Abstract
Enzymatic browning is a major factor affecting the quality of sugarcane juice, mainly due to the activities of polyphenol oxidase (PPO) and peroxidase (POD). Effect of bentonite (0–1%, w/v) on the activities of these enzymes, when employed alone and also in combination with acidulants, was determined. Bentonite alone could reduce the activities of PPO and POD enzymes to 160 and 24.2 u/mL, respectively. The PPO and POD activity was completely inhibited below pH 4.1 when ascorbic acid was used alone or in combination with bentonite. However, PPO and POD activity was inhibited to 60 and 51 u/mL, respectively, at pH 3.7 when citric acid was used individually and to 112 and 15.36 u/mL, respectively, when employed along with bentonite. In addition, color changes at 4 and 10 °C were measured during the storage of sugarcane juice.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3350-4) contains supplementary material, which is available to authorized users.
Keywords: Sugarcane juice, Bentonite, Ascorbic acid, Citric acid, Polyphenol oxidase (PPO) and peroxidase (POD), Browning
Introduction
Sugarcane (Saccharum officinarum L.) is a commercial crop, mostly used for manufacturing of jaggery and crystallized sugars. Fresh extracted sugarcane juice is gaining popularity as a delicious beverage. Sugarcane is cultivated in more than 110 countries in tropical and subtropical regions of the world. Sugarcane juice contains water (75–85%), non-reducing sugars (sucrose, 10–21%), reducing sugars (glucose and fructose, 0.3–3%), organic substances (0.5–1%), inorganic substances (0.2–0.6%) and nitrogenous bodies (0.5–1%). Sugarcane juice of 100 mL provides 40 kcal of energy, 10 mg of iron and 6 µg of carotene. It is rich in enzymes and has many medicinal properties (Krishnakumar and Devadas 2006), often used as a remedy for jaundice in folk medicine.
Enzymatic browning is a major factor contributing to quality loss in foods and beverages (Mc Evily et al. 1992). Mechanical stress during processing, results in cellular delocalization of enzymes and their substrates, leading to biochemical deteriorations, such as enzymatic browning, off-flavor development, and texture breakdown (Varoquaux 1991). Enzymatic browning is usually caused by the enzyme polyphenol oxidase (PPO, EC 1.10.3.1), which, in the presence of oxygen, converts phenolic compounds into dark colored pigments (Zawistowski et al. 1991). The contribution of other enzymes such as peroxidase (POD, EC 1.11.1.7) to total browning may also be relevant (Rolle and Chism 1987).
Normally, PPO is separated from its phenolic substrates in its native state in the plant tissue, which are located in the vacuole, so that browning only occurs when cells are damaged and compartmentation is lost (Vaughn et al. 1988).
Peroxidase is an iron-containing enzyme capable of oxidizing phenolics to quinones in the presence of hydrogen peroxide. Like PPO, the physiological role of peroxidase in plants is not well understood but it has been implicated in a number of primary and secondary metabolic functions including lignin biosynthesis, ripening and senescence, ethylene biosynthesis, hormone balance and membrane integrity. According to Bucheli and Robinson (1994), it appears that although cane juice exhibits significant peroxidase activity which can cause enzymatic browning, this enzyme does not contribute to color formation under normal extraction conditions, possibly because of insufficient levels of hydrogen peroxide.
The simplest method of inactivating PPO along with all other enzymes present is, application of heat, since enzymes being proteins are therefore easily denatured by heat. The color of the sugarcane juice was found to decrease with increasing temperature. PPO activity was found to decrease with increasing temperature with complete inactivation in juice extracted at 100 °C. Contrarily, POD was observed to be less sensitive to the heat treatment and almost half of the activity still remained in juice extracted at 100 °C (data not shown). This is consistent with previous reports on POD, which indicated that it is a relatively heat-stable enzyme (Burnette 1977).
The other widely used method for controlling the enzymatic browning is, the application of acids, especially the ones which naturally occur in tissues such as citric, malic, phosphoric and ascorbic acids. In general, their action is to lower the tissue pH and thus to decrease the rate of enzymatic browning. The optimum pH of phenolase lies within the range 6–7 and below pH 3 there is no enzymatic activity (Eskin et al. 1971).
Adsorption is another method and bentonite is found to be most efficient in color and turbidity removal (Erdogan et al. 1996). Also, bentonite is used in the clarification of apple juice (Koyuncu et al. 2007). According to FDA, bentonite is used in food at levels not to exceed current good manufacturing practice. Current good manufacturing practice results in no significant residue in foods (FDA 2016). Hitherto practically there is no literature available on the effect of bentonite in combination with acidulants on enzymes present in sugarcane juice. Hence the present study, first of its kind, is taken up, to evaluate the effectiveness of bentonite along with acidulants (citric acid and ascorbic acid) on major enzymes like PPO and POD in sugarcane juice, in order to improve the quality.
Materials and methods
Materials
Sugarcane (Saccharum officinarum) was procured from local market, Mysuru, Karnataka. o-dianisidine and hydrogen peroxide, ascorbic acid (99.9%) were obtained from Sigma-Aldrich, USA. Pyrocatechol was obtained from Loba chemicals, Mumbai, India. Bentonite and citric acid (99.7%) were procured from Hi-media, Mumbai, India. The stock solution of o-dianisidine was prepared freshly in methanol and solutions of hydrogen peroxide and other reagents were dissolved in distilled water. The common reagents used were all analytical grade (Merck, Mumbai, India).
Juice extraction
The canes were de-waxed after soaking in water for about an hour. Juice extracted from the canes was filtered through two layered muslin cloth. The filtered juice was treated with bentonite at different concentrations (0, 0.2, 0.4, 0.6, 0.8 and 1% w/v). pH was adjusted by the addition of acidulants viz., citric acid and ascorbic acid at different concentrations. The treated juice was centrifuged at 8000 rpm for 15 min and supernatant obtained was used for enzyme assay. Effect of heating (90 °C for 3–4 min), pH, titratable acidity, total soluble solids and ascorbic acid concentration was evaluated for the treated juice. Treated juice was then filled into 260 mL glass bottles and color changes during 10 days of storage at 4 and 10 °C were measured. Individual measurements were replicated three times and average values are reported.
PPO activity measurement
Polyphenol oxidase activity was assayed spectrophotometrically by adding 1 mL of reaction mixture, consisting of 0.9 mL of 0.5 M pyrocatechol in 0.1 M sodium acetate buffer (pH 6.5), and 0.1 mL of prepared enzyme. The changes in absorbance at 420 nm were recorded up to 3 min using UV–visible spectrophotometer (Shimadzu UV-160A, Japan) from the time enzyme extract was added. The enzyme activity was determined by measuring the slope of the reaction line at zero time (initial rate). The enzyme activity unit was defined as the change in absorbance per minute per milligram of protein extracted (specific activity) or the change in absorbance per minute per gram of tissue (Gonzlez et al. 2000).
POD activity measurement
Peroxidase activity was determined at 25 °C by measuring the initial rate of increase in absorbance at 437 nm. The reaction was started by adding 0.1 mL of enzyme extract, 2.7 mL of 0.2 M sodium phosphate buffer (pH 6.0), 0.1 mL of freshly prepared hydrogen peroxide 30% (v/v), and 0.1 mL of 20 mM o-dianisidine. The changes in absorbance at 437 nm were recorded up to 3 min from the time juice containing enzyme was added using UV–visible spectrophotometer. One unit of the activity was defined as the amount of enzyme that caused an absorbance change of 0.001 per min under standard conditions (Dogan et al. 2007).
Color measurement
The color values of sugarcane juice treated with bentonite (0, 0.4 and 0.8% w/v) along with acidulants (ascorbic acid, citric acid) were measured using a color meter (Hunter Lab Instruments, USA). Color was indicated using the CIE L* and a* coordinates, since greenness (a) and lightness (L) were the most appropriate parameters to indicate visible color change in sugarcane juice. Illuminant D65 and 2° observer angle were used. The instrument was calibrated using a standard white reflector plate (Mao et al. 2007).
Total soluble solids and titratable acidity
The total soluble solids were measured as ° brix by using hand held refractometer (Erma, Japan), which was adjusted to 0° brix with distilled water before analyzing the samples. titratable acidity was determined by quantifying the volume of 0.1 N NaOH, and expressed as citric acid or as ascorbic acid percentage (Ranganna 1986).
Ascorbic acid analysis
Ascorbic acid was analysed by titrimetric method using 2, 6 Dichlorophenol-indophenol dye. An aliquot of oxalic acid extract of the sample was titrated against the standardized dye to a pink end point which persists for at least 15 s (Ranganna 1986).
Statistical analysis
Mean and standard deviation were calculated with replication using the Microsoft Excel (Office Edition 2007). All the analyses have been done in triplicates and the data obtained were analyzed at a 5% level of significance. Student’s t test was used to analyze and compare the effect of citric and ascorbic acid on PPO and POD assay. Correlation coefficient was calculated to determine the dependence of the random variables from the experimental data.
Results and discussion
In order to ascertain the level of bentonite requirement for the pretreatment of sugarcane juice for controlling the enzymatic browning, its concentration was varied over a range. To increase the efficacy of the pretreatment, this adsorbent was employed along with acidulants, namely, ascorbic acid and citric acid. The results are discussed in the following sections.
Effect of adsorbent concentration
Currently, polyelectrolytes, bentonite, kaolin, gelatin, casein, agars, activated silica, phosphoric acid, aluminum polychloride, magnesium oxide are used as clarifying or fining agents. These compounds are reported to alter the stability, turbidity, colour and flavour of juices. Flocculation and agglutination of previously formed coagulants are improved by the addition of these clarifying agents (Prati and Moretti 2010) as they are high molecular weight compounds. Apple juice was clarified by flocculation and precipitation using bentonite and gelatin, which are commonly used clarifying agents in industries (Benitez and Lozano 2007).
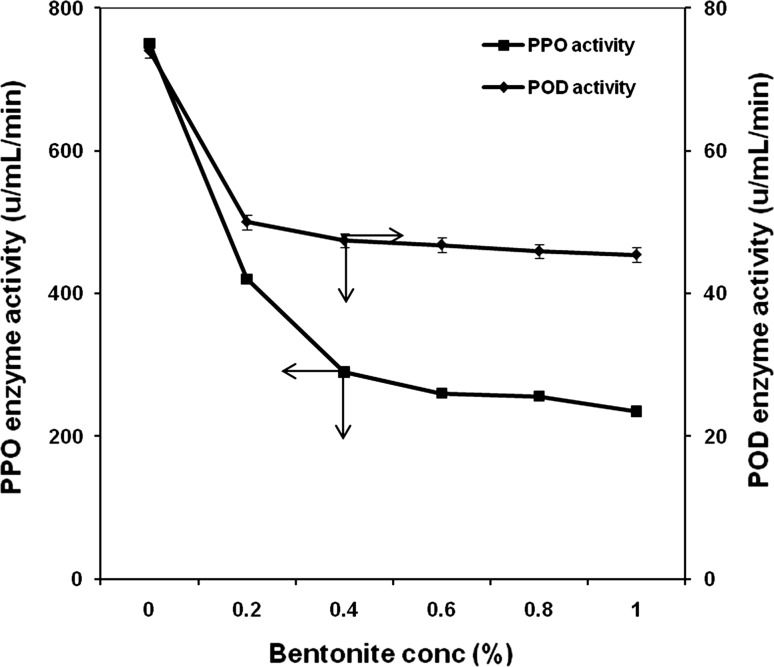
The effect of bentonite concentration on PPO and POD enzyme activity was studied at different concentrations (0–1%, w/v) and the results are shown in Fig. 1. Increase in bentonite concentration caused a progressive reduction in PPO and POD enzyme activity. PPO activity decreased to 420 u/mL (by 56%) and 290 u/mL (by 38%) after adding bentonite at 0.2 and 0.4% w/v concentration, above which there was no perceivable reduction in enzyme activity. Therefore, 0.4% w/v of bentonite was employed in further experimentation, unless otherwise mentioned. The activity of POD did not reduce to a large extent when compared to that of PPO, by the addition of bentonite at different concentrations.
Fig. 1.
Effect of adsorbent concentration on PPO and POD activity
Effect of acidulant concentration
Ascorbic acid is the most significant inhibitor of phenolase because it has no detectable flavour at the concentration used which would interfere with the acceptability of the final processed product (Taeufel and Voigt 1964). In addition to the advantage of vitamin value, it has no corrosive action upon metals (El-shimi 1993). The effect of ascorbic acid on PPO and POD activity is shown in Table 1. PPO activity was inhibited completely below pH 4.3 by addition of ascorbic acid, whereas POD activity was inhibited completely only below pH 3.9. Thus, maintaining sugarcane juice at pH 3.9 by the addition of ascorbic acid not only inhibited PPO but also completely inhibited POD activity.
Table 1.
Effect of acidulants on enzyme activity
| pH | Ascorbic acid | Citric acid | ||
|---|---|---|---|---|
| PPO activity (u/mL) | POD activity (u/mL) | PPO activity (u/mL) | POD activity (u/mL) | |
| 5.3 (control) | 750 ± 0.9 | 74.04 ± 1.2 | 990 ± 1.9 | 75 ± 2.4 |
| 4.9 | 420 ± 0.5* | 50.44 ± 1.1* | ||
| 4.6 | 220 ± 0.0* | 36.32 ± 2.1* | ||
| 4.3 | 50 ± 1.2* | 10.70 ± 0.0* | 350 ± 2.1* | 57 ± 2.1* |
| 4.1 | 0 | 0 | 290 ± 1.1* | 56 ± 1.1* |
| 3.9 | 0 | 0 | 160 ± 0.9* | 50 ± 1.0* |
| 3.7 | 0 | 0 | 60 ± 0.5* | 51 ± 1.2* |
Values are mean ± SD of triplicates
*Significantly different (p < 0.05) from control
Another acidulant, without toxic effect is citric acid, which is a phenolase Cu-chelating agent, and the inhibition of PPO was attributed to the chelating action. Earlier studies have used 10% citric acid that aided in clarification of sugarcane juice in addition to inhibition of growth of pathogenic microorganisms (Prati and Moretti 2010). Citric acid was found to be effective in extending shelf life, by inhibiting PPO activity thereby maintaining quality of chestnut slices during storage (Jiang et al. 2004). The effect of citric acid on the activity of PPO and POD in sugarcane juice is shown in Table 1. PPO and POD activity was inhibited to 60 u/mL (by 84%) and 50.5 u/mL (by 32.6%), respectively at the lowest pH 3.7, when citric acid was used as acidulant. Thus, it can be inferred that when compared to citric acid, ascorbic acid is preferable for inhibiting effectively both PPO and POD enzyme activity in sugarcane juice. The finding that both the enzyme activities decreased at lower pH is in agreement with the earlier report (Kunitake et al. 2014).
PPO and POD activity at different pH adjusted by the addition of citric and ascorbic acid have been assayed and analyzed. Activity of PPO and POD showed very good correlation with pH (correlation coefficient ≈ 0.99 for both the assays) in case of citric acid. Activity of PPO and POD also showed good correlation with pH (correlation coefficient 0.95 and 0.97 respectively) in the case of ascorbic acid.
Effect of heating on ascorbic acid concentration
Ascorbic acid in citrus juice concentrates was found to decrease with increasing temperature (Burdurlu et al. 2006). It is desirable to know the stability of the acidulant in sugarcane juice during processing (heating). Hence, thermal stability of ascorbic acid (which is generally known to be heat sensitive) was studied by measuring the concentration of ascorbic acid present before and on heating (90 °C for 3–4 min). As given in Table 2, concentration of ascorbic acid did not vary significantly on heating the sugarcane juice (concentration of ascorbic acid before heating—after heating; pH 5.3 (control) 0.35 ± 0–0.21 ± 0.1; pH 4.3: 4.8 ± 0.1–4.8 ± 0.3; pH 4.1: 7.86 ± 0.8–7.58 ± 0.2; pH 3.9: 12.2 ± 0.9; 11.5 ± 1.2; pH 3.7: 19.1 ± 1.1–17.2 ± 0.5 mg/100 mL). However, the concentration of ascorbic acid decreased during storage in transparent glass bottles, from initial 19.05 mg/100 mL to 15.41 and 15.07 mg/mL after storage for 10 and 20 days, respectively (data not shown).
Table 2.
Effect of bentonite in combination with ascorbic acid and citric acid on PPO and POD enzyme activity
| System details | Enzyme activity in u/mL | |||
|---|---|---|---|---|
| Ascorbic acid | Citric acid | |||
| PPO | POD | PPO | POD | |
| pH 5.3 (control) | 924 ± 0.4* | 41.12 ± 0.5* | 924 ± 0.4* | 41.12 ± 0.5* |
| 0.4% w/v Bentonite; pH 5.3 | 160 ± 0.2 | 24.16 ± 0.1 | 924 ± 0.89 | 41.12 ± 0.14 |
| 0.4% w/v Bentonite, pH 4.3 | 0 | 25.76 ± 0 | 160 ± 0.89 | 22.88 ± 0.17 |
| 0.4% w/v Bentonite, pH 4.1 | 0 | 13.28 ± 0 | 160 ± 0 | 21.92 ± 0.01 |
| 0.4% w/v Bentonite, pH 3.9 | 0 | 0 | 126 ± 0.1 | 18.88 ± 0.10 |
| 0.4% w/v Bentonite, pH 3.7 | 0 | 0 | 112 ± 0 | 15.36 ± 0.19 |
Values are mean ± SD of triplicates
*No acidulant
Titratable acidity decreased with an increase in pH following the general trend (Fig. S1). It was observed that, there was not much difference in pH and titratable acid value in acidulants treated juice before and after heating. Also, total soluble solid of sugarcane juice was ~ 19° Brix and on addition of bentonite and acidulants (ascorbic acid, citric acid) it showed ~ 20° Brix and ~ 18° Brix, respectively.
Effect of adsorbent in combination with acidulant
The effect of adsorbent (bentonite, 0.4% w/v) when employed in combination with the acidulants (ascorbic acid and citric acid) on PPO and POD activities is shown in Table 2. Complete inhibition of PPO activity was observed at pH 4.3 itself, whereas inhibition of POD activity was observed only at pH 3.9. Thus pH 3.9 was found to be most suitable for inhibiting the enzyme activity in sugarcane juice.
PPO activity reduced to 160 u/mL (by 83%) and 112 u/mL (by 88%) at pH 4.3 and 3.9 respectively when bentonite was employed in combination with citric acid. Similarly POD activity reduced to 22.88 and 15.36 u/mL at pH 4.3 and 3.9, respectively. Further, it may be noted that bentonite alone could act effectively in reducing enzyme activity.
PPO and POD activity in case of ascorbic acid and bentonite combination resulted in correlation coefficient of 0.93 and 0.94, respectively with pH. Significant difference between both the assay parameters was not observed, when the activities of PPO and POD (with pH in case of citric acid and ascorbic acid) were analyzed by the Student’s t test (tcal < ttab for p = 0.7 level of significance and df = 8). Although there was no significant variation with the change in acidifying agent in the media, only in case of ascorbic acid (at pH 3.9) complete inhibition of both the enzymes was observed, whereas there was residual enzyme activity in case of citric acid. This may be due to the blockage of initiation and/or propagation of free radical species that are required for the phenolic browning to occur, by ascorbic acid by virtue of its effective antioxidant capacity.
Color measurement
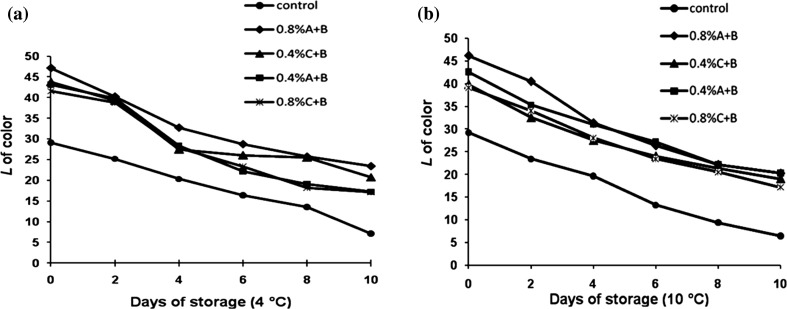
Color changes in sugarcane juice over a period of 10 days of storage were determined by measuring changes in L* (lightness) and a* (browning) coordinates. Both these parameters are frequently used in monitoring browning of sugarcane juice. Usually, a decrease in L* value and an increase in a* value are indicative of browning (Mao et al. 2007; Rojas-Grau et al. 2008).
Fresh sugarcane juice appeared olive-green and showed clear signs of browning during storage. Browning was observed in the control with a rapid decrease of ‘L’ value from the 1st day of storage as shown in Fig. 2. Bentonite (0–0.8% w/v) combined with acidulants treated juice showed reduced browning with an increase in L* value when compared to the control juice. Concentration of 0.8% (w/v) bentonite showed an increase in L* value when compared to 0.4% (w/v) concentration. However, bentonite when combined with ascorbic acid maintained higher L* values than citric acid during all storage time.
Fig. 2.
Lightness (L*) of sugarcane juice treated with bentonite stored at a 4 °C and b 10 °C compared to control. A ascorbic acid, B bentonite, C citric acid
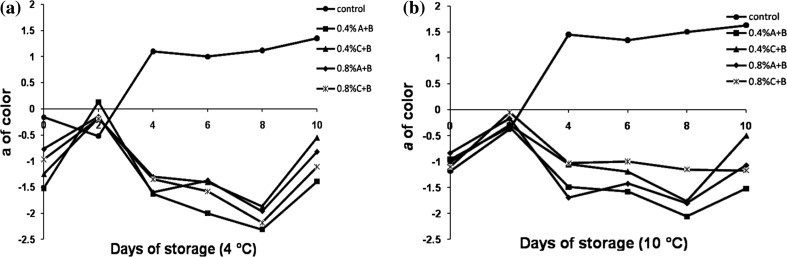
Browning in sugarcane juice is indicated by a rapid increase of ‘a’ value as shown in Fig. 3. Bentonite combined with acidulant showed negative ‘a’ value when compared to control (which showed positive ‘a’ value). Browning reaction, however, did not appear to take place in sugarcane juice subjected to pretreatment with adsorbent and acidulant during storage at 4 and 10 °C, in spite of minimal PPO and POD activity.
Fig. 3.
Browning (a*) in sugarcane juice treated with bentonite during storage at a 4 °C and b 10 °C compared to control. A ascorbic acid, B bentonite, C citric acid
Conclusion
Bentonite (0.4% w/v) was found to be effective in reducing PPO and POD activity in sugarcane juice by 69 and 39%, respectively. PPO activity was inhibited completely below pH 4.3 by addition of ascorbic acid, whereas POD activity was inhibited completely below pH 3.9. By the addition of citric acid, PPO and POD activity was inhibited by 84 and 32.6%, respectively at the lowest pH 3.7. Bentonite was found to be more effective in presence of acidulant and complete inhibition was observed in the presence of ascorbic acid. Whereas, in combination with citric acid, PPO and POD activity reduced by 88 and 63%, respectively at pH 3.7. These results gain importance when compared to presently practiced heat treatment process which causes thermal damage besides being energy intensive.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the Director, CFTRI, Mysore for the infrastructure facilities provided at the institute.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Benitez EI, Lozano JE. Effect of gelatin on apple juice turbidity. Lat Am Appl Res. 2007;37:261–366. [Google Scholar]
- Bucheli CS, Robinson SP. Contribution of enzymatic browning to color in sugarcane juice. J Agric Food Chem. 1994;42(2):257–261. doi: 10.1021/jf00038a006. [DOI] [Google Scholar]
- Burdurlu HS, Koca N, Karadeniz F. Degradation of vitamin C in citrus juice concentrates during storage. J Food Eng. 2006;74(2):211–216. doi: 10.1016/j.jfoodeng.2005.03.026. [DOI] [Google Scholar]
- Burnette FS. Peroxidase and its relationship to food flavour and quality: a review. J Food Sci. 1977;42:1–6. doi: 10.1111/j.1365-2621.1977.tb01204.x. [DOI] [Google Scholar]
- Dogan S, Turan P, Dogan M, Arslan O, Alkan M. Variations of peroxidase activity among Salvia species. J Food Eng. 2007;79:375–382. doi: 10.1016/j.jfoodeng.2006.02.001. [DOI] [Google Scholar]
- El-shimi NM. Control of enzymatic browning in apple slices by using ascorbic acid under different conditions. Plant Foods Hum Nutr. 1993;43:71–76. doi: 10.1007/BF01088098. [DOI] [PubMed] [Google Scholar]
- Erdogan B, Demirci S, Akay Y. Treatment of sugar beet juice with bentonite, sepiolite, diatomite and quartamin to remove color and turbidity. Appl Clay Sci. 1996;11:55–67. doi: 10.1016/0169-1317(96)00012-9. [DOI] [Google Scholar]
- Eskin NAM, Henderson HM, Toznsend RJ. Biochemistry of foods. New York, San-Fransisco, London: Academic Press; 1971. [Google Scholar]
- FDA (2016) Sec. 184.1155 Bentonite. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=184.1155. Accessed 1 Apr 2017
- Gonzlez EM, de Ancos B, Cano MP. Partial characterization of PPO and POD activities in blackberry fruits. J Agric Food Chem. 2000;48:5459–5464. doi: 10.1021/jf000169w. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Pen L, Li Jianrong. Use of citric acid for shelf life and quality maintenance of fresh-cut Chinese water chestnut. J Food Eng. 2004;63:325–328. doi: 10.1016/j.jfoodeng.2003.08.004. [DOI] [Google Scholar]
- Koyuncu H, Kulb AR, Çalımlıc A, Yıldızc N, Ceylan H. Adsorption of dark compounds with bentonites in apple juice. LWT Food Sci Technol. 2007;40(3):489–497. doi: 10.1016/j.lwt.2005.12.005. [DOI] [Google Scholar]
- Krishnakumar T, Devadas CT. Quality changes in sugarcane juice by delayed extraction and storage. Beverage Food World. 2006;33:77–78. [Google Scholar]
- Kunitake M, Ditchfield C, Silva C, Petrus R. Effect of pasteurization temperature on stability of an acidified sugarcane juice beverage. Ciênc Agrotec. 2014;38(6):554–561. doi: 10.1590/S1413-70542014000600004. [DOI] [Google Scholar]
- Mao LC, Xu YQ, Que F. Maintaining the quality of sugarcane juice with blanching and ascorbic acid. Food Chem. 2007;104:740–745. doi: 10.1016/j.foodchem.2006.09.055. [DOI] [Google Scholar]
- Mc Evily AJ, Iyengar R, Otwell WS. Inhibition of enzymatic browning in foods and beverages. Crit Rev Food Sci Nutr. 1992;32:253–273. doi: 10.1080/10408399209527599. [DOI] [PubMed] [Google Scholar]
- Prati P, Moretti RH. Study of clarification process of sugar cane juice for consumption. Ciênc Tecnol Aliment. 2010;30:776–783. doi: 10.1590/S0101-20612010000300033. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruits and vegetable products. 2. New Delhi: Tata McGraw Hill Publishing Company Limited; 1986. [Google Scholar]
- Rojas-Grau MA, Soliva- Fortuny R, Martin- Belloso O. Effect of natural antibrowning agents on color and related enzymes in fresh-cut fuji apples as an alternative to the use of ascorbic acid. J Food Sci. 2008;73:S267–S272. doi: 10.1111/j.1750-3841.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- Rolle R, Chism G. Physiological consequences of minimally processed fruits and vegetables. J Food Qual. 1987;10:157–177. doi: 10.1111/j.1745-4557.1987.tb00856.x. [DOI] [Google Scholar]
- Taeufel, Voigt Sodium chloride as inhibitor in enzymic browning of apples. Nahrung. 1964;8:80–91. doi: 10.1002/food.19640080110. [DOI] [Google Scholar]
- Varoquaux P. Ready-to-use fresh fruits and vegetables. Rev Gen Froid. 1991;81:33–43. [Google Scholar]
- Vaughn KC, Lax AR, Duke SO. Polyphenol oxidase: the chloroplast oxidase with no established function. Physiol Plant. 1988;72:659–665. doi: 10.1111/j.1399-3054.1988.tb09180.x. [DOI] [Google Scholar]
- Zawistowski J, Biliaderis CG, Eskin NAM. Polyphenol oxidases. In: Robinson DS, Eskin NAM, editors. Oxidative enzymes in foods. London: Elsevier; 1991. pp. 217–273. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.