Abstract
Effects of various sweeteners (sucrose, maltose syrup and honey) on individual anthocyanins, colour and turbidity in sour cherry (SCN) and strawberry nectars (SN) were investigated during 168 and 42 days of storage at 20 °C, respectively. In SCN, major anthocyanin was cyanidin-3-glucosylrutinoside (cyd-3-glu-rut), followed by cyanidin-3-rutinoside (cyd-3-rut), cyanidin-3-glucoside (cyd-3-glu) and cyanidin-3-sophoroside (cyd-3-soph). Maltose syrup increased stabilities of cyd-3-glu-rut (8%) and cyd-3-rut (4%), while honey reduced stabilities of all anthocyanins in SCN. Due to higher anthocyanin stability, maltose syrup for SCN was recommended. In SN, the major anthocyanin was pelargonidin-3-glucoside (pg-3-glu), followed by pelargonidin-3-rutinoside (pg-3-rut) and cyd-3-glu. Stabilities of anthocyanins (46–51%) and colour density (6–7%) in SN sweetened with honey were higher than that sweetened with sucrose and maltose syrup. Considering maximum wavelength (λmax), absorbance value at λmax (Amax), polymeric colour and colour density values together, copigmentation of anthocyanins occurred in SN sweetened with honey. This study is the first study showing copigmentation of anthocyanins with honey.
Keywords: Sweeteners, Copigmentation, Anthocyanin stability, Polymeric colour, Turbidity
Introduction
Nectars are produced from the unfermented but fermentable fruit purée, concentrated fruit purée and fruit concentrate by water addition with or without sugars, honey and/or syrups (Codex Alimentarius Commission 2005). In the world, the most common fruits used in nectar production are the apricots and peaches (Shahidi and Alasalvar 2016). However, SCN and SN, rich in anthocyanins, are also highly preferred nectars by consumers (Shahidi and Alasalvar 2016). Since the percentage of fruit ingredient in nectars is much lower (60–75%) than those of fruit juices, the amount of anthocyanins in nectars are also much lower than that in juices (Codex Alimentarius Commission 2005). Therefore, the degradation of the anthocyanins even in low quantities can cause the deterioration in nectars’ colour at significant level.
The main factors for anthocyanin degradation in fruit nectars are sugars and high storage temperatures (Tsai et al. 2004). In markets, fruit nectars are sold in the supermarkets mostly at room temperature (~ 20 °C) which can increase the degradation rate of anthocyanins (Iversen 1999). Thus, to minimize the colour deterioration in nectars due to anthocyanin degradation at room temperature, the type of sugar added to nectars should be carefully selected. Previous studies showed that sugars (sucrose, fructose, glucose and honey) had significant effects on the stabilities of anthocyanins in strawberries (Wrolstad et al. 1990), blood orange juice (Cao et al. 2009) or anthocyanin model systems (Tsai et al. 2004). However, to date, the effect of sugars on anthocyanins in SCN and SN during storage at 20 °C has not been investigated.
As compared with fruit juices, nectars have higher water contents (Codex Alimentarius Commission 2005) despite lower anthocyanin contents. And, the effects of sugars on anthocyanin stability depended on the content and availability of water in media, and the concentration and type of sugar (Tsai et al. 2004). While the increase in water availability led to the increase in anthocyanin degradation due to the nucleophilic attack of water, the increase in sugar (sucrose) content led to the decrease in anthocyanin degradation (Tsai et al. 2004) due to the reduction in water availability. Thus, the anthocyanins in the nectars are more likely to be exposed to water attack which converts the red flavylium ion into its colourless hemiacetal form. As a result, the colours of nectars more easily deteriorate than the colours of fruit juices.
Since sweeteners could show different effect on different types of anthocyanins, SCN (cyanidin-based anthocyanins) and SN (pelargonidin-based anthocyanins) containing different types of anthocyanins were selected in the present study. In the present study, the effects of sucrose, maltose syrup and honey on individual anthocyanins, colour density, polymeric colour, hyperchromic effect and bathochromic shift in SCN and SN were investigated during storage for the first time. Moreover, this study is the first study showing the copigmentation effect (which leads to increase in the stabilities of anthocyanins and colour density) of honey on anthocyanins.
Materials and methods
Chemical and reagents
Standards of anthocyanins were purchased from Sigma (St. Louis, MO, USA). All reagents used for liquid chromatography were HPLC grade and purchased from Merck (Darmstadt, Germany). All other reagents were analytical grade and obtained from Merck.
Sour cherry nectar (SCN)
SCN was prepared from the concentrate (65 °Bx) which was produced from Kütahya variety of sour cherries (Prunus cerasus L.) by Döhler Co. (Karaman, Turkey). The concentrate was stored at − 18 °C for 2 months prior to nectar preparation. SCN was prepared in accordance with General Standards for Fruit Juices and Nectars (Codex Alimentarius Commission 2005). In SCN, soluble solid content was 14 °Bx and fruit (juice) content was 35% (%, v:v).
Strawberry nectar (SN)
SN was also produced from the concentrate (65 °Bx) which was produced from Camarosa variety of strawberry (Fragaria × ananassa) by the same company (Döhler Co.). SN was also prepared in accordance with General Standards for Fruit Juices and Nectars (Codex Alimentarius Commission 2005). In SN, soluble solid content was 7.5 °Bx and fruit (juice) content was 40% (%, v:v).
Addition of sweeteners
In both nectars, sucrose, maltose syrup and honey were used as the sweeteners which were added to the nectars at the maximum concentration allowed by the Standard (Turkish Food Codex 2014). Sucrose, maltose syrup and honey were added at 20% by weight of the nectars (2 L). Since sucrose is the most preferred sweetener in the nectar production, nectars sweetened with sucrose was evaluated as “control group.”
Storage
SCN samples were stored at 20 ± 0.5 °C (Sanyo MIR 153 and 253, Gunma, Japan) for 168 days. Due to lower anthocyanin stability of SN, the SN samples were stored at the same temperature for 42 days.
pH and titratable acidity
Titration acidity was potentiometrically determined by the method proposed by IFU (1968). For this purpose, fruit nectar samples were titrated with 0.1 N adjusted NaOH solution until pH reaching to 8.1. Titration acidity of fruit nectars was calculated as “g/100 mL” in anhydrous citric acid.
Turbidity
Turbidity of the samples was determined by a turbidimeter (HACH 2100N, Loveland, CO, USA).
Anthocyanin profile
Purification
Anthocyanins in nectars were purified on a C18 cartridge (Waters Co., Milford, MA, USA) following the method described by Lee and Wrolstad (2004). All details of the purification were given in our previous study (Navruz et al. 2016).
Identification of anthocyanins
Anthocyanins in SCN were identified by LC–MS–MS (Thermo Scientific UltiMate 3000 Dionex, Sunnyvale, CA, USA) equipped with an ESI source. Mass/charge (m/z) ratios revealed by Bonerz et al. (2007) were used to identify the anthocyanins in the samples. Mass spectrometric analysis of the anthocyanins yielded molecular ions M+ at m/z 595 (cyd-3-rut), 611 (cyd-3-soph) and 757 (cyd-3-glurut). Anthocyanins were separated with an Inertsil ODS-4 reverse-phase column (2.1 mm × 50 mm, 2 μm) (GL Sciences, Inc., Tokyo, Japan) at 30 °C with a flow rate of 0.3 mL/min. The mobile phase consisted of 1% formic acid in water (eluent A, v/v) and 1% formic acid in methanol (eluent B, v/v). The other details of the analysis were given in our previous study (Navruz et al. 2016).
Anthocyanins in SN were identified by HPLC (Agilent 1200 series, Waldbronn, Germany) equipped with a photodiode array (PDA) detector, a binary pump, a degasser, a thermostatted autosampler and column compartment. The anthocyanins were separated on a C18 column (250 × 4.6 mm) (Phenomenex, Inc., Los Angeles, CA, USA) with a C18 (5 µm) guard column (4 × 3 mm, 5 µm) (Phenomenex, Inc.). The eluents used were (A) 100% acetonitrile and (B) O-phosphoric acid, acetic acid, acetonitrile and water (1:10:5:84; v/v/v/v) with a flow rate of 1 mL/min. The other details of the analysis were given in our previous study (Turfan et al. 2011). Identification of anthocyanins in SNs was carried out by comparing retention times and absorption spectra of unknown peaks, and spiking of external reference standards.
Changes in the contents of individual anthocyanins in SCN and SN during storage were determined by the HPLC method detailed above. Quantification of anthocyanins in SN was carried out using calibration curves of the following external reference standards: cyd-3-glu (0–30 mg/kg, y = 8.3723x + 4.0419, R2 = 0.999), pg-3-glu (0–200 mg/kg, y = 118.7389x − 980.5169, R2 = 0.999) and pg-3-rut (0–200 mg/kg, y = 119.7389x − 980.5169, R2 = 0.999), while contents of all individual anthocyanins in SCN were expressed as cyd-3-glu (0–200 mg/kg, y = 150.42x − 207.2, R2 = 0.999). The calibration curves for each anthocyanin standard contained 7 data points. The amount of the anthocyanins was calculated taking into account of recovery values (89–102%).
Limit of detection (LOD) and quantification (LOQ)
The limit of detection (LOD) and limit of quantification (LOQ) for anthocyanins were determined based on signal to noise (S/N) ratio. According to ICH guideline for the validation of analytical procedures, an acceptable S/N is 3:1 (or 2:1) for estimating the LOD and 10:1 for estimating the LOQ (Li et al. 2002).
Polymeric colour and colourimetric measurement
Bisulfite bleaching method was used to determine the percent polymeric colour and colour density (Giusti and Wrolstad 2005). Bathochromic effect, i.e., a shift in maximum wavelength (λmax, nm), and hyperchromic effect, i.e., an increase in the absorbance value at λmax, were determined on a UV–VIS double-beam spectrophotometer (ThermoScientific Evolution 201, Cambridge, England) with 1 cm path length disposable cuvettes (Brand Gmbh, Wertheim, Germany). Bathochromic and hyperchromic effects were evaluated as the indicators of copigmentation (Navruz et al. 2016).
All measurements were replicated two times.
Calculation of kinetic data
The reaction rate constants (k) and the half-life periods (t1/2) were calculated using the following equations:
where Co is the initial content of anthocyanins or initial values of colour properties (polymeric colour, colour density, Amax and λmax) in samples, Ct is anthocyanin content and colour properties after t min exposure of the samples to 20 °C (T).
Statistical analyses
Minitab statistical software [version 14 (Minitab Inc., State College, PA, USA)] was used for data analysis. Type of anthocyanins, colour properties and storage time were the main variables considered. Statistical differences among means were determined by the Duncan’s multiple range test at the 1 and 5% significance level.
Results and discussion
Titratable acidity and pH values of SCN and SN
Titratable acidity and pH of SCN sweetened with sucrose were 0.69 g/100 mL and 3.21, respectively (Table 1). While sweeteners and storage did not have significant effect on the pH (3.21–3.25) of SCNs and titratable acidities (0.32–0.39 g/100 mL) of SNs (p > 0.01), sweeteners and storage led to the changes in the pH (3.37–3.57) of SNs and titratable acidities (0.67–0.74 g/100 mL) of SCNs (p < 0.01).
Table 1.
pH, titratable acidity and turbidity values of SCN and SN
| Nectars | Storage | pH | Titratable acidity (%) | Turbidity (NTU) |
|---|---|---|---|---|
| SCN | ||||
| Sucrose | Before storage | 3.21 ± 0.00Aa | 0.69 ± 0.00Aab | 2.95 ± 0.01Bb |
| After storage | 3.25 ± 0.01Aa | 0.69 ± 0.00Aa | 5.15 ± 0.35Ab | |
| Maltose syrup | Before storage | 3.22 ± 0.00Aa | 0.67 ± 0.00Ab | 1.10 ± 0.00Bc |
| After storage | 3.25 ± 0.01Aa | 0.68 ± 0.00Aa | 3.65 ± 0.35Ac | |
| Honey | Before storage | 3.25 ± 0.01Aa | 0.74 ± 0.08Aa | 79.35 ± 0.92Ba |
| After storage | 3.25 ± 0.01Aa | 0.68 ± 0.01Ba | 154.50 ± 0.71Aa | |
| SN | ||||
| Sucrose | Before storage | 3.46 ± 0.01Aa | 0.38 ± 0.00Aa | 27.60 ± 0.02Bb |
| After storage | 3.44 ± 0.00Ab | 0.39 ± 0.00Aa | 58.30 ± 0.25Ab | |
| Maltose syrup | Before storage | 3.37 ± 0.01Ab | 0.37 ± 0.00Aa | 18.00 ± 0.13Bc |
| After storage | 3.45 ± 0.01Bb | 0.38 ± 0.01Aa | 49.50 ± 0.52Ac | |
| Honey | Before storage | 3.47 ± 0.00Aa | 0.32 ± 0.01Ab | 158.00 ± 0.23Ba |
| After storage | 3.57 ± 0.01Ba | 0.35 ± 0.01Aa | 194.50 ± 0.54Aa | |
A, B: Values with different letters within columns indicate significant difference (p < 0.01) between storage times
a, b: Values with different letters within columns indicate significant difference (p < 0.01) between sweeteners
Characterization of anthocyanins in SCN and SN and stabilities of anthocyanins during storage
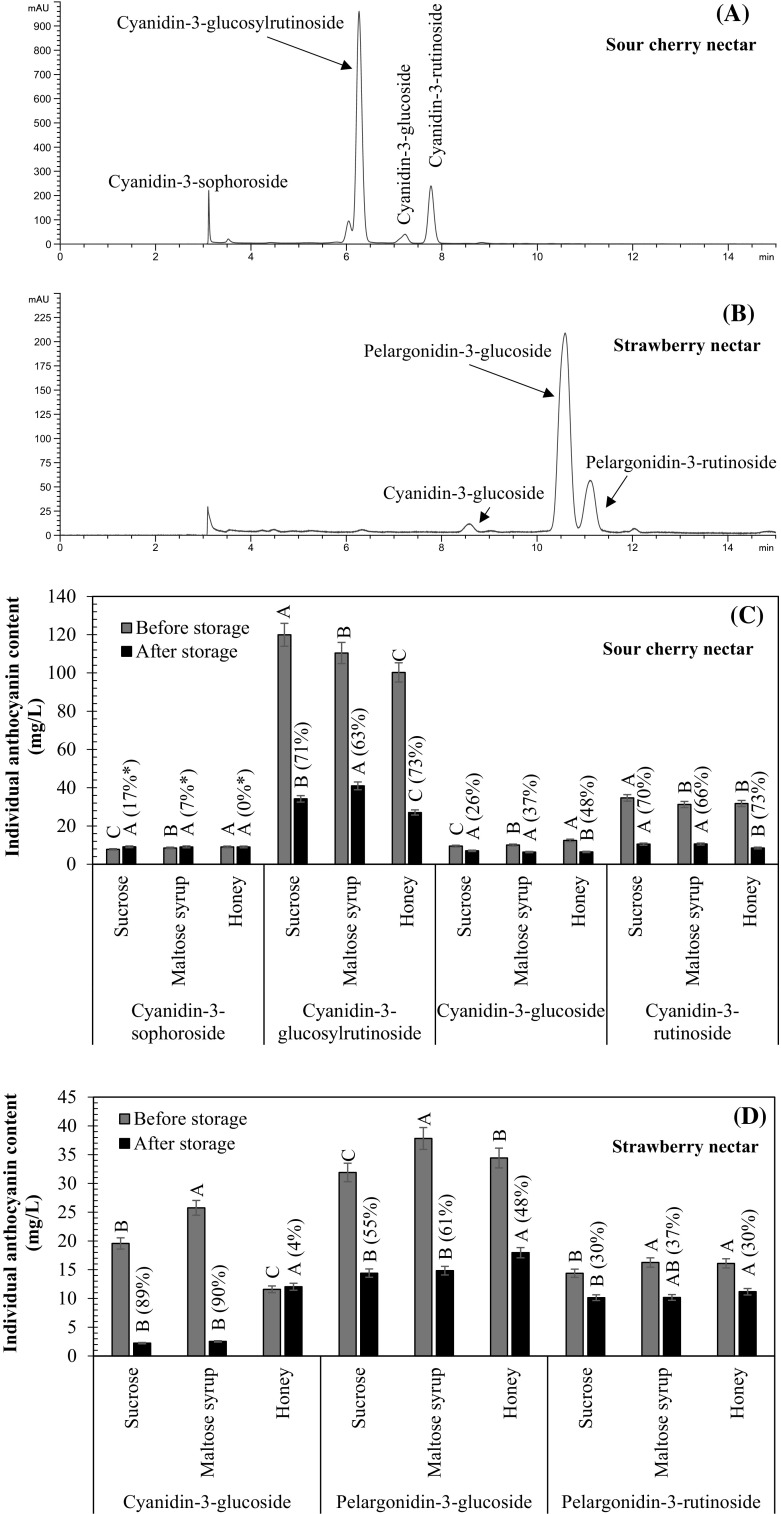
The anthocyanin profiles of SCN and SN sweetened with sucrose before storage are presented in Fig. 1A, B. Major anthocyanin in SCN was identified as cyd-3-glu-rut (58.7%), followed by cyd-3-rut (16.3%), cyd-3-glu (4.7%) and cyd-3-soph (3.4%). Similar anthocyanin profile was also reported in sour cherry juice (Bonerz et al. 2007; Obon et al. 2011) and sour cherry concentrate (Navruz et al. 2016). Different from SCN, three anthocyanins were identified in SN as pg-3-glu (49.1%), pg-3-rut (14.0%) and cyd-3-glu (3.0%). The same anthocyanins were identified in strawberries (Oszmiański and Wojdyło 2009).
Fig. 1.
Anthocyanin profiles and individual anthocyanins in SCN (A, C) and SN (B, D). A–C Values with different letters within columns indicate significant difference between sugars. *Values on the figures show the increase (in %) in the content of anthocyanin. The other values on the figures show the anthocyanin degradation in %
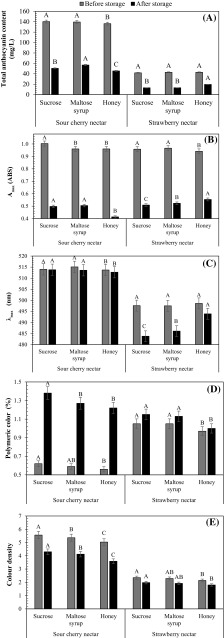
The total monomeric anthocyanin contents of SCN and SN sweetened with sucrose were 140.3 mg cyd-3-glu/L and 41.8 mg pg-3-glu/L (Fig. 2A). The changes in total monomeric anthocyanins of these nectars stored at 20 °C were given in Fig. 2A. The degradation of anthocyanins in these nectars during storage was fitted to first-order reaction model (R2 = 0.9746–9999; figure not shown). The calculated k and t1/2 values were given in Table 2. The t1/2 values of the SCNs were higher (1.7–4.2 times) than those of SNs. As explained before, the major anthocyanidin in anthocyanins of SCN is cyanidin, while that in SN is pelargonidin. Thus, higher anthocyanin stability in SCN was due to higher stability of cyanidin than that of pelargonidin at pH 3.0–3.5 (Cabrita et al. 2000).
Fig. 2.
Effect of storage on total monomeric anthocyanin content (A), Amax (B), λmax (C), polymeric colour (D) and colour density (E) in SCN and SN. A–C Values with different letters indicate significant difference between sugars
Table 2.
Kinetic parameters for the degradation of anthocyanins, reductions in Amax, λmax and colour intensity and formations of polymeric colour and turbidity in samples during storage
| Kinetic data | SCN | SN |
|---|---|---|
| Anthocyanin degradation during storage | ||
| Sucrose | ||
| − k × 103 (1/day) | 5.99 | 26.5 |
| t1/2 (days) | 115.8 | 26.2 |
| Maltose syrup | ||
| − k × 103 (1/day) | 5.30 | 27.4 |
| t1/2 (days) | 130.8 | 25.3 |
| Honey | ||
| − k × 103 (1/day) | 6.68 | 18.2 |
| t1/2 (days) | 103.8 | 38.1 |
| Reduction in A max values | ||
| Sucrose | ||
| − k × 103 (1/day) | 1.80 | 6.40 |
| t1/2 (days) | 385 | 108 |
| Maltose syrup | ||
| − k × 103 (1/day) | 1.60 | 6.20 |
| t1/2 (days) | 433 | 112 |
| Honey | ||
| − k × 103 (1/day) | 2.10 | 5.20 |
| t1/2 (days) | 330 | 133 |
| Reduction in λmax values | ||
| Sucrose | ||
| − k × 103 (1/day) | – | 0.30 |
| t1/2 (days) | – | 2310 |
| Maltose syrup | ||
| − k × 103 (1/day) | – | 0.20 |
| t1/2 (days) | – | 3466 |
| Honey | ||
| − k × 103 (1/day) | – | 0.10 |
| t1/2 (days) | – | 6931 |
| Increase in polymeric colour | ||
| Sucrose | ||
| k × 103 (1/day) | 4.80 | 2.50 |
| Maltose syrup | ||
| k × 103 (1/day) | 4.40 | 2.50 |
| Honey | ||
| k × 103 (1/day) | 4.60 | 1.10 |
| Reduction in colour density | ||
| Sucrose | ||
| − k × 103 (1/day) | 1.60 | 3.09 |
| t1/2 (days) | 433 | 224 |
| Maltose syrup | ||
| − k × 103 (1/day) | 1.80 | 3.07 |
| t1/2 (days) | 385 | 225 |
| Honey | ||
| − k × 103 (1/day) | 2.80 | 2.89 |
| t1/2 (days) | 247 | 240 |
| Increase in turbidity | ||
| Sucrose | ||
| k × 103 (1/day) | 4.14 | 15.2 |
| Maltose syrup | ||
| k × 103 (1/day) | 6.68 | 22.8 |
| Honey | ||
| k × 103 (1/day) | 3.91 | 12.4 |
In SCN sweetened with sucrose, cyd-3-soph and cyd-3-glu had the highest stability during storage (Fig. 1C). However, since sophoroside is a disaccharide formed from two glucose units, cyd-3-soph showed higher stability than cyd-3-glu. Similar to the effect of the number of sugar residues on anthocyanin stability in SCN, increase in the number of sugar residues also led to the increase in anthocyanin stability in SN. When this nectar was sweetened with sucrose, pg-3-rut and pg-3-glu showed the highest stability. As known, rutinose is a disaccharide formed from glucose and rhamnose. Thus, the presence of rhamnose as well as glucose in pelargonidin based anthocyanins increased the stability of anthocyanins during storage at 20 °C.
When SCN was sweetened with maltose syrup, the stabilities of cyd-3-glu-rut (8%) and cyd-3-rut (4%) increased, while the stability of cyd-3-glu (11%) decreased (Fig. 1C). Moreover, the use of maltose syrup as a sweetener led to increase (13%) in the stability of total monomeric anthocyanins (Table 2). These results clearly indicated that maltose syrup led to increase in the stability of cyanidin based anthocyanins containing rutinoside as a sugar residue during storage at 20 °C. Unlike the stabilizing effect of rutinoside on cyanidin based anthocyanins in SCN, the highest reduction in the stability of pelargonidin based anthocyanins in SN was determined in pg-3-rut (7%) (Fig. 1D). Thus, maltose syrup may be preferred for high anthocyanin stability in the presence of the anthocyanins containing cyanidin and rutinoside. On the contrary, maltose syrup should be avoided in the presence of the anthocyanins containing pelargonidin. Maltose syrup led to slight (3%) reduction in the anthocyanin stability of SN when compared with that of the sample sweetened with sucrose. Similarly, in a previous study (Torreggiani et al. 1999), anthocyanin content of “glucose, fructose and sucrose-containing strawberry juice” was only 4% higher than that of “maltose-containing strawberry juice” stored at − 10 °C for 8 months.
Studies indicated that maltose and sucrose had protective effect on anthocyanins (Lewis et al. 1995; Tsai et al. 2004). However, the results of the present study showed that the level of their protective effect depended on the individual anthocyanins, media (nectar type) and storage time. While anthocyanin stability in SCN sweetened with maltose syrup was higher than that in the same nectar sweetened with sucrose, the stability was higher when SN was sweetened with sucrose as compared to the same nectar sweetened with maltose syrup. The differences among anthocyanin stabilities of these nectars could have resulted from sucrose degradation products, especially fructose.
Dawber et al. (1966) showed that acid-catalyzed hydrolysis of sucrose at 20 °C is a unimolecular process which consists of two steps. Sucrose degradation products slowly form in the second step. Thus, the increase in storage time at 20 °C probably led to the increase in the contents of sucrose degradation products and as a result of this increase, anthocyanin stabilities in the nectars, whose pH values ranged from 3.37 to 3.54, decreased. Due to lower anthocyanin stability of strawberries (t1/2 values ranged from 25.3 to 38.1 days, Table 2), the SN could be stored at 20 °C for only 42 days. However, SCN (t1/2 values ranged from 103.8 to 130.8 days, Table 2) could be stored at the same temperature for as long as 168 days. Thus, due to longer storage time, the contents of sucrose degradation products (glucose and fructose) in SCN might be higher than those in SN. Since fructose had much more degradation effect on anthocyanins as compared to glucose (Kopjar and Piližota 2011), anthocyanin stability in SCN sweetened with sucrose could be lower than that sweetened with maltose syrup despite the opposite effects of these sweeteners on anthocyanin stability in SN. In fact, that the anthocyanin stabilities in SCN and SN after storage at 20 °C for 42 days was the same also confirmed this finding (data not shown).
Honey had also significant effect on anthocyanins of both nectars. Among the sweeteners used, the highest anthocyanin stability was observed in SN sweetened with honey, although honey cause a decrease (10%) in the anthocyanin stability of SCN when compared with the anthocyanin stabilities in the nectars sweetened with sucrose (Table 2). Total monomeric anthocyanin stability in SN sweetened with honey was 46% higher than that sweetened with sucrose. At the end of 42 days of storage at 20 °C, honey led to the highest contents and stabilities of individual anthocyanins in SN (Table 2). While the use of sucrose (89%) and maltose syrup (90%) decreased the content of cyd-3-glu in SN, honey protected this anthocyanin during storage and no significant change (4%) was detected in this anthocyanin content at the end of storage period (Fig. 1D). Moreover, honey led to the increase (7%) in the stability of pg-3-glu which is the major anthocyanin of SN (Fig. 1D). This may be due to copigmentation effects of phenolics [caffeic acid, benzoic acid, gallic acid, chlorogenic acid, trans-cinnamic acid, kaempferol and catechin (Moniruzzaman et al. 2014)], amino acids [proline, phenylalanine, tyrosine, lysine, arginine, glutamic acid, histidine and valine (Hermosín et al. 2003)] and/or organic acids [malic, maleic, citric, succinic and fumaric acids (Suarez-Luque et al. 2002)] in honey on cyd-3-glu and pg-3-glu in SN. In fact, previous studies (Eiro and Heinonen 2002; Sun et al. 2010) indicated that gallic acid, caffeic acid and chlorogenic acid, which are also present in honey, had copigmentation effects on pg-3-glu and cyd-3-glu. Moreover, changes in Amax and λmax values of SN during storage also confirmed the copigmentation effect of honey on anthocyanins in SN (Table 2 and Fig. 2B, C). The changes in these values were explained in “Effect of sweeteners on enhancement of colour” section in detail.
Different from the copigmentation effect of honey on anthocyanins in SN, honey did not show copigmentation effect on anthocyanins in SCN. All individual anthocyanins in SCN sweetened with honey showed the lowest stabilities (Table 2). Honey increased the degradation rate of anthocyanins (10–21%) in SCN. As known, copigmentation effect of a copigment depended on many factors such as anthocyanin type, ratio of anthocyanin to copigment, pH and media. The results of the present study showed that the contents and type of anthocyanins in SCN were not suitable for copigmentation with the copigments present in honey. Degradation effect of honey on anthocyanins in SCN could be attributed to: 1) degradation effect of fructose in honey on anthocyanins, 2) HMF formation especially from hexoses in honey. Honey contains fructose (38.2%), glucose (31.3%), maltose (7.3%) and sucrose (1.3%) (Manyi-Loh et al. 2011). As the storage time at 20 °C increased, HMF contents of honey also increased (Bulut and Kılıç 2009). Since HMF accelerates the degradation rate of anthocyanins (Boranbayeva et al. 2014), anthocyanin stability in SCN sweetened with honey could be the lowest during storage at 20 °C. Moreover, the results of the present study showed that copigmentation could have protected the anthocyanins in SN from the degradation effect of HMF, resulting from storage, as well as high fructose content of honey.
Effect of sweeteners on enhancement of colour
Changes in λmax (bathochromic shift) and the absorbance value at the λmax (Amax, hyperchromic effect) of SCN and SN sweetened with sucrose, maltose syrup and honey were investigated during storage at 20 °C (Fig. 2B–C). Sweeteners showed significant effect on Amax values of both nectars (p < 0.05). Before storage, the highest Amax values were found in SCN (1.023 ABS) and SN (0.986 ABS) sweetened with sucrose and maltose syrup, respectively. Storage led to the reduction in Amax value, regardless of the sweeteners used. During storage, the effect of sweeteners on anthocyanins showed differences. The lowest k value (1.6 × 10−3 1/day) for the reduction in Amax value during storage was found in SCN sweetened with maltose syrup. Reduction effect of sucrose on Amax value of SCN accelerated after 140 days of storage. The highest Amax value was found in SCN sweetened with maltose syrup, followed by sucrose and honey (data not shown) after 140 days of storage at 20 °C.
Unlike SCN, although the lowest Amax values were determined in the SN sweetened with honey until 7th day of storage at 20 °C, the highest Amax values in the same sample was determined after 21th day of storage at the same temperature. Thus, copigmentation effect of copigments in honey on anthocyanins of SN appeared at a significant level after 21th day of storage and the effects continued until the end of 42 days of storage. When storage ended, Amax value (0.574) of SN sweetened with honey was 8.5 and 5.7% higher than those of SNs sweetened with sucrose (0.529) and maltose syrup (0.543), respectively (Fig. 2B). In fact, t1/2 value (133 days) for Amax values of SN sweetened with honey was also higher than those sweetened with sucrose (23%) and maltose syrup (18%) (Table 2).
When the changes in λmax values of the samples were investigated (Fig. 2C), sucrose, maltose syrup and honey did not show significant effect on the λmax values of SCNs during storage. However, in SNs, the sweeteners caused the significant changes in λmax values. During storage, honey provided the highest λmax values. At the end of 42 days of storage, λmax value (493.9 nm) of SN sweetened with honey was 10.1 nm and 7.8 nm higher than those sweetened with sucrose and maltose syrup, respectively. Moreover, the highest and lowest stabilities for λmax values were determined in SN sweetened with honey (t1/2 = 6931 days, Table 2) and sucrose (t1/2 = 2310 days, Table 2), respectively. Thus, when the changes in Amax and λmax values are considered together, the results clearly indicated that while copigments in honey showed copigmentation effect on the anthocyanins in SN, no copigmentation effect was detected by the sweeteners used on the anthocyanins in SCN.
Effects of sweeteners on colour density and polymeric colour
Sweeteners also showed significant effect on the colour density and polymeric colour of SCN (p < 0.05). Before and after storage, the highest colour density and polymeric colour values were achieved in SCN sweetened with sucrose, while the lowest values were found in SCN sweetened with honey (Fig. 2D, E). During storage, colour density values of all samples decreased gradually. The reduction in colour density was fitted to first-order reaction kinetics. Sucrose (75%) and maltose syrup (56%) led to higher stability for colour density in SCN than honey. These results clearly showed that honey should not be preferred as a sweetener during the production of SCN to minimize the reduction in colour density. Moreover, polymeric colour formation rate (4.6 × 10−3 1/day, Table 2) in SCN sweetened with honey was found higher (4%) than that with maltose syrup, although the highest polymeric colour formation rate (4.8 × 10−3 1/day, Table 2) was found in the SCN sweetened with sucrose. As known, the increases in polymeric colour values of the samples could result from anthocyanin polymerization, copigmentation or anthocyanin degradation (Kopjar and Piližota 2011).
To explain the reason for the increases in the polymeric colour values, the relationships between polymeric colour and Amax, and monomeric anthocyanin contents were investigated. Strong negative correlations between polymeric colour and Amax (r = − 0.978 − (−) 0.987), and monomeric anthocyanin contents (r = –0.975 − (−)0.981) were found. Moreover, no significant changes were detected in λmax values of SCN during storage. When these results were evaluated together, the reason for polymeric colour formation in SCN could have been attributed to anthocyanin degradation, regardless of the sweeteners used.
In SN, honey resulted in the lowest polymeric colour and colour density values before storage (Fig. 2D). During storage, the slowest polymeric colour formation and the highest stability for colour density were achieved in the SN sweetened with honey (Table 2). The stability of colour density in SN sweetened with honey was 7% higher than SN sweetened with sucrose. Although no correlations were detected between colour density and λmax values of the SNs sweetened with sucrose and maltose syrup, good positive correlation (r = 0.740) was found between these values of the SN sweetened with honey. However, in all SNs, strong negative correlations (r = –0.882 − (−)0.906) were detected between the percentage of polymeric colour and λmax values. The high correlation coefficients indicated that changes in λmax values and polymeric colour values of the SN sweetened with sucrose and maltose syrup could have resulted from anthocyanin degradation. However, the changes in λmax values and polymeric colour values of the SN samples sweetened with honey led to the increase in colour density and thus the changes in λmax and polymeric colour values should have resulted from copigmentation.
Effects of sweeteners on turbidity during storage
The effects of sweeteners on the turbidity of the nectars were also investigated (Table 1). When sucrose was used as a sweetener, the turbidity of SCN was 2.95 NTU. However, this value was 27.60 NTU in SN. The turbidity of all SNs were higher than those in SCNs. In both nectars, maltose syrup led to the lowest (1.10–18 NTU) turbidity values, while honey led to the highest (79.35–158 NTU) turbidity values.
During storage, the turbidity values of all nectar samples increased. The increase in turbidity during storage at 20 °C was fitted to a first-order reaction model. The highest k values (6.68 × 10−3 1/day for SCN; 22.8 × 10−3 1/day for SN) for turbidity formation were determined in the nectars sweetened with maltose syrup (Table 2). The use of honey as a sweetener resulted in the reduction in k values for turbidity formation. The lowest k values for turbidity formation were found in the SN sweetened with honey (3.91 × 10−3 1/day for SCN; 12.4 × 10−3 1/day for SN).
Conclusion
Sucrose, maltose syrup and honey significantly affected the anthocyanin stability, colour density and turbidity formation in both SCN and SN during 168 and 42 days of storage at 20 °C, respectively. When maltose syrup is used as a sweetener, the stabilities at 20 °C of cyd-3-glu-rut and cyd-3-rut, which are the major anthocyanins in SCN, increased. Thus, maltose syrup should be preferred for the highest anthocyanin stability in SCN. Different from SCN, honey provided the highest stabilities for both pg-3-glu, which is the major anthocyanin in SN, and cyd-3-glu in SN. Moreover, at the end of storage, the highest Amax and λmax values as well as the highest stabilities of colour density and monomeric anthocyanins were determined in SN sweetened with honey. All results showed that honey had copigmentation effect on anthocyanins in SN. As compared with sucrose and maltose syrup, honey caused a decrease in the content of cyd-3-glu before storage. This was probably due to the copigmentation between copigments in honey and cyd-3-glu in SN before storage. However, further studies are needed to explain which copigments in honey reacted with anthocyanins in SN. Although turbidity values of both nectars sweetened with honey were very high (79–158 NTU), honey should be preferred as a sweetener in SN to increase the anthocyanin stability and colour density. As known, turbidity is not a significant quality criteria for SN produced from purée.
Acknowledgements
This study is in part of Miss Ertan’s master thesis and was funded by “The Scientific and Technological Research Council of Turkey” (Grant # 213O249). The authors would like to thank Mrs. Ayşe Navruz, M.S., for helping in the preparation of phenolic extracts.
References
- Bonerz D, Würth K, Dietrich H, Will F. Analytical characterization and the impact of ageing on anthocyanin composition and degradation in juices from five sour cherry cultivars. Eur Food Res Technol. 2007;224:355–364. doi: 10.1007/s00217-006-0328-7. [DOI] [Google Scholar]
- Boranbayeva T, Karadeniz F, Yılmaz E. Effect of storage on anthocyanin degradation in black mulberry juice and concentrates. Food Bioprocess Tech. 2014;7:1894–1902. doi: 10.1007/s11947-014-1296-8. [DOI] [Google Scholar]
- Bulut L, Kılıç M. Kinetics of hydroxymethylfurfural accumulation and colour change in honey during storage in relation to moisture content. J Food Process Preserv. 2009;33:22–32. doi: 10.1111/j.1745-4549.2008.00233.x. [DOI] [Google Scholar]
- Cabrita L, Frøystein NÅ, Andersen ØM. Anthocyanin trisaccharides in blue berries of Vaccinium padifolium. Food Chem. 2000;69:33–36. doi: 10.1016/S0308-8146(99)00230-7. [DOI] [Google Scholar]
- Cao S, Liu L, Lu Q, Xu Y, Pan S, Wang K. Integrated effects of ascorbic acid, flavonoids and sugars on thermal degradation of anthocyanins in blood orange juice. Eur Food Res Technol. 2009;228:975–3983. doi: 10.1007/s00217-009-1015-2. [DOI] [Google Scholar]
- Codex Alimentarius Commission (2005) General standards for fruit juices and nectars. http://www.fao.org/fao-who-codexalimentarius/shproxy/zh/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B2472005%252FCXS_247e.pdf. Accessed 1 Feb 2018
- Dawber JG, Brown DR, Reed RA. Acid-catalyzed hydrolysis of sucrose: a student study of a reaction mechanism. J Chem Educ. 1966;43:34–35. doi: 10.1021/ed043p34. [DOI] [Google Scholar]
- Eiro MJ, Heinonen M. Anthocyanin color behavior and stability during storage: effect of intermolecular copigmentation. J Agric Food Chem. 2002;50:7461–7466. doi: 10.1021/jf0258306. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. In: Handbook of food analytical chemistry. Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P, editors. New York: Wiley; 2005. [Google Scholar]
- Hermosín I, Chicón RM, Cabezudo MD. Free amino acid composition and botanical origin of honey. Food Chem. 2003;83:263–268. doi: 10.1016/S0308-8146(03)00089-X. [DOI] [Google Scholar]
- IFU Analysen . Nr: 3 (Determination of titratable acidity) Zurich: Internationale Fruchtsaft Union Juris Verlag; 1968. [Google Scholar]
- Iversen CK. Black currant nectar: effect of processing and storage on anthocyanin and ascorbic acid content. J Food Sci. 1999;64:37–41. doi: 10.1111/j.1365-2621.1999.tb09856.x. [DOI] [Google Scholar]
- Kopjar M, Piližota V. Prevention of thermal degradation of anthocyanins in blackberry juice with addition of different sugars. CyTA-J Food. 2011;9:237–242. doi: 10.1080/19476337.2010.522735. [DOI] [Google Scholar]
- Lee J, Wrolstad RE. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J Food Sci. 2004;69:564–573. doi: 10.1111/j.1365-2621.2004.tb13651.x. [DOI] [Google Scholar]
- Lewis CE, Walker JR, Lancaster JE. Effect of polysaccharides on the colour of anthocyanins. Food Chem. 1995;54:315–319. doi: 10.1016/0308-8146(95)00026-F. [DOI] [Google Scholar]
- Li TL, Chung-Wang YJ, Shih TC. Determination and confirmation of chloramphenicol residues in swine muscle and liver. J Food Sci. 2002;67:21–28. doi: 10.1111/j.1365-2621.2002.tb11352.x. [DOI] [Google Scholar]
- Manyi-Loh CE, Ndip RN, Clarke AM. Volatile compounds in honey: a review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int J Mol Sci. 2011;12:9514–9532. doi: 10.3390/ijms12129514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman M, Yung An C, Rao PV, Hawlader MNI, Azlan SABM, Sulaiman SA, Gan SH. Identification of phenolic acids and flavonoids in monofloral honey from Bangladesh by high performance liquid chromatography: determination of antioxidant capacity. Biomed Res Int. 2014;2014:1–11. doi: 10.1155/2014/737490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navruz A, Türkyılmaz M, Özkan M. Colour stabilities of sour cherry juice concentrates enhanced with gallic acid and various plant extracts during storage. Food Chem. 2016;197:150–160. doi: 10.1016/j.foodchem.2015.10.098. [DOI] [PubMed] [Google Scholar]
- Obon JM, Diaz-Garcia MC, Castellar MR. Red fruit juice quality and authenticity control by HPLC. J Food Compost Anal. 2011;6:760–771. doi: 10.1016/j.jfca.2011.03.012. [DOI] [Google Scholar]
- Oszmiański J, Wojdyło A. Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur Food Res Technol. 2009;228:623–631. doi: 10.1007/s00217-008-0971-2. [DOI] [Google Scholar]
- Shahidi F, Alasalvar C. Handbook of functional beverages and human health. Boca Raton, FL: CRC Press; 2016. [Google Scholar]
- Suarez-Luque S, Mato I, Huidobro JF, Simal-Lozano J, Sancho MT. Rapid determination of minority organic acids in honey by high-performance liquid chromatography. J Chromatogr A. 2002;955:207–214. doi: 10.1016/S0021-9673(02)00248-0. [DOI] [PubMed] [Google Scholar]
- Sun J, Cao X, Liao X, Hu X. Comparative analyses of copigmentation of cyanidin 3-glucoside and cyanidin 3-sophoroside from red raspberry fruits. Food Chem. 2010;120:1131–1137. doi: 10.1016/j.foodchem.2009.11.031. [DOI] [Google Scholar]
- Torreggiani D, Forni E, Guercilena I, Maestrelli A, Bertolo G, Archer GP, Champion D. Modification of glass transition temperature through carbohydrates additions: effect upon colour and anthocyanin pigment stability in frozen strawberry juices. Food Res Int. 1999;32:441–446. doi: 10.1016/S0963-9969(99)00106-4. [DOI] [Google Scholar]
- Tsai PJ, Hsieh YY, Huang TC. Effect of sugar on anthocyanin degradation and water mobility in a roselle anthocyanin model system using 17O NMR. J Agric Food Chem. 2004;52:3097–3099. doi: 10.1021/jf0306587. [DOI] [PubMed] [Google Scholar]
- Turfan Ö, Türkyılmaz M, Yemiş O, Özkan M. Anthocyanin and colour changes during processing of pomegranate (Punica granatum L., cv. Hicaznar) juice from sacs and whole fruit. Food Chem. 2011;129:1644–1651. doi: 10.1016/j.foodchem.2011.06.024. [DOI] [Google Scholar]
- Turkish Food Codex (Türk Gıda Kodeksi) (2014) Turkish food codex standards for fruit juices and similar products (Meyve suyu ve benzeri ürünler tebliği, Tebliğ No: 2014/34)
- Wrolstad RE, Skrede G, Lea PER, Enersen G. Influence of sugar on anthocyanin pigment stability in frozen strawberries. J Food Sci. 1990;55:1064–1065. doi: 10.1111/j.1365-2621.1990.tb01598.x. [DOI] [Google Scholar]