Abstract
Different types of nanoparticles have been synthesized to protect carotenoids against exposition of external factors such as light, heat and oxygen; and processing conditions; to increase stability and to improve the bioavailability of nanoencapsulated carotenoid. The type of nanostructure synthesized (nanoemulsions, liposomes, solid lipid nanoparticles, nanostructured lipid carrier, and polymeric nanoparticles) influences on the synthesis and nanoparticles stability, which reflect in physic-chemical characteristics such as polydispersity index, zeta potential, and encapsulation efficiency. Different nanostructures can be used to improve stability of carotenoids; however, currently, polymeric nanocapsules are the nanostructure most utilized due to its stability during storage, high efficiency to encapsulate and to control the release of the carotenoid encapsulated. Due to these considerations, they have been focus of researchers for future studies regarding to application of carotenoids nanoencapsulated by food industries. The focus of this review is the presentation of different carotenoids delivery systems and the use of techniques to evaluate parameters that might limit the application of this innovative and potential technology in cosmetic, pharmaceutical and food industry.
Keywords: Carotenoid, Nanotechnology, Stability, Nanoparticle
Introduction
Carotenoids are bioactive compounds with biological and chemical importance. Carotenoids are fat-soluble pigments that confer yellow, orange and red colour to vegetables. These compounds are present in nature such as β-carotene, α-carotene, lutein, zeaxanthin, lycopene, astaxanthin, and bixin.
Carotenoids are isoprenoid compounds with polyene chains that may contain up to 15 conjugated double bonds. This chemical characteristic confers to these compounds instability to light, oxygen and heat and functionality such as provitamin A characteristic, antioxidant activity and anticancer activities (Lerfall 2016). Therefore, carotenoids are considered human health promoters and have an important role in human nutrition and health.
Currently, carotenoids have been used to fortify functional food (Nagarajaiah and Prakash 2015), as a supplement in pharmaceutical products and to topic use in cosmetic industry. These products usually undergo a stabilization process during its production and, for that reason, the influence of processing on carotenoid is an interesting issue, mainly for the food industry (Sáiz-Abajo et al. 2013).
Nanotechnology has improved stability; solubility and bioavailability of bioactive compounds to the application in food, cosmetic and pharmaceutical field (Okonogi and Riangjanapatee 2015). This technology has great potential in herbal products and functional foods to provide innovation on characteristics of foods, such as texture, taste, colouring strength and processability and aggregating improvements in human health (Ezhilarasi et al. 2012).
Nanotechnology refers to the obtention of material scales (nanometer—10 to 1000 nm), which produces particles with the greater mass per surface area and most biologically active. Several studies have tried to encapsulate carotenoids to increase their antioxidant properties and avoid degradation (Da Silva et al. 2017; Tan et al. 2014).
The industry has utilized novel applications of nanotechnologies in different sectors. It includes nanoparticles, such as micelles, nanoliposomes, nanoemulsions, biopolymeric nanoparticles and solid lipid nanoparticles, as well as the development of nanosensors, which aim to ensure pharmaceutical and food safety (Sozer and Kokini 2009).
These nanostructured vehicles must be evaluated to ensure safety and stability during their application (Gaumet et al. 2008). The most important parameters in this evaluation are size, polydispersity index (PDI), zeta potential, morphology, pH, drug loading and encapsulation efficiency (Couvreur et al. 2002).
Thus, the aim of this review was to collect information concerning the parameters considered important in the characterization, stability and functionality evaluation of nano-size vehicles; and to present the different types of nanostructures used as vehicles for carotenoids. Furthermore, the readers may understand how the use of nanotechnology evolved regarding carrier of bioactive compounds meanly concerning to encapsulation of carotenoids for potential application in food industry.
Parameters of characterization and stability evaluation of nanoencapsulated compounds
Nanoencapsulation have been utilized to improve the stability of compounds and thus, to increase its application mainly for pharmaceutical and food industry. Different nanoencapsulation methods and types of nano-sized vehicles are utilized as delivery systems such as polymeric carriers and lipid nanostructures (Hejri et al. 2013; Müller et al. 2000).
After synthesis of these nanostructured vehicles, the evaluation of parameters such as size, polydispersity index (PDI), zeta potential, morphology, pH and release profile is essential to stability evaluation, safety to the biomedical application (Couvreur et al. 2002; Gaumet et al. 2008). The choice of techniques for characterization and stability evaluation is dependent on the nature of stability issues and product dosage form (Wu et al. 2011). Table 1 summarizes the techniques utilized to characterize and evaluate nanocapsules and nanoparticles.
Table 1.
Parameters used to characterization of nanostructures
| Parameter | Technique | Principle | Importance |
|---|---|---|---|
| Size and polydispersity index | Laser diffraction (LD) | Light interaction | Ensure that particle exclusively on the nanometer scale was achieved (Lobato et al. 2013) |
| Brunauer–Emmett–Teller (BET) | Adsorption | Allow the evaluation of surface area utilizing pore structure analysis (Akbari et al. 2011) | |
| X-ray diffraction peak broadening analysis (DPBA) | X-ray | This method is capable of yielding the crystallite size distribution (Akbari et al. 2011) | |
| Dynamic light scattering (DLS) | Light interaction | Allow the description of particle size distribution and destabilization phenomena (Venturini et al. 2011) | |
| Morphology | Scanning electron microscopy (SEM) | Microcopy | Allow to obtention of information regarding to structure, wall thickness estimative and polymer porosity (Burghardt and Droleskey 2005) |
| Transmission electron microscopy (TEM) | Microscopy | ||
| Atomic force microscopy (AFM) | Microscopy | It is more appropriate for surface analysis (Gaumet et al. 2008) | |
| Zeta potential | Zeta potential analysis (ζ) | Electrophoresis mobility | Determine particle stability in suspension, macromolecule and material surface (Honary and Zahir 2013; Win and Feng 2005) |
| Loading capacity | Ultrafiltration | Particle size | Reduce the quantity of carrier required for the administration to the target site (Lim et al. 2013) |
| Tangential filtration | |||
| Ultracentrifugation | Density | ||
| Encapsulation efficiency | Ultracentrifugation | Density | Allow to evaluate the efficiency of the nanoformulations to encapsulate compounds and to quantify the compounds administered to the target size (Li et al. 2016) |
| Ultrafiltration | Particle size | ||
| Dialyses method | Particle size | ||
| Release profile | Sample and Separate (SS) | Diffusion | Provide information concerning the dosage form used to assess product safety and efficacy (D’Souza 2014) |
| Continuous flow (CF) | |||
| Dialysis method (DM) | Physical separation |
Size and size distribution
Size (mean diameter or z-average) and size distribution are important parameters to nanoencapsulation evaluation due to their relation to distribution, physicochemical changes of the encapsulated compounds, viscosity, surface area and packing density of the nanoparticles (Gaumet et al. 2008). The particle size allows the selection of adequate colloidal preparations for parenteral administration and also possibly useful as sustained-release injections to a target site (Onoue et al. 2014). Moreover, the nanoparticle size control is a parameter that must be ensured, during storage due to the fact of physical stability is related to the periodic determination of the mean diameter (Wu et al. 2011).
The size distribution or PDI correspond the particles uniformity in suspension, in which PDI values higher than 0.5 indicate a broad distribution and between 0.1 and 0.25 show a narrow size distribution (Wu et al. 2011). The PDI is estimated considering the particle mean size, the refractive index of the solvent, the measurement angle and the variance of the distribution.
Several methods can determine the nanoparticle size and size distribution such as laser diffraction (LD); surface area analysis (BET) and X-ray diffraction peak broadening (Akbari et al. 2011). However, the most used technique to nanostructures is dynamic light scattering (DLS) (Venturini et al. 2011), which allows the description of particle size distribution and destabilization phenomena.
Dynamic light scattering (DLS), also referred to dynamic light scattering and quasi-elastic light scattering, is a rapid method for determining the mean size, the size distribution and PDI. The method consists in the particle interaction with light generally at an observation angle of 90°, and the calculation model is based on the equivalent sphere principle, in which each particle is viewed as a sphere (Gaumet et al. 2008).
Zeta potential (ζ)
Zeta potential is the method most frequently utilized to characterize the surface charge. Zeta potential corresponds to the electrical potential in colloidal systems, which determines particle stability in suspension, macromolecule or material surface (Honary and Zahir 2013). This parameter is influenced by nanocapsule composition and the dispersion medium (Wu et al. 2011). Zeta potential may also be applied to investigate if a biologically active compound is linked to the core or only adsorbed into the surface and to evaluate the adsorption of plasma proteins onto the particles (Couvreur et al. 1995).
Zeta potential is measured based on the electrophoretic mobility that corresponds to the boundary of the surrounding liquid layer attached to the moving particles in the medium (Wu et al. 2011). Values higher than 30 mV and lower than − 30 mV promote high stability and prevent particles aggregation (Honary and Zahir 2013).
Morphology
Morphology is another important parameter for the characterization of nanoparticles where the microscopy is used to evaluate nanoparticles integrity and stability under different conditions. However, a detailed nanostructures morphology characterization is difficult due to their small size and complexity of the composition (Couvreur et al. 2002).
Many types of electron microscopy can be applied to observe nanoparticle morphology and structure. Among direct visualization techniques, Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM) and Atomic Force Microscope (AFM) are widely used for assessment of particle morphology (Wu et al. 2011).
Currently, transmission electron microscopy (TEM) is the most used technique, which is performed after freeze-fracture of nanoparticles. TEM provides information regarding structure, wall thickness estimative and polymer porosity (Couvreur et al. 2002). The mean advantage of TEM is the potential resolution provided by electron beams accelerated at high voltage that presents efficient wavelengths shorter by a factor of 105 than visible light (Burghardt and Droleskey 2005). Despite to the morphology is an important parameter of evaluation; the applied techniques require a significant number of particles to achieve statistical size distribution and additional sample preparation that alter particle properties.
Loading capacity, encapsulation efficiency and release profile
After the preparation of nanocarrier, parameters such as loading capacity, efficiency and release profile must be evaluated. The loading capacity is a parameter highly variable dependent upon the fabrication process and the type of carrier lipid utilized for the selected carotenoid (Gutiérrez et al. 2013). Nanostructured systems with maximal compound loading and high entrapment efficiency, can reduce the quantity of carrier required for the administration of sufficient amount of active compound to the target site (Lim et al. 2013).
The loading capacity corresponds to the percentage of a compound by weight in the final formulation. This parameter can be measured after preparation and separation of the nanocapsules from continuous phase (Couvreur et al. 1995). Loading capacity can be measured by ultracentrifugation, size exclusion chromatography, ultrafiltration or tangential filtration. However, the last technique is the most used due to the tendency of nanoparticles clog membranes utilized in the quantification of compound loaded (Couvreur et al. 2002).
The encapsulation efficiency is a parameter regarded key step in characterizing the quality of the nanostructures (Li et al. 2016) and it corresponds to the percentage of the concentration of encapsulated compound by the total concentration of the compound added in the formulation (Couvreur et al. 2002). As the loading capacity, the encapsulation efficiency can be determined by ultracentrifugation, size exclusion chromatography or tangential filtration. Another method frequently utilized to determine encapsulation efficiency is the dialysis method (Li et al. 2016). The release kinetics provides critical information concerning the dosage form used to assess product safety and efficacy. This parameter also provides details on the release mechanism and kinetics, enabling a rational and scientific approach to product development (Langer 1990). The release studies are performed at 37 °C to simulate the physiological conditions. However, elevated temperatures and different pH have been explored to characterize compound release from a variety of dosage forms (D’Souza et al. 2005).
The release profile is currently evaluated using some methods such as sample and separate (SS), continuous flow (CF), dialysis membrane (DM) methods and novel techniques such as voltammetry and turbidimetry. Currently, the method most used, to assess drug release from nano-sized dosage forms, is the dialysis method (DM) that consists in the use of dialyzes membranes which allow for ease of sampling at periodic intervals (D’Souza 2014). In the dialyzes method, the nanostructures are added into a dialyzes bag containing release media that is subsequently sealed and placed in a vessel containing release media, agitated to minimize unstirred water layer effects (Muthu and Singh 2009). Mathematical models to characterize compounds release have been discussed due to the possibility to elucidate release mechanisms and can be used to guide formulation development efforts; however, this field has been little explored (D’Souza 2014).
Types of nano carotenoids delivery systems
Carotenoids are often utilized as additives in food products despite these compounds exhibit very low water solubility or even water insolubility (Moraru et al. 2003). Also, carotenoids present high melting point, chemical instability, and a low bioavailability, limiting the application of these compounds in pharmaceutical and food industry.
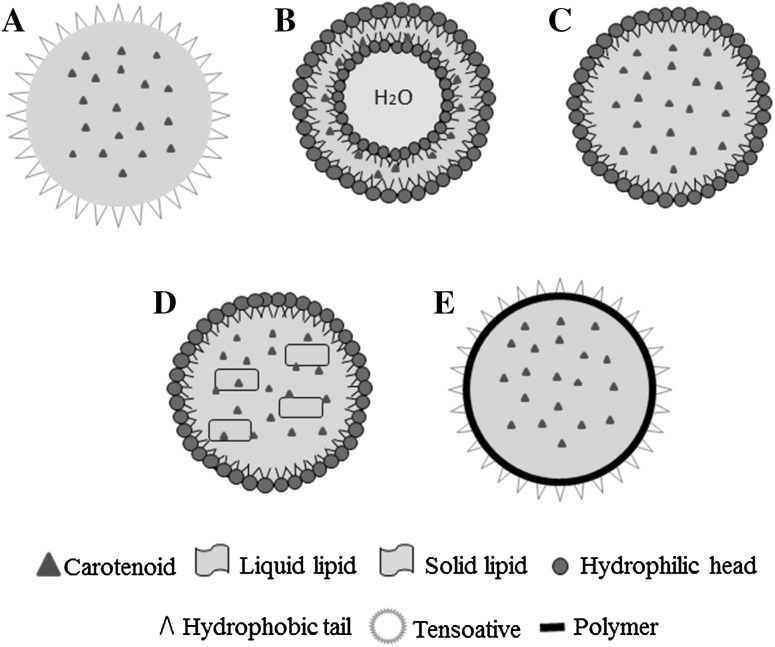
In this context, nanoencapsulated compounds have been developed, as presented in Table 2, to increase the stability, apparent solubility, industrial application and mainly for the design of carotenoids delivery systems, improving its bioavailability (Gutiérrez et al. 2013). Delivery system corresponds to technology in which a bioactive compound is entrapped in a carrier to control the rate of bioactive release (Fathi et al. 2012). There are many types of nano-sized vehicles (Fig. 1) and in general, some steps are involved in the encapsulation of bioactive compounds such as the formation of the wall around the material to be encapsulated and ensuring that undesired leakage does not occur (Mozafari et al. 2008).
Table 2.
Characteristics of carotenoids encapsulated utilizing different types of nanocarriers
| Carotenoid | Carrier system | Size (nm) | Zeta potential (mV) | Encapsulation (%) | References |
|---|---|---|---|---|---|
| Astaxanthin | Nanostructured lipid carriers (NLC) | 85–138 | − 22 to − 35 | No reported | Tamjidi et al. (2014) |
| Polymeric nanosphere | 21,168–312 | 35.3–18 to − 30 | No reported 98 | Wang et al. (2017) and Tachaprutinun et al. (2009) | |
| Polymeric nanocapsule | 98–157 | − 4 to 28 | 59–76 | Anarjan et al. (2012) | |
| Bixin | Solid lipid nanoparticle (SLN) | 135.5–352.8 | − 17.9 to − 36.5 | 17.96 | Rao et al. (2014) |
| Polymeric nanocapsule | 195 | − 14 | 100 | Lobato et al. (2013) | |
| β-carotene | Liposome | 1900–2500 | − 38 to − 74 | 100 | Toniazzo et al. (2014) |
| Nanoemulsion | 132–184 | No reported | 100 | Yuan et al. (2008) | |
| Nanostructured lipid carriers (NLC) | 8–15 | No reported | 100 | Hejri et al. (2013) | |
| Solid lipid nanoparticle (SLN) | 168 | No reported | No reported | Qian et al. (2013) | |
| Polymeric nanocapsule | 166 | − 18 | 100 | da Silva et al. (2016) | |
| Polymeric nanosphere | 32–206 | − 8 to − 32 | No reported | Yin et al. (2009) | |
| 102–793 | − 24 to 9 | 15–65 | Cao-Hoang et al. (2011) | ||
| Canthaxanthin | Liposome | 174–425 | No reported | No reported | Xia et al. (2015) |
| Lycopene | Nanoemulsion | 100–200 | − 33 to − 42 | 51–65 | Ha et al. (2015) |
| Nanostructured lipid carriers (NLC) | 150–160 | − 73 to − 75 | 100 | Okonogi and Riangjanapatee (2015) | |
| Polymeric nanocapsule | 193 | − 12 | 100 | dos Santos et al. (2015) | |
| Lutein | Nanoelmulsion | 150 | No reported | 100 | Vishwanathan et al. (2009) |
| Nanostructured lipid carriers (NLC) | 150–350 | − 40 to − 63 | No reported | Mitri et al. (2011) | |
| Polymeric nanocapsule | 191–195 | − 5 | 99 | Brum et al. (2017) | |
| Polymeric nanosphere | 200 | No reported | 100 | Hong et al. (2016) | |
| 72–143 | No reported | No reported | Tan et al. (2016b) |
Fig. 1.
Types of nano-delivery systems used to encapsulate carotenoids: a nanoemulsion, b nanoliposome, c solid lipid nanoparticle (SLN), d nanostructured lipid carrier (NLC) and e polymeric nanocapsule
The carotenoids can be encapsulated in lipid-based carriers and polymeric nanocapsules, utilizing different polymers as wall material such as poly(lactic acid) (PLA) and its copolymers, poly(lactide-co-glycolide) (PLGA); and poly(ε-caprolactone) (PCL) due to their biocompatibility and biodegradability characteristics. The use of materials considered “generally recognized as safe” (GRAS) is essential to produce safe nanocapsules under the conditions of its intended purpose, along with nutritional quality and stability to food or pharmaceutical applications.
Nanoemulsions
Nanoemulsion, also denominated as ultrafine emulsion or mini-emulsion, corresponds to an emulsion with disperse-phased droplets (oil) and a continuous phase (water), generally presenting diameter between 50 and 200 nm (Sanguansri and Augustin 2006). Nanoemulsions are characterized by specifying molecular constituents; quantity of these constituents and the sizes of the droplet structures (Mason et al. 2006).
Nanoemulsions (Fig. 1a) can have lipid cores separated from the aqueous phase by a monomolecular layer of a surfactant material, allowing for nanoencapsulation of oil-based bioactive (Sanguansri and Augustin 2006). Nanoemulsions can be prepared using high energy (high-pressure, homogenization, microfluidization, and ultrasonication), low energy (solvent diffusion) or combined methods (high-shear stirring) (Fathi et al. 2012). During the preparation of nanoemulsion, some criteria are considered essential to stability and size of particles such as the choice of surfactant, the surfactant concentration and the flexibility of interface (Bhosale et al. 2014). The influence of different emulsifiers such as polysorbates Tween 20, 40, 60 and 80; decaglycerol monolaurate and sodium caseinate in the formation of nano-vehicles to carotenoids were tested by Yin et al. (2009) and Tan et al. (2016b). The authors verified that emulsifiers act as a protective barrier and support in antioxidant activity of encapsulated compounds.
Encapsulation into nanoemulsions of bioactive compounds such as carotenoids (Yuan et al. 2008) and flavonoids (Li et al. 2012) represents the first effective approach to improve the dispersion and bioavailability. Moreover, encapsulation also protects these compounds against degradation (Donsì et al. 2011).
Nanoemulsion containing β-carotene was synthesized to evaluate the stability of carotenoid during storage at 4 and 25 °C (Yuan et al. 2008). These authors observed that refrigeration was an important factor in the stability of β-carotene nanoemulsions presenting stable range diameter of 132–184 nm and gradual degradation of carotenoid during storage (14–25%), with slightly greater loss occurred at 25 °C than at 4 °C.
Lutein nanoemulsion was prepared and compared with supplements regarding bioavailability (Vishwanathan et al. 2009). The lutein nanoemulsions presented diameter of 150 nm and were significantly more bioavailable even at 10–40% lower doses than supplements proving that nanoemulsions could improve bioavailability even at physiological doses.
Lycopene was also incorporated in nanoemulsion to protect the antioxidant activity and improve the bioaccessibility of lycopene in tomato extract, presenting sizes between 100 and 200 nm (Ha et al. 2015). In this study, lycopene nanoemulsion exhibited high antiradical efficiency, antioxidant activity, high in vitro bioaccessibility and high aqueous stability.
Nanoemulsions present various advantages: improving the bioavailability of compound, small-sized droplets, increase absorption rate, improvement of solubility of lipophilic compound, rapid and efficient penetration of the compound, less amount of energy requirement and proving of aqueous dosage form for water insoluble compounds. Although nanoemulsions present many advantages, the use of a large surfactant and co-surfactant concentration necessary for stabilizing the nanoemulsion due to its low storage stability and limited solubilizing capacity for high-melting substances limit its application (Mishra et al. 2014).
Nanoliposomes
Liposomes are structures constituted of phospholipid that presents concentric lipid bilayers alternating with aqueous compartments. These structures encapsulate hydrophilic and lipophilic compounds with sizes in the nanometer to micrometer range (Gregoriadis 1973; Mozafari et al. 2008; Muller and Landfester 2015). The use of liposomes as a delivery system was established for the first time by Gregoriadis (1973) to eliminate the inability of some compounds to penetrate target cells, and liposomes were employed as drug carriers to actinomycin D.
Nano-sized liposomes (Fig. 1b) can be produced by different methods such as mechanical (ultrasonication, extrusion, high-pressure homogenization and membrane homogenization) and non-mechanical methods (depletion of mixed detergent-lipid micelles and reversed-phase evaporation) (Fathi et al. 2012). Choosing the best preparation method depends on some parameters such as the physicochemical properties of encapsulated compounds and liposomal ingredients. The concentration and toxicity of encapsulated compounds; potential modifications to the features of the liposome on release and processes involved during delivery of the liposome are also parameters evaluated (Gómez-Hens and Fernández-Romero 2006; Mozafari et al. 2008).
Liposomes have been used as carriers for bioactive compounds to improve their solubility, stability, and bioavailability. The interaction between carotenoid (β-carotene, lutein, canthaxanthin, and lycopene) and lipid bilayer; and effects of carotenoid incorporation on the physical properties of liposome was evaluated by Xia et al. (2015). This study showed that the carotenoids affect morphologic characteristics of liposomes, increasing liposome size (20–425 nm) and that carotenoid modulation on the properties of liposomes is concentration-dependent.
Another study evaluated the modulating effects of liposomes encapsulation (10–100 nm) on the carotenoids bioaccessibility. The results presented that carotenoid bioaccessibility depended strongly on the incorporating ability of carotenoids into a lipid bilayer and loading content (Tan et al. 2014).
Liposomes also can be combined with biopolymers such as chitosan (chitosomes) to encapsulate carotenoids due to the capacity of chitosan coating to improve the carotenoid encapsulating efficiency of liposomes and increase the ability of liposomes to protect β-carotene and lutein (Tan et al. 2016a). Gums (guar and xanthan gum) were utilized as stabilizing in β-carotene-loaded liposome formulations applied in yogurt. It was possible observed that liposomes protected the carotenoid incorporated and the mixture of gums was highly effective in avoiding liposome aggregation (Toniazzo et al. 2014).
The application of liposomes as carrier presents some advantages such reduction in toxicity of encapsulated compounds, increasing to stability, efficacy, and therapeutic index and improving pharmacokinetic effects (Akbarzadeh et al. 2013). However, characteristics such as low solubility, short half-life, low encapsulation efficiency, batch-to-batch irreproducibility and difficulties in controlling liposome size can be limit the manufacture and development of liposomes (Akbarzadeh et al. 2013; Gómez-Hens and Fernández-Romero 2006).
Solid lipid nanoparticles (SLN)
Solid lipid nanoparticle (SLN) is the denomination for nanometric-size dispersion of lipids that should be solid at body temperatures (37 °C). SLN is composed of a solid lipid core with a compound linked to lipid matrix and a surfactant and cosurfactant which stabilize the lipophilic components in an aqueous phase (Arana et al. 2015; Weiss et al. 2008). The mobility of bioactive compounds can be controlled by the physical state of the lipidic matrix and for this, crystallized lipids to increase the stability of bioactive compound incorporated (Weiss et al. 2008).
The SLN was introduced in 1991 as an alternative carrier system to traditional colloidal carriers for drug delivery, such as emulsions and liposomes (Müller et al. 2000). In general, SLN (Fig. 1c) can be prepared by different techniques: high-pressure homogenization, solvent emulsification–evaporation, breaking of oil-in-water microemulsion and preparation via water-in-oil-in-water double emulsion (Üner and Yener 2007).
High-pressure homogenization is the most used method to the production of SLN due to its efficiency and reliability. These nanoparticles can be obtained by hot homogenization or cold homogenization technique (Müller et al. 2000; Üner and Yener 2007). In both techniques, the heated lipid solubilizes the compound. For the hot homogenization method, the mixture lipid and compound are dispersed under stirring in a hot aqueous surfactant solution at the same temperature. Then, this pre-emulsion is homogenized in a controlled high pressure; the nanoemulsion is cooled down to room temperature, the lipid recrystallizes and leads in solid lipid nanoparticles (Müller et al. 2000).
Different bioactive compounds are encapsulated utilizing SLN (Arana et al. 2015; Qian et al. 2013; Rao et al. 2014). Between these compounds, carotenoids have been the most studied. Calendula officinalis L. (Asteraceae) is a medicinal plant extract that consists to carotenes, mainly, and SLN containing this extract was synthesized to evaluate its toxicity and healing efficacy on a conjunctival epithelium cell. The SLN presented a diameter between 67 and 523 nm and zeta potential in a range of − 35 to − 48 mV and proved that nanoparticles were safe and improved epithelium repair on the ocular surface (Arana et al. 2015).
Bixin was loaded in solid lipid nanoparticles, utilizing different lipid matrices. The nanoparticles were prepared by technique fusion–emulsification, obtaining particles with size ranged from 135 to 352 nm, PDI of 0.185–0.572 and zeta potential of − 18 to − 37 mV and this SLN showed a hepatoprotective effect in rats (Rao et al. 2014).
Qian et al. (2013) evaluated the stability of β-carotene encapsulated by SLN. The authors synthesized nanoparticles (d < 200 nm) homogenizing lipid (cocoa butter and hydrogenated palm oil), Polysorbate 80 and water and observed that after 8 days of storage, the SLN contained 50.3 ± 1.2% of the β-carotene content.
The SLN presents some advantages regarding other colloidal carriers such as incorporation of lipophilic and hydrophilic compounds, avoidance of organic solvents and the possibility to large scale production. However, SLN has low loading capacity, presence of others colloidal structures and stability problems due to transformations of the physical state of the lipid (Mehnert and Mäder 2001).
Nanostructured lipid carrier (NLC)
Nanostructured lipid carrier (NLC) is a new generation of lipid solids nanoparticles that consist of different lipids blended to form the lipid matrix with a particular nanostructure (Radtke and Muller 2001). The NLC was developed by first time in 2001 by Radtke and Muller (2001) to reduce the limitations of SLN based on preparation methods described for SLN. The method of obtention of NLCs (Fig. 1d) depends on the type of compound encapsulated, especially its solubility and stability, and the lipid matrix (Kaur et al. 2015).
Currently, bioactive compounds NLCs are increasingly introduced as ingredients for food applications such as carotenoids (Hejri et al. 2013; Mitri et al. 2011), and unsaturated fatty acids (Zhu et al. 2015). For example, the β-carotene loaded nanostructured lipid carriers were obtained by solvent diffusion method for using in foods and oral administration. These NLCs presented small diameter (8–15 nm) and high β-carotene retention, proving that NLCs can be produced and employed as appropriate carriers for bioactive compounds in foods (Hejri et al. 2013).
Lutein was also loaded in NLCs produced by high-pressure homogenization to protect skin from photodamage (Mitri et al. 2011). In this study, NLCs showed diameter range 150–350 nm, a zeta potential range − 40 to − 63 mV, and the encapsulated lutein remained in the skin, protecting the skin against UV.
In another study, astaxanthin was loaded in NLCs to the utilization of this active ingredient in food formulations, evaluating physicochemical characteristics and storage stability (Tamjidi et al. 2014). The astaxanthin-NLC presented particle size of 94 nm, zeta potential of − 24 mV and stability for more than 1 month.
NLCs were developed to overcome the limitations of SLNs, and its main advantages are high entrapment of lipophilic and hydrophilic compounds, extended release, high stability, simple preparation and scale-up and controlled particle size (Kaur et al. 2015). Despite the potential of NLCs, some limitations were detected such as cytotoxic effects and irritative and sensitizing action of surfactants (Kaur et al. 2015).
Polymeric nanoparticles
Polymeric nanoparticles represent the most current and promise model of delivery systems in the pharmaceutical, medical and food field. These nanostructures can be classified as nanocapsules or nanospheres. Polymeric nanoparticles were synthesized for the first time by Birrenbach and Speiser (1976) to the application as adjuvants (enhancers of the immune response) in immunology.
Nanocapsules (Fig. 1) can be defined as a vesicular system in which specific compound, solubilized in an aqueous or oil core, is covered by a single polymeric membrane whereas nanospheres are a solid polymeric sphere in which particular compound is dispersed in polymer surface (Couvreur et al. 2002).
Polymeric nanoparticles can be synthesized by different methods, classified into two broad categories: polymerization of the monomer and preformed polymer (Couvreur et al. 1995). The method most utilized currently is preformed polymer that consist of the injection of an organic phase containing the polymers, solvent accessible to remove (acetone or ethanol), oil, triglycerides and the compound of interest into in an aqueous phase containing water and a tensoative compound under magnetic stirring (Quintanar-Guerrero et al. 1998). In addition, different polymers can be utilized as wall material to the obtention of polymeric nanoparticles such as poly(lactic acid) (PLA) and its copolymers, poly(lactide-co-glycolide) (PLGA); and poly(ε-caprolactone) (Coradini et al. 2014).
Polymeric nanoparticles have been used to protect and increase to stability of carotenoids such as bixin (Lobato et al. 2013), lycopene (Dos Santos et al. 2015), lutein (Brum et al. 2017) and β-carotene (Cao-Hoang et al. 2011; Da Silva et al. 2016). Bixin nanocapsules were produced to improve the stability of carotenoid during storage and presented mean diameter of 195 nm and physical stability during 119 days of storage at 25 °C (Lobato et al. 2013). In others study, bixin nanoencapsulated, in the same condition, was evaluated in relation to stability under photosensitization and heating (65–95 °C) and the results showed that bixin encapsulated was more stable than bixin free in both experiments of photosensitization and heating (Lobato et al. 2015).
The stability of β-carotene was tested in nanocapsules containing a blend of carotenoids from carrots, and it was observed that parameters such diameter (166 nm) and zeta potential (− 18 mV) remained stable after 100 days of storage (Da Silva et al. 2016). Nanospheres containing synthetic β-carotene and polylactic acid were also synthesized to increase the stability of this carotenoid and verified the formation of stable structures of 102 nm of diameter and oxidation stability (Cao-Hoang et al. 2011).
The lycopene nanocapsules stability was evaluated during storage and observed that nanocapsules presented satisfactory stability compared to free lycopene, showing 50% content after 14 days of storage at room temperature (25 °C), 40% after 84 days of storage at 5 °C and stability under high temperature (60, 70 and 80 °C) and photosensitization (5, 15 and 25 °C) (Dos Santos et al. 2015, 2016). Lutein was nanoencapsulated using poly-ɛ-caprolactone (PCL) and the authors obtained nanocapsules stables ranged from 191 to 195 nm (Brum et al. 2017). In another study, lutein was nanoencapsulated with chitosan to improve the solubility, and the results showed that lutein nanospheres with 200 nm of diameter presented higher solubility than no-nanoencapsulated lutein (Hong et al. 2016).
The polymeric nanoparticles have attracted the attention for applications due to advantages such as high stability, high encapsulation efficiency and controlled release of encapsulated compounds (Singh et al. 2011). In addition, nanocapsules present a central cavity that avoids the direct contact of the compounds with tissues and therefore irritation after administration (Couvreur et al. 2002). Thus, this nanostructure has been the most utilized to encapsulate carotenoids conferring more stability, safety, and long-lasting use.
Conclusion
Nanotechnology is a promising technology used to protect and improve the carotenoids application in food, cosmetic, and pharmaceutical field. Different nanostructures can be used to encapsulate carotenoids such as nanoemulsions, liposomes, and solid lipid nanoparticle; however, currently, polymeric nanocapsules are the nanostructure most utilized due to its stability during storage and high efficiency to encapsulate and to control the release of the carotenoid encapsulated. The nanoencapsulation reduce the degradation rate of carotenoids and it can increase the application of these fat-soluble compounds, in the different research fields. However, most studies concerning nanostructures of carotenoids are related to synthesis and characterization, with reduced attention on the biological application and on the release kinetics of carotenoid. Thus, in future, additional studies regarding different carotenoids nanocarrier and the behavior of the compounds nanoencapsulated in food systems and also in vivo will be required to enable their wide application by the food industry.
Acknowledgements
The authors acknowledge CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil) for the Grant (Proc. 00.889.834/0001-08).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Akbari B, Tavandashti MP, Zandrahimi M. Particle size characterization of nanoparticles: a practical approach. Iran J Mater Sci Eng. 2011;8(2):48–56. [Google Scholar]
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anarjan N, Tan CP, Nehdi IA, Ling TC. Colloidal astaxanthin: preparation, characterisation and bioavailability evaluation. Food Chem. 2012;135(3):1303–1309. doi: 10.1016/j.foodchem.2012.05.091. [DOI] [PubMed] [Google Scholar]
- Arana L, Salado C, Vega S, Aizpurua-Olaizola O, Idl Arada, Suarez T, Usobiaga A, Arrondo JL, Alonso A, Goñi FM, Alkorta I. Solid lipid nanoparticles for delivery of Calendula officinalis extract. Colloid Surf B. 2015;135:18–26. doi: 10.1016/j.colsurfb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Bhosale RR, Osmani RA, Ghodake PP, Shaikh SM, Chavan SR. Nanoemulsion: a review on novel profusion in advanced drug delivery. Indian J Pharm Biol Res. 2014;2(1):122. doi: 10.30750/ijpbr.2.1.19. [DOI] [Google Scholar]
- Birrenbach G, Speiser PP. Polymerized micelles and their use as adjuvants in immunology. J Pharm Sci. 1976;65(12):1763–1766. doi: 10.1002/jps.2600651217. [DOI] [PubMed] [Google Scholar]
- Brum AAS, dos Santos PP, da Silva MM, Paese K, Guterres SS, Costa TMH, Pohlmann AR, Jablonski A, Flôres SH, Rios AdO. Lutein-loaded lipid-core nanocapsules: physicochemical characterization and stability evaluation. Colloid Surf A. 2017;522:477–484. doi: 10.1016/j.colsurfa.2017.03.041. [DOI] [Google Scholar]
- Burghardt RC, Droleskey R. Transmission electron microscopy current protocols in microbiology. New York: Wiley; 2005. [DOI] [PubMed] [Google Scholar]
- Cao-Hoang L, Fougère R, Waché Y. Increase in stability and change in supramolecular structure of β-carotene through encapsulation into polylactic acid nanoparticles. Food Chem. 2011;124(1):42–49. doi: 10.1016/j.foodchem.2010.05.100. [DOI] [Google Scholar]
- Coradini K, Lima FO, Oliveira CM, Chaves PS, Athayde ML, Carvalho LM, Beck RCR. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur J Pharm Biopharm. 2014;88(1):178–185. doi: 10.1016/j.ejpb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Couvreur P, Dubernet C, Puisieux F. Controlled drug delivery with nanoparticles: current possibilities and future trends. Eur J Pharm Biopharm. 1995;41(1):2–13. [Google Scholar]
- Couvreur P, Barratt G, Fattal E, Vauthier C. Nanocapsule technology: a review. Crit Rev Ther Drug Carrier Syst. 2002;19(2):99–134. doi: 10.1615/CritRevTherDrugCarrierSyst.v19.i2.10. [DOI] [PubMed] [Google Scholar]
- D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. 2014;2014:1–12. [Google Scholar]
- D’Souza SS, Faraj JA, DeLuca PP. A model-dependent approach to correlate accelerated with real-time release from biodegradable microspheres. AAPS J. 2005;6(4):E553–E564. doi: 10.1208/pt060470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva MM, Nora L, Cantillano RFF, Paese K, Guterres SS, Pohlmann AR, Costa TMH, Rios AdO. The production, characterization, and the stability of carotenoids loaded in lipid-core nanocapsules. Food Bioprocess Tech. 2016;9(7):1148–1158. doi: 10.1007/s11947-016-1704-3. [DOI] [Google Scholar]
- Da Silva MM, Paese K, Guterres SS, Pohlmann AR, Rutz JK, Cantillano RFF, Nora L, Rios AdO. Thermal and ultraviolet–visible light stability kinetics of co-nanoencapsulated carotenoids. Food Bioprod Process. 2017;105:86–94. doi: 10.1016/j.fbp.2017.05.004. [DOI] [Google Scholar]
- Donsì F, Sessa M, Mediouni H, Mgaidi A, Ferrari G. Encapsulation of bioactive compounds in nanoemulsion-based delivery systems. Proc Food Sci. 2011;1:1666–1671. doi: 10.1016/j.profoo.2011.09.246. [DOI] [Google Scholar]
- Dos Santos PP, Paese K, Guterres SS, Pohlmann AR, Costa TH, Jablonski A, Flôres SH, Rios AdO. Development of lycopene-loaded lipid-core nanocapsules: physicochemical characterization and stability study. J Nanopart Res. 2015;17(2):1–11. [Google Scholar]
- Dos Santos PP, Paese K, Guterres SS, Pohlmann AR, Jablonski A, Flôres SH, Rios AdO. Stability study of lycopene-loaded lipid-core nanocapsules under temperature and photosensitization. Lebensm Wiss Technol. 2016;71:190–195. doi: 10.1016/j.lwt.2016.03.036. [DOI] [Google Scholar]
- Ezhilarasi PN, Karthik P, Chhanwal N, Anandharamakrishnan C. Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol. 2012;6(3):628–647. doi: 10.1007/s11947-012-0944-0. [DOI] [Google Scholar]
- Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Technol. 2012;23(1):13–27. doi: 10.1016/j.tifs.2011.08.003. [DOI] [Google Scholar]
- Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69(1):1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Gómez-Hens A, Fernández-Romero JM. Analytical methods for the control of liposomal delivery systems. Trend Anal Chem. 2006;25(2):167–178. doi: 10.1016/j.trac.2005.07.006. [DOI] [Google Scholar]
- Gregoriadis G. Drug entrapment in liposomes. FEBS Lett. 1973;36(3):292–296. doi: 10.1016/0014-5793(73)80394-1. [DOI] [PubMed] [Google Scholar]
- Gutiérrez FJ, Albillos SM, Casas-Sanz E, Cruz Z, García-Estrada C, García-Guerra A, García-Reverter J, García-Suárez M, Gatón P, González-Ferrero C, Olabarrieta I, Olasagasti M, Rainieri S, Rivera-Patiño D, Rojo R, Romo-Hualde A, Sáiz-Abajo M-J, Mussons M-L. Methods for the nanoencapsulation of β-carotene in the food sector. Trends Food Sci Technol. 2013;32(2):73–83. doi: 10.1016/j.tifs.2013.05.007. [DOI] [Google Scholar]
- Ha TV, Kim S, Choi Y, Kwak H-S, Lee SJ, Wen J, Oey I, Ko S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015;178:115–121. doi: 10.1016/j.foodchem.2015.01.048. [DOI] [PubMed] [Google Scholar]
- Hejri A, Khosravi A, Gharanjig K, Hejazi M. Optimisation of the formulation of β-carotene loaded nanostructured lipid carriers prepared by solvent diffusion method. Food Chem. 2013;141(1):117–123. doi: 10.1016/j.foodchem.2013.02.080. [DOI] [PubMed] [Google Scholar]
- Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1) Trop J Pharm Res. 2013;12(2):255–264. [Google Scholar]
- Hong DY, Lee J-S, Lee HG. Chitosan/poly-γ-glutamic acid nanoparticles improve the solubility of lutein. Int J Biol Macromol. 2016;85:9–15. doi: 10.1016/j.ijbiomac.2015.12.044. [DOI] [PubMed] [Google Scholar]
- Kaur S, Nautyal U, Singh R, Singh S, Devi A. Nanostructure lipid carrier (NLC): the new generation of lipid nanoparticles. APJHS. 2015;2(2):76–93. [Google Scholar]
- Langer R. New methods of drug delivery. Science. 1990;249(4976):1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- Lerfall J. Carotenoids: occurrence, properties and determination. In: Caballero B, Finglas P, Toldrá F, editors. Encyclopedia of food and health. 1. Trondheim: Elsevier; 2016. pp. 663–669. [Google Scholar]
- Li Y, Zheng J, Xiao H, McClements DJ. Nanoemulsion-based delivery systems for poorly water-soluble bioactive compounds: influence of formulation parameters on polymethoxyflavone crystallization. Food Hydrocolloid. 2012;27(2):517–528. doi: 10.1016/j.foodhyd.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li X, Li S, Weng Y, Wang K, Zhang T, Chen S, Lu X, Jiang Y, Xu J, Liang X. Development and validation of a method for determination of encapsulation efficiency of CPT-11/DSPE-mPEG2000 nanoparticles. Med Chem (Los Angeles) 2016;6:345–348. [Google Scholar]
- Lim E-K, Jang E, Lee K, Haam S, Huh Y-M. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics. 2013;5(2):294–317. doi: 10.3390/pharmaceutics5020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato KBdS, Paese K, Forgearini JC, Guterres SS, Jablonski A, Rios AdO. Characterisation and stability evaluation of bixin nanocapsules. Food Chem. 2013;141(4):3906–3912. doi: 10.1016/j.foodchem.2013.04.135. [DOI] [PubMed] [Google Scholar]
- Lobato KBdS, Paese K, Forgearini JC, Guterres SS, Jablonski A, Rios AdO. Evaluation of stability of bixin in nanocapsules in model systems of photosensitization and heating. Lebensm Wiss Technol. 2015;60(1):8–14. doi: 10.1016/j.lwt.2014.09.044. [DOI] [Google Scholar]
- Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys Condens Mater. 2006;18(41):R635. doi: 10.1088/0953-8984/18/41/R01. [DOI] [Google Scholar]
- Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–196. doi: 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Soni GC, Mishra R. Nanoemulsion: a novel drug delivery tool. Int J Pharm Res Rev. 2014;3(7):32–43. [Google Scholar]
- Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. Int J Pharm. 2011;414(1–2):267–275. doi: 10.1016/j.ijpharm.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Moraru CI, Panchapakesan CP, Huang QR, Takhistov P, Liu S, Kokini JL. Nanotechnology: a new frontier in food science. Food Technol. 2003;57(12):24–29. [Google Scholar]
- Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18(4):309–327. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- Muller LK, Landfester K. Natural liposomes and synthetic polymeric structures for biomedical applications. Biochem Biophys Res Commun. 2015;468(3):411–418. doi: 10.1016/j.bbrc.2015.08.088. [DOI] [PubMed] [Google Scholar]
- Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Muthu MS, Singh S. Poly (d, l-lactide) nanosuspensions of risperidone for parenteral delivery: formulation and in vitro evaluation. Curr Drug Deliv. 2009;6(1):62–68. doi: 10.2174/156720109787048302. [DOI] [PubMed] [Google Scholar]
- Nagarajaiah SB, Prakash J. Nutritional composition, acceptability, and shelf stability of carrot pomace-incorporated cookies with special reference to total and β-carotene retention. Cogent Food Agric. 2015;1(1):1–10. [Google Scholar]
- Okonogi S, Riangjanapatee P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int J Pharm. 2015;478(2):726–735. doi: 10.1016/j.ijpharm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Onoue S, Yamada S, Chan H-K. Nanodrugs: pharmacokinetics and safety. Int J Nanomed. 2014;9:1025–1037. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C, Decker EA, Xiao H, McClements DJ. Impact of lipid nanoparticle physical state on particle aggregation and β-carotene degradation: potential limitations of solid lipid nanoparticles. Food Res Int. 2013;52(1):342–349. doi: 10.1016/j.foodres.2013.03.035. [DOI] [Google Scholar]
- Quintanar-Guerrero D, Allemann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm. 1998;24(12):1113–1128. doi: 10.3109/03639049809108571. [DOI] [PubMed] [Google Scholar]
- Radtke M, Muller RH. NLS™ nanostructured lipid carriers: the new generation of lipid drug carriers. New Drugs. 2001;2:48–52. [Google Scholar]
- Rao MP, Manjunath K, Bhagawati ST, Thippeswamy BS. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection—Preparation, characterisation and in vivo evaluation. Int J Pharm. 2014;473(1–2):485–492. doi: 10.1016/j.ijpharm.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Sáiz-Abajo M-J, González-Ferrero C, Moreno-Ruiz A, Romo-Hualde A, González-Navarro CJ. Thermal protection of β-carotene in re-assembled casein micelles during different processing technologies applied in food industry. Food Chem. 2013;138(2–3):1581–1587. doi: 10.1016/j.foodchem.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Sanguansri P, Augustin MA. Nanoscale materials development—a food industry perspective. Trends Food Sci Technol. 2006;17(10):547–556. doi: 10.1016/j.tifs.2006.04.010. [DOI] [Google Scholar]
- Singh S, Pandey VK, Tewari RP, Agarwal V. Nanoparticle based drug delivery system: advantages and applications. Indian J Sci Technol. 2011;4(3):177–180. [Google Scholar]
- Sozer N, Kokini JL. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009;27(2):82–89. doi: 10.1016/j.tibtech.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov Food Sci Emerg. 2014;26:366–374. doi: 10.1016/j.ifset.2014.06.012. [DOI] [Google Scholar]
- Tachaprutinun A, Udomsup T, Luadthong C, Wanichwecharungruang S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int J Pharma. 2009;374(1–2):119–124. doi: 10.1016/j.ijpharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Tan C, Zhang Y, Abbas S, Feng B, Zhang X, Xia S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloid Surf B. 2014;123:692–700. doi: 10.1016/j.colsurfb.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Tan C, Feng B, Zhang X, Xia W, Xia S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocolloid. 2016;52:774–784. doi: 10.1016/j.foodhyd.2015.08.016. [DOI] [Google Scholar]
- Tan TB, Yussof NS, Abas F, Mirhosseini H, Nehdi IA, Tan CP. Forming a lutein nanodispersion via solvent displacement method: the effects of processing parameters and emulsifiers with different stabilizing mechanisms. Food Chem. 2016;194:416–423. doi: 10.1016/j.foodchem.2015.08.045. [DOI] [PubMed] [Google Scholar]
- Toniazzo T, Berbel IF, Cho S, Fávaro-Trindade CS, Moraes ICF, Pinho SC. β-carotene-loaded liposome dispersions stabilized with xanthan and guar gums: physico-chemical stability and feasibility of application in yogurt. Lebensm Wiss Technol. 2014;59((2, Part 2)):1265–1273. doi: 10.1016/j.lwt.2014.05.021. [DOI] [Google Scholar]
- Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomed. 2007;2(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- Venturini CG, Jäger E, Oliveira CP, Bernardi A, Battastini AMO, Guterres SS, Pohlmann AR. Formulation of lipid core nanocapsules. Colloid Surf A. 2011;375(1–3):200–208. doi: 10.1016/j.colsurfa.2010.12.011. [DOI] [Google Scholar]
- Vishwanathan R, Wilson TA, Nicolosi RJ. Bioavailability of a nanoemulsion of lutein is greater than a lutein supplement. Nano Biomed Eng. 2009;1(1):38–49. doi: 10.5101/nbe.v1i1.p38-49. [DOI] [Google Scholar]
- Wang Q, Zhao Y, Guan L, Zhang Y, Dang Q, Dong P, Li J, Liang X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017;227:9–15. doi: 10.1016/j.foodchem.2017.01.081. [DOI] [PubMed] [Google Scholar]
- Weiss J, Decker E, McClements DJ, Kristbergsson K, Helgason T, Awad T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008;3(2):146–154. doi: 10.1007/s11483-008-9065-8. [DOI] [Google Scholar]
- Win KY, Feng S-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–469. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Xia S, Tan C, Zhang Y, Abbas S, Feng B, Zhang X, Qin F. Modulating effect of lipid bilayer–carotenoid interactions on the property of liposome encapsulation. Colloid Surf B. 2015;128:172–180. doi: 10.1016/j.colsurfb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Yin L-J, Chu B-S, Kobayashi I, Nakajima M. Performance of selected emulsifiers and their combinations in the preparation of β-carotene nanodispersions. Food Hydrocolloid. 2009;23(6):1617–1622. doi: 10.1016/j.foodhyd.2008.12.005. [DOI] [Google Scholar]
- Yuan Y, Gao Y, Mao L, Zhao J. Optimisation of conditions for the preparation of β-carotene nanoemulsions using response surface methodology. Food Chem. 2008;107(3):1300–1306. doi: 10.1016/j.foodchem.2007.09.015. [DOI] [Google Scholar]
- Zhu J, Zhuang P, Luan L, Sun Q, Cao F. Preparation and characterization of novel nanocarriers containing krill oil for food application. J Funct Food. 2015;19:902–912. doi: 10.1016/j.jff.2015.06.017. [DOI] [Google Scholar]