Abstract
The present study focused on the effect of olive oil on Ostwald ripening of flavor nanoemulsions. The release of the aroma compounds from the nanoemulsion system was also investigated. The results showed that the droplets size of the nanoemulsions decreased sharply first and then kept stable with the increase of Tween 80. The optimum surfactant/cosurfactant (Km) ratio was determined at 7:1. The average particle size of nanoemulsion was 39.22 nm. The polydispersity index (PDI) was 0.242 nm, and the particle size distribution was in the range of 20–150 nm at the optimum Km. The stability of the nanoemulsions was improved after the addition of olive oil, and it increased noticeably with the increase of olive oil. The addition of olive oil could help to stabilize the emulsions and hamper Ostwald ripening. All the 11 aroma compounds in the nanoemulsions were detected after 24-h storage. While only 5 aroma compounds were found after 48-h storage, and α-pinene and β-myrcene were the only two aroma compounds detected after 72-h storage with low contents of 1.41 and 0.5 mg/L. The addition of olive oil inhibited the release of the aroma compounds from the nanoemulsion system. The released ethyl acetate was reduced by 48% after the addition of 10% olive oil. Significant decrease on the release of α-pinene and nonanal was observed after the addition of 3% olive oil. And the decrease was also observed on the release of β-myrcene, D-limonene, α-terpineol, decanal and eugenol when the olive oil content was ≥ 5%. However, benzyl alcohol, β-ionone and 1-octanol showed no significant changes with the increase of olive oil. This indicated that the addition of olive oil could provide greater retention of the aroma compounds in the nanoemulsions.
Keywords: Olive oil, Aroma compounds, Nanoemulsions, Ostwald ripening
Introduction
Flavors and fragrances are one kind of important food additives used in food, especially in beverages. Most of the compounds in flavors and fragrances have the limitations in application due to the lack of compatibility and solubility in most food environments, volatility and instability during processing and storage (Hashtjin and Abbasi 2015). Therefore emulsions are used in beverages to make a good dispersion of these water-insoluble flavors and fragrances into an aqueous beverage. Nanoemulsion is a colloidal dispersion with particle size in the range of 10–100 nm, and it can significantly improve the solubility and bioavailability of the lipophilic compounds (Wooster et al. 2008; Velikov and Pelan 2008). The nanoemulsion systems have many advantages compared with conventional emulsions for practical applications in the chemical, pharmaceutical and cosmetic industries due to its high kinetic stability, low viscosity, high transparency, and high stability against sedimentation, creaming, coalescence, and flocculation (McClements 2005; Solans et al. 2005; Jafari et al. 2007; Barradas et al. 2015). It is also reported that the low turbidity of the prepared flavor nanoemulsions was obtained, and this indicated that the flavor nanoemulsions could be used in clear beverage (Zhang et al. 2016). Several other studies have also focused on the investigation of flavor nanoemulsions (Guttoff et al. 2015).
Nanoemulsion is a thermodynamical and unstable system that tends to breakdown over time (Jiang et al. 2014). Flavor nanoemulsions are always associated with Ostwald ripening due to the Laplace’s Law effect, which is the main reason that affects the stability of the nanoemulsions (Gong and Zhu 2010). Ostwald ripening is the phenomenon that small particles become smaller and the large particles become larger over time (Taylor 1998; Kabalnov 2001). The insoluble dispersed phase is one of the factors that can slow the Ostwald ripening rate (McClements et al. 2012). Ostwald ripening is driven by a minimal difference between the size of emulsified oil with different solubility and their chemical potential gradient (Nazarzadeh et al. 2013). Wooster et al. (2008) found that the entropy gained from oil demixing was bad to Ostwald ripening due to the thermodynamic barrier. Sadovoy et al. (2013) found that sunflower oil provided greater retention and hampered the release of aromas in layer-by-layer assembled multilayer shells nanoemulsion. It is important to prevent Ostwald ripening in the preparation of physically stable flavor nanoemulsions (Chang and McClements 2014).
At present, the determination of the optimum conditions for the formation of the nanoemulsion systems is significant to investigate in order to achieve their application in practical applications, such as the surfactant and oil type, phase concentrations (Gutiérrez et al. 2008; Komaiko and McClements 2014). It is reported that the construction of pseudo-ternary phasediagrams is the best way to evaluate all types of formulations which can arise from dispersion of surfactants, water and oil, and also to study the ratios of these factors in a mixture (Streck et al. 2014). The diagrams can provide contour maps, in which the information of the surfactant, oil, water, and surfactant compositions can be provided (Mehta et al. 2009).
The flavors and fragrances used in food industries are unstable when exposed to high temperatures, oxygen, light and humidity. The nanoemulsions are used to incorporate the flavors and fragrances in these systems in order to protect the aroma compounds from evaporation, degradation, and prolong the release of these compounds (Barradas et al. 2015). In this study, the effect of olive oil on Ostwald ripening of flavor nanoemulsions was studied. And the release of the aroma compounds from the nanoemulsion systems was also investigated.
Materials and methods
Reagents and reference samples
Olive oil was purchased from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China). Tween 80 and absolute ethyl alcohol were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Following aroma standards were tested: ethyl acetate (1); α-pinene (2); β-myrcene (3); D-limonene (4); benzyl alcohol (5); 1-octanol (6); nonanal (7); α-terpineol (8); decanal (9); eugenol (10); β-ionone (11); and they were provided by Sigma Chemical Company (Saint Louis, MO, USA).
Effect of the surfactant on the average diameters of nanoemulsions
The preparation of oil phase: 31 mg of each aroma compounds were mixed together, and 3.44% olive oil was added. The surfactant Tween 80 was added with the ratio of surfactant/olive oil (SOR) at 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, respectively.
The preparation of aqueous phase: the absolute ethyl alcohol was used as cosurfactant. The absolute ethyl alcohol was weighted with the ratio of surfactant/cosurfactant (Km) at 7:1, and it was mixed with 5 mL deionized water. Then 0.01 g NaCl was added to the mixture. And the mixture was diluted with deionized water to 10 mL.
The preparation of nanoemulsions: the oil phase was slowly stirred with a glass rod and 10 mL of aqueous phase was added at a rate of about 1.0 mL/min. Finally, the mixture was placed on a magnetic stirrer at a speed of 1500 r/min and it was stirred for 6 h with a stirring rotor in it.
Optimizing surfactant/cosurfactant (Km) value
The pseudo-ternary phase diagram of the nanoemulsions was established to determine the optimal surfactant/cosurfactant (Km) value. The surfactant Tween 80 and the co-surfactant absolute ethyl alcohol were mixed according to a Km value of 9:1, 7:1, and 5:1 respectively to obtain a mixture. Then the mixture and the olive oil were mixed at the ratio of 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9. Then the preparation of the nanoemulsions was performed according to the method described above.
Effects of olive oil on the stability, droplet sizes and aroma release of the nanoemulsions
The preparation of oil phase: 31 mg of each aroma compounds were mixed together, and 1, 3, 5, 10, 15 and 20% olive oil were added respectively. The surfactant Tween 80 was added with the ratio of surfactant/olive oil (SOR) at 6:4. The preparation of aqueous phase was performed according to the method described above with a Km value of 7:1. Then the preparation of the nanoemulsions was performed according to the method described above. The prepared nanoemulsions were stored at 28 °C for 72 h. And the droplet size of the nanoemulsions was determined every 12 h. The released aroma compounds from the nanoemulsions during storage were determined by SPME–GC–MS. All the experiments were performed in triplicate.
Droplet size of the nanoemulsions
The average particle diameters and particle size distributions of olive oil-based nanoemulsions were measured by dynamic light scattering (Zetasizer Nano-ZS90, Malvern Instruments, Malvern, UK). The measurements were carried out at a scattering angle of 173° using a 658 nm laser. Each measurement was recorded in an average of 15 scans. The samples were diluted approximately 1000-fold with deionized water to prevent multiple scattering effects. The particle size and the size distribution of the nanoemulsions were reported as mean particle diameter and the polydispersity index (PDI), respectively.
Aroma release
The SPME device equipped with a 50/30 μm DVB/CAR/PDMS fiber (Supelco, Bellfonte, PA, USA) was used to extract the aroma compounds in the samples. A volume of 5 mL of the nanoemulsion was placed into a 20 mL headspace vial, and was equilibrated for 15 min in a water bath at 37 °C. After equilibrating for 10 min, the fiber head was lifted and the headspace extraction was executed for 30 min. Desorption was carried out at the temperature of 250 °C for 5 min. The fibre was activated in the injection port of the gas chromatograph at 250 °C for 30 min before use. The aroma compounds were measured by an Agilent 6890 N gas chromatograph coupled to an Agilent 5975B mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) and equipped with a J&W HP-5 fused silica capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness). The electron ionization mode with a 70 eV voltage was used. The flow rate of helium was 1.2 mL/min. The injector temperature was 250 °C. And the column was held at 40° for 1 min, increased from 40 to 220 °C at 6 °C/min, and then it was held at 220 °C for 1 min, and increased to 250 °C at a rate of 30 °C/min.
Quantitative determinations were obtained via an external standard method. The standard curves of these aroma compounds were determined by the linear relationship between a series of concentration gradients of aroma compounds (n × 1/6, n × 2/6, n × 3/6, n × 4/6, n × 5/6, n × 6/6, n × 7/6 (n = 50 μg/mL)) and their GC peak areas. The equations of the standard curves of 11 aroma compounds were: ethyl acetate (y = 8.9 × 106 x − 3 × 107, R2 = 0.998), α-pinene (y = 4×106 x − 2 × 106, R2 = 0.995), β-myrcene (y = 4×106 x + 102,042, R2 = 0.999), D-limonene (y = 3×106 x + 3×107, R2 = 0.998), benzyl alcohol (y = 2×106 x + 4×107, R2 = 0.998), 1-octanol (y = 1×106 x + 6×107, R2 = 0.997), nonanal (y = 995107 x + 4×107, R2 = 0.997), α-terpineol (y = 1×106 x + 3×107, R2 = 0.998), decanal (y = 1×106 x + 4×107, R2 = 0.999), eugenol (y = 897889 x + 3×107, R2 = 0.997), β-ionone (y = 1×106 x + 4×107, R2 = 0.995). The contents of the aroma compounds were calculated on the basis of the GC peak areas related to the corresponding standard curves.
Statistical analysis
The data were expressed as mean ± standard deviation (SD) for the measurements in triplicate. The data were analyzed by one-way ANOVA in SPSS 12.0 for Windows (SPSS, Inc., Chicago, IL). Multivariate analysis was conducted by principal component analysis (PCA).
Results and discussion
Effect of the surfactant concentrations on the average diameters of nanoemulsions
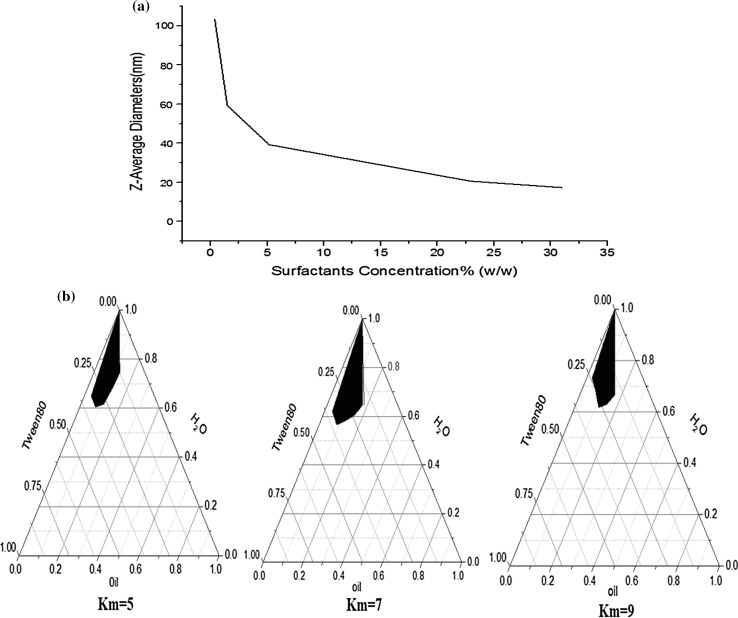
Surfactant concentration is an important factor that effects the surface coverage of the droplet and the O/W interfacial tension. As shown in Fig. 1a, the average diameter of the nanoemulsions decreased with the increase of the surfactant concentration. The average diameter was 42.48 nm when the SOR was 3:7, and it was reduced by 55% when compared with the average diameter of 95.4 nm at the SOR of 1:9. And it was 22.9 nm when the SOR was at 6:4 with a surfactant concentration of 5.16%, which was reduced by about 76% compared with the average diameter at SOR 1:9. It became relatively stable when the SOR was more than 8:2. Tween 80 is an emulsifier with small molecule, and it can form a layer between the two phases and this will reduce the interfacial tension which is in favor of the formation of the droplets (Jo and Kwon 2013). Usually, small droplet with great surface area requires more emulsifier to cover the surface area. Then all the droplets in the nanomulsions were fully covered by the emulsifier with certain concentrations of emulsifier, and the excessive emulsifier was not utilized (Jo and Kwon 2013; Li et al. 2015). This can be explained that droplets size decreased sharply first and then kept stable with the increase of Tween 80.
Fig. 1.
Effect of the surfactants concentration on nanoemulsions diameters (a) and the pseudo-ternary phase diagram of the nanoemulsions (b)
Optimizing surfactant/cosurfactant (Km) value
The pseudo-ternary phase diagram of the nanoemulsion systems was established to determine the optimal surfactant/cosurfactant (Km) value. As shown in Fig. 1b, the oil–water interfacial tension was reduced greatly with the presence of surfactant. The presence of cosurfactant resulted in further decline of the interfacial tension due to producing mixed adsorption on interfacial tension (Liu et al. 2009). The existence of cosurfactant in nanoemulsion system incorporating with surfactant could form hybrid membranes, which could enhance the flexibility of the interface. And it is easily to bend and more easily trend to form nanoemulsions. In other word, as the role of a cosurfactant, absolute ethanol is mainly used to reduce the interfacial tension, which can result in the increase of liquidity in the interface membrane and the adjustment of HLB value of the surfactant.
However, exceeding cosurfactant was not conducive to form nanoemulsions. And it could destroy the stability of the nanoemulsions. Pesudo-ternary phase diagram was studied to find out the optimum Km for nanoemulsions in the present study. As it can be seen from Fig. 1b, the area of nanoemulsion was the largest at the Km of 7:1. It can be explained that the cosurfactant is completely embedded in the surfactant at the optimum mass ratios. This indicated that it has the maximum oil load at this time and can be diluted indefinitely by water.
The results of the diameter distributes of the nanoemulsions at the optimum km of 7:1 are shown in Table 1. The average particle size of the nanoemulsion was 39.22 nm, and the polydispersity index (PDI) was 0.242 nm. The particle size distribution was found in the range of 20–150 nm. Only one peak was observed at 50.45 nm. Among all these particles, the particles which were smaller than 83 nm accounted for 90%, and the particles which were smaller than 97 nm accounted for 95%. This indicated that the range of the diameter distributes of the nanoemulsions was narrow and the particle size was uniform at the optimum km.
Table 1.
Diameter distributes of the nanoemulsions
| Cumulant result | Distribution results | Undersize results | ||||||
|---|---|---|---|---|---|---|---|---|
| Size (d.nm) | % Int | σ | % pd | Di (%) | Size (d.nm) | |||
| Z-average (nm) | 39.22 | |||||||
| Pd Index | 0.242 | Peak 1 | 50.45 | 100.0 | 22.65 | 44.9 | 50 | 45.7 |
| Polydispersity (nm) | 19.3 | Peak 2 | 0.000 | 0.0 | 0.000 | 0 | 90 | 83.3 |
| % Polydispersity | 49.2 | Peak 3 | 0.000 | 0.0 | 0.000 | 0 | 95 | 96.9 |
| Derived kcps | 497,038.2 | |||||||
Effect of olive oil on the stability and droplet size
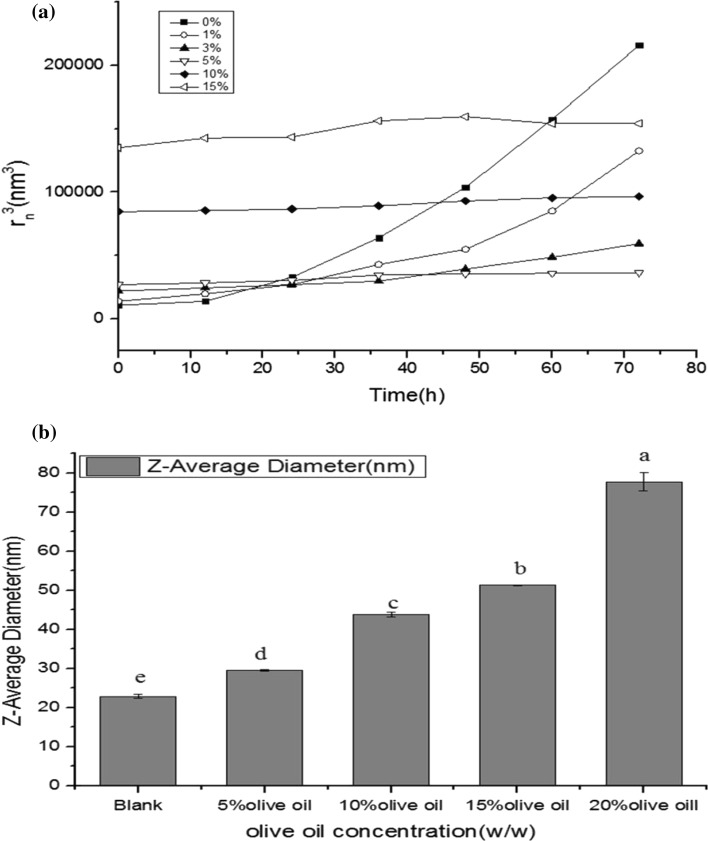
As shown in Fig. 2a, the droplet size of the nanoemulsions without olive oil increased significantly during storage at 28 °C. This indicated that the nanoemulsion system was very unstable without the addition of olive oil. Similarly, the droplet size of the nanoemulsions with 1% olive oil increased significantly after 24 h’ storage. And it was also unstable at this olive oil concentration. The nanoemulsions with 3% olive oil were more stable than that with 1% olive oil. And the stability of the nanoemulsions was the best when the concentration of the olive oil was 5%. Although the droplet size of the nanoemulsions was stable during the storage when the concentration of the olive oil was 10% and 15%, it was bigger than the droplet size of the nanoemulsions with 5% olive oil. If the cube of the diameters (r3n) is approximate proportional to the time (t), the line slopes are the Ostwald ripening rates (Mcclements 2011). Generally, the increase of olive oil concentrations resulted in the decrease of Ostwald ripening. The nanoemulsions showed a reduced Ostwald ripening rate at low olive oil content (1–5%) compared with the nanoemulsions without olive oil, but it was not enough to inhibit the increase of particle size. While there was no obvious changes on particle size at relative high olive oil content during storage (10–15%).
Fig. 2.
Effects of different concentrations of olive oil on the stability (a) and droplet sizes (b) of the nanoemulsions
Figure 2b showed the effects of different contents of olive oil on the droplet sizes of the nanoemulsions. The results showed that the droplet size increased with the increase of olive oil content. The droplet size and distribution are important parameters to nanoemulsion stability (Mikulcová et al. 2016). The droplet size decides the water solubility of the nanoemulsions, which plays a vital role on Ostwald ripening (Heurtault et al. 2003). As demonstrated previously, Ostwald ripening has little effect on low solubility compounds. Incorporating oil with high solubility into relatively water-insoluble oil can inhibit the Ostwald ripening (Liang et al. 2012). The solubility of plant oil in water is far lower than the solubility of tributyrin in water which contains much more nonpolar triglycerides (Coupland et al. 1996). Proper proportions of plant oil are contributive to the stability of nanoemulsions by reducing the solubility of dispersed phase. Wooster et al. (2008) studied the effect of the addition of proper peanut oil which had a larger Vm than n-alkane oils on the nanoemulsion, and they found that the bigger the diameter of the dispersed phase was, the smaller the Ostwald ripening rate was, and there was a linear relationship between them.
Effect of storage on the release of aroma compounds
As shown in Table 2, all the 11 aroma compounds were detected after 24-h storage. The released content of α-pinene was the highest at the level of 32.28 μg/mL, followed by β-myrcene (30.12 μg/mL) and D-limonene (26.35 μg/mL). While the lowest content of ethyl acetate at the level of 6.36 μg/mL was found. α-Pinene, β-myrcene, D-limonene, eugenol and β-ionone were the five aroma compounds detected after 48 h, and their contents decreased by 35, 45, 79, 18 and 47% respectively. While the other 6 aroma compounds were not detected after 48 h. Only α-pinene and β-myrcene were found at low concentrations of 1.41 and 0.5 μg/mL after 72 h.
Table 2.
Effect of storage on the release of aroma compounds from the nanoemulsions
| Compounds | Water solubility at 25 °C (mg/L) | Vapor pressure, mm Hg (25 °C) | Contents (μg/mL) | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||
| 1 | Ethyl acetate | 80,000 | 111.7 ± 0.1 | 6.36 ± 2.27 | nd | nd |
| 2 | α-Pinene | 2.49 | 3.5 ± 0.1 | 32.28 ± 3.72 | 21.12 ± 5.25 | 1.41 ± 0.08 |
| 3 | β-Myrcene | 5.6 | 2.3 ± 0.1 | 30.12 ± 2.65 | 16.71 ± 3.48 | 0.5 ± 0.12 |
| 4 | D-limonene | 13.8 | 1.5 ± 0.2 | 26.35 ± 2.22 | 5.66 ± 2.86 | nd |
| 5 | Benzyl alcohol | 4.29 | 0.2 ± 0.4 | 21.76 ± 5.53 | nd | nd |
| 6 | 1-Octanol | 540 | 0.1 ± 0.8 | 13.42 ± 6.32 | nd | nd |
| 7 | Nonanal | 96 | 0.5 ± 0.4 | 14.63 ± 2.69 | nd | nd |
| 8 | α-Terpineol | 1817 | 0.2 ± 0.4 | 12.37 ± 1.08 | nd | nd |
| 9 | Decanal | 43.52a | 0.2 ± 0.4 | 18.95 ± 3.14 | nd | nd |
| 10 | Eugenol | 2460 | 0.0 ± 0.5 | 10.63 ± 4.35 | 8.72 ± 1.25 | nd |
| 11 | β-Ionone | 7.986a | 0.0 ± 0.5 | 8.28 ± 3.26 | 4.36 ± 1.02 | nd |
aWater solubility calculated from corresponding octanol–water partition coefficients and applicable correction factors using the Estimation Programs Interface Suite (Royal Society of Chemistry). For nonpolar solutes, the oil–water and octanol–water partition are nearly identical21
nd Not detected
Binks et al. (2010) found that the lower water solubility of aroma compounds, the higher their retardation in lipid phase in O/W emulsions and the aroma compounds stayed longer in lipid phase. So there was less transformation from dispersed phase to continuous phase. And more contents of α-pinene, β-myrcene and D-limonene were kept in dispersed phase. Hence, aroma compounds with lower water solubility had higher equilibrium pressures. And they were detected at high levels after 24 h’ storage. On the other hands, the content of the released ethyl acetate was found the lowest at the level of 6.36 μg/mL, and this compound had the highest water solubility of 80,000 mg/L. The reason is that high water solubility makes it easy to transfer from dispersed phase to continuous phase which results in the quick release from the system during storage. β-Ionone has a low water solubility of 7.986 mg/L, but the released content of this compound was only 8.28 μg/mL after 24-h storage. And the reason might be that β-ionone reached low saturation vapor pressure (0.0 ± 0.5 mm Hg) in the headspace, and the released content could not reflect its water solubility.
Effect of olive oil on the release of aroma compounds
As shown in Table 3, the release of most of the aroma compounds decreased with the increase of the content of olive oil. For ethyl acetate, there were no significant changes on its released content at low olive oil content (1–5%) compared with control group (0% olive oil). While the released ethyl acetate was reduced by 48% at 10% olive oil addition compared with the control group. For α-pinene and nonanal, the released contents of these two compounds did not have significant differences (p < 0.05) until the olive oil content was ≥ 3%. Significant differences (p < 0.05) on the released contents of β-myrcene, D-limonene, α-terpineol, decanal and eugenol were observed when the olive oil content was ≥ 5%. However, benzyl alcohol, β-ionone and 1-octanol showed no significant changes with the increase of the olive oil.
Table 3.
Effect of different concentrations of olive oil on the release of aroma compounds from the nanoemulsions
| Compounds | Contents (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 0% Olive oil | 1% Olive oil | 3% Olive oil | 5% Olive oil | 10% Olive oil | ||
| 1 | Ethyl acetate | 16.04 ± 1.50 | 16.42 ± 1.53 | 14.85 ± 1.17 | 11.23 ± 1.46 | 8.30 ± 0.98a |
| 2 | α-Pinene | 38.36 ± 1.22 | 37.55 ± 1.77 | 30.10 ± 2.87ab | 31.46 ± 2.61a | 26.43 ± 1.31ab |
| 3 | β-Myrcene | 35.6 ± 2.06 | 34.42 ± 3.65 | 36.38 ± 1.57 | 20.67 ± 2.35ab | 18.37 ± 2.99a |
| 4 | D-limonene | 34.52 ± 3.01 | 32.24 ± 2.78 | 33.58 ± 2.47 | 26.35 ± 1.05ab | 25.11 ± 1.89a |
| 5 | Benzyl alcohol | 27.55 ± 3.63 | 27.64 ± 3.65 | 27.11 ± 2.24 | 28.48 ± 3.52 | 26.82 ± 3.61 |
| 6 | 1-Octanol | 18.51 ± 2.26 | 19.36 ± 4.51 | 19.04 ± 0.97 | 19.33 ± 3.27 | 17.85 ± 1.09 |
| 7 | Nonanal | 20.55 ± 3.52 | 20.73 ± 1.62 | 15.42 ± 1.52ab | 15.44 ± 1.68a | 14.42 ± 2.72a |
| 8 | α-Terpineol | 18.30 ± 4.25 | 17.26 ± 2.57 | 17.08 ± 1.29 | 14.32 ± 3.52a | 15.02 ± 2.48a |
| 9 | Decanal | 23.97 ± 2.32 | 23.45 ± 1.66 | 21.45 ± 0.84 | 17.68 ± 3.77ab | 18.32 ± 2.24a |
| 10 | Eugenol | 17.75 ± 4.98 | 16.37 ± 2.14 | 17.38 ± 1.42 | 16.88 ± 2.74 | 12.15 ± 1.07ab |
| 11 | β-Ionone | 13.50 ± 1.36 | 12.27 ± 3.17 | 10.86 ± 2.96 | nd | nd |
aThe aroma compounds in different olive oil content and the corresponding blank control group were in significant difference (p < 0.05)
bThe aroma compounds in different olive oil content and the former olive oil content were in significant difference (p < 0.05)
nd Not detected
Sadovoy et al. (2013) found that the process of the aroma molecule released from emulsion includes three steps: aroma molecule transfers from dispersed phase to continuous phase; diffusing on the emulsion surface; and finally evaporating into the air. The aroma content in the nanoemulsion system consists of aroma in the headspace, continuous phase and dispersed phase, respectively. The releasing rate of the aroma compounds with low water solubility is affected by the transferring through a stabilizing shell, lipid phase, stagnant vapor layer (Binks et al. 2010). As a carrier, olive oil can help the nanoemulsion system to hamper the transferring of the aromas from dispersed phase to continuous phase. In addition, there are many nonpolar triacylglycerol, which is consisted of one glycerinum molecule and three fatty acids. Ester group can form the large delocalized π bond, and two electrons evenly distribute to two oxygen molecules, so it has low water solubility and high nonpolar solubility. Most of the aroma compounds are strong nonpolar molecules. According to the principle that the similar substance is more likely to be dissolved by each other, olive oil can embed the aroma molecules. In conclusion, the evaporation rates of the aromas decreased after the addition of olive oil. In the present study, the release of most of the aroma compounds was inhibited by increasing content of olive oil. Egle et al. (2005) found that xanthan could create a hydrophobic interaction to hold flavor compounds and inhibited their release. We concluded that the higher content of olive oil, the stronger hydrophobic interaction in nanoemulsions, and this resulted in the less release of the aroma compounds.
Principal component analysis
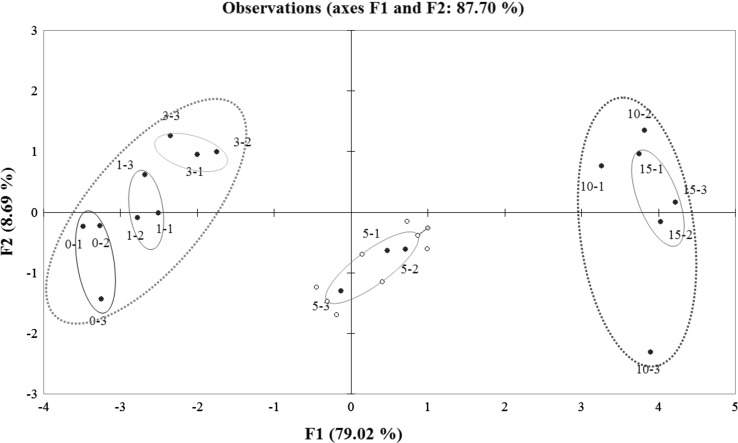
Figure 3 shows the PCA results from the analysis of the released aroma compounds (after 24-h storage) in nanoemulsion systems with different olive oil concentrations. As shown in Fig. 3, two principal components with 79.02% and 8.69% of the total variance were obtained, and they accounted for 87.70%. The group of data points associated with 0–3, 5 and 10% olive oil showed significant differences, and they were clearly separated from each other. Nanoemulsions with high content of olive oil (10%, 15%), were grouped in the right quadrant. This indicated that there were positively and strongly correlated with PC1. Nanoemulsions with low contents of olive oil (0%, 1%, 3%) were located in the negative part of the first dimension. And 5% olive oil exhibited a negative correlation with PC2. And this indicated that a separation on the released aroma contents with different olive oil was achieved.
Fig. 3.
PCA plot for aroma release from the nanoemulsions at 24 h for different olive oil concentrations. Olive oil concentrations are 0% (0–1, 0–2, 0–3), 1% (1–1, 1–2, 1–3), 3% (3–1, 3–2, 3–3), 5% (5–1, 5–2, 5–3), 10% (10–1, 10–2, 10–3) and 15% (15–1, 15–2, 15–3)
Conclusion
This study focused on the influence of olive oil on Ostwald ripening and the release of aromas from nanoemulsions. The average diameter of the nanoemulsions decreased with the increase of the surfactant concentrations. The pseudo-ternary phase diagram showed that the optimum km of the flavor nanoemulsions was 7:1. When the concentration of the olive oil was 5%, the stability of the nanoemulsions was the best. The droplet size of the nanoemulsions increased with the increase of olive oil content. The released content of α-pinene was observed the highest after 24-h storage, followed by β-myrcene and D-limonene. The release of most of the aroma compounds was hampered with the increase of olive oil, while the released of benzyl alcohol, β-ionone and 1-octanol showed no significant changes with the increase of olive oil.
Acknowledgements
This study was supported by the National Key Reasearch and Development Plan of China (2017YFD0400101), National Natural Science Foundation of China (Program No. 31671824), the Major Scientific and Technological Innovation Project in Hubei Province (2015ABA035, 2016ABA112).
References
- Barradas TN, Campos VEBD, Senna JP, Tebaldi BS, Coutinho CDSC, Tebaldi BS, Silva KGDHE, Mansur CRE. Development and characterization of promising o/w nanoemulsions containing sweet fennel essential oil and non-ionic sufactants. Colloid Surf A: Physicochem Eng Asp. 2015;480:214–221. doi: 10.1016/j.colsurfa.2014.12.001. [DOI] [Google Scholar]
- Binks BP, Fletcher PDI, Holt BL, Pascal B, Kenneth W. Selective retardation of perfume oil evaporation from oil-in-water emulsions stabilized by either surfactant or nanoparticles. Langmuir. 2010;26:18024–18030. doi: 10.1021/la103700g. [DOI] [PubMed] [Google Scholar]
- Chang Y, McClements DJ. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods, influence of the oil phase, surfactant, and temperature. J Agric Food Chem. 2014;62:2306–2312. doi: 10.1021/jf500160y. [DOI] [PubMed] [Google Scholar]
- Coupland JN, Weiss J, Lovy A, Mcclements DJ. Solubilization kinetics of triacyl glycerol and hydrocarbon emulsion droplets in a micellar solution. J Food Sci. 1996;61:1114–1117. doi: 10.1111/j.1365-2621.1996.tb10942.x. [DOI] [Google Scholar]
- Egle B, Jens AN, Meyer AS. Effect of xanthan on flavor release from thickened viscous food model systems. J Agric Food Chem. 2005;53:3577–3583. doi: 10.1021/jf048111v. [DOI] [PubMed] [Google Scholar]
- Gong W, Zhu JB. Advances in the research of optimization methods and instability mechanisms of nanoemulsion. Chin J New Drugs. 2010;19:1036–1040. [Google Scholar]
- Gutiérrez JM, González C, Maestro A, SolèI Pey CM, Nolla J. Nanoemulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13:245–251. doi: 10.1016/j.cocis.2008.01.005. [DOI] [Google Scholar]
- Guttoff M, Saberi AH, McClements D. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem. 2015;171:117–122. doi: 10.1016/j.foodchem.2014.08.087. [DOI] [PubMed] [Google Scholar]
- Hashtjin AM, Abbasi S. Optimization of ultrasonic emulsification conditions for the production of orange peel essential oil nanoemulsions. J Food Sci Technol. 2015;52(5):2679–2689. doi: 10.1007/s13197-014-1322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurtault B, Saulnier P, Pech B, Benoıt JP, Proust JE. Interfacial stability of lipid nanocapsules. Colloid Surf B. 2003;30:225–235. doi: 10.1016/S0927-7765(03)00096-1. [DOI] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82:478–488. doi: 10.1016/j.jfoodeng.2007.03.007. [DOI] [Google Scholar]
- Jiang Y, Wei J, Baoshan G, Xiaohui Q, Yongjun L. Preparation of d-limonene oil-in-water nanoemulsion from an optimum formulation. J Oleo Sci. 2014;63:1133–1140. doi: 10.5650/jos.ess14041. [DOI] [PubMed] [Google Scholar]
- Jo YJ, Kwon YJ. Characterization of β-carotene nanoemulsions prepared by microfluidization technique. Food Sci Biotechnol. 2013;23:107–113. doi: 10.1007/s10068-014-0014-7. [DOI] [Google Scholar]
- Kabalnov A. Ostwald ripening and related phenomena. J Disper Sci Technol. 2001;22:1–12. doi: 10.1081/DIS-100102675. [DOI] [Google Scholar]
- Komaiko J, McClements DJ. Optimization of isothermal low-energynanoemulsion formation: hydrocarbon oil, non-ionic surfactant, and water systems. J Colloid Interface Sci. 2014;425:59–66. doi: 10.1016/j.jcis.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Li W, Chen H, He Z, Han C, Liu S, Li Y. Influence of surfactant and oil composition on the stability and antibacterial activity of eugenol nanoemulsions. LWT-Food Sci Technol. 2015;62:39–47. doi: 10.1016/j.lwt.2015.01.012. [DOI] [Google Scholar]
- Liang R, Xu S, Shoemaker FC, Li Y, Zhong F, Huang Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J Agric Food Chem. 2012;60:7548–7555. doi: 10.1021/jf301129k. [DOI] [PubMed] [Google Scholar]
- Liu GX, Zhang JY, Wu X, Li JY, Liu Y, Zhou XZ, Wei XJ, Niu JR, Hu HW. Basic study on different co-surfactant o/w pharmaceutical microemulsions. Chin J Hosp Pharm. 2009;29:177–180. [Google Scholar]
- McClements DJ. Food emulsions: principles, practices, and techniques. 2. Boca Raton: CRC Press; 2005. [Google Scholar]
- Mcclements DJ. Edible nanoemulsions, fabrication, properties, and functional performance. Soft Matter. 2011;7:2297–2316. doi: 10.1039/C0SM00549E. [DOI] [Google Scholar]
- McClements D, Henson L, Popplewell M, Decker E, Choi S. Inhibition of Ostwald ripening in model beverage emulsions by addition of poorly water soluble triglyceride oils. J Food Sci. 2012;71(1):33–38. doi: 10.1111/j.1750-3841.2011.02484.x. [DOI] [PubMed] [Google Scholar]
- Mehta SC, Somasundaran P, Kulkarni R. Variation in emulsion stabilizationbehavior of hybrid silicone polymers with change in molecular structure: phasediagram study. J Colloid Interface Sci. 2009;333:635–640. doi: 10.1016/j.jcis.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Mikulcová V, Bordes R, Kašpárková V. On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles. Food Hydrocoll. 2016;61:780–792. doi: 10.1016/j.foodhyd.2016.06.031. [DOI] [Google Scholar]
- Nazarzadeh E, Anthonypillai T, Sajjadi S. On the growth mechanisms of nanoemulsions. J Colloid Interf Sci. 2013;397:154–162. doi: 10.1016/j.jcis.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Sadovoy AV, Lomova MV, Antipina MN, Braun NA, Sukhorukov GB, Kiryukhin MV. Layer-by-layer assembled multilayer shells for encapsulation and release of fragrance. ACS Appl Mater Inter. 2013;5:8948–8954. doi: 10.1021/am401871u. [DOI] [PubMed] [Google Scholar]
- Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10:102–110. doi: 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- Streck L, de Araújo MM, de Souza I, Fernandes-Pedrosa MF, do Egito EST, de Oliveira AG, da Silva-Júnior AA. Surfactant–cosurfactant interactions and process parameters involved in the formulation of stable and small droplet-sized benznidazole-loaded soybean O/W emulsions. J Mol Liq. 2014;196:178–186. doi: 10.1016/j.molliq.2014.03.033. [DOI] [Google Scholar]
- Taylor P. Ostwald ripening in emulsions. Adv Colloid Interfac. 1998;75:107–163. doi: 10.1016/S0001-8686(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Velikov KP, Pelan E. Colloidal delivery systems for micronutrients and nutraceuticals. Soft Matter. 2008;4:1964–1980. doi: 10.1039/b804863k. [DOI] [Google Scholar]
- Wooster TJ, Matt G, Peerasak S. Impact of oil type on nanoemulsion formation and ostwald ripening stability. Langmuir. 2008;24:12758–12765. doi: 10.1021/la801685v. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bing L, Reineccius GA. Comparison of modified starch and Quillaja saponins in the formation and stabilization of flavor nanoemulsions. Food Chem. 2016;192:53–59. doi: 10.1016/j.foodchem.2015.06.078. [DOI] [PubMed] [Google Scholar]