Abstract
The aim of this work was to explore the use of protein isolate from tomato seed enriched with the sucrose and the ascorbic acid as a medium for the growth of kefir mixture culture to develop a new non-dairy functional food. Unstructured mathematical and logistic models were proposed to describe cell growth, kefiran production, nutriment consumption and antioxidant activity. It was found that the maximal cell mass in the culture reached 8.38 g L−1 after 24 h of fermentation. A significant amount of kefiran was also produced (0.65 g L−1). The kefir culture growth significantly decreased protein content and enhanced the antioxidant activity during varied fermentation through the production of bio active peptides. After 24 h of fermentation, IC50 value for protein isolate was estimated to be about 10.48 µg mL−1. The proposed models adequately described the changes during fermentation and as observed as a promising approach for the formulation of tomato seed-based functional foods. The preservation of the isolate was also investigated through a spray-drying process. The effect of spray-drying on the viability of lactic acid bacteria and stability of protein content and the antioxidant activity of the powder was also carried out. Results showed that the spray-drying method has great potential for the synthesis of powder from the fermented isolate that are rich in desirable properties. However, it was appropriate to preserve the powder for 10 days at 37 °C for the preservation of protein functionality.
Keywords: Protein isolate, Kefir, Functional food, Growth kinetics, Antioxidant activity, Spray-drying
Introduction
Nowadays, there has been a growing interest to develop new functional foods, defined as all products containing probiotic microorganisms specified as “live microorganisms, which when administered in adequate amounts confer a health benefit on the host” (Prado et al. 2008). The yogurt is undoubtedly the fermented milk product best known and consumed in the worldwide. However, due to the problems of lactose intolerance and cholesterol content associated with the consumption of fermented dairy products, there has recently been an intensive research addressed to the non-dairy fermented foods (Granato et al. 2010). In this respect, cereal-based products, vegetables and fruits offer an alternative to the production of fermented foods due to their functional benefits (Cagno et al. 2013). Among vegetable probiotic beverages, there have been recent a proposal for beet-based drink (Yoon et al. 2005), cabbage juice (Yoonm et al. 2006), carrot juice (Nazzaro et al. 2008) and tomato-based drink. Furthermore, either studies have been performed to address the conversion of agricultural or food industry wastes to new products with added value (Moayedi et al. 2016). In this case, tomato seed, the major byproduct of tomato processing industries, representing about 60% of the wastes, contains a substantial amount of protein ranging from 20 to 30% (Sogi et al. 2002; Mechmeche et al. 2017a, b). According to Savadkoohi and Farahnaky (2012), tomato seed proteins are of high nutritional value and have nutraceutical properties and also are good sources of bio active peptides which can be used as a health enhancing ingredient in functional foods. By the way, tomato seed protein-rich isolate offer an alternative to the production of fermented foods due to their functional benefits (Moayedi et al. 2016). The authors carried out the optimization of defatted tomato waste protein fermentation by means of Bacillus subtilis in a submerged system. To the best of our knowledge, no study has been conducted on the fermentation kinetics of kefir culture in tomato seed rich protein isolate. For this reason, the current study examines in detail the fermentation of defatted tomato seed protein isolate by means of kefir. In fact, kefir is an ancient food attributed to exceptional health promoting and curative properties since the beginning of recorded history (Corona et al. 2016). It was used for the treatment of tuberculosis, cancer and gastrointestinal disorders (Randazzo et al. 2016). Water kefir is a non-dairy kefir prepared with a sucrose solution with or without fruit extracts fermented by kefir grains, consisting of a consortium of yeasts, mainly Kluyveromyces, Candida and Saccharomyces, and lactic acid bacteria (LAB), including the genera Lactobacillus, Lactococcus, Leuconostoc and Streptococcus. All these microorganisms are embedded in a resilient water-soluble branched glucogalactan matrix named kefiran (Rodrigues et al. 2005). In food microbiology, there has been an increasing interest in modeling the kinetics of beneficial microorganisms in the last 10 years to predict their behavior in food systems (Lee et al. 2015). A number of mathematical models have been used to describe the sigmoidal curves of bacterial growth, the logistic model and others (Charalampopoulosa et al. 2009). These equations can fit cell growth over time and take into account growth inhibition in the stationary phase of growth and may lead also to the development of better strategies for the optimization of the fermentation process to ensure its economic viability.
Based on the several positive effects of kefir as well as protein-rich isolate from tomato seed on human health, this work was aimed to develop a new non-dairy functional food. Cell growth, kefiran production, nutriment consumption and antioxidant activity will be monitored and the results will be fitted to an unstructured mathematical model. An essay on the preservation of the isolate is also investigated through a spray-drying process for increasing its shelf life and maintaining its nutritional quality.
Materials and methods
Tomato seed material
Tomato wastes, consisting mainly of skins and seeds, were provided from a Tunisian tomato-processing factory. The skins were removed by immersing wastes in water and the remaining seeds were air-dried (Sogi et al. 2002). Then, dried seeds were ground in Retsch AS 200 Basic blender (Retsch GmbH, Germany). The tomato seed meal was then defatted for 6 h in a Soxhlet extraction apparatus using the hexane (1 g meal:10 mL hexane) at room temperature (20 °C) and further placed in a fume hood to remove the residual hexane until there was no hexane odor in order to obtain a defatted tomato seed meal. Defatted meal was then stored in airtight sample bottles in the refrigerator at 4 °C until further analysis.
Preparation of tomato seed isolate (TSI)
1 g of the defatted tomato seed meal was extracted for 49.76 h with 82.81 mL of deionized water according to Mechmeche et al. (2017a). The pH of the suspension (7.5–11.5) was kept constant during extraction by adjusting with 0.5 N NaOH and the temperature was regulated at 50 °C by a water bath. Then, Tomato Seed Isolate (TSI) was obtained after 25 min of centrifugation (2000×g) to remove the raw material. TSI was filtered through Whatman filter paper no 1 and stored at freezing conditions until be used in the experiments.
Microorganisms
The fermentation was carried out with the freeze-dried kefir microbial mixture (Genesis Laboratories, USA) containing approximately 109 CFU/g of LAB (Lactococcus lactis sp lactis, Lactococcus lactis sp lactis biovar diacetylactis, Lactococcus lactis sp cremoris, Leuconostoc mesenteroides sp cremoris, Lactobacillus kefyr) and Candida kefyr and Saccharomyces unisporus spp., as declared by the producer.
Production of fermented tomato seed isolate (FTSI)
The conditions of fermentation of tomato seed isolate by the kefir microbial mixture were optimized earlier using response surface methodology (RSM) and a central composite rotatable design (CCRD) (Mechmeche et al. 2017b). The maximal cells culture and the biomass production could be achieved through the inoculation of 0.22 g L−1 of kefir with the addition of 6.75 g L−1 of sucrose as carbon source and of 196 mg L−1 of ascorbic acid as vitamin. The fermentation of TSI was carried out in triplicate at 37 °C for 24 h.
Microbiological analysis
Samples were withdrawn at different time intervals during the cultivation in a centrifugation tube. Cell dry weight was determined by gravimetric methods in duplicate. The culture broth was centrifuged in 10 mL falcon tubes at 9000 rpm for 15 min to precipitate the cells. The supernatant was collected in another falcon tube for kefiran analysis. The cells were then centrifuged again under the same condition. After the second centrifugation, the supernatant was discarded and the cells were dried at 65 °C in an oven for 48 h.
Kefiran determination
Extracellular kefiran was recovered from the culture supernatant and determined according to Piermaria et al. (2009). Kefiran was precipitated overnight by the addition of an equal volume of cold absolute ethanol at 4 °C. The resulting precipitate was collected by centrifugation at 9000 rpm for 15 min, dissolved in hot distilled water and precipitated with ethanol. This step was repeated for three times to obtain pure kefiran. The supernatant of the centrifugation was discarded and the kefiran was dried at 65 °C in an oven for 48 h.
Analytical methods
Fermented Tomato Seed Isolate (FTSI) was subjected to several determinations periodically every 2 h. Total sugar was estimated by the phenol–sulphuric-acid method. The soluble protein content was assessed according to the Bradford method using the bovine serum albumin as a standard. Free radical-scavenging ability of FTSI was determined using a stable 2, 2-diphenyl-2-picrylhydrazyl radical (DPPH·) according to the method of Kao and Chen (2006) with some modifications. The spectrophotometric analysis of ABTS· + scavenging activity was also determined according to the method of Re et al. (1999).
Mathematical modelling
Fitting procedures and parametric estimations calculated from the results were carried out by minimization of the sum of quadratic differences between observed and model-predicted values, using the nonlinear least-squares (quasi-Newton) method provided by the macro ‘Solver’ of the Microsoft Excel XP spreadsheet. Statistica 6.0 software (StatSoft, Inc. 2001) was used to evaluate the significance of the estimated parameters by fitting the experimental values to the proposed mathematical models, and the consistency of these equations.
Growth kinetics of kefir culture mixture in tomato seed isolate can be expressed by the modified Gompertz model, which is described by the Eq. (1):
| 1 |
where A, µ and the productivity can be expressed by the Eqs. (2), (3) and (4):
| 2 |
| 3 |
| 4 |
Kefiran, sugar and protein were described by aligning a modified Weibull function as proposed by Mafart et al. (2002) (Eq. (5)):
| 5 |
Spray-drying
Spray-drying was performed with a Mini Spray-dryer Model 190 Bûchi (Bûchi, Göppingen, Germany), using compressed air from an in-house supply (~ 80 psi), a two-fluid nozzle (0.50 mm) atomized the protein solution. The air was filtered through a 0.22 µm Milidisk filter (Millipore) before entering the nozzle, and the flow rate was controlled by a variable area flow meter (Cole Parmer, 150 mm). A peristaltic pump (1–100 rpm, Masterflex, Cole Parmer) pumped liquid protein feed to the nozzle using silicone tubing (3 mm i.d.). Cooling water was circulated through a jacket around the nozzle. Based on a full factorial experimental design, the effects of the feed ratio, atomizer speed, and inlet air temperature on properties of spray-dried fermented tomato seed isolate were investigated (Data not shown). The following standard operating conditions were used in this study: an inlet temperature (Tinlet) of 90 °C, a drying air flow rate of 0.60 m3 min−1, an atomizing air flow rate of 0.90 m3 h−1, and a liquid feed rate of 5 mL min−1. Operation under these standard conditions resulted in an outlet temperature (Toutlet) of approximately 53 °C.
Powder analysis
The powder produced during experiment was kept in closed vessels until the analysis stage every 5 days during 20 days of storage. Plate count agar was used as the media for total mesophylic counts pour plate, incubated at 30 °C for 3 days. Lactic acid bacteria growth was determined by direct counting of colony forming units (CFU/mL) by plating 0.1 mL of serial dilutions on MRS agar, and incubating at 37 °C for 24 h. Total coliforms were plated on Deoxycholate Agar and cell counting was carried after 48 h at 37 °C. Clostridium perfringens were determined using TSC agar and incubated at 46 °C for 48 h.
The protein content was investigated by Bradford method. The free radical-scavenging ability antioxidant activity was evaluated by DPPH· and ABTS· + tests. Results are expressed as percentage on a dry matter basis.
Statistical analysis
Statistical analyses were carried out using STAT GRAPHICS Centurion XV. Statistical differences were determined using ANOVA followed by Least Significant Difference (LSD) testing. Differences were considered statistically significant when P < 0.05.
Results and discussion
Growth kinetics modelling
Cultivation was carried out for 24 h to investigate the kinetics of cell growth, kefiran production, sugar and protein consumption by kefir microorganisms in protein rich isolate from tomato seed enriched with sucrose as carbon source and ascorbic acid as vitamin. In fact, the use of sucrose as one of the most suitable carbon sources and the ascorbic acid as vitamin improve the production of kefiran relative to cell growth (Tayuan et al. 2011).
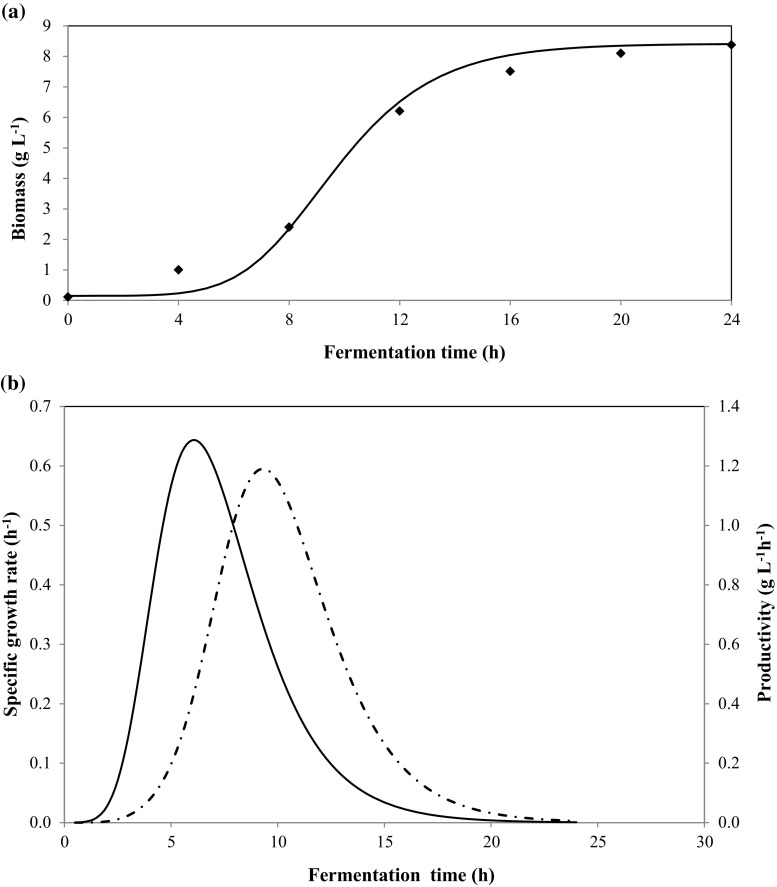
According to the results, a significant growth of the kefir culture mixture was observed between 0 and 24 h (Fig. 1a). It can be clearly observed that the biomass was not produced during the first 4 h of cultivation, which can be considered as the lag phase where the cells were adapted to the new environment. After that, the cells grew exponentially and reached their maximal cell mass (8.38 g L−1) after 24 h of fermentation. To describe cell growth in terms of biomass with time, a non-linear algebraic equation was used. In fact, the model parameters of the growth kinetics were estimated from fitting the experimental data to the unstructured Gompertz model. The performance of the developed model was evaluated by comparing the predicted growth responses to the observed responses obtained from experiment (Lee et al. 2015). Hence, the use of the modified Gompertz equation (Eq. (1)) to describe the growth kinetics of kefir microorganisms was useful in quantifying the lag period (λ = 3.54 h), the maximum specific growth rate (µmax = 0.65 h−1) and the log increase in population (A = 4.02) reached in the culture, which are parameters with biological meaning. In this order, the values predicted are highly correlated with the experimental data, with a regression coefficient R2 = 99.30%. The specific growth rate and the productivity formed per unit time reached to its maximum levels, respectively after about 5.80 and 9.20 h of fermentation to have a peak value, then decreased (Fig. 2b). The maximal productivity was found to be about 1.19 g L−1 h−1. By the way, the kefir microorganisms used in the study showed good growth in TSI, which indicates that the fermentation conditions were appropriate for growth and that the media provides all the required nutrients. The same findings were achieved by Randazzo et al. (2016) on Mediterranean fruit juices and by Corona et al. (2016) on vegetable juices.
Fig. 1.
Biomass production (a) and specific growth rate (–) and productivity (—) (b) at 37 °C during 24 h of the fermentation of tomato seed protein isolate by Kefir. Continuous lines for model simulated; Points for experimental data
Fig. 2.
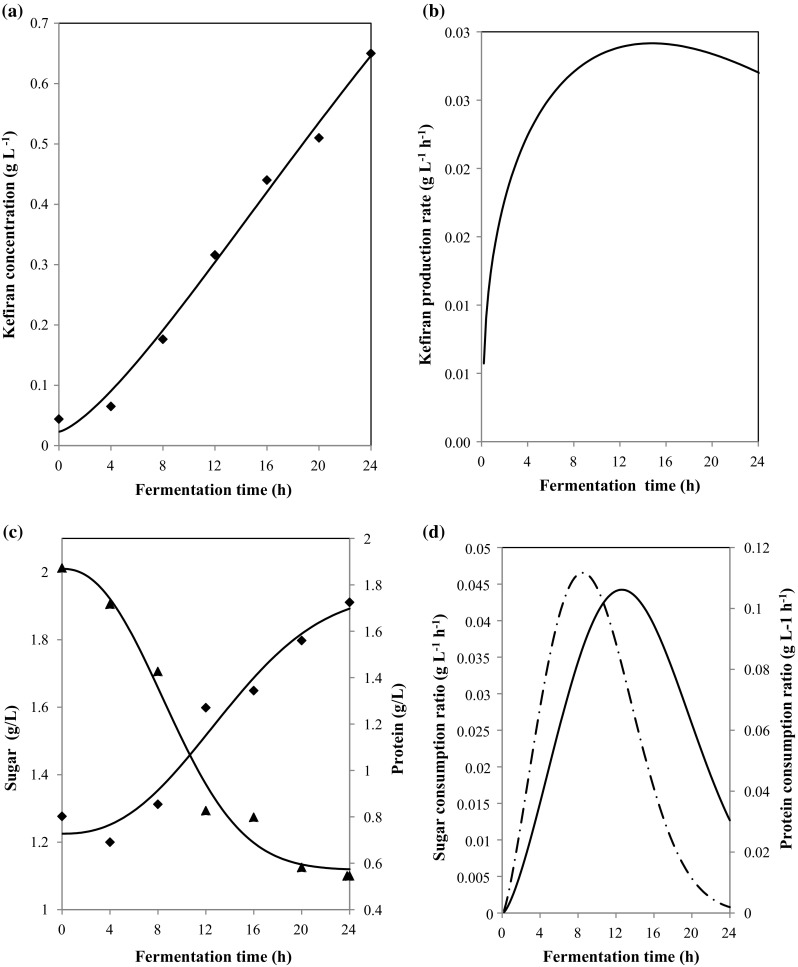
Experimental data (points) and model predictions (continuous lines) for kefiran concentration (a), rates of kefiran production (b), sugar and protein concentration (c) and Sugar and protein consumption rates (d) during 24 h of the fermentation of tomato seed protein isolate by kefir
Regarding the kefiran concentrations, the production was important (Fig. 2a), when the maximum concentration (0.65 g L−1) was seen after 24 h of fermentation. The kinetic modelling of kefiran production could be conducted using the Weibull models (Eq. (5)). In fact, the use of these models was useful and they could describe the change of kefiran concentrations (Table 1) with time because of the high determination coefficients (R2 = 99.20%). In addition, the low RMSE values (0.003) indicated a good fit to the experimental data. Based on this, we have expressed the kefiran production rate in terms of fermentation time (Fig. 2 b). A significant difference (P < 0.05) in the kefiran production rate was detected in the different time of fermentation. Whereas, the higher rate was observed at 24 h of fermentation (0.027 g L−1 h−1), which was significantly different from the rate at the beginning of the culture (0.006 g L−1 h−1). In fact, carbon source is considered a very important component of the cultivation medium since it is generally used as a source for energy and biosynthesis of kefiran. Previously, lactose was conventionally used as a good source for the high production of kefiran. However, Dailin et al. (2016) showed that the medium containing sucrose exhibited significant effect on cell growth and kefiran production. In this case, the plots of sugar concentrations as observed and model fit showed a good agreement between the model solutions and the experimental data obtained by a regression coefficient R2 = 96.50% (Table 1). This confirms that the Weibull model gives a better solution and has high accuracy and could describe the change of sugar concentration with time. Certainly, kefir mixture culture was able to gain its nutritional requirements from tomato seed meal and to grow well in the medium composed of defatted meal enriched with the sucrose. Moreover, the effect of nitrogen sources on the production of kefiran and cell growth has been also investigated. According to Cheirsilp et al. (2003), the higher the C/N ratio is, the lower the total kefiran production. With regard to the protein content, it can be noted that the values decreased significantly (P < 0.05) in the different time of fermentation (Fig. 2c). So, in order to evaluate the protein concentrations and to describe their kinetics, the Weibull equation (Eq. (5)) was also used. For an ideal approximation of modeled kinetics, R2 approaches to 1 and RMSE becomes 0. As shown by the values of the statistical parameters (Table 1), the Weibull fitting was found to be well suitable to describe the degradation kinetics of protein. This finding was in agreement with the results of Limón et al. (2015) who reported that the concentration of peptides and amino acids decreased when the lactic fermentation of kidney bean progressed after 48 h. In fact, phosphate salts are inorganic compounds that are generally used in the production medium to enhance bacterial growth. Gunter and Ovodov (2005) showed that cell growth and production of polysaccharides were limited by the absence of phosphate. Moreover, Duguid and Wilkinson (1953) proved that the maximal cell growth of Aerobacter aerogenes and polysaccharide production could be obtained at phosphate concentration of 0.12 g L−1. The Weibull equation was first used to fit the experimental data in order to estimate then the rate of nutriment (sugar and protein) consumption VS and VP. According to the obtained results (Fig. 2d), the maximum protein consumption rate was observed in 8.20 g L−1 h−1. Then, the effect of sugars and protein concentration on the specific growth rate were evaluated (Fig. 3c, d). A hyperbolic shape of the trend was observed using the Weibull model. Data showed that rate increases with glucose concentration up to 1.29 g L−1 and then they remain constant. Sharma and Mishra (2014) also reported an improvement of specific growth rate with glucose concentration up to 0.50 g L−1 in vegetable juice following the Monod’s model description. Maximum specific growth rate as a function of protein concentration was also determined in this work (Fig. 3c, d) and an hyperbolic shapes of the trends were detected for the protein rich isolates from tomato seed. In order to prove that protein-rich isolate from tomato seed can serve as a good media for the growth of kefir mixture culture, which has the ability to use both sugars and protein as a source of carbon source, a correlations between nutriment concentration, biomass and kefiran production were tabulated (Fig. 3e, f). Analyzing the correlation between the sugar and protein concentrations and the biomass production, a high correlation was obtained for the fermentation carried out in the protein rich isolate (R2 > 91%). An insignificant difference between the regression coefficient for sugar concentration (R2 = 91%) and protein concentration (R2 = 98.30%) confirming that these had good correlation with the biomass production (Fig. 3e). A high correlation between sugar and protein concentrations and the kefiran production were also observed (Fig. 3f)). It was concluded that protein isolate from tomato seed being a good source of nutrients resulted in production of both biomass and kefiran for 24 h. Statistical analysis showed an insignificant difference (P > 0.05) between the biomass and the kefiran production confirming that there was not a dominance of biomass production against kefiran production.
Table 1.
Parametric estimations corresponding to the kinetic models applied to the kefiran, sugar, protein and antioxidant activity variation during growth of Kefir mixture culture in the protein isolate from tomato seed at 37 °C for 24 h
| Variables | δ | p | V | R2 | RMSE |
|---|---|---|---|---|---|
| Kefiran | 3.25 | 1.37 | 0.03 | 99.20 | 0.03 |
| Sugar | 15.70 | 2.39 | 0.04 | 96.50 | 0.01 |
| Protein | 10.85 | 2.26 | 0.11 | 98.30 | 0.03 |
| IC50 | 13.11 | 1.35 | 1.96 | 96.80 | 2.78 |
δ, p shape parameters; V Maximum rate; R2 regression coefficient (%); RMSE Root Mean Square Error
Fig. 3.
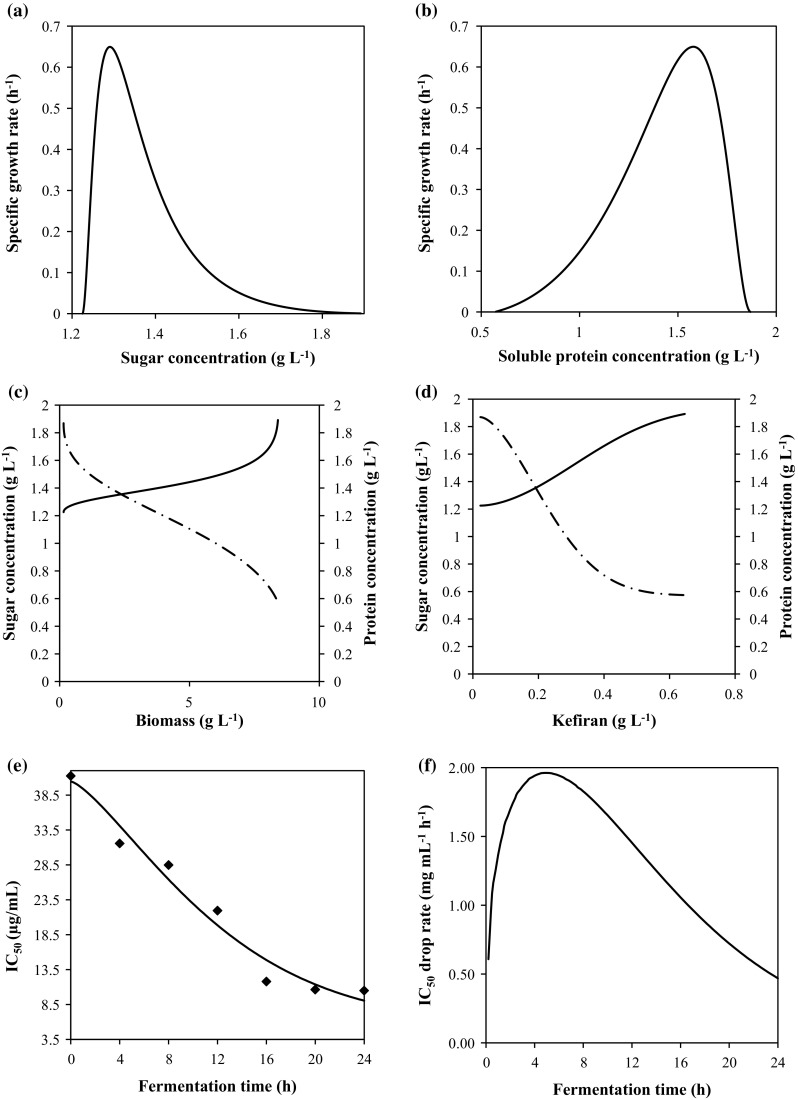
Specific growth rate as a function of sugar concentration (a) and as a function of protein concentration (b) and Sugar (–) and protein concentration (—) as a function of biomass production (c) and as a function of exopolysaccharide concentration (d). Experimental data (points) and model predictions (continuous lines) for IC50 (e) and rates of IC50 drop (f) during 24 h of the fermentation of tomato seed protein isolate by kefir
Antioxidant activity modelling
It has been reported that bio active peptides in protein isolate exert their antioxidant activities through different mechanisms. Therefore, the evaluation of the antioxidant capacity of the isolate would give better description on this activity. In this study, the antioxidant capacity of the isolate was assessed by using both the DPPH and ABTS free radical scavenging methods. In fact, FTSI showed a significant increase of radical scavenging activity during 24 h of fermentation. The free radical scavenging activity in all samples increased significantly in a linear mode with time of fermentation (Fig. 3e). After 24 h of fermentation, the highest (P < 0.05) antioxidant capacity was observed. IC50 value for protein isolate was 10.48 µg mL−1 after 24 h of fermentation (Fig. 3e). In fact, the enhancement of radical scavenging activity may be attributed to the production of different bio active peptides during fermentation of the isolate, which exhibited antioxidant properties (Elfahri et al. 2014). These results were in line with those reported, who showed that the fermentation of tomato waste proteins by proteolytic B. subtilis produced hydrolysates with good antioxidant activity. Based on this, a kinetic modelling for the antioxidant capacity in terms of IC50 with time could be achieved using the Weibull models. It is clear that the values predicted by the Eq. (5) are highly correlated with the experimental data because of the elevated regression coefficient (R2 = 96.80%) and the low RMSE value (Table 1). According to the results presented in Fig. 3f, we can note that the maximum IC 50 drop rate (1.96 µg mL−1 h−1) was observed after 5 h of fermentation. Overall, tomato seed isolate showed a high antioxidant activity and could be used as good sources of protein. It could be used in food processing in the formulation of functional foods and nutraceuticals to prevent damage related to oxidative stress in human disease conditions (Korhonen and Pihlanto 2003). Moreover, natural antioxidants are desirable because they can be used at higher concentrations without the toxic side-effects associated with the use of synthetic equivalents. The peptides were considered safe and healthy with high activity, easy absorption, low cost in comparison to animal proteins and no or little negative side-effects (Sarmadi and Ismail 2010). In addition, no anti-nutritional factor or harmful constituent have been reported in tomato seed that make it better source of protein over other non-conventional sources (Sogi et al. 2002). In this case, the production of functional foods from fermented tomato seed isolate constitutes a viable alternative for transforming this agro-industrial waste stream into a useful ingredient. The dehydration of the fermented isolate seems to be a convenient alternative for long-term storage or usage. However, the nutritional value must be well-preserved in the drying process. But, the quality attributes of a functional food may change during the spray drying process. In addition, poor storage conditions of functional foods may lead to loss of bioactive ingredients and undesirable colour and odour changes (Harbourne et al. 2011). Therefore, it is of great importance to measure the rate of change of a given quality parameter with storage after spray-drying process.
Effect of spray-drying process on physical properties of the fermented isolate powder
Spray-drying is a unit operation by which a liquid product is atomized in a hot gas current to instantaneously obtain a powder (Gharsallaoui et al. 2007). The spray method is well-trusted in practical uses, which has been confirmed by its use in the manufacturing of dried food, fertilizers, oxide ceramics, and pharmaceuticals (Nandiyanto and Okuyama 2011). Moreover, Dehydration, or drying, involves transient heat and mass transfer accompanied by physical, chemical, and phase change transformations. Unfortunately, these transformations may cause changes in the product’s quality as well as the mechanisms of heat and mass transfer (Chen et al. 2016). In this case, the effect of spray-drying on the viability of lactic acid bacteria, the stability of the protein content and the antioxidant activity of the obtained powder was carried out after spray-drying. Results showed that the powder contains after drying 7.33 Log CFU/g of lactic acid bacteria (Fig. 4a). The powder stored at 37 °C was also analyzed every 5 days during 20 days of conservation in order to evaluate the limit period of storage. It was found also that survival of lactic acid flora varied during the storage. After 20 days of conservation, the lactic acid bacteria flora reached 5.69 Log CFU/g (Fig. 4a). The loss of viability of the dried culture was by about 22.44% since the cells are exposed to various stresses. In addition, the evaluation of the microbial diversity in the powder after spray-drying showed the absence of total coliform and clostridium perfringens. Soluble protein content analysis of sample after spray-drying revealed the presence of 12.64 mg g−1 of powder (Fig. 4b). During the first 5 days of storage, soluble protein decreased slightly from 12.64 to 11.31 mg g−1. After 20 days of storage, the protein content decreased by about 34.65%. Moreover, the analysis of the antioxidant activity showed that the powder showed IC50 of 38.69 and 41.95 µg g−1, respectively by DPPH and ABTS assays after the spray-drying (Fig. 4c, d). During conservation, results showed that the ability of the fermented powder to trap the free radicals decreased to be about 89.50 and 87.63 µg g−1 respectively by DPPH and ABTS tests. This could be due to the decrease of the viability of lactic acid bacteria flora which exhibited a high antioxidant activity as well as the deterioration of the present bioactive molecules in the powder. Overall, we can note that the spray-drying method has great potential for the synthesis of powder from the fermented tomato seed isolate that are rich in desirable properties. According to the results, it was appropriate to conserve the powder for 10 days at 37 °C for the preservation of the protein functionality. In this case, Phisut (2012) developed a spray drying technique of fruit juice powder and demonstrated that the powders, produced had good quality, low water activity, easier transport and storage. While, some factors influencing the properties of product and have an impact on protein functionality. In fact, the biological value of dried proteins varies with the drying procedure. Prolonged exposures to high temperatures can affect the functional properties or render the protein less useful in the diet. In addition, different reactions may take place during storage of the powder, some antioxidants may disappear and/or new molecules can be produced affecting the antioxidant activity (Phisut 2012).
Fig. 4.
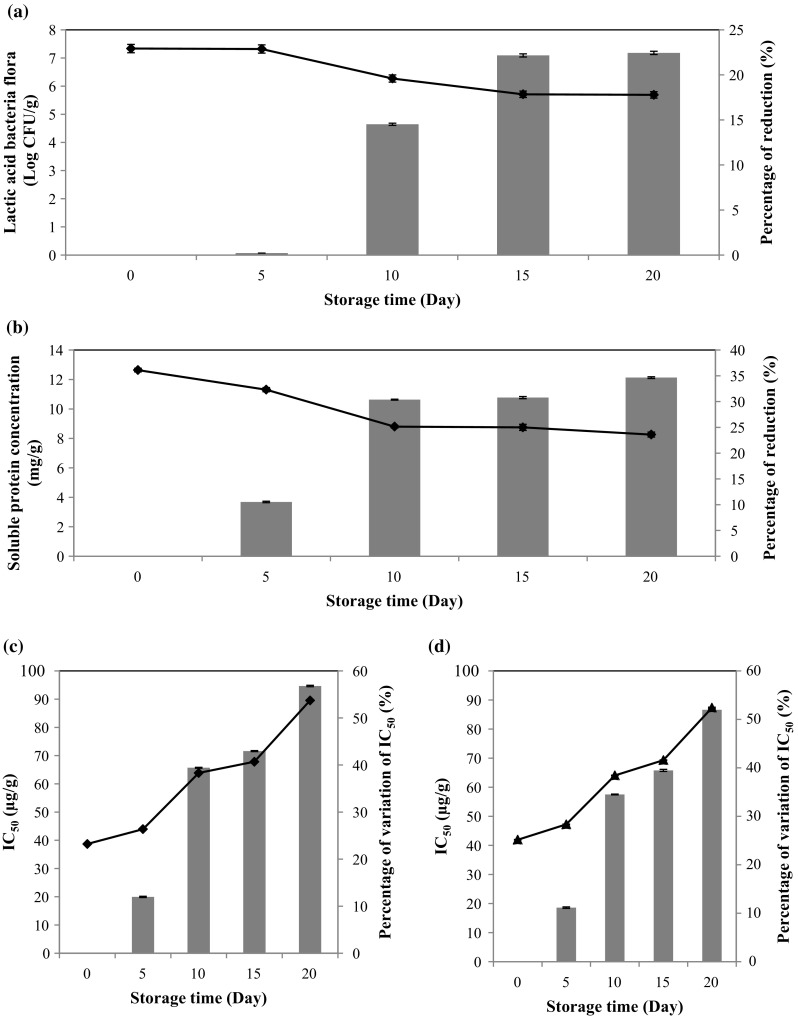
Viability of Lactic acid bacteria (a), soluble protein content (b), the antioxidant activity by DPPH (c) and ABTS methods (d) and the percentages of variations (■) as a function of storage time in the powder of the fermented tomato seed isolate
Conclusion
Taking into account the increasing complexity of the needs of different typologies of consumers, including vegan vegetarian and subjects with intolerance/allergy to dairy products, we applied an integrated technological approach in this work to elaborate a new functional food from the fermented tomato seed isolate by commercial kefir microorganisms. TSI has proven to be suitable substrate for the fermentation. The mathematical models used herein allowed description of the microbial kinetics, kefiran production, nutriments consumption and antioxidant activity. The new product might represent important foods providing live microorganisms to vegan people with a limited availability of fermented products. However, several aspects needed to be considered in the design of a novel fermented food such as the stability of the final product during storage. The Spray-drying process of the fermented isolate seems to be a convenient alternative for the preservation of protein functionality.
References
- Cagno RD, Coda R, Angelis MD, Gobbetti M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013;33:1–10. doi: 10.1016/j.fm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Charalampopoulosa D, Vázquezb JA, Pandiellac SS. Modelling and validation of Lactobacillus plantarum fermentations in cereal-based media with different sugar concentrations and buffering capacities. Biochem Eng J. 2009;44:96–105. doi: 10.1016/j.bej.2008.11.004. [DOI] [Google Scholar]
- Cheirsilp B, Shoji H, Shimizu H, Shioya S. Interaction between Lactobacillus kefiranofaciens and Saccharomyces cerevisiae in mixed culture for kefiran production. J Biosci Bioeng. 2003;96:279–284. doi: 10.1016/S1389-1723(03)80194-9. [DOI] [PubMed] [Google Scholar]
- Chen ZG, Guo XY, Wu T. A novel dehydration technique for carrot slices implementing ultrasound and vacuum drying methods. Ultrason Sonochem. 2016;30:28–34. doi: 10.1016/j.ultsonch.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Corona O, Randazzo W, Alessandro M, Guarcello R, Nicola F, Erten H, Moschetti G, Settanni L. Characterization of kefir-like beverages produced from vegetable juices. LWT—Food Sci Technol. 2016;66:572–581. [Google Scholar]
- Dailin DJ, Elsayed EH, Othman NZ, Malek R, Phin HS, Aziz R, Wadaan M, El Enshasy HA. Bioprocess development for kefiran production by Lactobacillus kefiranofaciens in semi industrial scale bioreactor. Saudi J Biol Sci. 2016;23(4):495–502. doi: 10.1016/j.sjbs.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid JP, Wilkinson JF. The influence of cultural conditions on polysaccharide production by Aerobacter aerogenes. J Gen Microbiol. 1953;9:174–189. doi: 10.1099/00221287-9-2-174. [DOI] [PubMed] [Google Scholar]
- Elfahri KR, Donkor ON, Vasiljevic T. Potential of novel Lactobacillus helveticus strains and their cell wall bound proteases to release physiologically active peptides from milk proteins. Int Dairy J. 2014;38:37–46. doi: 10.1016/j.idairyj.2014.03.010. [DOI] [Google Scholar]
- Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: a review. Food Res Int. 2007;40:1107–1121. doi: 10.1016/j.foodres.2007.07.004. [DOI] [Google Scholar]
- Granato D, Branco GF, Nazzaro F, Cruz AG, Faria JA. Functional foods and nondairy probiotic food development: trends, concepts, and products. Compr Rev Food Sci Food Saf. 2010;9(3):292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- Gunter EA, Ovodov YS. Effect of calcium, phosphate and nitrogen on cell growth and biosynthesis of cell wall polysaccharides by Silene vulgaris cell culture. J Biotechnol. 2005;117:385–393. doi: 10.1016/j.jbiotec.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Harbourne N, Marete E, Jacquier JC, O’Riordan D. Stability of phytochemicals as sources of anti-inflammatory nutraceuticals in beverages: a review. Food Res Inter. 2011;50(2):480–482. doi: 10.1016/j.foodres.2011.03.009. [DOI] [Google Scholar]
- Kao T, Chen B. Functional components in soybean cake and their effects on antioxidant activity. J Agric Food Chem. 2006;54:7544–7555. doi: 10.1021/jf061586x. [DOI] [PubMed] [Google Scholar]
- Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. Int Dairy J. 2003;16:945–960. doi: 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- Lee S, Lee H, Kima S, Lee J, Ha J, Gwak E, Oh MH, Park BY, Kimc JS, Choi KH, Yoon Y. Probabilistic models to describe the effect of NaNO2 in combination with NaCl on the growth inhibition of Lactobacillus in frankfurters. Meat Sci. 2015;110:302–309. doi: 10.1016/j.meatsci.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Limón RI, Peñas E, Torino MI, Martínez-Villaluenga C, Dueñas M, Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015;172:343–352. doi: 10.1016/j.foodchem.2014.09.084. [DOI] [PubMed] [Google Scholar]
- Mafart P, Couvert O, Gaillard S, Leguerinel I. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int J Food Microbiol. 2002;72:107–113. doi: 10.1016/S0168-1605(01)00624-9. [DOI] [PubMed] [Google Scholar]
- Mechmeche M, Kachouri F, Chouabi M, Ksonini H, Setti K, Hamdi M. Optimization of extraction parameters of protein isolate from tomato seed using response surface methodology. Food Anal Method. 2017;10:809–819. doi: 10.1007/s12161-016-0644-x. [DOI] [Google Scholar]
- Mechmeche M, Ksontini H, Hamdi M, Kachouri F. Production of bioactive peptides in tomato seed protein isolate fermented by water kefir culture: optimization of the fermentation conditions. Int J Pept Res Ther. 2017 [Google Scholar]
- Moayedi A, Hashemi M, Safari M. Valorization of tomato waste proteins through production of antioxidant and antibacterial hydrolysates by proteolytic Bacillus subtilis: optimization of fermentation conditions. J Food Sci Technol. 2016;53(1):391–400. doi: 10.1007/s13197-015-1965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandiyanto ABD, Okuyama K. Progress in developing spray-drying methods for the production of controlled morphology particles: from the nanometer to submicrometer size ranges. Adv Powder Technol. 2011;22:1–19. doi: 10.1016/j.apt.2010.09.011. [DOI] [Google Scholar]
- Nazzaro F, Fratianni F, Sada A, Orlando P. Synbiotic potential of carrot juice supplemented with Lactobacillus spp. and inulin or fructooligosaccharides. J Sci Food Agric. 2008;88(13):2271–2276. doi: 10.1002/jsfa.3343. [DOI] [Google Scholar]
- Phisut N. Spray drying technique of fruit juice powder: some factors influencing the properties of product. Int Food Res J. 2012;19(4):1297–1306. [Google Scholar]
- Piermaria JA, Pinotti A, Garcia MA, Abraham AG. Films based on kefiran, an expopolysaccharide obtained from kefir grain: development and characterization. Food Hydrocoll. 2009;23:684–690. doi: 10.1016/j.foodhyd.2008.05.003. [DOI] [Google Scholar]
- Prado FC, Parada JL, Pandey A, Soccol CR. Trends in non-dairy probiotic beverages. Food Res Int. 2008;41(2):111–123. doi: 10.1016/j.foodres.2007.10.010. [DOI] [Google Scholar]
- Randazzo W, Corona O, Guarcello R, Francesca N, Germanà MA, Erten H, Moschetti G, Settanni L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016;54:40–51. doi: 10.1016/j.fm.2015.10.018. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Bio Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues KL, Caputo LRG, Carvalho JCT, Evangelista J, Schneedorf JM. Antimicrobial and healing activity of kefir and kefiran extract. Int J Antimicrob Agents. 2005;25(5):404–408. doi: 10.1016/j.ijantimicag.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;3:49–56. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Savadkoohi S, Farahnaky A. Dynamic rheological and thermal study of the heat-induced gelation of tomato-seed proteins. J Food Eng. 2012;113:479–485. doi: 10.1016/j.jfoodeng.2012.06.010. [DOI] [Google Scholar]
- Sharma V, Mishra HN. Unstructured kinetic modeling of growth and lactic acid production by Lactobacillus plantarum NCDC 414 during fermentation of vegetable juices. LWT—Food Sci Technol. 2014;59:1123–1128. [Google Scholar]
- Sogi DS, Garg SK, Bawa AS. Functional properties of seed meals and protein concentrates from tomato-processing waste. J Food Sci. 2002;67:2997–3001. doi: 10.1111/j.1365-2621.2002.tb08850.x. [DOI] [Google Scholar]
- Tayuan C, Tannock GW, Rodtong S. Growth and exopolysaccharide production by Weissella sp. from low-cost substitutes for sucrose. Afr J Microbiol Res. 2011;5(22):3693–3701. [Google Scholar]
- Yoon KY, Woodams EE, Hang YD. Fermentation of beet juice by beneficial lactic acid bacteria. LWT—Food Sci Technol. 2005;38(1):73–75. [Google Scholar]
- Yoonm KY, Woodams EE, Hang YD. Production of probiotic cabbage juice by lactic acid bacteria. Bioresour Technol. 2006;97(12):1427–1430. doi: 10.1016/j.biortech.2005.06.018. [DOI] [PubMed] [Google Scholar]