Abstract
The present work reports the development of ion chromatography conductivity based detection analytical method for the determination of some inorganic anions (chloride, sulfate and phosphate) in vegetable oils. The analytes were extracted from samples prior to injection into the chromatographic system, employing a simple two-step procedure. In the first step, 4.5 g of the sample was vigorously mixed with 15 mL of deionized water and then mixed for 15 min on a horizontal roller. Afterwards, the mixture was sonicated for 15 min in an ultrasonic bath. Then, the mixture was centrifuged for 15 min at 5000 rpm and, after filtration through a 0.22 μm membrane, the aqueous phase was used for the determination of the analytes. A clean-up step was introduced in the analysis of olive oils in order to correct an increase of the baseline of the chromatograms. The limits of detection and quantification of the proposed method were, respectively, 0.005 and 0.02 μg g−1 for chloride, 0.02 and 0.06 μg g−1 for phosphate and 0.008 and 0.03 μg g−1 for sulfate. Vegetable oils from corn, canola, soybean, sunflower and olive were analyzed and recovery tests (94.8 ± 10.1% mean recovery) were performed to attest the accuracy of the proposed method.
Keywords: Inorganic anions, Ion chromatography, Vegetable oils, Extraction
Introduction
Vegetable oils have a variety of commercial uses, such as in the cosmetic, pharmaceutical, chemical and food industries (Dugo et al. 2007). Monitoring of vegetable oils has been very important in the tracking of heavy metals (Robaina et al. 2012; Leonardis et al. 2000; Ooms and Pee 1983; Pehlivan et al. 2008), in order to meet the demands and requirements of international and national regulations, but only a few efforts have been made to evaluate inorganic anions in this kind of sample (Dugo et al. 2007; Buldini et al. 1997a; Lemos et al. 2015).
The determination of inorganic anions in vegetable oils is important for nutritional and toxicological assessments. For instance, chloride is an ordinary food preservative and its quantification is necessary for the quality control of the final product. It is found in a wide variety of foods and plays an important role in the metabolic acid–base equilibrium. On the other hand, chloride presents adverse effects when ingested in high concentrations (Suetrong et al. 2016).
Sulfate is not considered a toxic anion, but can be employed as an indicator of the use of pesticides, such as copper sulfate, in the soils where the original vegetables were cultivated. Also, sulfate can result from the oxidation of sulfite, a substance commonly employed in foodstuff preservation (Buldini et al. 1997b).
Phosphate compounds are regularly used in agriculture as fertilizers. Inorganic phosphate is also employed in food processing, especially for flavor stabilization and preservation and, for this reason, can appear in high concentrations in some types of foods. The excess intake of phosphate must be avoided, because it can cause adverse effects such as the inhibition of calcium absorption in the intestine and disturbance of the mineral equilibrium in the body (Ellinger 1972). In this context, the development of simple strategies for the determination of anions in vegetable oils is needed.
The determination of anions in oils is a difficult task, especially because commonly used analytical techniques are not compatible with this kind of matrix. There are some reports in the current literature on this subject. Lemos et al. (2015) developed a method for the determination of some inorganic anions (fluoride, chloride, nitrate and sulfate) and formate in virgin olive oils by capillary electrophoresis with capacitively coupled contactless conductivity detection (CE-C4D). The anions were extracted from the samples before injection into the instrument, but only formate could be quantified in the five samples analyzed.
Silveira et al. (2014) proposed a methodology for chloride, sulfate, phosphate, acetate and formate determination in biodiesel by ion chromatography. The analytes were extracted from the samples using an ultrasound-assisted approach and the method was successfully applied to the analysis of biodiesel samples derived from different fat sources.
Buldini et al. (1997a) proposed an interesting strategy for sample preparation of vegetable oils prior to the determination of anions by ion chromatography. They removed the complex organic matrix by saponification followed by UV irradiation. Nevertheless, the procedure was slow, requiring 1.5 h for sample saponification and approximately 1 h of UV irradiation to complete the degradation of the matrix. Dugo et al. (2007) also employed IC for anions separation and determination, but they extracted the analytes from the sample using a carbonate buffer solution (pH 8.0) heated at 70 °C.
The goal of the present work was to develop a simple and rapid method for the extraction of chloride, sulfate and phosphate from edible oils followed by their determination in the extracts by ion chromatography with conductivity detection.
Materials and methods
Apparatus
All determinations of inorganic anions in aqueous solutions (sample extracts and standard solutions) were carried out using an Ion Chromatography System (Dionex, Sunnyvale, Ca, USA), model ICS-2100, equipped with an integrated eluent (potassium hydroxide) generator, model RFIC-EG (EGC III KOH cartridge), and an AERS 500 2 mm membrane conductivity suppressor. Conductivity signals were measured with a DS6 heated conductivity cell and the chromatograms were acquired and registered using Chromeleon software, version 7.2, also supplied by Dionex. The separation of the anions was performed with an Ion-Pac AS15 analytical column (2 × 250 mm, 7.5 μm particle size) and using an IonPac AG15 guard column (2 × 50 mm, 7.5 μm particle size) to protect the analytical column. Both analytical and guard column were supplied by Dionex (Sunnyvale, CA, USA).
The elution of the anions was performed in isocratic mode using a 33 mM potassium hydroxide solution pumped at a flow rate of 0.33 mL min−1. Under these conditions chloride, sulfate and phosphate peaks appeared at 5.03, 7.83 and 18.82 min, respectively. The samples (and standard solutions) were injected using a 25 µL loop injector and a current of 27 mA was set in the conductivity suppressor cell.
The extractions were performed with the aid of a horizontal roller mixer, model MR-II, supplied by Biomixer (São Paulo, Brazil), and an ultrasonic bath, model UltraCleaner 1600, supplied by Unique (São Paulo, Brazil).
Reagents and solutions
The deionized water used in this work was obtained using a Direct Q3 water system from Millipore (Bedford, MA, USA), and always had a resistivity higher than 18 MΩ cm.
Aqueous stock standard solutions of the inorganic anions, at a nominal concentration of 1000 mg L−1, were purchased from Sigma Aldrich (St. Louis, MO, USA). All of the solutions employed in this work were prepared by dilution of the stock solutions with deionized water.
Material decontamination
Plastic flasks were decontaminated by soaking them in a 10% v/v HNO3 solution for at least 24 h. After soaking, the flasks were rinsed with purified water, dried in a dust-free environment and stored in a clean place until use.
Edible oil samples
The oil samples analyzed in this work (corn, canola, sunflower, soybean, extra virgin olive and extra soft olive oils) were purchased at a local supermarket in the city of Niterói, Rio de Janeiro, Brazil. The bottles were wrapped with aluminum foil to protect them from light and stored in the dark at room temperature to reduce lipid oxidation.
Sample extraction procedure
An aliquot of 4.5 g of each oil sample was transferred into a clean polyethylene tube and 15 mL of deionized water was added. The tubes were capped, and the mixtures were shaken vigorously for 30 s and sonicated, one at a time, for 15 min in an ultrasonic bath. Afterwards, the mixtures were placed on a roller mixer for 15 min at 110 rpm. Finally, they were centrifuged for 15 min at 5000 rpm to separate the oil and water phases. The water phase containing the extracted analytes was collected and filtered through a polyvinylidene fluoride (PVDF) membrane with a pore diameter of 0.22 µm. The final extracts obtained from the treatment of extra virgin olive oils were also cleaned-up by percolating them through a solid phase extraction (SPE) cartridge containing 500 mg of C18 supplied by Applied Separations (Allentown, PA, USA).
Analyte recovery assays
Recovery assays were performed in order to evaluate the accuracy of the extraction procedure. In this experiment, standard stock solutions containing known concentrations of the three anions (chloride, sulfate and phosphate) were prepared in methanol and added to the oil samples. Then, the spiked samples were submitted to the optimized extraction procedure. The amounts recovered were calculated by obtaining the difference in relation to the non-spiked samples.
Results and discussion
The determination of inorganic anions in vegetable oils is important from a toxicological and technological point of view. However, the concentrations of the anions found are, in general, very low and their determination pose an analytical challenge, since the treatment of samples must be compatible with the instrumentation used for the measurements. In this work, a simple extraction procedure for determination of chloride, sulfate and phosphate in soybean, corn, canola, sunflower, extra virgin olive and extra soft olive vegetable oils by ion chromatography has been optimized. The parameters studied were: (1) the volume of the extraction phase (maintaining a constant mass of sample), (2) the time of agitation, and (3) the addition of surfactants for better emulsification. Additionally, a clean-up step was tested in order to make the extracts suitable for injection into the chromatographic system.
Evaluation of the volume of extractant solution
In extraction procedures, the ratio mass of sample/volume of extractant solution is one of the most important parameters to be evaluated, since the whole process is dependent on the distribution of the analytes between the phases. The use of higher volumes of solution for extraction, in general, results in a more efficient transfer of the analytes from the sample to the extractant phase. On the other hand, an excessive volume of extractant solution will cause an inappropriate dilution of the extract, which can increase the limits of detection and quantification. Therefore, the evaluation of this parameter is of fundamental importance.
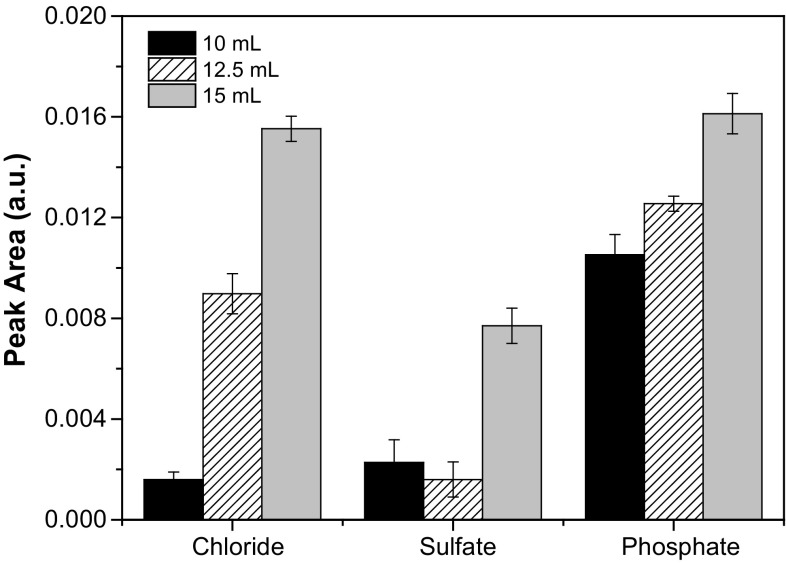
To evaluate the optimum volume of extractant (water) we tested different volumes: 10, 12.5 and 15 mL, maintaining a constant mass of sample of 4.5 g. Additionally, we tested if additional repeated extractions improved the process.
The obtained results clearly showed that the use of 15 mL of extractant in a single step facilitated the migration of the three targeted ions from the sample to the aqueous phase, since the highest response was observed under these conditions for all analytes (Fig. 1). Thus, we considered that the most efficient extraction was achieved using 15 mL and this volume was used for all further experiments. It is important to note that all solutions were sonicated for 15 min before the separation of the phases in order to promote a more intimate contact between the oil (sample) and the water (extractant) (Luque de Castro and Priego-Capote 2007; Abismail et al. 1999; Behrend et al. 2000; Behrend and Schubert 2001; Gaikwad and Pandit 2008).
Fig. 1.
Effect of the volume of extractant phase (deionized water) on the analytical signal obtained for chloride, sulfate and phosphate anions. Mass of sample = 4.5 g. Results are expressed as mean ± standard deviation (n = 3)
Influence of the extraction time
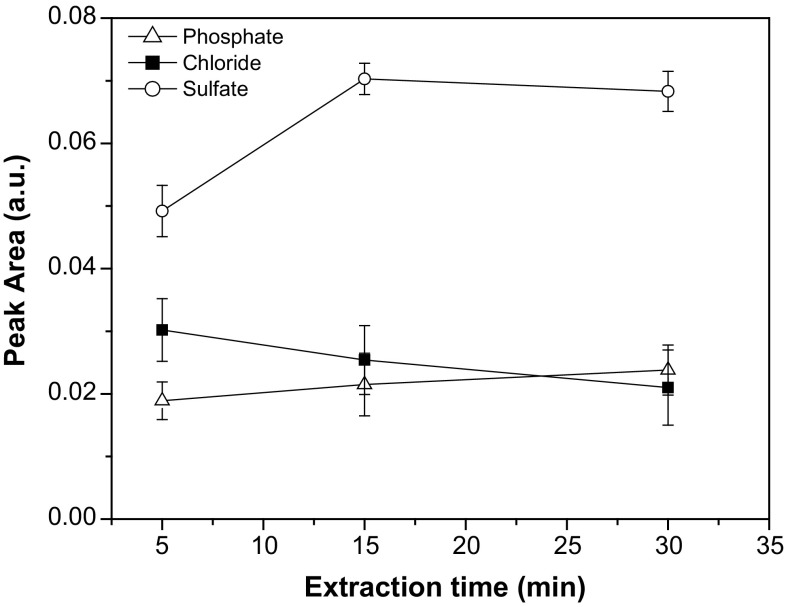
Sometimes, extraction processes are strongly dependent on the time of contact between the phases involved, especially when the transfer of the targeted substances is slow. This behavior seems to be more important when the extraction is carried out with two immiscible phases (Cassella et al. 2012), as in the present case, since their contact is not so intimate. Therefore, we evaluated the influence of the extraction time on the extraction process. All mixtures were sonicated for 15 min before application of the extraction time.
As can be seen in Fig. 2, the extraction time only presented noticeable effect on the sulfate extraction, having no significant influence on the extraction of either chloride or phosphate. The highest response for sulfate was observed after 15 min of shaking on a roller mixer, indicating that the transfer of this anion from the sample to the aqueous phase is slow and dependent on a certain time of contact between the two phases. Therefore, a 15 min extraction time was selected for the method in all further experiments.
Fig. 2.
Influence of the extraction time on the analytical signal for chloride, sulfate and phosphate anion in the aqueous extract. Results are expressed as mean ± standard deviation (n = 3)
Influence of the addition of surfactant
Finally, we tested the possibility of using surfactants to enhance the contact between the oil and water phases. Surfactants alter the interfacial tension between the two liquids and, consequently, could promote the formation of stable emulsions, in which water would be dispersed through the oil as very small droplets (Schramm 1992). The dispersion of the extractant in the form of small droplets could increase the extraction efficiency, since the contact area between the two phases would be very large (Pereira et al. 2013; Caldas et al. 2013).
This experiment was performed by comparing the extraction efficiency using 1% m/v solutions of Triton X-100™ and Triton X-114™ with that obtaining using only deionized water as extractant. No statistical differences (differences lower than 5%) could be observed among the signals obtained using the three different extractants. Additionally, a remarkable increase in the blank values (especially for chloride) was observed when we employed surfactant solutions for extraction, which resulted in a deterioration of the limits of detection and quantification. This is very likely associated with the presence of the analytes as contaminants in the surfactants chosen for the experiments. In the light of these results, we decided to perform extractions using only deionized water.
Influence of the clean-up of the extracts
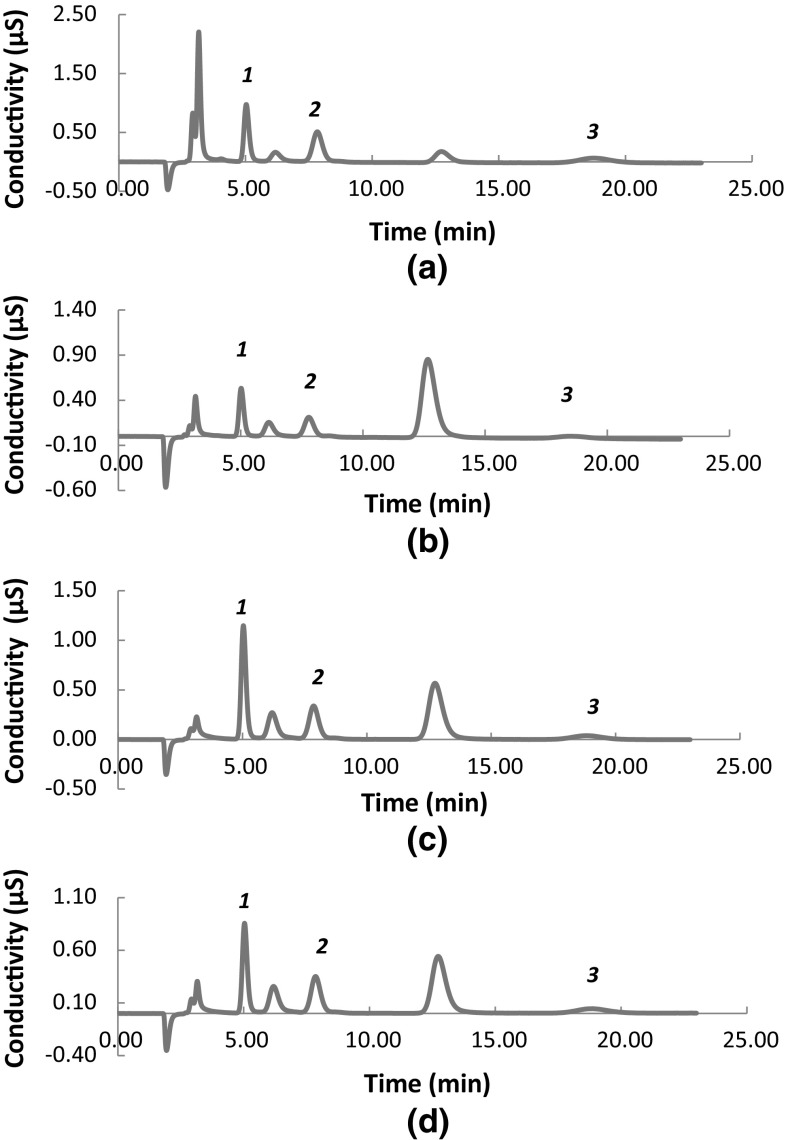
After we established the optimal experimental conditions for the extraction, we tested the possible application of the method for the analysis of different vegetable oils. We applied the method for the extraction of chloride, sulfate and phosphate from soybean, canola, corn and sunflower oils. The chromatograms of the extracts can be seen in Fig. 3. These chromatograms showed that an excellent separation of the anions present in the extracts could be achieved with no remarkable variations in the baseline of the chromatograms. In this context, an easy integration of the peaks could be performed, which allowed their quantification in the extracts by using the external calibration approach.
Fig. 3.
Chromatograms of the aqueous extraction of soybean oil (a), canola oil (b), corn oil (c) and sunflower oil (d). 1=chloride ion, 2= sulfate ion and 3= phosphate ion. The isocratic elution was performed with a 33 mM potassium hydroxide solution and a flow rate of 0.33 mL min−1
On the other hand, when we analyzed olive oil extracts, the same behavior was not observed. In this case, a considerable increase of the baseline was verified, which created some difficulties in peak integration. In order to overcome this problem, we tested the application of a clean-up procedure based on the SPE technique. The SPE procedure was performed by simple percolation of the obtained extract through a cartridge containing 500 mg of C18. The percolated extract was collected and injected into the chromatographic system. The chromatograms obtained after clean-up of the extracts did not present any increase of the baseline. Also, the clean-up procedure was efficient in eliminating any oil residue and obtaining a clearer solution. Therefore, we applied the SPE clean-up in the analysis of the olive oil samples.
Analytical features of the method
Once all conditions were established, the analytical features of the method were derived. Analytical curves were constructed for the three analytes, using standard solutions prepared in deionized water in the range of 0.050–0.50 mg L−1 for chloride and sulfate, and 0.060–0.50 mg L−1 for phosphate. From these curves, we calculated the limits of detection (LOD) and quantification (LOQ), for the three anions, considering the standard deviation (σ) of ten measurements of the blank solution and the slope of each analytical curve (S). According to Miller and Miller (2010), LOD can be calculated using the expression LOD = 3σ/S, whereas the LOQ can be calculated using the expression LOQ = 10σ/S. The analytical features for each analyte are shown in Table 1.
Table 1.
Analytical features of the proposed methodology for the determination of chloride, sulfate and phosphate in vegetable oils by ion chromatography
| Parameter | Chloride | Sulfate | Phosphate |
|---|---|---|---|
| Limit of detection (LOD) | 0.005 μg g−1 | 0.008 μg g−1 | 0.02 μg g−1 |
| Limit of quantification (LOQ) | 0.02 μg g−1 | 0.03 μg g−1 | 0.06 μg g−1 |
| Precision (%) | 5.2 | 4.8 | 3.6 |
| Working range (analytical curve) | 0.050–0.50 mg L−1 | 0.050–0.50 mg L−1 | 0.060–0.50 mg L−1 |
| Typical analytical curvea | y = 0.756x + 0.0043 | y = 0.4877x − 0.0005 | y = 0.1963x − 0.0028 |
| r2 | 0.9996 | 0.9996 | 0.9994 |
ay = peak area and x = analyte concentration in mg L−1
The precision of the method was calculated through five independent analysis of the extra virgin olive oil sample. It was expressed in terms of relative standard deviation and the results are also shown in Table 1.
The optimized method presented superior performance, in terms of limits of detection and quantification, when compared to other methods reported in the current literature for the determination of chloride, sulfate and phosphate in vegetable oils by ion chromatography. Additionally, the total time devoted to sample preparation is shorter than the time needed in such methods, as can be see in Table 2.
Table 2.
Comparison of the method proposed in this work with other methods already developed for the determination of chloride, sulfate and phosphate in vegetable oils by ion chromatography
| References | Remarks | |||
|---|---|---|---|---|
| Chloride | Phosphate | Sulfate | Sample preparation conditions | |
| Buldini et al. (1997a, b) | 10 μg kg−1 (LOD) | 35 μg kg−1 (LOD) | 30 μg kg−1 (LOD) | Total sample preparation time = 90 min (30 min mixing/extraction + 60 min photolysis). Heating at 95 °C for mixing, at 50 °C for extraction and then at 85 °C for photolysis |
| Dugo et al. (2007) | 10 μg kg−1 (LOD) 33 μg kg−1 (LOQ) |
20 μg kg−1 (LOD) 97 μg kg−1 (LOQ) |
25 μg kg−1 (LOD) 83 μg kg−1 (LOQ) |
Total sample preparation time = 45 min (30 min extraction + 15 min sonication + 5 min centrifugation). Heating at 70 °C in the extraction and sonication steps |
| Silveira et al. (2014)a | 0.10 mg kg−1 (LOD) 0.30 mg kg−1 (LOQ) |
0.81 mg kg−1 (LOD) 2.47 mg kg−1 (LOQ) |
0.09 mg kg−1 (LOD) 0.26 mg kg−1 (LOQ) |
Total sample preparation time = 65 min (20 min stirring + 30 min heating + 15 min sonication). At heating step the temperature was 85 °C |
| This work | 0.005 μg g−1 (LOD) 0.02 μg g−1 (LOQ) |
0.02 μg g−1 (LOD) 0.06 μg g−1 (LOQ) |
0.008 μg g−1 (LOD) 0.03 μg g−1 (LOQ) |
Total sample preparation time = 30 min (15 min sonication + 15 min shaking on a roller mixer). No heating |
aIn this work, the developed analytical method was applied in the analysis of biodiesel samples instead of vegetable oils
Application
The developed method was applied for the determination of chloride, sulfate and phosphate in different samples of vegetable oils (Table 3). Only phosphate in corn, canola and extra virgin olive oils could not be quantified.
Table 3.
Concentrations of chloride, sulfate and phosphate found in vegetables oil samples after application of the proposed method
| Oil | Analyte concentration found (µg g−1) | ||
|---|---|---|---|
| Chloride | Sulfate | Phosphate | |
| Soybean | 0.047 ± 0.012 | 0.39 ± 0.010 | 0.72 ± 0.01 |
| Corn | 0.11 ± 0.03 | 0.079 ± 0.013 | < LQ |
| Canola | 0.12 ± 0.01 | 0.10 ± 0.03 | < LQ |
| Sunflower | 0.087 ± 0.022 | 0.18 ± 0.03 | 0.060 ± 0.013 |
| Extra virgin olive | 0.22 ± 0.01 | 0.22 ± 0.01 | < LQ |
| Extra soft olive | 0.37 ± 0.02 | 0.21 ± 0.01 | 0.84 ± 0.03 |
Results are expressed as mean ± standard deviation (n = 3)
We evaluated the accuracy of the method through a recovery test. As described in the experimental section, the recovery test was performed by spiking the samples with known amounts of each analyte and applying the developed method to the analysis of the fortified samples. Table 4 shows the recovery percentages obtained for the studied oils. The results are expressed as mean ± standard deviation of three independent analyses.
Table 4.
Recovery results of chloride, sulfate and phosphate anions from corn, soybean, sunflower and canola oil after apply a extraction procedure
| Type of oil | Anion | Amount added (µg) | Recovery (%) |
|---|---|---|---|
| Soybean | Cl− | 2.5 5.0 10.0 |
86.5 88.8 93.6 |
| SO42− | 2.5 5.0 10.0 |
103 90.4 95.3 |
|
| PO43− | 2.5 5.0 10.0 |
107 80.0 92.7 |
|
| Corn | Cl− | 2.5 5.0 10.0 |
84.6 91.8 108 |
| SO42− | 2.5 5.0 10.0 |
74.4 88.2 99.6 |
|
| PO43− | 2.5 5.0 10.0 |
93.0 95.4 98.9 |
|
| Sunflower | Cl− | 2.5 5.0 7.5 |
73.0 89.0 90.3 |
| SO42− | 2.5 5.0 7.5 |
88.9 94.4 93.3 |
|
| PO43− | 2.5 5.0 7.5 |
93.5 84.5 81.2 |
|
| Canola | Cl− | 2.5 5.0 7.5 |
98.2 105 140 |
| SO42− | 2.5 5.0 7.5 |
100 95.0 95.5 |
|
| PO43− | 2.5 5.0 7.5 |
98.7 91.0 101 |
|
| Extra virgin olive | Cl− | 4.5 7.5 12.0 |
102 100 100 |
| SO42− | 4.5 7.5 12.0 |
95.0 98.5 98.2 |
|
| PO43− | 4.5 7.5 12.0 |
85.2 92.5 99.8 |
|
| Extra soft olive | Cl− | 4.5 7.5 12.0 |
110 109 98.0 |
| SO42− | 4.5 7.5 12.0 |
87.5 103 91.3 |
|
| PO43− | 4.5 7.5 12.0 |
87.0 87.2 90.0 |
The recovery percentages varied from 73.0 to 140%, with a mean value of 94.8 ± 10.1%. These results indicated that the extraction procedure developed in the present work was suitable for the preparation of vegetable oil samples for the determination of chloride, sulfate and phosphate by ion chromatography.
Conclusion
The extraction procedure proposed for the extraction of chloride, sulfate and phosphate anions from vegetable oils was simple, rapid and efficient. The extraction with water ensured the highest mass transfer of anions from the oil phase to the extraction phase and made the process environmentally friendly. Another advantage was the reduced analysis time achieved by the simultaneous extraction and quantification of the analytes.
Some of the parameters significantly affected the method. Both the mass ratio of the sample/extractant volume and the mixing time presented a noticeable influence on the extraction efficiency. Though, it was possible to perform the whole extraction of the analytes in no more than 30 min (15 min mixing + 15 min ultrasound agitation).
The results showed that the method can be applied for the determination of chloride, sulfate and phosphate in vegetable oils of different origins (corn, canola, soybean, sunflower and olive). A clean-up step of the extracts was needed in the analysis of olive oil samples in order to eliminate a baseline increase and facilitate the integration of the peaks.
Acknowledgements
The authors are grateful to Faperj (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro) and to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the financial support, and to OEA-GCUB (Organização dos Estados Americanos-Grupo de Coimbra de Universidades Brasileiras) to the scholarship granted to Adriana F. Campos.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abismail B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C. Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem. 1999;6:75–83. doi: 10.1016/S1350-4177(98)00027-3. [DOI] [PubMed] [Google Scholar]
- Behrend O, Schubert H. Influence of hydrostatic pressure and gas content on continuous ultrasound emulsification. Ultrason Sonochem. 2001;8:271–276. doi: 10.1016/S1350-4177(01)00088-8. [DOI] [PubMed] [Google Scholar]
- Behrend O, Ax K, Schubert H. Influence of continuous phase viscosity on emulsification by ultrasound. Ultrason Sonochem. 2000;7:77–85. doi: 10.1016/S1350-4177(99)00029-2. [DOI] [PubMed] [Google Scholar]
- Buldini PL, Cavalli S, Trifiro A. State-of-the-art ion chromatographic determination of inorganic ions in food. J Chromatogr A. 1997;789(1–2):529–548. doi: 10.1016/S0021-9673(97)00963-1. [DOI] [PubMed] [Google Scholar]
- Buldini PL, Ferri D, Sharma JL. Determination of some inorganic species in edible vegetable oils and fats by ion chromatography. J Chromatogr A. 1997;789(1–2):549–555. doi: 10.1016/S0021-9673(97)00822-4. [DOI] [Google Scholar]
- Caldas LFS, Brum DM, de Paula CER, Cassella RJ. Application of the extraction induced by emulsion breaking for the determination of Cu, Fe and Mn in used lubricating oils by flame atomic absorption spectrometry. Talanta. 2013;110:21–27. doi: 10.1016/j.talanta.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Cassella RJ, Brum DM, Robaina NF, Rocha AA, Lima CF. Extraction induced by emulsion breaking for metals determination in diesel oil by ICP-MS. J Anal At Spectrom. 2012;27:364–370. doi: 10.1039/C2JA10279J. [DOI] [Google Scholar]
- Dugo D, Pellicano MT, La Pera L, Lo Turco V, Tamborrino A, Clodoveo ML. Determination of inorganic anions in commercial seed oils and in virgin olive oils produced from de-stoned olives and traditional extraction methods, using suppressed ion exchange chromatography (IEC) Food Chem. 2007;102:599–605. doi: 10.1016/j.foodchem.2006.05.039. [DOI] [Google Scholar]
- Ellinger RH, in: Furia TE (ed) (1972) Handbook of food additives. CRC Press, Colombus
- Gaikwad SG, Pandit AB. Ultrasound emulsification: effect of ultrasonic and physicochemical properties on dispersed phase volume and droplet size. Ultrason Sonochem. 2008;15:554–563. doi: 10.1016/j.ultsonch.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Lemos MAT, Cassella RJ, Jesus DP. A simple analytical method for determining inorganic anions and formate in virgin olive oils by capillary electrophoresis with capacitively coupled contactless conductivity detection. Food Control. 2015;57:327–332. doi: 10.1016/j.foodcont.2015.04.026. [DOI] [Google Scholar]
- Leonardis A, Macciola V, Felice M. Copper and iron determination in edible vegetable oils by graphite furnace atomic absorption spectrometry after extraction with diluted nitric acid. Int J Food Sci Technol. 2000;35:371–375. doi: 10.1046/j.1365-2621.2000.00389.x. [DOI] [Google Scholar]
- Luque de Castro MD, Priego-Capote F. Ultrasound-assisted preparation of liquid samples. Talanta. 2007;72:321–334. doi: 10.1016/j.talanta.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Miller JC, Miller JN. Statistics and chemometrics for analytical chemistry. 6. Essex: Pearson Education Limited; 2010. [Google Scholar]
- Ooms R, Pee WV. Determination of trace metal content in corn oil by atomic absorption spectroscopy. J Am Oil Chem Soc. 1983;60:957–960. doi: 10.1007/BF02660207. [DOI] [Google Scholar]
- Pehlivan E, Arslan G, Gode F, Altun T, Ozcan MM. Determination of some inorganic metals in edible vegetable oils by inductively coupled plasma atomic emission spectroscopy (ICP-AES) Grasas Aceites. 2008;59:239–244. doi: 10.3989/gya.2008.v59.i3.514. [DOI] [Google Scholar]
- Pereira FM, Zimpeck RC, Brum DM, Cassella RJ. Novel extraction induced by emulsion breaking as a tool for the determination of trace concentrations of Cu, Mn and Ni in biodiesel by electrothermal atomic absorption spectrometry. Talanta. 2013;117:32–38. doi: 10.1016/j.talanta.2013.08.042. [DOI] [PubMed] [Google Scholar]
- Robaina NF, Brum DM, Cassella RJ. Application of the extraction induced by emulsion breaking for the determination of chromium and manganese in edible oils by electrothermal atomic absorption spectrometry. Talanta. 2012;99:104–112. doi: 10.1016/j.talanta.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Schramm LL. Emulsions: fundamentals and applications in the petroleum industry. Washington: American Chemical Society; 1992. [Google Scholar]
- Silveira ELC, Caland LB, Tubino M. Simultaneous quantitative analysis of the acetate, formate, chloride, phosphate and sulfate anions in biodiesel by ion chromatography. Fuel. 2014;124:97–101. doi: 10.1016/j.fuel.2014.01.095. [DOI] [Google Scholar]
- Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit Care. 2016;20:315. doi: 10.1186/s13054-016-1499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]