Matlab 7.0 Version.
Results and discussion
Physicochemical properties of the pineapple wines
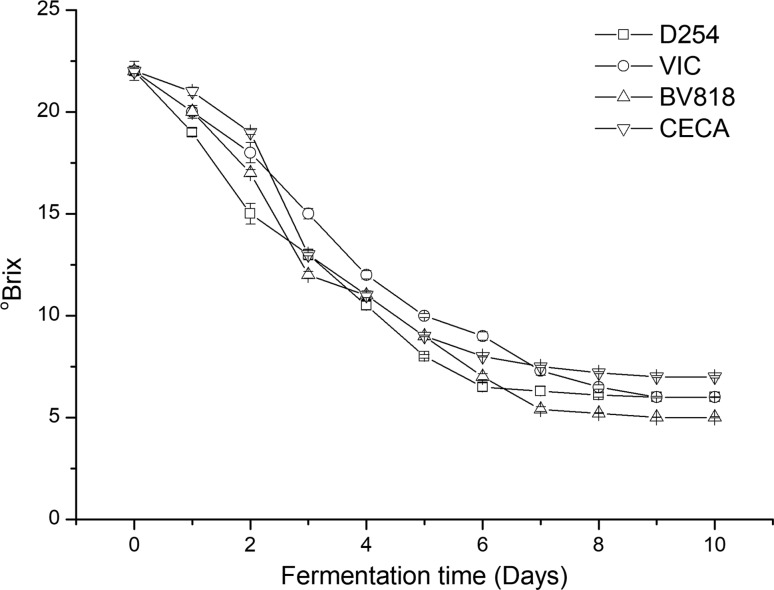
Pineapple wines were produced through dry wine processing technology. °Brix version fermentation curves of the four commercial S. cerevisiae strains were shown for pineapple wines are shown in Fig. 1. The °Brix value of the pineapple wines produced by different yeast strains rapidly decreased in the initial stage, but differences in the time of fermentation were observed. In the wines produced by D254 and CECA, the °Brix value gradually decreased during the 6-day fermentation period and reached a stable °Brix value of approximately 6–7%, indicating the completion of fermentation. BV818- and VIC-produced wines with similar trends over 7 and 8 days and reached constant °Brix values of 5 and 6%, respectively.
Fig. 1.
°Brix changes in pineapple wines during fermentation
The physicochemical properties of the pineapple wines produced by the different yeast strains are shown in Table 1. The reducing sugar was less than 2 g/L in the four pineapple wines, indicating that these were dry wines. The ethanol contents of the four pineapple wines varied from 10.8 to 11.0% (P > 0.05). The pineapple wines differed in titratable acidity from 5.66 to 6.16 g/L, and the highest amount was observed in the pineapple wine produced by CECA. D254-fermented pineapple wine showed the lowest content of volatile acidity, whereas no obvious difference was observed in the other three pineapple wines.
Table 1.
General physicochemical properties of the pineapple wines fermented by four yeast strains
| D254 | VIC | BV818 | CECA | |
|---|---|---|---|---|
| Reducing sugar (g/L) | 0.91 ± 0.08b | 1.09 ± 0.11b | 0.42 ± 0.06a | 0.45 ± 0.12a |
| Titratable acidity (g/L) | 5.66 ± 0.11a | 5.92 ± 0.18ab | 5.77 ± 0.12a | 6.16 ± 0.15b |
| Volatile acidity (g/L) | 0.62 ± 0.04a | 0.78 ± 0.04b | 0.78 ± 0.06b | 0.87 ± 0.05b |
| Ethanol (% vol) | 11.0 ± 0.04a | 10.8 ± 0.06a | 10.9 ± 0.05a | 11.0 ± 0.04a |
Values shown represent averages of triplicate samples (data are mean ± SD)
Values with different superscript roman letters (a, b) in the same row are significantly different according to the Duncan test (P < 0.05)
Acidity and ethanol play important biochemical roles in determining wine quality (Iorizzo et al. 2014). Appropriate acidity and ethanol contents are beneficial to the sensory quality and stability of fruit wines. Some acids in pineapple wine are naturally present in pineapples, while others are fermentation byproducts. The different acidity contents could be caused by various metabolic abilities of wine yeast strains used in this work in pineapple wine making. These results were consistent with those in cherry wine production (Sun et al. 2011) and persimmon wine production (Zhu et al. 2014).
Volatile compounds of the pineapple wines
Table 2 shows the concentration and type of the identified volatile compounds in the pineapple wines produced by different S. cerevisiae strains.
Table 2.
Mean concentration (μg/L) of volatile compounds detected in the wines fermented by four yeast strains
| Code | Compounds | Odour descriptiona | D254 | VIC | BV818 | CECA | Mean value |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| A1 | 2-Methyl-1-propanol | Wine, solvent, bitter | 212.9 | 164.4 | 21.2 | 319.3 | 179.5 |
| A2 | 3-Methyl-1-butanol | Whiskey, malt, burnt | 1505.4 | 1041.0 | 1771.4 | 1189.0 | 1376.7 |
| A3 | 2-Methylbutan-1-ol | Wine, onion | 1236.6 | 727.8 | 519.0 | 626.5 | 777.5 |
| A4 | 2,3-Butanediol | 19.5 | 183.9 | 410.5 | 20.7 | 158.7 | |
| A5 | Trans-3-hexen-1-ol | Moss, fresh | 3.7 | 2.1 | – | 1.9 | 1.9 |
| A6 | Heptan-1-ol | Chemical, green | – | – | 8.4 | – | 2.1 |
| A7 | 2-Ethylhexan-1-ol | Rose, green | 1.5 | 0.5 | – | – | 0.5 |
| A8 | 1-Octanol | Chemical, metal, burnt | 8.6 | 3.2 | 1.5 | 2.0 | 3.8 |
| A9 | 2-Phenylethanol | Honey, spice, rose, lilac | 2387.1 | 1572.2 | 1238.1 | 2120.5 | 1829.5 |
| A10 | 3,7-Dimethyl-6-octen-1-ol | – | – | 8.0 | 2.0 | 2.5 | |
| Subtotal | 5357.3 | 3695.1 | 3978.1 | 4281.9 | 4332.6 | ||
| Esters | |||||||
| E1 | Ethyl acetate | Pineapple | 309.7 | 237.2 | 111.4 | 242.2 | 225.1 |
| E2 | Ethyl propionate | Fruit | 3.8 | 3.5 | – | 2.5 | 2.5 |
| E3 | Methyl butyrate | Ether, fruit, sweet | 11.0 | 16.9 | – | – | 7.0 |
| E4 | Ethyl isobutyrate | Sweet, rubber | 12.2 | – | – | – | 3.1 |
| E5 | Isobutyl acetate | Fruit, apple, banana | 8.3 | 6.7 | – | 1.2 | 4.1 |
| E6 | Ethyl butyrate | Apple | 97.8 | – | 27.1 | – | 31.2 |
| E7 | Isoamyl acetate | Banana | 1967.7 | 1207.0 | 463.8 | 162.0 | 950.1 |
| E8 | 2-Methylbutyl acetate | Fruit | – | 900.0 | – | – | 225.0 |
| E9 | Ethyl 3-hydroxybutyrate | Marshmallow | – | – | 1.9 | 2.0 | 1.0 |
| E10 | Ethyl hexanoate | Apple peel, fruit | 128.0 | 80.6 | 157.1 | 31.9 | 99.4 |
| E11 | Ethyl benzoate | Camomile, flower, celery, fruit | 0.7 | – | – | – | 0.2 |
| E12 | Diethyl succinate | Wine, fruit | 1.0 | 0.5 | 0.7 | – | 0.6 |
| E13 | Ethyl octanoate | Fruit, fat | 329.0 | 172.2 | 504.8 | 135.5 | 285.4 |
| E14 | 2-Phenethyl acetate | Rose, honey, | 120.4 | 125.6 | 115.2 | 86.7 | 112.0 |
| E15 | Ethyl decanoate | Grape | 58.7 | 32.0 | 76.5 | 42.9 | 52.5 |
| E16 | Ethyl laurate | Leaf | 22.3 | 7.2 | 32.4 | 20.9 | 20.7 |
| E17 | Ethyl myristate | Ether | 1.1 | 1.0 | 4.3 | 0.8 | 1.8 |
| Subtotal | 3071.7 | 2790.4 | 1495.2 | 728.6 | 2021.5 | ||
| Acids | |||||||
| AC1 | Acetic acid | Sour | 210.6 | 123.3 | 98.1 | 134.3 | 141.6 |
| AC2 | Isobutyric acid | Rancid, butter, cheese | 11.3 | – | – | – | 2.8 |
| AC3 | Isovaleric acid | Sweat, acid, rancid | 12.7 | 2.0 | 3.6 | 10.4 | 7.2 |
| AC4 | 2-Methylbutyric acid | Cheese, sweat | 16.0 | 22.8 | 15.8 | 27.1 | 20.4 |
| AC5 | Hexanoic acid | Sweat | 89.1 | 50.1 | 91.2 | 52.0 | 70.6 |
| AC6 | Octanoic acid | Sweat, cheese | 115.1 | 63.3 | 105.7 | 57.5 | 85.4 |
| AC7 | Decanoic acid | Rancid, fat | 4.3 | 2.3 | 11.6 | 5.3 | 5.9 |
| Subtotal | 459.1 | 263.8 | 326.0 | 286.6 | 333.9 | ||
| Aldehydes and ketones | |||||||
| AK1 | Acetaldehyde | Pungent, ether | 4.1 | 2.2 | 1.1 | 55.2 | 15.7 |
| AK2 | Acetal | Fruit, cream | 0.8 | 2.5 | – | 7.3 | 2.7 |
| AK3 | Benzaldehyde | Almond, burnt sugar | 1.0 | 0.7 | – | – | 0.4 |
| AK4 | Octanal | Fat, soap, lemon, green | – | – | – | 2.1 | 0.5 |
| AK5 | Phenylacetaldehyde | Honey, sweet | 3.4 | 1.4 | 2.0 | 0.0 | 1.7 |
| AK6 | Acetophenone | Must, flower, almond | – | – | 3.0 | 2.0 | 1.3 |
| AK7 | Nonanal | Fat, citrus, green | 2.6 | 7.7 | 15.2 | 17.7 | 10.8 |
| AK8 | Decanal | Soap, orange peel, tallow | 3.2 | 2.0 | 3.6 | 1.2 | 2.5 |
| Subtotal | 15.1 | 16.5 | 24.9 | 85.5 | 35.5 | ||
| Lactones | |||||||
| L1 | γ-Butyrolactone | Caramel, sweet | 36.9 | 34.0 | 107.6 | 46.6 | 56.3 |
| L2 | γ-Caprolactone | Coumarin, sweet | 18.8 | 4.3 | 16.8 | 9.6 | 12.4 |
| L3 | γ-Octanoic lactone | Coconut | 7.0 | 3.4 | 4.5 | 3.9 | 4.7 |
| L4 | 1,5-Octalactone | Peach | 7.8 | 3.9 | 10.4 | 2.6 | 6.2 |
| Subtotal | 70.5 | 45.6 | 139.3 | 62.7 | 79.5 | ||
| Sulphur compounds | |||||||
| S1 | 3-Methylthiopropanol | Sweet, potato | 368.8 | 55.0 | 350.5 | 250.0 | 256.1 |
| S2 | Methyl 3-methylthiopropionate | Vegetative, radish and horseradish | 163.4 | 58.3 | 62.0 | 39.9 | 80.9 |
| S3 | Ethyl 3-methylthiopropionate | Pineapple citrus | 92.2 | 99.4 | 101.9 | 69.9 | 90.9 |
| S4 | Dihydro-2-methyl-3(2H)-thiophenone | Cabbage, onion, must | – | – | – | 5.6 | 1.4 |
| Subtotal | 624.4 | 212.7 | 514.4 | 365.4 | 429.2 | ||
| Phenols | |||||||
| P1 | Chavicol | Medicine, phenol | 7.2 | 4.6 | 0.8 | 1.2 | 3.5 |
| P2 | 2-Methoxy-4-vinylphenol | Clove, curry | 3.1 | 1.9 | 1.8 | 1.1 | 2.0 |
| P3 | Eugenol | Clove, honey | 2.2 | 0.6 | – | – | 0.7 |
| Subtotal | 12.5 | 7.1 | 2.6 | 2.3 | 6.1 | ||
| Styryl derivatives | |||||||
| SD1 | Styrene | Balsamic, gasoline | 77.1 | 36.7 | 26.7 | 45.5 | 46.5 |
| SD2 | 2,4-Dimethylstyrene | 1.7 | – | 5.0 | 0.8 | 1.9 | |
| Subtotal | 78.8 | 36.7 | 31.7 | 46.3 | 48.4 | ||
| Furans | |||||||
| F1 | 2-Methylfuran | Ether | 0.9 | – | – | 1.6 | 0.6 |
| F2 | 2,4-Dimethylfuran | – | – | – | 4.0 | 1.0 | |
| F3 | 4-Hydroxy-2,5-dimethylfuran-3-one | Caramel | – | – | 15.1 | – | 3.8 |
| Subtotal | 0.9 | – | 15.1 | 5.6 | 5.4 | ||
| Terpenes | |||||||
| T1 | Limonene | Lemon, orange | 11.7 | 4.1 | 67.5 | 6.2 | 22.4 |
| Subtotal | 11.7 | 4.1 | 67.5 | 6.2 | 22.4 | ||
| Total | 9720.0 | 7072.0 | 6594.8 | 5871.1 | 7314.5 | ||
Values shown represent averages of triplicate samples (maximum SD: ± 10%)
– not detected
Alcohols accounted for more than 50% of the total volatile content in the four pineapple wines and were the most abundant group in terms of the amounts of the identified volatile compounds. This result differed from that of Pino and Queris (2010), who reported that esters account for the largest proportion of the total aroma in pineapple wine. This discrepancy may be due to the differences in pineapple variety, yeast strains, fermentation techniques and the test methods. The four pineapple wines presented quantitative and qualitative differences in alcohol composition. 2-Phenylethanol, which produces a honey, spicy, rose and lilac aroma, was the most abundant alcohol in D254-, CECA- and VIC-fermented wines, but a 32.3% decrease was found in VIC-fermented wine compared with the mean concentration. The wine produced by BV818 showed the highest concentration of 3-methyl-1-butanol, which can generate a whiskey, malt and burnt aroma. Its concentration was 28.7% higher than the mean level. Although eight types of alcohols were observed in the four pineapple wines, heptan-1-ol was found only in BV818-fermented wine (Table 3). A small amount of 2-ethylhexan-1-ol, which provides a rose and green aroma, was detected in D254- and VIC-fermented wines, but 3,7-dimethyl-6-octen-1-ol was absent. Yeasts contribute significantly to the unique higher alcohol profiles and contents in fruit wines during fermentation. Higher alcohols produced by yeast are converted from α-keto acids via the decarboxylation and reduction of an aldehyde group. α-Keto acids can be synthesized from pyruvate via the Harris pathway from a carbon source or through the degradation of branched-chain amino acids (BCAAs) via the Ehrlich pathway (Ma et al. 2017). The significant differences in the total alcohol contents of the four wines could be attributed to the variation in alcohol metabolic activities of yeast strains. Higher alcohols are considered favorable components when their total concentration is lower than 300 mg/L (Duarte et al. 2010). Although the total alcohol contents of strains VIC, BV818, CECA and D254 gradually increased, higher alcohols could positively influence the aroma and flavour of the four pineapple wines.
Table 3.
The specific volatiles produced by a specific yeast
| Code | Compounds | Odour description | D254 (μg/L) | VIC (μg/L) | BV818 (μg/L) | CECA (μg/L) |
|---|---|---|---|---|---|---|
| Alcohols | ||||||
| A6 | Heptan-1-ol | Chemical, green | – | – | 8.4 | – |
| Esters | ||||||
| E4 | Ethyl isobutyrate | Sweet, rubber | 12.2 | – | – | – |
| E8 | 2-Methylbutyl acetate | Fruit | – | 900.0 | – | – |
| E11 | Ethyl benzoate | Camomile, flower, celery, fruit | 0.7 | – | – | – |
| Acids | ||||||
| AC2 | Isobutyric acid | Rancid, butter, cheese | 11.3 | – | – | – |
| Aldehydes and ketones | ||||||
| AK4 | Octanal | Fat, soap, lemon, green | – | – | – | 2.1 |
| Sulphur compounds | ||||||
| S4 | Dihydro-2-methyl-3(2H)-thiophenone | Cabbage, onion, must | – | – | – | 5.6 |
| Furans | ||||||
| F2 | 2,4-Dimethylfuran | – | – | – | 4.0 | |
| F3 | 4-Hydroxy-2,5-dimethylfuran-3-one | Caramel | – | – | 15.1 | – |
Esters, which are mostly formed through the esterification of alcohols with fatty acids during fermentation and aging, are a group of major aromatic compounds in wines. In this study, esters were the most abundant group in terms of the type of the identified volatiles. This result was consistent with that of Pino and Queris (2010), who observed that esters are the most abundant volatile constituents in pineapple wine. The amounts and types of esters in the four pineapple wines considerably varied. Isoamyl acetate, which contributes to banana odour, is considered a positive component of wines. Although the concentration of isoamyl acetate in the four pineapple wines were significantly higher than its odour threshold (30 μg/L), its concentration in D254-fermented wine was 0.6, 3.2 and 11.1 times higher than that in VIC-, BV818- and CECA-fermented wines, respectively. The wine produced by strain BV818 presented the highest amounts of ethyl hexanoate and ethyl octanoate, and their concentrations were 58.0 and 76.9% higher than the mean contents, respectively. These two components can exude intense fruity aromas. Among the four wines, D254-fermented wine possessed the most diverse esters, that is, fifteen types. Nevertheless, this number was lower than that identified by Dellacassa et al. (2017) but was two types higher than that reported by Pino and Queris (2010). Ethyl isobutyrate, which yields a sweet and rubber aroma, and ethyl benzoate, which produces a camomile, floral, celery and fruity aroma, were unique to the wine produced by strain D254 (Table 3). 2-Methylbutyl acetate, which has a characteristic fruity aroma, was uniquely found in the wine produced by strain VIC (Table 3). Ethyl 3-hydroxybutyrate, which contributes to wine with a marshmallow-like odour, was present in the wines produced by BV818 and CECA but was absent in the wines produced by D254 and VIC. The former two wines contained less types (eleven types) of ester without ethyl propionate and diethyl succinate. Esters can be synthesized via lipase synthesis, alcohol acyltransferase, or alcohol dehydrogenase pathway and hydrolyzed to their corresponding alcohols and acids by esterase (Li et al. 2017). The highest total concentration of esters was formed by D254, followed by VIC, BV818 and CECA. This result suggested that the ability of D254 to accumulate esters was stronger than that of the other strains.
Fatty acid, which can contribute to fruity, cheesy, fatty and rancid aromas, is mainly formed during fermentation. The amount and number of acids were lower than those of alcohols and esters in the four pineapple wines. Acetic acid and hexanoic acid were quantitatively the most representative components of this group. The wine produced by D254 contained the most diverse acids with seven types (Table 3). Fatty acid can be synthesized by malonyl-CoA and acetyl-CoA precursors, and the carbon chain is shortened through β-oxidation. D254-fermented wine contained the highest total acids, corresponding to the highest concentration (250.6 μg/L) of volatile fatty acids found in this work. It is harmful to the flavour of wine whereby acids confer a sweaty, sour and thin taste at high levels (Hernanz et al. 2009). In this work, the contents of all acid components were much lower than their odour thresholds. Therefore, acids contributed actively to pineapple wine flavour.
Carbonyl compounds mainly include aldehydes and ketones that are formed during yeast fermentation (Nyanga et al. 2013). Seven compounds, namely, acetaldehyde, acetal, octanal, phenylacetaldehyde, acetophenone, nonanal and decanal, of this group have not been detected in pineapple wine in other studies. Nonanal contributes to fruit wines with a fat, citrus and green odour. The nonanal contents in BV818- and CECA-fermented wines were 40.7 and 63.9% higher than the mean value, respectively, indicating that the abilities of BV818 and CECA to yield nonanal from pineapple juice were stronger than those of the other two strains. CECA- and BV818-fermented wines showed the highest concentrations of acetaldehyde and decanal, which were 251.6 and 44.0% higher than their mean levels, respectively. BV818-produced wine exhibited the lowest type of aldehydes and ketones. Phenylacetaldehyde, which is characterized by a honey and sweet aroma, was not found in CECA-fermented wine. Nevertheless, octanal was uniquely produced by CECA (Table 3), and its wine had the highest total amounts of aldehydes and ketones.
Lactones enrich the aroma and flavour of wines (Steingass et al. 2015). Four lactones were identified in the four pineapple wines. The concentrations of γ-butyrolactone and 1,5-octalactone in BV818-fermented wine were 91.9 and 67.6% higher than their mean levels, respectively. Therefore, intense caramel, sweet and peach aromas might be exuded in BV818-fermented wine. D254-fermented wine showed the highest amounts of γ-caprolactone and γ-octanoic lactone. The former one compound has been detected by Dellacassa et al. (2017) in pineapple wine. In fresh pineapples, the presence of γ-octanoic lactone and other lactones, such as δ-octalactone, γ-decalactone, δ-decalactone and γ-dodecalactone has been reported (Steingass et al. 2014). Lactones can be strongly related to fatty acid metabolism. The order of the total content of lactones was interestingly consistent with that of acids, suggesting that the four wine yeast strains exhibited different capabilities of producing lactones and acids.
Sulphur compounds can be produced through sulfate assimilatory or dissimilatory reduction pathways in microorganisms (Sun et al. 2011). Specific sulphur compounds produce a wide range of aromas and increase wine complexity. 3-Methylthiopropanol (sweet and potato odour), methyl 3-methylthiopropionate (vegetative, radish and horseradish odour) and ethyl 3-methylthiopropionate (pineapple citrus odour) were found in the four pineapple wines, and the latter two compounds were identified as the main esters in two pineapple varieties (Zheng et al. 2012). The highest levels of 3-methylthiopropanol and methyl 3-methylthiopropionate were found in D254-fermented wine and were 44.0 and 102.0% higher than their mean values, respectively. The highest amount of ethyl 3-methylthiopropionate was detected in BV818-fermented wine and was 12.1% higher than the mean value. The amount of methyl 3-methylthiopropionate in D254-fermented wine was similar to its odour threshold (180 μg/L) and the value that previously reported by Dellacassa et al. (2017), who also identified these three components in pineapple wine. High content of methyl 3-methylthiopropionate may contribute an unpopular sense to wine, and this compound is formed through the Stickland reaction of methionine (Steingass et al. 2014). Differences in the amino acid metabolism of yeasts could account for the various contents of methyl 3-methylthiopropionate in the four wines. Dihydro-2-methyl-3(2H)-thiophenone was found only in CECA-fermented wine. Moreover, the highest diversity of sulphur compounds (four types) was observed in CECA-fermented wine. These results confirmed that S. cerevisiae is responsible for the metabolism of volatile sulphur compounds (Swiegers and Pretorius 2007).
Volatile phenols can positively affect the aroma and flavour of fruit wines at low concentrations. Small amount of phenols, including chavicol, 2-methoxy-4-vinylphenol and eugenol, were quantified at various concentrations in the tested wines. Eugenol, which is characterized with a clove and honey odour, was present in the wines produced by D254 and VIC but was absent in the wines fermented by BV818 and CECA.
Two styryl derivatives, namely, styrene and 2,4-dimethylstyrene, were identified. The wine produced by strain VIC only contained styrene, which could negatively influence fruit wines with a balsamic and gasoline odour.
Furan compounds formed by the degradation of carbohydrates can enhance the mild and sweet aroma of wines (Perestrelo et al. 2006). Three furans were identified in this study. 2-Methylfuran, which produces an ether-like flavour, was found in D254- and CECA-fermented wines. 2,4-Dimethylfuran was unique to the wine produced by CECA (Table 3). 4-Methoxy-2,5-dimethylfuran-3(2H)-one, which releases a caramel flavour, was uniquely produced by BV818 (Table 3).
Terpenes are mainly released by their nonodorous glycosides during alcoholic fermentation (Sun et al. 2011). Only limonene was detected in the wine samples. Limonene could contribute to the wines with a pleasant lemon and orange odour. The highest limonene concentration was found in the pineapple wine produced by strain BV818, its concentration was much higher than that reported by Dellacassa et al. (2017). This result indicated that strain BV818 could relatively strongly hydrolyse glycosides. This finding was corresponded with the point described by Loscos et al. (2007), who reported that terpene levels are highly dependent on yeasts.
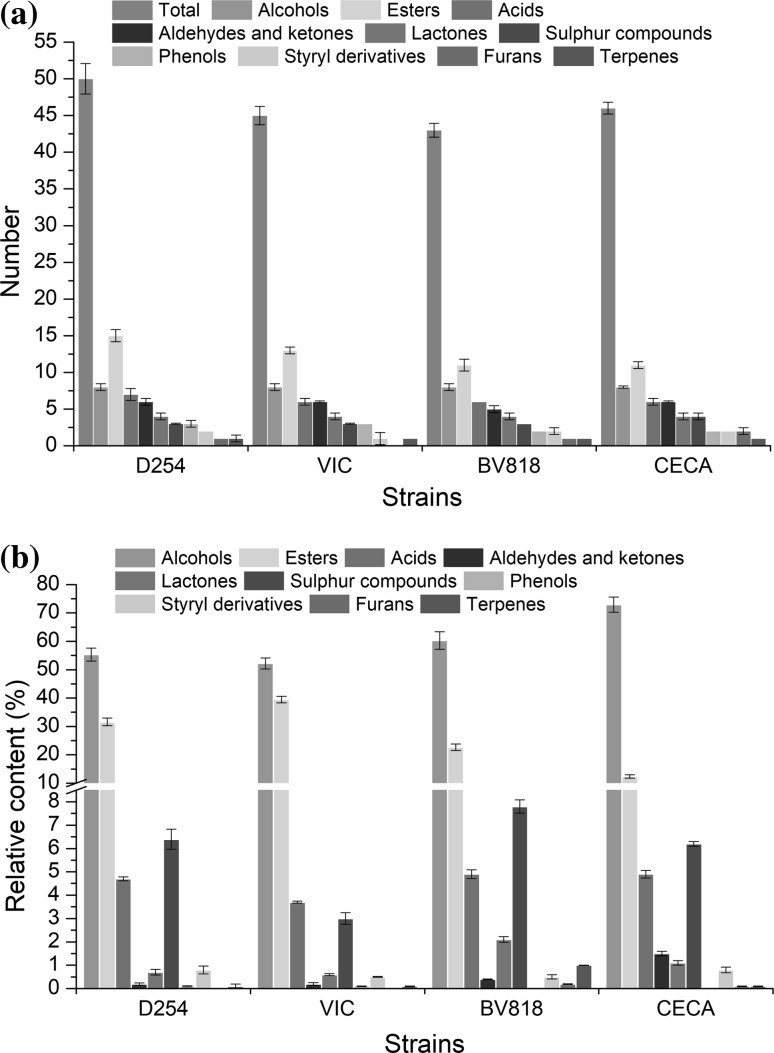
Figure 2 illustrates the overall variation in the number and relative content of volatile compounds in the four pineapple wines. Qualitatively, the greatest difference in the compounds in the four pineapple wines was observed in esters, which were the main components accounted for the variation in the sum of the volatile compounds (Fig. 2a). This result revealed that the capacity of yeast strain to yield esters was an important factor that influenced the integrity and complexity of the aroma of the pineapple wines. Wine aroma is formed by a large number of volatile compounds possessing different aroma characteristics. The pineapple wines produced by the different yeast strains were characterized with aroma descriptors belonging to seven groups: floral, fruity, green, sweet, spicy, fatty (or chemical) and others. The complexity of wine aroma depends not only on the number of aromatic compounds but also on the proportion of aromatic compounds (Pino and Queris 2010). For the relative content of volatile compounds shown in Fig. 2b, the relative content of esters was the highest in VIC-fermented wine. The relative contents of lactones and sulphur compounds yielded by BV818 was the highest. The relative contents of higher alcohols and carbonyl compounds were the highest in CECA-produced wine. The relative content of most volatile compounds yielded by D524 was moderate, but the total concentrations of alcohols, esters, acids and sulphur compounds were the highest. The distinguishing volatile compounds produced by yeast strains could lead to the differences in the flavour characteristic of the final fruit wine. The highest types and total concentrations of volatile compounds were produced by D254, suggesting that the ability of D254 to yield volatile compounds in pineapple juice was stronger than that of the other strains.
Fig. 2.
Variation of volatile compounds in the four pineapple wines. Variation of a the number, b the relative content of volatile compounds in the pineapple wines produced by four yeast strains
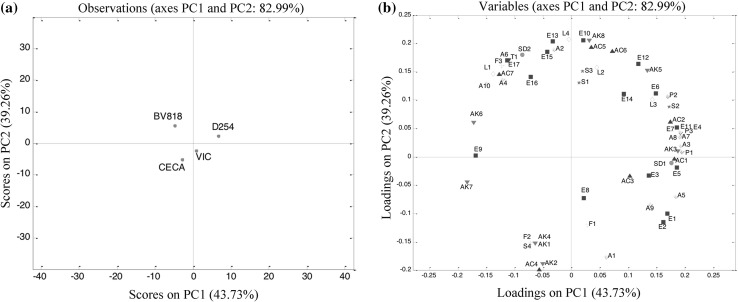
PCA of volatile compounds
PCA was initially applied to the 59 volatile compounds described in Table 2. The percentage of the cumulative contribution of the variance of the first two PCs was 82.99%. PC1 and PC2 represented 43.73 and 39.26% variabilities of the volatile compounds, respectively. Figure 3a profiling the score scatter plot on PC1 and PC2, showed separated wine samples. The characteristic aroma of VIC-fermented wine was relatively close to that of CECA-fermented wine. The two wines were obviously different from D254- and BV818-fermented wines in terms of their characteristic aroma. Figure 3b illustrates the corresponding loading plot that established the relative importance of each volatile. In this study, the aroma quality of the four wines was influenced by the identified volatile components. Eight groups of compounds including three alcohols (A3, A7 and A8), five esters (E4, E6, E7, E11 and E14), two acids (AC1 and AC2), two carbonyl compounds (AK3 and AK5), one lactone (L3), three phenols (P1, P2 and P3), one sulphur compound (S2) and one styryl derivative (SD1) were associated with D254-fermented wine. BV818-produced wine was more related to seven groups of compounds including four alcohols (A2, A4, A6 and A10), four esters (E13, E15, E16 and E17), one acid (AC7), one lactone (L1), one styryl derivative (SD2), one furan (F3) and one terpene (T1). VIC-fermented wine was related to 2-methyl-1-propanol (A1) and 2-methylbutyl acetate (E8). CECA-fermented wine was associated with AK1, AK2, AK4 and AK7 in carbonyl compounds, F2 in furan, and S4 in sulphur compound.
Fig. 3.
Principal components analysis of the volatile compounds in pineapple wines fermented by four yeast strains. a Distinction between the samples (scores), b relation between the volatile compounds (loadings)
Odour activity values
The pineapple wines produced by different yeasts displayed a wide concentration range of volatile compounds, and the contribution of the compounds to the flavour of fruit wines depended on OAVs (Table S2 in supplementary material). Eleven types of compounds showed OAVs > 1 in this test (Table 4). The pineapple wines produced by strains D254, VIC and BV818 possessed four esters with OAVs > 1. The highest OAVs of ethyl butyrate and isoamyl acetate were found in the wine produced by D254. Likewise, the highest OAVs of ethyl hexanoate and ethyl octanoate were detected in the wine produced by BV818. 2-Methylbutyl acetate was uniquely found in VIC-fermented wine and had OAV > 1. The OAVs of phenylacetaldehyde in D254-, VIC- and BV818-fermented wines were also > 1. Thus, intense fruity and sweet odours could be expected in D254-, VIC- and BV818-fermented wines. An OAV of > 1 of nonanal was found in CECA-fermented wine, but undesirable odours (fatty, citrus and green) could be increased. The OAVs of γ-butyrolactone in the wines produced by D254, BV818 and CECA were above 1, but the highest OAV was observed in the wine produced by BV818. The OAVs of > 1 of 4-hydroxy-2,5-dimethylfuran-3-one and limonene were uniquely produced by BV818. Therefore, the wine produced by strain BV818 could exude intense caramel, sweet, lemon and orange aromas. Ethyl 3-methylthiopropionate is considered a characteristic aroma compound in pineapple (Zheng et al. 2012). The concentrations of ethyl 3-methylthiopropionate in the four wines were much higher than its odour threshold, suggesting that the characteristic aromas of pineapple fruit could be retained during fermentation.
Table 4.
OAVs (> 1) for the main volatile compounds in the wines fermented by four yeast strains
| Code | Compounds | Odour threshold (μg/L) | D254 | VIC | BV818 | CECA |
|---|---|---|---|---|---|---|
| E6 | Ethyl butyrate | 20.0 | 4.9 | – | 1.4 | – |
| E7 | Isoamyl acetate | 30.0 | 65.6 | 40.2 | 15.5 | 5.4 |
| E8 | 2-Methylbutyl acetate | 160.0 | – | 5.6 | – | – |
| E10 | Ethyl hexanoate | 14.0 | 9.1 | 5.8 | 11.2 | 2.3 |
| E13 | Ethyl octanoate | 20.0 | 16.5 | 8.6 | 25.2 | 6.8 |
| AK5 | Phenylacetaldehyde | 1.0 | 3.4 | 1.4 | 2.0 | – |
| AK7 | Nonanal | 15.0 | 0.2 | 0.5 | 1.0 | 1.2 |
| L1 | γ-Butyrolactone | 35.0 | 1.1 | 1.0 | 3.1 | 1.3 |
| S3 | Ethyl 3-methylthiopropionate | 7.0 | 13.2 | 14.2 | 14.6 | 10.0 |
| F3 | 4-Hydroxy-2,5-dimethylfuran-3-one | 10.0 | – | – | 1.5 | – |
| T1 | Limonene | 25.0 | 0.5 | 0.2 | 2.7 | 0.2 |
PCA of the main volatiles with OAVs > 1
PCA was applied to the eleven volatile compounds with OAVs of > 1 described in Table 4. The percentage of the cumulative contribution of the variance of PCs was 86.12%. Figure S1a in supplementary material illustrates that the pineapple wines fermented with different yeast strains were differentiated by their aroma profiles. In Figure S1b in supplementary material, ethyl butyrate (E6), isoamyl acetate (E7) and phenylacetaldehyde (AK5) were mainly related to D254-produced wine. Ethyl octanoate (E13), γ-butyrolactone (L1), 4-hydroxy-2,5-dimethylfuran-3-one (F3) and limonene (T1) were the main influencing compounds in BV818-produced wine. 2-Methylbutyl acetate (E8) was strongly associated with VIC-produced wine. Nonanal (AK7) was linked to CECA-produced wine.
The main contributors of the flavour of wines are aromatic compounds that considerably differ in type or concentration (Feng et al. 2015). The maximum sum of the characteristic compounds with OAVs > 1 was found in BV818-produced wine (Table 3). Four groups of compounds (esters, lactones, furans and terpenes) strongly affected the aroma of BV818-fermented wine. Seven volatiles with OAVs > 1 and three unique components were detected in D254-fermented wine (Tables 3, 4). These results suggested that fermentation with strains BV818 and D254 might effectively to enhance the complexity and intensity of the aroma and flavour of pineapple wine.
Quantitative descriptive analysis
The sensory evaluation of the four pineapple wines mainly included fruity, sweet, green, fatty and global aromas (Figure S2 in supplementary material). The pineapple wines produced by strains D254 and BV818 were characterized by an intense fruity note, which is a fundamental trait of pineapple wines and usually considered an important indicator of fruit wine quality. The intense fruity aroma could be attributed to the large quantities of ethyl butyrate, isoamyl acetate, ethyl hexanoate, ethyl 3-methylthiopropionate and limonene. The highest and lowest “sweet” scores were displayed in BV818- and VIC-fermented wines, respectively. Although the strongest fatty nose was yielded by BV818, it did not affect the global aroma of BV818-fermented wine. Wines produced by VIC and CECA showed a similarity in sense except the “green” descriptor. These results were consistent with PCA, suggesting that the flavour properties of VIC- and CECA-fermented wines were relatively similar in this test.
mRNA levels
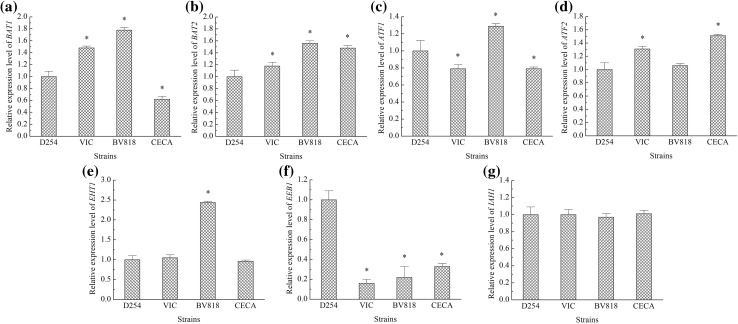
In essence, the formation of volatile compounds is closely related to the transcription of genes in a metabolic network and the expression of proteins in cells. Alcohols and esters accounting for more than 80% of the total volatile content were the most two abundant groups of the identified volatile compounds. To investigate the difference in the metabolic activity of yeasts in pineapple juice, the mRNA levels of seven genes involved in higher alcohol and ester metabolism were examined. In the Ehrlich pathway, mitochondrial and cytosolic branched-chain amino acid transferases (BCAATases) respectively encoded by BAT1 and BAT2 catalyze the transamination of BCAAs to their corresponding α-keto acids (Ma et al. 2017). Alcohol O-acetyltransferases involved in acetate ester synthesis are mainly encoded by ATF1 and ATF2, while those for ethyl ester synthesis are encoded by EHT1 and EEB1 (Sumby et al. 2010). Isoamyl acetate-hydrolyzing esterase encoded by IAH1 participates in the hydrolysis of isoamyl acetate to isoamyl alcohol and acetic acid (Li et al. 2017).
In Fig. 4, the expression levels of BAT1, BAT2, ATF1, ATF2, EHT1 and EEB1 changed in the four yeast strains to varying degrees, suggesting that the four yeast strains had different metabolic activities in pineapple juice. These differences in metabolic activities brought about the changed contents of alcohols and esters found in the four pineapple wines. In yeast, the metabolism pathways of esters and alcohols are not unique. Although the expression level of IAH1 did not obviously change, its level was not enough to prevent the modification of the contents of isoamyl acetate, isoamyl alcohol and acetic acid in the four wines.
Fig. 4.
The expression levels of seven genes involved in alcohol and ester metabolism. a BAT1, b BAT2, c ATFI, d ATF2, e EHT1, f EEB1, g IAH1. Significant difference (indicated with * upon the columns) of the strains (VIC, BV818 and CECA) from strain D254 was confirmed for P < 0.05 (n = 3)
Conclusion
The results demonstrated that different S. cerevisiae strains possess various capacities to release or synthesize volatile compounds during pineapple wine fermentation. In terms of global aroma, strain D254 that yielded the highest total number and concentration of volatile compounds could be used as a starter culture for the making of intense pineapple wine. BV818-produced wine had the greatest amounts of volatiles with OAVs > 1, and scored the highest in terms of global aroma. Thus, BV818 might be the appropriate strain that could provide characteristic aromas and enhance the wine complexity. Strain VIC could produce the highest relative content of volatile esters. CECA-fermented wine was characterized by high relative contents of alcohols, aldehydes and ketones. However, VIC and CECA were not ideal starter cultures because of their low sense scores. This study provided further knowledge on the aroma characteristic of pineapple wine and established a basis for the making of pineapple wine with the desired flavour profile.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by the Natural Science Foundation of Hainan province (Grant Number 317056) and the Scientific Research Foundation of Hainan University (grant number KYQD1660).
Author contributions
XL carried out the experiments and drafted the manuscript. QKW participated in the wine fermentation and physicochemical properties analysis. XPH did PCA for the revised manuscript. WYW and YXZ participated in the analysis of volatiles. SXL and CFL conceived the study and reviewed the final manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Xue Lin, Email: linxiaoxuelx@163.com.
Qingke Wang, Email: 81508624@qq.com.
Xiaoping Hu, Email: 77541963@qq.com.
Wuyang Wu, Email: 2420526022@qq.com.
Yexin Zhang, Email: 962348617@qq.com.
Sixin Liu, Email: liusixinlsx@163.com.
Congfa Li, Phone: +86-898-66278326, Email: congfa@vip.163.com.
References
- Bamidele OP, Fasogbon MB. Chemical and antioxidant properties of snake tomato (Trichosanthes cucumerina) juice and Pineapple (Ananas comosus) juice blends and their changes during storage. Food Chem. 2017;220:184–189. doi: 10.1016/j.foodchem.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Chanprasartsuk OO, Prakitchaiwattana C, Sanguandeekul R, Fleet GH. Autochthonous yeasts associated with mature pineapple fruits, freshly crushed juice and their ferments; and the chemical changes during natural fermentation. Bioresour Technol. 2010;101:7500–7509. doi: 10.1016/j.biortech.2010.04.047. [DOI] [PubMed] [Google Scholar]
- Dellacassa E, Trenchs O, Fariña L, Debernardis F, Perez G, Boido E, Carrau F. Pineapple (Ananas comosus L. Merr.) wine production in Angola: characterisation of volatile aroma compounds and yeast native flora. Int J Food Microbiol. 2017;241:161–167. doi: 10.1016/j.ijfoodmicro.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Duarte WF, Dias DR, Oliveira JM, Vilanova M, Teixeira JA, Almeida e Silva JB, Schwan RF. Raspberry (Rubus idaeus L.) wine: yeast selection, sensory evaluation and instrumental analysis of volatile and other compounds. Food Res Int. 2010;43:2303–2314. doi: 10.1016/j.foodres.2010.08.003. [DOI] [Google Scholar]
- Feng Y, Liu M, Ouyang Y, Zhao X, Ju Y, Fang Y. Comparative study of aromatic compounds in fruit wines from raspberry, strawberry, and mulberry in central Shaanxi area. Food Nutr Res. 2015;59:29290. doi: 10.3402/fnr.v59.29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carpintero EG, Sánchez-Palomo E, Gómez Gallego MA, González-Viñas MA. Effect of cofermentation of grape varieties on aroma profiles of la mancha red wines. J Food Sci. 2011;76:C1169–C1180. doi: 10.1111/j.1750-3841.2011.02374.x. [DOI] [PubMed] [Google Scholar]
- Hernanz D, Gallo V, Recamales AF, Melendez-Martinez AJ, Gonzalez-Miret ML, Heredia FJ. Effect of storage on the phenolic content, volatile composition and colour of white wines from the varieties zalema and colombard. Food Chem. 2009;113:530–537. doi: 10.1016/j.foodchem.2008.07.096. [DOI] [Google Scholar]
- Iorizzo M, Macciola V, Testa B, Lombardi SJ, De LA. Physicochemical and sensory characteristics of red wines from the rediscovered autochthonous Tintilia grapevine grown in the Molise region (Italy) Eur Food Res Technol. 2014;238:1037–1048. doi: 10.1007/s00217-014-2186-z. [DOI] [Google Scholar]
- Jiang B, Zhang Z. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of China. Molecules. 2010;15:9184–9196. doi: 10.3390/molecules15129184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang JH, Zhang CY, Ma HX, Xiao DG. Regulation of Saccharomyces cerevisiae genetic engineering on the production of acetate esters and higher alcohols during Chinese Baijiu fermentation. J Ind Microbiol Biotechnol. 2017;44:949–960. doi: 10.1007/s10295-017-1907-2. [DOI] [PubMed] [Google Scholar]
- Loscos N, Hernandez-Orte P, Cacho J, Ferreira V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J Agric Food Chem. 2007;55:6674–6684. doi: 10.1021/jf0702343. [DOI] [PubMed] [Google Scholar]
- Ma L, Huang S, Du L, Tang P, Xiao D. Reduced production of higher alcohols by Saccharomyces cerevisiae in red wine fermentation by simultaneously overexpressing BAT1 and deleting BAT2. J Agric Food Chem. 2017;65:6936–6942. doi: 10.1021/acs.jafc.7b01974. [DOI] [PubMed] [Google Scholar]
- Moyano L, Zea L, Villafuerte L, Medina M. Comparison of odor-active compounds in sherry wines processed from ecologically and conventionally grown Pedro Ximenez grapes. J Agric Food Chem. 2009;57:968–973. doi: 10.1021/jf802252u. [DOI] [PubMed] [Google Scholar]
- Ndip RN, Akoachere JF, Dopgima LL, Ndip LM. Characterization of yeast strains for wine production: effect of fermentation variables on quality of wine produced. Appl Biochem Biotechnol. 2001;95:209–220. doi: 10.1385/ABAB:95:3:209. [DOI] [PubMed] [Google Scholar]
- Nyanga LK, Nout MJ, Smid EJ, Boekhout T, Zwietering MH. Fermentation characteristics of yeasts isolated from traditionally fermented masau (Ziziphus mauritiana) fruits. Int J Food Microbiol. 2013;166:426–432. doi: 10.1016/j.ijfoodmicro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Perestrelo R, Fernandes A, Albuquerque FF, Marques JC, Câmara JS. Analytical characterization of the aroma of Tinta Negra Mole red wine: identification of the main odorants compounds. Anal Chim Acta. 2006;563:154–164. doi: 10.1016/j.aca.2005.10.023. [DOI] [Google Scholar]
- Pino JA, Queris O. Analysis of volatile compounds of pineapple wine using solidphase microextraction techniques. Food Chem. 2010;122:1241–1246. doi: 10.1016/j.foodchem.2010.03.033. [DOI] [Google Scholar]
- Roda A, Lucini L, Torchio F, Dordoni R, De Faveri DM, Lambri M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017;229:734–742. doi: 10.1016/j.foodchem.2017.02.111. [DOI] [PubMed] [Google Scholar]
- Rodríguez ME, Lopes CA, Barbagelata RJ, Barda NB, Caballero AC. Influence of Candida pulcherrima Patagonian strain on alcoholic fermentation behaviour and wine aroma. Int J Food Microbiol. 2010;138:19–25. doi: 10.1016/j.ijfoodmicro.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Steingass CB, Grauwet T, Carle R. Influence of harvest maturity and fruit logistics on pineapple (Ananas comosus [L.] Merr.) volatiles assessed by headspace solid phase microextraction and gas chromatography-mass spectrometry (HS-SPME-GC/MS) Food Chem. 2014;150:382–391. doi: 10.1016/j.foodchem.2013.10.092. [DOI] [PubMed] [Google Scholar]
- Steingass CB, Langen J, Carle R, Schmarr HG. Authentication of pineapple (Ananas comosus [L.] Merr.) fruit maturity stages by quantitative analysis of γ- and δ-lactones using headspace solid-phase microextraction and chirospecific gas chromatography-selected ion monitoring mass spectrometry (HS-SPME-GC-SIM-MS) Food Chem. 2015;168:496–503. doi: 10.1016/j.foodchem.2014.07.071. [DOI] [PubMed] [Google Scholar]
- Sumby KM, Grbin PR, Jiranek V. Microbial modulation of aromatic esters in wine: current knowledge and future prospects. Food Chem. 2010;121:1–16. doi: 10.1016/j.foodchem.2009.12.004. [DOI] [Google Scholar]
- Sun SY, Jiang WG, Zhao YP. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds and polyphenols in cherry wines. Food Chem. 2011;127:547–555. doi: 10.1016/j.foodchem.2011.01.039. [DOI] [PubMed] [Google Scholar]
- Swiegers JH, Pretorius IS. Modulation of volatile sulfur compounds by wine yeast. Appl Microbiol Biotechnol. 2007;74:954–960. doi: 10.1007/s00253-006-0828-1. [DOI] [PubMed] [Google Scholar]
- Velázquez R, Zamora E, Álvarez ML, Hernández LM, Ramírez M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front Microbiol. 2015;6:1222. doi: 10.3389/fmicb.2015.01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LY, Sun GM, Liu YG, Lv LL, Yang WX, Zhao WF, Wei CB. Aroma volatile compounds from two fresh pineapple varieties in China. Int J Mol Sci. 2012;13:7383–7392. doi: 10.3390/ijms13067383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JC, Niu YW, Feng T, Liu SJ, Cheng HX, Xu N, Yu HY, Xiao ZB. Evaluation of the formation of volatiles and sensory characteristics of persimmon (Diospyros kaki L.f.) fruit wines using different commercial yeast strains of Saccharomyces cerevisiae. Nat Prod Res. 2014;28:1887–1893. doi: 10.1080/14786419.2014.955492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.