Abstract
In this study, we evaluated the effects of sweeping frequency ultrasound (SFU) pretreatment on the angiotensin-converting enzyme (ACE) inhibitory activity of zein hydrolysates and enzymatic hydrolysis thermodynamics. The solubility, surface hydrophobicity (Ho), degree of hydrolysis (DH) of zein and ACE inhibitory activity of hydrolysates were determined. After SFU pretreatment, the solubility and Ho of zein were significantly increased. During the hydrolysis process, ultrasonic pretreatment significantly increased the DH of zein and the ACE-inhibitory activity of zein hydrolysates by 19.37 and 133.76%, respectively. First-order kinetics could be used to explain both traditional and ultrasonic-assisted hydrolysis. In contrast to traditional hydrolysis, the reaction rate constants of SFU-assisted hydrolysis were largely increased by 82.76, 17.81, 23.96, and 21.26% at hydrolysis temperatures of 293, 303, 313, and 323 K, respectively. For the thermodynamic parameters, SFU pretreatment decreased activation energy, enthalpy of activation, entropy of activation, and free energy of activation by 19.52, 20.63, 6.16, and 7.02% respectively. In conclusion, SFU pretreatment markedly enhanced the hydrolysis of zein, and this method could be applied to the protein proteolysis industry to produce zein peptides with high ACE inhibitory activity.
Keywords: Zein, Sweeping frequency ultrasound, Hydrolysis, Angiotensin-converting enzyme inhibitory activity, Thermodynamics
Introduction
Zein is a co-product of corn wet-milling processing, accounting for 68% of corn gluten meal (Jin et al. 2016). Because of its special structure, zein has mainly been used as a drug carrier and for tissue engineering (Paliwal and Palakurthi 2014). Moreover, recent animal experiments and human trials have suggested that zein hydrolysates, i.e., corn peptides, have beneficial effects on alcohol metabolism (Ma et al. 2012), lipid metabolism (Lee and Chang 2001), and hypertension (Huang et al. 2011). Unfortunately, the water insolubility of zein and its extensive intermolecular interactions have created barrier for enzymes to attack the protein bonds, which seriously limit the release of functional peptides and reduces the bioavailability of zein. Therefore, developing a more efficient technique to overcome these technical shortcomings is necessary.
Ultrasound technology, as an emerging non-thermal processing physical technology, has been extensively used in the food industry. Due to mechanical, cavitational, and thermal effects, high-power ultrasound is used to induce physical and chemical changes in biological matrices (Shirsath et al. 2012). In particular, acoustic cavitation is considered to be the basic process responsible for initiating most acoustic chemical reactions in liquid (Ashokkumar et al. 2007). The collapse of cavitation bubbles can produce violent physical forces (shear forces, micro jets, shock waves, etc.) and free radicals (Suslick 1990; Kadam et al. 2015). In recent years, ultrasound has been increasingly investigated in nanoemulsion preparation (Kadkhodaee and Povey 2008), ultrasound-assisted extraction (Ma et al. 2008), improving the functional properties of protein and preparing bioactive peptides (Yu et al. 2012). Sweeping frequency ultrasound (SFU) is an advanced ultrasound technology with a sweeping frequency (fi ± δ kHz), which will increase from fi − δ to fi + δ kHz and decrease from fi + δ to fi − δ with the same linear speed (Jin et al. 2015b). Compared with power ultrasound technology, sweeping frequency ultrasound (SFU) has many advantages, including uniform energy distribution and processing material resonance. SFU pretreatment before hydrolysis can significantly increase the degree of hydrolysis (DH) and angiotensin-converting enzyme (ACE) inhibitory activity of peptides (Ren et al. 2013; Jin et al. 2015b). Some studies have shown that improvement of DH and ACE inhibitory activities is caused by various changes in the secondary structures and microstructures of wheat gluten (Zhang et al. 2016). Furthermore, Qu et al. (2012) explored the mechanism of ultrasound pretreatment on enzymatic hydrolysis of wheat germ protein from hydrolysis kinetics. However, no studies have evaluated the kinetics and thermodynamics of SFU-assisted hydrolysis in the production of ACE inhibitory peptides derived from zein.
Therefore, in this study, we aimed to investigate changes in the surface hydrophobicity and solubility of zein after pretreatment with SFU, assess the effects of SFU pretreatment on the DH of zein and ACE inhibitory activity of zein hydrolysates, and explore changes in the thermodynamics of zein hydrolysis caused by SFU pretreatment.
Materials and methods
Materials
Zein (protein content: 98%) was purchased from Gaoyou Rixing Pharmaceutical Adjuvant Co. Ltd. (Yangzhou, China). Alcalase (activity: 132,507 U/mL) was obtained from Novozymes Biotechnology Co. Ltd. (Shanghai, China). ACE was extracted from pig’s lung using Hippuryl–His–Leu (Sigma-Aldrich Trading Co. Ltd., Shanghai, China) as a substrate (Zhou et al. 2013). Other reagents used were of analytical grade.
Ultrasound pretreatment of zein
An aliquot of zein suspension (200 mL, 2 g zein) was sealed in high-pressure-resistant bags and treated by SFU equipment, which consists of upper and lower plates, each one can deliver its own frequency separately (internal dimensions: 362 mm × 294 mm × 502 mm; Shangjia Biotechnology Co., Wuxi, Jiangsu, China) with 5.4 L water. The pretreatment parameters were optimized in our previous study, as follows: sweeping cycle, 500 s; power, 600 W; sweeping frequency, 40 ± 2 kHz; pulsed on-time/off-time, 10/3 s and sonication time, 40 min. The sonication temperature was maintained at 25 °C by circulating water bath and monitored by a thermometer. The control samples were prepared using a magnetic stirring apparatus instead of ultrasound, and other conditions remained unchanged.
In order to study the effects of SFU on the physicochemical properties of zein, ultrasound-treated and control samples were centrifuged at 2500 × g for 10 min to obtain supernatants for analysis of solubility and surface hydrophobicity (Ho).
Enzymatic hydrolysis of zein
Before enzymatic hydrolysis, the zein suspension was maintained at 20, 30, 40 and 50 °C Next, alcalase (E/S = 2000 U/g) was added to start the reaction and the pH was kept at 9.0 by continuously adding 1 M NaOH during the hydrolysis process. The hydrolysis times were 2, 4, 6, 8, and 10 min. To terminate the hydrolysis, the mixture was kept in boiling water for 10 min, after which the pH was adjusted to 7.0. Next, 6% (v/v) trichloroacetic acid was added into the mixture at a 1:1 ratio to precipitate dissolved protein. Finally, the hydrolysates were cooled and centrifuged at 5030 × g for 15 min to obtain supernatants for further analysis.
Solubility and Ho
Using bovine serum albumin as standard, the crude protein content of the supernatant was determined according to the Lowry method (Waterborg 2009). Solubility was expressed as the percentage of protein in the supernatant compared with the total initial protein.
Ho of zein dispersions was determined by Kato and Nakai (1980) with slight modifications, using 1-anilino-8-naphthalene-sulfonate (ANS) as a fluorescent probe. Ultrasound-pretreated and control zein supernatants were concentrated and adjusted with 0.01 M phosphate-buffered saline (PBS) to 0.5 mg/mL. Next, 50 μL ANS (8.0 mM in PBS) was added to 5 mL zein supernatant and mixed at 25 °C. The relative fluorescence intensity was monitored immediately using a Cary Eclipse spectrophotometer at an excitation wavelength of 700 nm (slit: 5.0 nm), emission wavelength of 360–750 nm, and scanning speed of 5 nm/s. Ho was expressed as the relative fluorescence intensity at a protein concentration of 0.5 mg/mL.
Degree of hydrolysis
The DH of zein was determined using the pH-state method (Liang et al. 2017), which was calculated using Eq. (1):
| 1 |
where B is the NaOH volume consumed (mL), Nb is the normality of the NaOH (mol/L), Mp is the protein weight (g), α is the average dissociation degree of α-NH2 in substrate (0.99 for alcalase), and htot is the total number of peptide bonds in the protein substrate (9.2 mmol/g for zein) (Kong and Xiong 2006).
To describe the thermodynamics of zein hydrolysis, the hydrolyzed zein concentration, i.e., the concentration of zein hydrolysates, was also assayed by the Lowry method (Waterborg 2009).
ACE inhibitory activity
The ACE inhibitory activity of zein hydrolysates was measured as previously reported (Vermeirssen et al. 2002), with slight modifications. An aliquot (45 μL) of substrate borate buffer (1.0 mM N-[3-(2-Furyl) acryloyl]-l-phenylalanylglycyl-glycine (FAPGG), 80 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 0.1 M borate buffer, 300 mM NaCl; pH 8.3) was mixed with 40 μL sample solution in microliter plate wells. The reaction was initiated by adding 20 μL ACE (0.1 U/mL). After reacting for 30 min at 37 °C, the absorbance of the reacting solution was determined immediately at 340 nm. The control was operated in the same manner using water instead of sample. The ACE inhibitory activity was calculated as follows:
| 2 |
where I is ACE inhibitory activity (%), AC is the absorbance of the control group without protein hydrolysates, AB is the absorbance of the blank group without ACE, and AS is the absorbance of the sample group with ACE and protein hydrolysates.
Hydrolysis reaction kinetics
The first-order kinetic model described by Takahashi et al. (2002) was fitted to study zein hydrolysis kinetics. The kinetic model was integrated and expressed as below:
| 3 |
where Ct is the zein concentration in the reaction solution at a given time t min (µg/mL), C0 is the initial zein concentration in reaction solution (µg/mL), t is the hydrolysis time (min), and k is the reaction rate constant.
The decrease in zein concentration was difficult to measure during hydrolysis, but could be indirectly determined by changes in the amount of zein hydrolysates. At a certain pressure and temperature, C0 = Q∞ and Ct = (Q∞ − Qt). Equation (4) could be converted to:
| 4 |
where Q∞ is the ultimate concentration of zein hydrolysate (µg/mL), obtained under hydrolysis condition of pH 9.0 at 50 °C for 10 h (Jin et al. 2015a); Qt is the zein hydrolysate concentration at a given time t min (µg/mL). The kin could then be obtained by calculating the slope of ln (Q∞ − Qt) against t.
Hydrolysis thermodynamics
The Arrhenius equation (Damodaran et al. 2007) was used to describe the relationship between the reaction rate constant k and the temperature T, which was rearranged as follow:
| 5 |
where k indicates the reaction rate constant (1/min), A indicates the pre-exponential factor (1/min), Ea indicates the activation energy (J/mol), R indicates the universal gas constant (8.314 J/mol K), and T indicates the absolute temperature (K). Ea can be calculated through the plot of lnk against 1/T.
According to the Eyring transition state theory (Qu et al. 2013), the relationship between enthalpy of activation (), entropies of activation (), Gibbs free energy () of the hydrolysis and the reaction temperature (T), and the reaction rate constant (k) could be expressed by the following equation:
| 6 |
where kB is the Boltzman constant (1.38 × 10−23 J/K), h is the Planck constant (6.6256 × 10−34 J/s), is the Gibbs free energy of activation (J/mol), is the enthalpy of activation (J/mol), and is the entropy of activation (J/mol K).
After logarithm transformation, Eq. (7) was expressed as:
| 7 |
,, and could be calculated by the plot of ln(k/T) against 1/T.
Statistical analysis
All experiments were repeated at least three times, and the data were presented as means and standard deviations. The obtained data was subjected to the least significant difference (LSD) test, and differences with P values of less than 0.05 were considered significant. All experimental data were linearly fitted to obtain the required equation. All graphs and calculations were conducted using OriginPro8.5 and SPSS 18.0.
Analysis of variance and Tukey’s test were performed under the significance level of P < 0.05 with the aid of the statistical software SPSS 18.0 (IBM Corporation, NY, USA).
Results and discussion
Effects of SFU pretreatment on the DH and solubility of zein
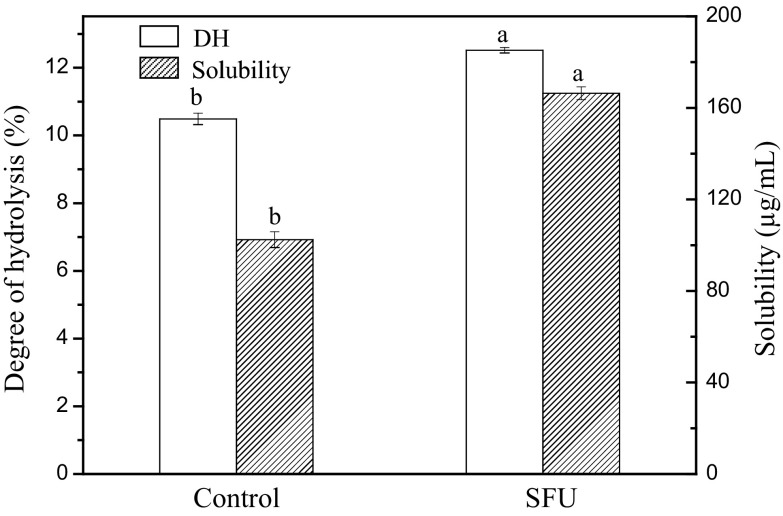
The solubility and DH in control and SFU-assisted hydrolysis of zein are presented in Fig. 1. The results showed that SFU pretreatment improved the solubility and DH of zein significantly (P < 0.05). Solubility is the amount of protein in a sample that dissolves into solution, which is a good indicator of protein function (Zayas 1997). After SFU pretreatment, the solubility of zein increased from 102.4 to 166 µg/mL. This result was consistent with the findings for ultrasound-pretreated proteins, such as soy protein isolate (Hu et al. 2013), black bean protein isolates (Jiang et al. 2014), and milk protein concentrate (Sun et al. 2014). SFU pretreatment with 40 ± 2 kHz may produce a strong cavitation effect accompanied by local high pressure gradients and high temperature of liquid layers in the vicinity (Ren et al. 2013), which may lead to protein decomposition, chemical bond breakage, and formation of free radicals. SFU pretreatment also had mechanical effects on zein, causing macromolecular proteins and medium to move at different high speeds and greatly increasing the change of collision.
Fig. 1.
Effects of sweeping frequency ultrasound (SFU) pretreatment on the degree of hydrolysis (DH) and solubility of zein. The same indexes marked with different letters indicate significant differences (P < 0.05, n = 3)
The 19.25% enhancement in the DH of zein could be attributed to the increased solubility and changes in molecular conformation of the protein. Due to the dense spatial three-dimensional structure of zein and predominantly hydrophobic nonpolar amino acid composition, alcalase did not hydrolyze zein effectively (Paliwal and Palakurthi 2014). After SFU pretreatment, the structure of the protein macromolecules became loose; more peptide bonds buried inside the protein were exposed (Shanmugam et al. 2012; Jin et al. 2015b), which increased the chance of the enzyme coming in contact with the active site of the protein.
Effects of SFU pretreatment on the Ho of zein and the ACE inhibitory activity of zein hydrolysates
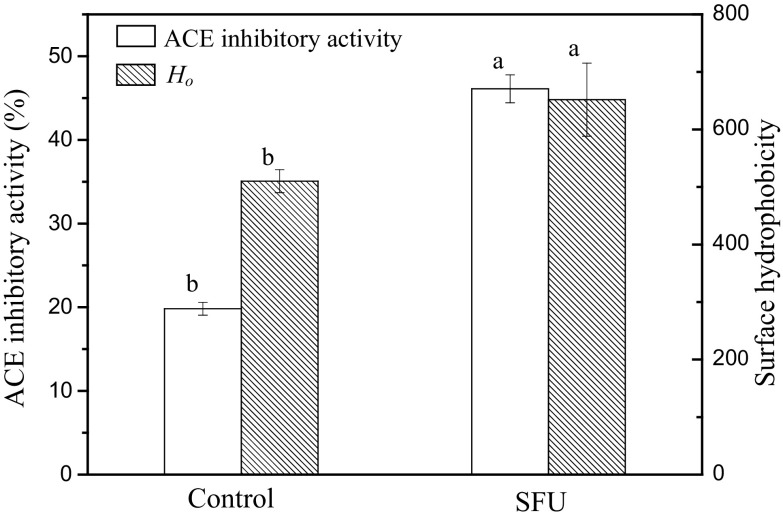
Figure 2 shows Ho and ACE inhibitory activity in both traditional and SFU-assisted hydrolysis. After ultrasound pretreatment, the Ho of zein increased significantly (P < 0.05). These findings were consistent with previous findings suggesting that ultrasonic treatment could increase Ho in corn gluten meal (Jin et al. 2015b) and black bean protein isolates (Jiang et al. 2014). Thus, SFU pretreatment induced greater unfolding of protein molecules and increased the exposure of hydrophobic groups on the protein macromolecules.
Fig. 2.
Effects of sweeping frequency ultrasound (SFU) pretreatment on the surface hydrophobicity (Ho) of zein and ACE inhibitory activities of zein hydrolysates. The same indexes marked with different letters indicate significant differences (P < 0.05)
Because zein has a high proportion of hydrophobic amino acids and proline, it is a good source for preparation of ACE inhibitory peptides (Yang et al. 2007). The ACE inhibitory activity of zein hydrolysates after SFU pretreatment dramatically increased from 19.81 to 46.11%. The results demonstrated that ultrasound pretreatment could improve the ACE inhibitory activity of zein hydrolysates effectively. During the hydrolysis process, the exposure of more internal hydrophobic groups and regions caused by ultrasound treatment may result in the release of peptides with more hydrophobic amino acids, playing an important role in improving the ACE inhibitory activity of peptides (Zhang et al. 2016). Additionally, the ACE inhibitory activities of peptides are highly influenced by the molecular weight and amino acid composition of the peptide. Indeed, Wang et al. (2014) reported that increasing DH during hydrolysis could produce peptides with higher ACE inhibitory activity. Therefore, the increases in DH and Ho of zein could contribute to the improvement of ACE inhibitory activity in zein hydrolysates.
Effects of SFU pretreatment on reaction rate constant k
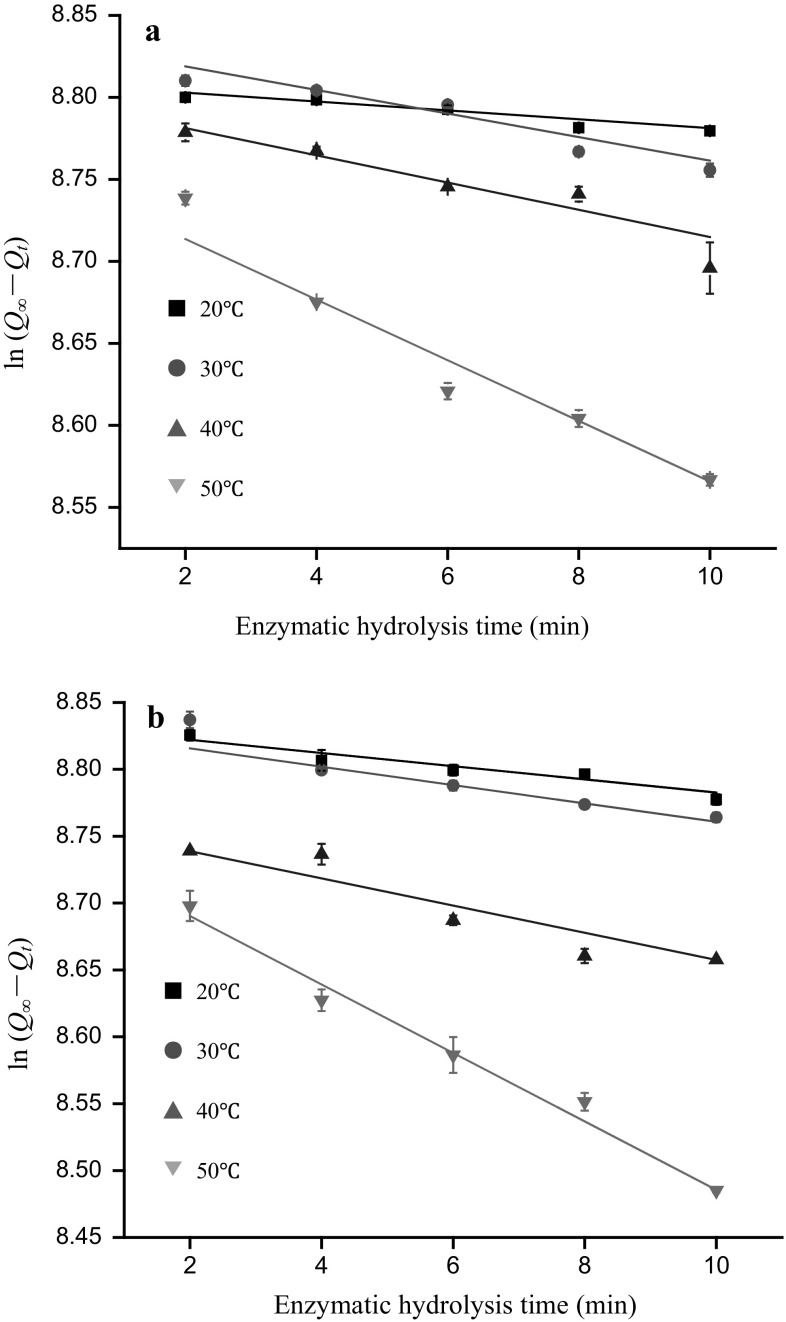
The reaction rate constant k is an important physical parameter in chemical reaction kinetics and depends on reaction temperature, protein structure, and catalysts. Because ultrasound pretreatment significantly increased the solubility, Ho, and DH of zein, the effects of ultrasonic on k were further investigated. Figure 3 shows the plots of ln(Q∞ − Qt) versus t in traditional and SFU-pretreated hydrolysis at temperatures varying from 293 to 323 K. As shown in Fig. 3, all of the correlation coefficients were larger than 0.90, which meant there was a good linear relationship between the ln(Q∞ − Qt) and the time at different temperatures for traditional and SFU-pretreated hydrolysis. Therefore, first-order kinetics was appropriate for depicting both traditional and SFU-pretreatment hydrolysis at a certain temperature range.
Fig. 3.
The relationship curves between ln(Q∞ − Qt) and reaction time t for traditional hydrolysis (a) and sweeping frequency ultrasound pretreatment (b)
The reaction rate constant k was obtained by calculating the slope of ln(Q∞ − Qt) against t, as shown in Table 1. As the reaction temperature increased by 10 K, the reaction rate constant increased 2–4 times. This could be related to the increased activity of the enzyme at higher temperatures and the higher collision frequency between the protein and enzyme (Jin et al. 2015a). The reaction rate constants ku of the hydrolysis after SFU pretreatment were increased by 82.76, 17.81, 23.96, and 21.26% significantly at temperatures of 293, 303, 313, and 323 K, respectively, compared with traditional hydrolysis. This could be attributed to the temperatures, intense high pressures, and shear forces generated by ultrasound cavitation efficacies, leading to structural changes in proteins and exposure of the active center (McClements 1995). Under the appropriate temperature, the enzyme could combine easily with the active center of the protein, thereby improving the reaction rate constant. Nevertheless, the increased ratio of reaction rate constants k caused by SFU pretreatment decreased as the hydrolysis temperature increased. The results showed that as the hydrolysis temperature increased, the thermal effects dominated; the increase of k induced by ultrasound was not as pronounced as that at lower temperature. In accordance with the studies of Qu et al. (2013), our findings further confirmed that the contribution of ultrasonic effects to the improvement of the reaction rate constant was higher under lower temperatures than under higher temperatures.
Table 1.
Reaction rate constants for traditional hydrolysis and sweeping frequency ultrasound pretreatment
| T (K) | Traditional hydrolysis | Ultrasound pretreatment | ||
|---|---|---|---|---|
| Kt (1/min) | Ku (1/min) | |||
| 293 | 0.0029b ± 0.0004 | 0.9297 | 0.0053a ± 0.0003 (82.76%)* | 0.934 |
| 303 | 0.0073b ± 0.0005 | 0.9354 | 0.0086a ± 0.0002 (17.81%)* | 0.9165 |
| 313 | 0.0096b ± 0.0009 | 0.9072 | 0.0119a ± 0.0007 (23.96%)* | 0.9045 |
| 323 | 0.0207b ± 0.0002 | 0.9571 | 0.0251a ± 0.0003 (21.26%)* | 0.9848 |
Values followed by different letters are significantly different at P < 0.05
The values of reaction rate constant k were compared by columns
*Increased initial reaction rates for sweeping frequency ultrasound pretreatment compared with traditional enzymatic hydrolysis
Effects of SFU pretreatment on hydrolysis thermodynamics
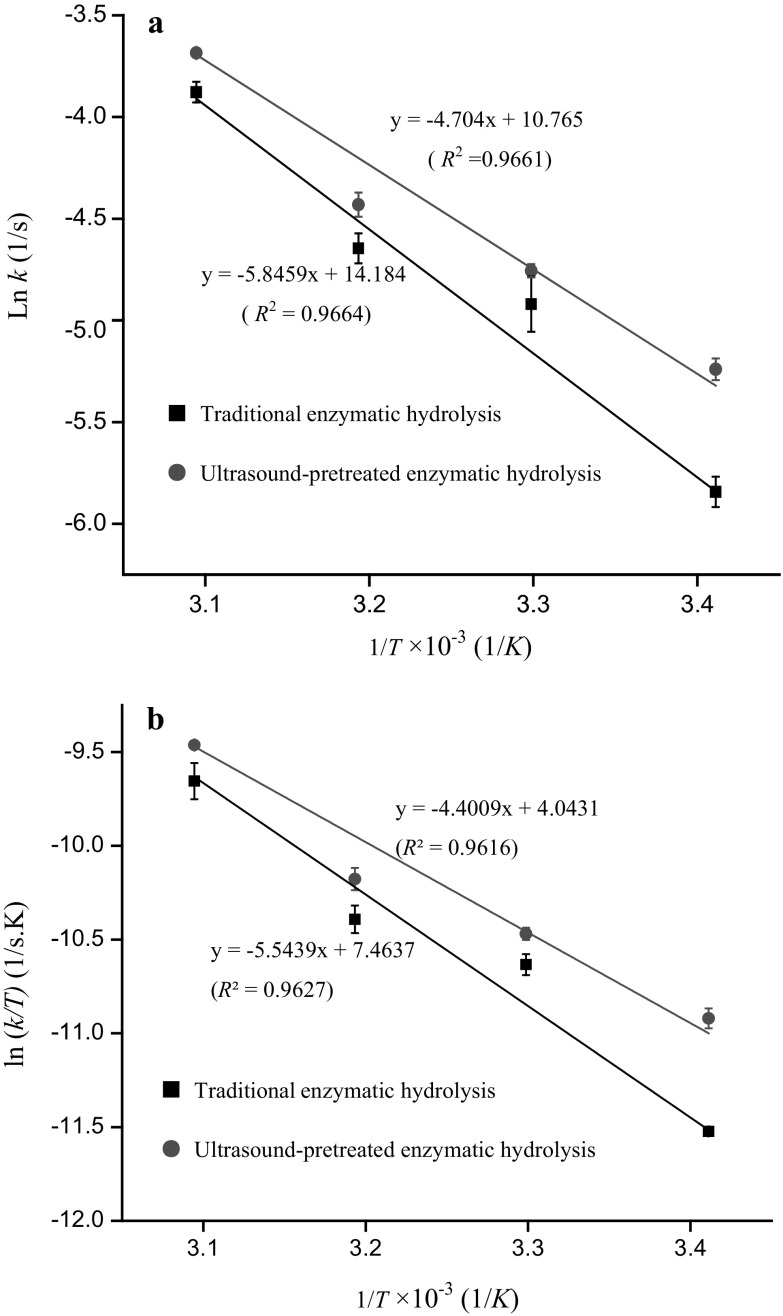
From the reaction rate constants obtained above, the thermodynamic parameters could be calculated according to Eqs. (6–7), and the results are summarized in Table 2. Activation energy (Ea), defined as the minimum energy required for molecules to convert from normal stable status to reactive status, represents the difficulty of chemical reactions (Subhedar and Gogate 2014). Arrhenius plots of ln k against 1/T are shown in Fig. 4a, and the corresponding slopes are represented as the Ea. For traditional and SFU-pretreated hydrolysis, the values of Ea were 48.55 and 39.06 kJ/mol, respectively, revealing that ultrasound pretreatment decreased significantly the energy barrier needed by the hydrolysis reaction by 19.52%. Therefore, SFU pretreatment could cause proteolysis to occur easily and allow the activate state to be reached more efficiently.
Table 2.
Thermodynamic parameters for traditional hydrolysis and sweeping frequency ultrasound pretreatment
| Hydrolysis | Ea (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol K) | ΔG (kJ/mol) |
|---|---|---|---|---|
| Traditional hydrolysis | 48.55 ± 1.97a | 46.05 ± 0.99a | − 154.47 ± 3.02a | 93.63 ± 1.11a |
| SFU pretreatment | 39.06 ± 1.21b (− 19.52%)* | 36.55 ± 1.35b (− 20.63%)* | − 163.98 ± 2.98b (− 6.16%)* | 87.06 ± 1.34b (− 7.02%)* |
Values followed by different letters are significantly different at P < 0.05
The values of , and were compared by rows
*Decreases in thermodynamic parameters for sweeping frequency ultrasound pretreatment compared with traditional enzymatic hydrolysis
Fig. 4.
The relationship between ln k (a), ln(k/T) (b) and 1/T in traditional and sweeping frequency ultrasound-pretreated hydrolysis
The Eyring plot of ln k/T versus reciprocal absolute temperature (1/T) is presented in Fig. 4b. The enthalpies of activation () and entropies of activation () were estimated from the slopes and intercepts of the curves, respectively. After obtaining and , Gibbs free energy () was easily calculated by Eq. (6). When the change in enthalpy is positive, the hydrolysis reaction will be of endothermic nature. A lower enthalpy means a lower energy cost when converting the substrate into a product. Compared with traditional hydrolysis, the of hydrolysis with SFU pretreatment was reduced by 20.63%, demonstrating that ultrasound pretreatment favored the formation of activated enzyme–substrate complexes and a transition state. Entropy reflects the extent of variation of local disorder between the ground state and the transition state of enzymatic hydrolysis (Jin et al. 2015a). Moreover, the 6.16% decrease in with SFU pretreatment indicated that the enzyme and zein showed reduced disorder after ultrasound irradiation. In enzyme catalysis, will be the energy barrier between the ground state of the complex of substrate binding with enzyme and the activated state of the complex of substrate binding with enzyme (Cheng et al. 2017). Notably, decreased 7.02%, which was dependent on the declines in both and . The results above showed a similar trend according to the research of Qu et al. (2013), who reported that the thermodynamic parameters , , and of the hydrolysis reaction after ultrasonic irradiation were reduced by 74.1, 34.3, and 1.4%, respectively. These remarkable decreases in , , and could be attributed to ultrasonically induced changes in the structure of zein, such as exposure of the internal hydrophobic groups and protein regions (Ma et al. 2011), breakage of noncovalent bonds, and changes in protein microstructures and three-dimensional structures (Jin et al. 2015a).
Conclusion
The effects of SFU pretreatment on the ACE inhibitory activity of peptides and hydrolysis thermodynamics were investigated. Under SFU pretreatment at a frequency of 40 ± 2 kHz, the DH of zein and the ACE inhibitory activities of zein hydrolysates were significantly improved compared with those after traditional hydrolysis, potentially because of the increase of solubility and Ho of zein. The results of kinetic and thermodynamic analysis indicated that SFU pretreatment accelerated the enzymatic hydrolysis rate by increasing reaction rate constants and reducing activation energy, enthalpy of activation, entropy of activation, and free energy of activation. Thus, our results showed that SFU-assisted hydrolysis of zein may be an efficient and environmentally friendly method for preparing peptides with high ACE inhibitory activity.
Acknowledgements
The authors wish to express their appreciation for the support obtained from the National Natural Science Foundation of China (Grant Nos. 31501427 & 31771977), the Key Research and Development Program of Jiangsu Province (Grant Nos. BE2016334 & BE2017353), China Postdoctoral Science Foundation (Grant No. 2016M601742), Foundation of Key Laboratory of Agricultural Products Physical Processing in Jiangsu Province (Grant No. JAPP2014-3), the Senior Professional Research Start-up Fund of Jiangsu University (Grant No. 14JDG180) and Young Backbone Teachers Program of Jiangsu University, the Nature Science Foundation of the Jiangsu Provincial Education Department (Grant No. 11KJB550001), Research-Innovation Program of Post-graduate in General Universities of Jiangsu, China (Grant No. KYLX15_1093), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Some parts of this article come from a PhD thesis (Ren 2014).
Conflict of interest
The author declares that they have no conflict of interest.
Human and animal statement
This article does not contain any studies involving human or animal subjects.
References
- Ashokkumar M, Lee J, Kentish S, Grieser F. Bubbles in an acoustic field: an overview. Ultrason Sonochem. 2007;14(4):470–475. doi: 10.1016/j.ultsonch.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Liu Y, Wu J, Ofori Donkor P, Li T, Ma H. Improving the enzymolysis efficiency of potato protein by simultaneous dual-frequency energy-gathered ultrasound pretreatment: thermodynamics and kinetics. Ultrason Sonochem. 2017;37:351–359. doi: 10.1016/j.ultsonch.2017.01.034. [DOI] [PubMed] [Google Scholar]
- Damodaran S, Parkin KL, Fennema OR. Fennema’s food chemistry. Boca Raton: CRC Press; 2007. [Google Scholar]
- Hu H, Wu J, Li-Chan ECY, Zhu L, Zhang F, Xu X, Fan G, Wang L, Huang X, Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30(2):647–655. doi: 10.1016/j.foodhyd.2012.08.001. [DOI] [Google Scholar]
- Huang W, Sun J, He H, Dong H, Li J. Antihypertensive effect of corn peptides, produced by a continuous production in enzymatic membrane reactor, in spontaneously hypertensive rats. Food Chem. 2011;128(4):968–973. doi: 10.1016/j.foodchem.2011.03.127. [DOI] [Google Scholar]
- Jiang L, Wang J, Li Y, Wang Z, Liang J, Wang R, Chen Y, Ma W, Qi B, Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res Int. 2014;62:595–601. doi: 10.1016/j.foodres.2014.04.022. [DOI] [Google Scholar]
- Jin J, Ma H, Qu W, Wang K, Zhou C, He R, Luo L, Owusu J. Effects of multi-frequency power ultrasound on the enzymolysis of corn gluten meal: kinetics and thermodynamics study. Ultrason Sonochem. 2015;27:46–53. doi: 10.1016/j.ultsonch.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Jin J, Ma H, Wang K, Yagoub Ael G, Owusu J, Qu W, He R, Zhou C, Ye X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason Sonochem. 2015;24:55–64. doi: 10.1016/j.ultsonch.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Jin J, Ma H, Wang B, Yagoub Ael G, Wang K, He R, Zhou C. Effects and mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason Sonochem. 2016;30:44–51. doi: 10.1016/j.ultsonch.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Kadam SU, Tiwari BK, Álvarez C, O’Donnell CP. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci Technol. 2015;46(1):60–67. doi: 10.1016/j.tifs.2015.07.012. [DOI] [Google Scholar]
- Kadkhodaee R, Povey MJ. Ultrasonic inactivation of Bacillus α-amylase. I. Effect of gas content and emitting face of probe. Ultrason Sonochem. 2008;15(2):133–142. doi: 10.1016/j.ultsonch.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kato A, Nakai S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. BBA Prot Struct. 1980;624(1):13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Kong B, Xiong YL. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J Agric Food Chem. 2006;54(16):6059–6068. doi: 10.1021/jf060632q. [DOI] [PubMed] [Google Scholar]
- Lee H-M, Chang U-J. Effect of corn peptide on the lipid metabolism in rats Korean. J Food Culture. 2001;16(5):416–422. [Google Scholar]
- Liang Q, Ren X, Ma H, Li S, Xu K, Oladejo AO. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J Food Qual. 2017 [Google Scholar]
- Ma Y, Ye X, Hao Y, Xu G, Xu G, Liu D. Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrason Sonochem. 2008;15(3):227–232. doi: 10.1016/j.ultsonch.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Ma H, Huang L, Jia J, He R, Luo L, Zhu W. Effect of energy-gathered ultrasound on Alcalase. Ultrason Sonochem. 2011;18(1):419–424. doi: 10.1016/j.ultsonch.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhang W, Yu G, He H, Zhang Y. The primary structure identification of a corn peptide facilitating alcohol metabolism by HPLC–MS/MS. Peptides. 2012;37(1):138–143. doi: 10.1016/j.peptides.2012.07.004. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Advances in the application of ultrasound in food analysis and processing. Trends Food Sci Technol. 1995;6(9):293–299. doi: 10.1016/S0924-2244(00)89139-6. [DOI] [Google Scholar]
- Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Control Release. 2014;189:108–122. doi: 10.1016/j.jconrel.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Qu W, Ma H, Jia J, He R, Luo L, Pan Z. Enzymolysis kinetics and activities of ACE inhibitory peptides from wheat germ protein prepared with SFP ultrasound-assisted processing. Ultrason Sonochem. 2012;19(5):1021–1026. doi: 10.1016/j.ultsonch.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Qu W, Ma H, Liu B, He R, Pan Z, Abano EE. Enzymolysis reaction kinetics and thermodynamics of defatted wheat germ protein with ultrasonic pretreatment. Ultrason Sonochem. 2013;20(6):1408–1413. doi: 10.1016/j.ultsonch.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Ren X (2014) Effects of sweeping frequency ultrasound on properties of zein and preparation of ACE-inhibitory peptides. Ph.D. Thesis Jiangsu University Jiangsu, China
- Ren X, Ma H, Mao S, Zhou H. Effects of sweeping frequency ultrasound treatment on enzymatic preparations of ACE-inhibitory peptides from zein. Eur Food Res Technol. 2013;238(3):435–442. doi: 10.1007/s00217-013-2118-3. [DOI] [Google Scholar]
- Shanmugam A, Chandrapala J, Ashokkumar M. The effect of ultrasound on the physical and functional properties of skim milk. Innov Food Sci Emerg. 2012;16:251–258. doi: 10.1016/j.ifset.2012.06.005. [DOI] [Google Scholar]
- Shirsath S, Sonawane S, Gogate P. Intensification of extraction of natural products using ultrasonic irradiations: a review of current status. Chem Eng Process. 2012;53:10–23. doi: 10.1016/j.cep.2012.01.003. [DOI] [Google Scholar]
- Subhedar PB, Gogate PR. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J Catal B Enzym. 2014;101:108–114. doi: 10.1016/j.molcatb.2014.01.002. [DOI] [Google Scholar]
- Sun Y, Chen J, Zhang S, Li H, Lu J, Liu L, Uluko H, Su Y, Cui W, Ge W, Lv J. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J Food Eng. 2014;124:11–18. doi: 10.1016/j.jfoodeng.2013.09.013. [DOI] [Google Scholar]
- Suslick KS. Sonochemistry. Science. 1990;247(4949):1439–1446. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ogata A, Yang WH, Hattori M. Increased hydrophobicity of carboxymethyl starch film by conjugation with zein. Science. 2002;247(4949):1439–1446. doi: 10.1271/bbb.66.1276. [DOI] [PubMed] [Google Scholar]
- Vermeirssen V, Van Camp J, Verstraete W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J Biochem Biophys Methods. 2002;51(1):75–87. doi: 10.1016/S0165-022X(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Wang B, Atungulu GG, Khir R, Geng J, Ma H, Li Y, Wu B. Ultrasonic treatment effect on enzymolysis kinetics and activities of ACE-inhibitory peptides from oat-isolated protein. Food Biophys. 2014;10(3):244–252. doi: 10.1007/s11483-014-9375-y. [DOI] [Google Scholar]
- Waterborg JH. The Lowry method for protein quantitation. In: Walker JM, editor. The protein protocols handbook. 2nd edn. Totowa, NJ: Humana Press; 2009. pp. 7–10. [Google Scholar]
- Yang Y, Tao G, Liu P, Liu J. Peptide with angiotensin I-converting enzyme inhibitory activity from hydrolyzed corn gluten meal. J Agric Food Chem. 2007;55(19):7891–7895. doi: 10.1021/jf0705670. [DOI] [PubMed] [Google Scholar]
- Yu L, Sun J, Liu S, Bi J, Zhang C, Yang Q. Ultrasonic-assisted enzymolysis to improve the antioxidant activities of peanut (Arachin conarachin L.) antioxidant hydrolysate. Int J Mol Sci. 2012;13(7):9051–9068. doi: 10.3390/ijms13079051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas JF. Solubility of proteins. Functionality of proteins in food. Berlin: Springer; 1997. pp. 6–75. [Google Scholar]
- Zhang Y, Wang B, Zhou C, Atungulu GG, Xu K, Ma H, Ye X, Abdualrahman MA. Surface topography, nano-mechanics and secondary structure of wheat gluten pretreated by alternate dual-frequency ultrasound and the correlation to enzymolysis. Ultrason Sonochem. 2016;31:267–275. doi: 10.1016/j.ultsonch.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Zhou C, Ma H, Yu X, Liu B, Yagoub Ael G, Pan Z. Pretreatment of defatted wheat germ proteins (by-products of flour mill industry) using ultrasonic horn and bath reactors: effect on structure and preparation of ACE-inhibitory peptides. Ultrason Sonochem. 2013;20(6):1390–1400. doi: 10.1016/j.ultsonch.2013.04.005. [DOI] [PubMed] [Google Scholar]