Abstract
This study was performed to determine the effect of the addition of Laurus nobilis L. essential oil (EO) (at 0.01% v/v) and of the packaging material (brown and transparent glass or PET) on the oxidative stability of Algerian extra-virgin olive oil (EVOO) stored for 90 days at 25 ± 2 °C under continuous exposure to fluorescent light (~ 900 lux). Control and enriched EVOO was analysed after 30, 60 and 90 days for various parameters. Bio-enrichment of EVOO with EO combined with packaging in brown glass enabled maintenance of the highest amounts of chlorophyll and carotenoids after 90 days of accelerated photo-oxidation. The lowest total phenols content was found in EVOO without EO packed in transparent glass. EO enrichment and brown packaging preserved higher levels of antioxidant activity but could not preserve the oxidation indices until the end of the period of light exposition.
Keywords: Essential oil, Bio-enrichment, Olive oil, Packaging, Photooxidation
Introduction
At present, consumers have an ever-increasing awareness of the importance of maintaining a healthy lifestyle by consuming foods without unsafe additives, such as preservatives, artificial flavours and colouring agents. At the same time, both industry and consumers are interested in food products with a long shelf-life.
Virgin olive oils are valuable vegetable oils extracted from fresh and healthy olive fruits (Olea europeae L.) by mechanical or other physical processes performed in conditions that should not lead to any chemical change. Specific quality and purity criteria for the different categories of virgin olive oils are established by the International Olive Council, with the most restrictive quality criteria being set for extra-virgin olive oil (EVOO).
Olive oil is highly consumed throughout the world, and its consumption has been reported to have potential protective effects against several pathologies, especially those related to cancer (Fernández-Arroyo et al. 2012).
Unfortunately, due to EVOO’s unsaturated fatty acid profile, one of the most severe quality problems of EVOO is its oxidative rancidity due to oxidation of unsaturated fatty acids that react with singlet oxygen (1O2) produced by the photo-oxidation of olive oil in the presence of photosensitizers (such as chlorophyll) and the subsequent formation of fatty acid hydroperoxides and carbonyl compounds that possess unpleasant tastes and odours (Piscopo and Poiana 2012).
Beyond olive oil’s chemical composition, the susceptibility of olive oil to oxidation also depends on its processing, the packaging material used to store it (such as plastics, glass, and tin), storage conditions (such as light exposure and temperature) and duration of storage and exposures. Recent studies (Rizzo et al. 2014; Serrano et al. 2016) have shown that olive oil is highly susceptible to photooxidative degradation when stored in PET bottles and exposed to different intensities of fluorescent light and daylight. Protection against light and addition of appropriate natural or synthetic antioxidants are necessary to preserve olive oil from oxidation. Antioxidants can, in fact, increase shelf-life of food products by retarding lipid oxidation (Djenane et al. 2016), and the addition of a natural preserving additive can be exploited to retain product safety and quality for long periods of time. For example, Esposto et al. (2015) investigated how adding an olive phenolic extract affected the quality of vegetable oils during frying.
Aromatic plants are known to show antioxidant activity; particularly, their essential oils have been used since ancient times in food flavouring, pharmaceuticals, cosmetics and perfumery. Essential oils from aromatic plants, such as rosemary, thyme and laurel, have been reported as being capable of protecting olive oil from thermal oxidation (Ayadi et al. 2009; Sousa et al. 2015).
The aim of the present work was to advance current knowledge of how adding laurel EO to flavoured EVOO and packaging materials used to package EVOO may influence oil preservation. Therefore, this study investigated the potential for how enrichment of EVOO with Laurus nobilis essential oil in combination with using particular packaging materials (PET and glass, transparent or brown) might preserve oxidative status, antiradical activity and content of pigments under accelerated exposure to light.
Materials and methods
Raw materials
Commercial L. nobilis EO, which was obtained through hydro-distillation and certified as 100% organic, was purchased from Florame Aromathérapie (St Rémy de Provence, France). The oil was maintained in glass opaque flasks at 4 ± 1 °C and was characterized for its chemical profile by GC–MS analysis and for its free radical scavenging activity by the DPPH assay.
EVOO was collected from industrial oil mills during the 2014/2015 olive-oil year from the Chemlal variety in the province of M’Chedallah region, located on the southern slope of the Djurdjura mountain chain (North-Center, Algeria: 440 m (average) of altitude) at geographic coordinates Latitude 36° 21′ 56″ (North), Longitude 4° 16′ 16″ (East). EVOO was characterized for free acidity, peroxide value (PV), coefficients of specific extinction at 232 and 270 nm (K232 and K270), chlorophyll and carotenoids content, total phenols content and free radical scavenging activity (through using the DPPH assay).
Accelerated oxidation study
Different EVOO samples were prepared to assess how EO addition and packaging type might influence the oxidative stability of oil subjected to conditions causing accelerated oxidation.
EO was added to EVOO at a 0.01% (v/v) ratio. This dosage had been selected through preliminary sensory trials (data not shown) with six different levels of EO addition from 0.01 to 1%. A group of 15 experienced and official tasters participated on a voluntary basis and were asked to identify and define the sensory appearance of the olive oil samples using Annex XII of EU Commission Regulation (1991) EEC/2568/91.
Aliquots of 200 mL of EVOO were dispensed into 250 mL bottles of different materials: transparent (clear) polyethylene terephthalate, TPET; brown (amber) PET, BPET; brown (amber) glass, BG; and transparent glass, TG. TPET and BPET were supplied by Pro.Form Packaging, Sétif, Algeria, TG and BG were supplied by Groupe ENAVA – Entreprise Nationale des Verres & Abrasifs, Spa, Algeria. PET bottles were cylindrical with a body diameter of 5 cm. The TG bottle was cylindrical and measured 5.5 cm in diameter, while the BG bottle had a square base of approximately 5 × 5 cm.
For each type of oil (with and without EO) and packaging material, one hermetically-sealed bottle was used at each sampling time (Caponio et al. 2013). Each bottle was exposed horizontally to a continuous fluorescent light intensity of 900 lux (cool white fluorescent tubes, OSTRAM-L 40 W/19-1, Germany, placed 90 cm above samples) for 90 days at 25 ± 2 °C. The bottles were rotated every 24 h to minimize possible temperature abuse and differences in light intensity at the surfaces of samples.
After 30, 60 and 90 days, EVOO samples were analysed as indicated for the original EVOO.
Analysis of essential oil
EO was characterized by gas chromatography-mass spectrometry (GC/MS) analysis using a Hewlett- Packard 6800 series GC system (Agilent Technologies) coupled with a quadrupole mass spectrometer (model HP 5973) equipped with a non-polar HP5 MS capillary column (5% phenyl methyl siloxane, 30 m × 0.25 mm, 0.33 μm film thickness) (Centre de Recherche en Analyses Physico-chimiques, Algiers, Algeria). For GC/MS detection, an electron ionization system with ionization energy of 70 eV was used over a scan range of 30–550 atomic mass units (amu). Helium was the carrier gas at a flow rate of 0.5 mL/min. Injector and detector MS transfer line temperatures were set at 250 and 280 °C, respectively. The temperature of the ion source was 230 °C. The column temperature was initially kept at 60 °C for 8 min, where it was subsequently gradually increased to 280 °C at 2 °C/min and finally held isothermally for 30 min. The volume of injections was 0.20 μL of a hexane-oil solution, injected by splitless mode.
Retention indices of all of the constituents were determined by the Kovats method. Identification of the components was conducted by visual interpretation, comparing their retention indices and mass spectra with data published in the literature (Adams 2001) using the Wiley 7N, NIST 02, and NIST 98 libraries. The results were also confirmed by the comparison of retention indices relative to C7-C29 n-alkanes assayed under the same conditions as EO. The composition percentage of the EO (as % of the identified compounds) was computed by the normalization method from the GC peak areas, calculated as the mean value of two injections from EO.
Free radical scavenging activity of EO was measured by the 2,2-diphenyl-1picryl-hydrazil (DPPH, from Alfa Aesar, Ward Hill, MA, USA) assay according to the method reported by Sahin et al. (2004) using Butylated hydroxytoluene (BHT, supplied by Sigma, St. Louis, MO, USA) as a reference lipophilic antioxidant compound. For the analysis, different concentrations of EO and BHT into ethanol were prepared and tested to evaluate the IC50 index, that is, the concentration (mg/L) required to inhibit 50% of DPPH radical formation.
Analysis of virgin olive oil
Oxidative status
The acidity (free fatty acids expressed as a percentage by weight of oleic acid), the peroxide value (PV, expressed as mEq O2/kg, milliequivalents of active oxygen per kg) and extinction coefficients at 232 and 270 nm (K232 and K270) were determined to evaluate EVOO’s oxidation. The analytical methods described in the European Union Commission Regulations (1991) EEC/2568/91 were adopted.
Chlorophyll and carotenoids contents
Chlorophyll and carotenoids content was determined as described by Minguez-Mosquera et al. (1991). Olive oil was diluted with hexane, and its absorbance (in 1 cm cell) was read at 670 nm (chlorophyll fraction) and at 470 nm (carotenoid fraction). The specific extinction coefficient (100 mLg-1 cm−1) of 613 for pheophytin (the major component of chlorophylls) and of 2000 for lutein (the major carotenoid) was later used to calculate the pigments content as mg/kg.
Total phenols content and free radical scavenging activity
Total phenols content (TPC) of EVOO samples was quantified based on the Folin-Ciocalteu assay according to the procedure described by Gutfinger (1981). The results were expressed as mg of gallic acid equivalents (GAE) per kg of olive oil by means of a calibration curve obtained from a gallic acid standard (supplied by Sigma, St. Louis, MO, USA).
Free radical scavenging activity was determined through DPPH assay according to the procedure described by Kalantzakis et al. (2006). EVOO samples were diluted in ethyl acetate (10% w/v) and analysed. The radical scavenging activity was expressed as the percent reduction in DPPH absorbance (RSA%).
Statistical analysis
All of the analytical determinations were performed at least in triplicate, and the results were expressed as the mean ± standard deviation of the replicates.
The effect of EO addition, packaging type, storage time and their first- and second-order interactions with the evaluated parameters were assessed by three-way ANOVA using STATISTICA software version 6. Differences were considered significant at p < 0.05. In the case of a significant difference, the means were discriminated by applying Tukey’s post hoc test at a 95% confidence level.
Results and discussion
Analysis of essential oil
GC/MS analysis of L. nobilis EO permitted identification of 45 compounds corresponding to 99.46% of the revealed constituents (Table 1). The predominant fraction of 71.6% consisted of oxygenated monoterpenes. The major components were 1-8 cineol (39.69%), camphene (14.21%), sabinene (10.05%), -pinene (6.72%), linalool (6.08%), methyleugenol (3.7%), terpinen-4-ol (2.95%), linalyl propanoate (2.44%) and eugenol (1.34%). This composition is highly similar to the composition of L. nobilis EO reported by Da Silveira et al. (2014).
Table 1.
Percentage composition of L. nobilis essential oil used in this study evaluated by GC/MS analysis
| No. | Compound | RT | RI | Area (%) | type | formula |
|---|---|---|---|---|---|---|
| 1 | α-Thujene | 9.73 | 925 | 0.53 | MTH | C10H16 |
| 2 | α-Pinene | 10.19 | 939 | 6.72 | MTH | C10H16 |
| 3 | Camphene | 10.97 | 945 | 0.6 | MTH | C10H16 |
| 4 | Sabinene | 12.70 | 973 | 10.05 | MTH | C10H16 |
| 5 | β-Pinene | 12.85 | 978 | 4.26 | MTH | C10H16 |
| 6 | β- Myrcene | 13.76 | 995 | 0.67 | MTH | C10H16 |
| 7 | α-Phellandrene | 14.57 | 1007 | 0.18 | MTH | C10H16 |
| 8 | Delta 3-Carene | 14.93 | 1010 | 0.2 | MTH | C10H16 |
| 9 | α-Terpinene | 15.46 | 1014 | 0.36 | MTH | C10H16 |
| 10 | p-Cymene | 16.05 | 1026 | 0.15 | MTH | C10H14 |
| 11 | 1,8-Cineole | 16.87 | 1029 | 39.69 | OM | C10H18O |
| 12 | cis- β-Ocimene | 17.14 | 1039 | 0.2 | MTH | C10H16 |
| 13 | trans- β-Ocimene | 17.80 | 1042 | 0.38 | MTH | C10H16 |
| 14 | g-Terpinene | 18.45 | 1045 | 0.73 | OM | C10H16 |
| 15 | Trans-Sabinene Hydrate | 19.26 | 1050 | 0.19 | OM | C10H18O |
| 16 | α-Terpinolene | 20.47 | 1063 | 0.16 | MTH | C10H16 |
| 17 | 4-Thujanol | 21.56 | 1078 | 0.1 | OM | C10H18O |
| 18 | Linalool | 22.00 | 1080 | 6.08 | OM | C10H18O |
| 19 | 2-Cyclohexen-1-Ol | 23.16 | 1089 | 0.08 | OTHER | C6H10O |
| 20 | Cis-Sabinene Hydrate | 24.50 | 1101 | 0.07 | OM | C10H18O |
| 21 | Camphor | 26.40 | 1111 | 0.12 | OM | C10H18O |
| 22 | α-Terpineol | 26.58 | 1126 | 0.26 | OM | C10H18O |
| 23 | Terpinen-4-ol | 27.27 | 1182 | 2.95 | OM | C10H18O |
| 24 | Linalyl propanoate | 28.41 | 1190 | 2.44 | OM | C10H18O |
| 25 | Estragol | 28.62 | 1195 | 0.06 | OM | C10H12O |
| 26 | Cis- Geraniol | 30.83 | 1227 | 0.11 | OM | C10H18O |
| 27 | Linalyl Acetate | 32.50 | 1254 | 0.11 | OM | C12H20O2 |
| 28 | Bornyl acetate | 34.42 | 1285 | 0.38 | OM | C12H20O2 |
| 29 | 2-Undecanone | 35.18 | 1297 | 0.09 | OM | C11H22O |
| 30 | Delta-terpinyl acetate | 36.61 | 1347 | 0.56 | OM | C12H20O2 |
| 31 | Camphene | 39.11 | 1357 | 14.21 | OM | C10H16 |
| 32 | Eugenol | 39.84 | 1367 | 1.34 | OM | C10H12O2 |
| 33 | β-Elemene | 41.50 | 1394 | 0.3 | ST | C15H24 |
| 34 | Methyleugenol | 42.86 | 1409 | 3.7 | OM | C11H14O2 |
| 35 | Trans-Caryophyllene | 43.14 | 1426 | 0.52 | ST | C15H24 |
| 36 | α-Caryophyllene | 45.27 | 1459 | 0.06 | ST | C15H24 |
| 37 | Germacrene D | 46.99 | 1486 | 0.06 | ST | C15H24 |
| 28 | Germacrene B | 47.94 | 1556 | 0.12 | ST | C15H24 |
| 39 | β-Cubebene | 49.05 | 1386 | 0.05 | ST | C15H24 |
| 40 | d-Cadinene | 49.59 | 1526 | 0.09 | ST | C15H24 |
| 41 | Cis-α—Bisabolene | 50.78 | 1540 | 0.06 | ST | C15H24 |
| 43 | Elemicin | 51.88 | 1552 | 0.07 | OST | C12H16O3 |
| 44 | β-Caryophyllene | 53.10 | 1600 | 0.37 | ST | C15H24 |
| 45 | b-Eudesmol | 57.12 | 1654 | 0.03 | OST | C15H26O |
MTH Monoterpenes hydrocarbons, OM oxygenated monoterpenes, ST sesquiterpenes; OST: oxygenated sesquiterpenes, Others; RT retention time, RI retention index
Mediouni Ben Jemâa et al. (2012) mentioned that three L. nobilis EO from Algeria, Tunisia and Morocco showed quantitative, rather than qualitative, differences in their chemical composition that depended on their cultivation locations. Many factors, such as geographical origins, climatic conditions, seasonal variations and extraction techniques might play important roles in the chemical characterization of EO, influencing the proportions of the main constituents.
Radical scavenging activity of L. nobilis EO tested by using the DPPH assay showed an IC50 of 24.73 ± 0.42 mg/L, which was close to the IC50 of 27.99 ± 0.66 mg/L that was measured for the reference lipophilic antioxidant BHT. Antioxidant activity of EO from aromatic plants is primarily attributed to the active compounds present in the highest amount in EO but also to the presence of minor constituents, which may act synergistically. For example, eugenol, methyleugenol and elemicin (1.34%, 3.7% and 0.07% of our EO, respectively) are reported to play important roles in antioxidant effectiveness (Park et al. 2003).
1,8-cineol, the main compound identified in our L. nobilis EO, exhibited a higher antioxidant activity in soybean oil (Maestri et al. 1997).
The composition analysis and DPPH results suggest that our EO could be used as a potential source of natural antioxidants for lipid food systems.
Accelerated oxidation study
Initial oil showed the following chemical profile: acidity 0.28 ± 0.01%; PV 2.50 ± 0.21; K232 2.23 ± 0.07; K270 0.14 ± 0.00; total phenols 1036.72 ± 0.26 mgGAE/kg; total carotenoids 1.55 ± 0.02 mg/kg; chlorophylls 2.60 ± 0.07 mg/kg.
Statistical analysis (Table 2) revealed that there was a three-way interaction between addition of essential oil, storage time under fluorescent light and packaging type in all of the evaluated parameters except for K232. Additionally, the effects of single factors, and of both their first-order and second-order interactions were significantly influential, except for the interaction of EO with packaging type with regard to content of carotenoids.
Table 2.
Results of a three-way ANOVA for the influence of essential oil addition (EO), storage time (time), packaging type (Pack) and their first- and second-order interactions on the composition of each bottle of oil
| Factor | p value | ||||||
|---|---|---|---|---|---|---|---|
| PV | Acidity | K232 | K270 | Carotenoids | Chlorophyll | Total phenols | |
| EO | < 0.05 | < 0.05 | 0.287 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Pack | < 0.05 | < 0.05 | 0.349 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Time | < 0.05 | < 0.05 | 0.320 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| EO * Pack | < 0.05 | < 0.05 | 0.397 | < 0.05 | 0.234 | < 0.05 | < 0.05 |
| EO * Time | < 0.05 | < 0.05 | 0.362 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Pack * Time | < 0.05 | < 0.05 | 0.427 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| EO * Pack * Time | < 0.05 | < 0.05 | 0.463 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
Oxidative status
The results for acidity, PV, K232 and K270 in all the EVOO samples at 30, 60 and 90 days of accelerated photooxidation are reported in Table 3.
Table 3.
Evaluation of the oxidative status of extra-virgin olive oil, with or without L. nobilis essential oil (0.1% v/v), stored under accelerated photooxidation conditions from 30 to 90 days in different packaging materials
| Virgin olive oil with essential oil | Virgin olive oil without essential oil | |||||||
|---|---|---|---|---|---|---|---|---|
| PET | BPET | TG | BG | PET | BPET | TG | BG | |
| Acidity (% oleic acid) | ||||||||
| 30 days | 0.25 ± 0.00a | 0.25 ± 0.00a | 0.24 ± 0.00a | 0.24 ± 0.00a | 0.29 ± 0.00abc | 0.28 ± .0.01ab | 0.27 ± 0.01a | 0.26 ± 0.00a |
| 60 days | 0.25 ± 0.00a | 0.25 ± 0.00a | 0.24 ± 0.04a | 0.24 ± 0.00a | 0.37 ± 0.00de | 0.34 ± 0.00cd | 0.43 ± 0.03f | 0.33 ± 0.00bcd |
| 90 days | 0.50 ± 0.03g | 0.56 ± 0.02h | 0.53 ± 0.04gh | 0.67 ± 0.02i | 0.54 ± 0.00gh | 0.42 ± 0.00ef | 0.56 ± 0.04h | 0.64 ± 0.05i |
| Peroxides value (meq O2/kg) | ||||||||
| 30 days | 5.00 ± 0.00b | 3.00 ± 0.03a | 3.00 ± 0.00a | 3.00 ± 0.01a | 4.07 ± 0.07b | 3.00 ± 0.05a | 4.93 ± 0.09b | 3.00 ± 0.09a |
| 60 days | 10.00 ± 0.00e | 8.00 ± 0.14c | 13.00 ± 0.49g | 10.00 ± 0.07e | 10.00 ± 0.70e | 10.00 ± 0.84e | 9.00 ± 0.12d | 11.00 ± 0.63f |
| 90 days | 16.00 ± 0.28i | 13.00 ± 0.49g | 16.00 ± 0.21i | 13.00 ± 0.35g | 16.00 ± 0.84i | 13.00 ± 063g | 17.00 ± 0.71j | 15.00 ± 0.62h |
| K232 | ||||||||
| 30 days | 2.75 ± 0.03a | 2.74 ± 0.04a | 2.72 ± 0.00a | 2.75 ± 0.03a | 2.91 ± 0.05a | 2.90 ± 0.14a | 2.73 ± 0.04a | 2.82 ± 0.03a |
| 60 days | 2.99 ± 0.00a | 2.81 ± 0.06a | 2.91 ± 0.06a | 3.03 ± 0.04a | 3.03 ± 0.00a | 2.91 ± 0.07a | 2.85 ± 0.06a | 2.91 ± 0.19a |
| 90 days | 3.03 ± 0.04a | 2.83 ± 0.01a | 2.92 ± 0.01a | 2.83 ± 0.01a | 3.03 ± 0.14a | 2.99 ± 0.08a | 2.94 ± 0.15a | 2.99 ± 0.21a |
| K270 | ||||||||
| 30 days | 0.15 ± 0.00a | 0.15 ± 0.00a | 0.17 ± 0.00ab | 0.15 ± 0.00a | 0.19 ± 0.00bc | 0.18 ± 0.01bc | 0.20 ± 0.01c | 0.19 ± 0.01bc |
| 60 days | 0.17 ± 0.00ab | 0.19 ± 0.00bc | 0.20 ± 0.00c | 0.18 ± 0.00bc | 0.29 ± 0.00gh | 0.23 ± 0.01d | 0.26 ± 0.01ef | 0.24 ± 0.01de |
| 90 days | 0.20 ± 0.00c | 0.18 ± 0.00bc | 0.18 ± 0.00bc | 0.19. ± 0.00bc | 0.35 ± 0.02h | 0.25 ± 0.01def | 0.27 ± 0.00fg | 0.25 ± 0.01def |
Values are reported as the mean ± S.D. of replicates (n = 3). Same letter, under the same parameter, indicates the parameters are not significantly different according to ANOVA and Tukey’s post hoc test (p < 0.05)
TPET Transparent polyethylene terephthalate, BPET brown PET, TG transparent glass, BG brown glass
Acidity of all the EVOO samples always remained below the 0.8% limit set by the EU (2013) Reg. 1348/2013 for extra-virgin olive oil. However, this parameter increased during the study, showing development of rancidity as the result of free fatty acid hydrolysis (Yildirim 2009) in agreement with previously reported results. In fact, Guil-Guerrero and Urda-Romacho (2009) reported that several extra-virgin olive oil varieties bottled in dark or transparent glass for 1 year experienced increased acidity throughout their storage. Pristouri et al. (2010) observed that the acidity of olive oil was affected by the packaging material, head space, oxygen, light transmission, temperature and storage time (12 month). Further, Fadda et al. (2012) measured an increased acidity in olive oil extracted with traditional or innovative technology, bottled in dark glass bottles and stored in the dark at a temperature of 20 °C. In contrast, Savarese et al. (2013) reported an almost-constant increase in acidity for extra-virgin olive oil bottled in red or transparent PET and kept in dark or light (300 lux) conditions during prolonged periods of storage (12 months).
Addition of EO showed a protective effect. In the enriched samples acidity started increasing only after 60 days, showing an increased time required for oxidation induction. This finding agrees with the findings from the study by Sousa et al. (2015), who found that flavouring olive oil with dried red chili pepper, laurel and oregano spices (enrichment level of 10 g/L of olive oil) did not significantly increase acidity, while the addition of fresh garlic induced a significant increase from 0.6 to 0.8% after 3 months of storage at room temperature (where it was also protected from light exposure and in a static position).
PV increased during storage, but it always remained below the limits set by the EU Regulation (2013) 1348/2013 for extra-virgin and virgin olive oil (20 mEq O2/kg). However, PV did not follow the same trend as was observed relating to acidity (Table 3). In fact, the brown packaging reduced formation of peroxides in both in plastic and glass, suggesting, in this case, that light has a higher catalysing effect compared to oxygen (PET is more permeable to oxygen than glass) on the reactions leading to formation of peroxides. Del Nobile et al. (2003) showed that while glass containers can completely prevent oxygen permeation, PET is only able to slow down the oxygen exchange, which may invalidate the reliability of PET as a competitor for glass containers in storing olive oil. Rizzo et al. (2014), found that the PV of a monovarietal EVOO packed in PET did not significantly increase over 120 days of storage in the dark or under one fluorescent lamp, whereas storage conditions that accelerated light exposure, such as exposure under 4 fluorescent lamps, caused PV to increase. Under this condition, coloured PET exhibited a higher ability of light shielding than clear PET that lead to lower levels of PV. Pristouri et al. (2010) reported that the PV of EVOO bottled in clear PET and stored under fluorescent light increased from 12.92 ± 0.44 to 20.61 ± 0.20 after 12 months. Kanavouras and Coutelieris (2006) found that plastic containers had a particularly strongly protective role when oil was stored in light; PET provided better light transmission resistance with respect to transparent glass, thereby offering greater protection in the presence of light.
The specific extinction coefficients (K232 and K270) allow evaluation of the degree of olive oil oxidation, indicating the presence of conjugated dienes (K232) and trienes systems (K270), which are both related to oxidation reactions. According to legislation for EVOO, K232 must be lower than 2.5 (2.6 for VOO), and K270 lower than 0.22 (0.25 for VOO). In our study K232 was always above the legal limit, except for the control sample at time zero, while K270 remained below the legal limit only in those samples enriched with EO.
The conjugated dienes systems increased slightly after 30 days of light exposure and later remained constant in every sample, showing that there was no effect with respect to EO, material and time of exposure. This finding indicates accumulation of primary oxidation products and negligible compared to the formation of secondary products, which is characteristic in the initial phase of oxidative degradation. Different results are reported in the literature. Asensio et al. (2013) reported that the incorporation of essential oils of oregano spices into olive oil resulted in an increased K232 after 126 days of light exposure. Samaniego-Sanchez et al. (2012) found that storing olive oil in the dark at room temperature (20 °C) or refrigerated temperature (4 °C) and in different containers (glass, PET, and Tetra-Brik) resulted in an increased K232.
In contrast, the K270, which indicates conjugated trienes (primary oxidation products) but also carbonyl compounds (secondary oxidation products), increased significantly in all of the samples. Olive oil with EO stored in brown PET and glass showed the lowest increase, while the highest increase was found in olive oil without EO stored in transparent PET. The increase in carbonyl compounds is due to the primary oxidation product’s evolution into secondary oxidation products (such as the formation of hydroperoxides), and the results clearly show that EO establishes an inhibitory effect.
Chlorophyll and carotenoids contents
Table 4 reports the variation in the concentration of pigments during the accelerated oxidation test. Generally, both chlorophyll and carotenoids decreased significantly in all the samples, and enrichment with laurel EO provided a certain (but not always significant) level of protection against degradation of pigments.
Table 4.
Evaluation of total phenols (expressed as GAE- gallic acid equivalents), total carotenoids and chlorophyll content of extra-virgin olive oil, with or without L. nobilis essential oil (0.1% v/v), stored in accelerated photooxidation conditions from 30 to 90 days in different packaging materials
| Virgin olive oil with essential oil | Virgin olive oil without essential oil | |||||||
|---|---|---|---|---|---|---|---|---|
| PET | BPET | TG | BG | PET | BPET | TG | BG | |
| Total phenols (mgGAE/kg) | ||||||||
| 30 days | 739.64 ± 1.03s | 753.76 ± 5.19t | 648.77 ± 1.64q | 896.42 ± 3.30v | 284.36 ± 0.52i | 411.95 ± 0.71n | 350.75 ± 1.62m | 685.96 ± 2.26r |
| 60 days | 564.97 ± 4.33p | 297.08 ± 2.26j | 527.30 ± 2.61o | 782.01 ± 6.29u | 201.50 ± 1.76f | 287.19 ± 1.55i | 271.65 ± 2.19h | 329.56 ± 0.77l |
| 90 days | 193.03 ± 1.13e | 296.61 ± 3.17j | 230.69 ± 6.36g | 306.02 ± 3.39k | 109.22 ± 0.84b | 131.82 ± 1.42c | 84.74 ± 0.70a | 141.24 ± 0.56d |
| Total carotenoids (mg/kg) | ||||||||
| 30 days | 0.77 ± 0.02ijk | 0.92 ± 0.05l | 0.72 ± 0.05ghij | 1.12 ± 0.05m | 0.72 ± 0.07ghij | 0.81 ± 0.06jkl | 0.77 ± 0.02ijk | 1.05 ± 0.08m |
| 60 days | 0.59 ± 0.00ef | 0.85 ± 0.03kl | 0.60 ± 0.02efg | 0.66 ± 0.02fghi | 0.64 ± 0.01fgh | 0.75 ± 0.03hijk | 0.41 ± 0.02bc | 0.64 ± 0.02fgh |
| 90 days | 0.40 ± 0.00bc | 0.54 ± 0.04def | 0.43 ± 0.00bcd | 0.63 ± 0.04efgh | 0.21 ± 0.00a | 0.41 ± 0.07bc | 0.32 ± 0.02ab | 0.51 ± 0.01cde |
| Chlorophyll (mg/kg) | ||||||||
| 30 days | 0.55 ± 0.03de | 0.97 ± 0.02i | 0.73 ± 0.04g | 1.51 ± 0.06l | 0.83 ± 0.01h | 1.02 ± 0.02ij | 0.63 ± 0.02ef | 1.14 ± 0.08k |
| 60 days | 0.52 ± 0.02d | 0.63 ± 0.04ef | 0.71 ± 0.06fg | 1.07 ± 0.02jk | 0.52 ± 0.02d | 0.63 ± 0.04ef | 0.48 ± 0.04cd | 0.79 ± 0.04gh |
| 90 days | 0.42 ± 0.02bc | 0.53 ± 0.04d | 0.48 ± 0.01cd | 0.79 ± 0.00gh | 0.22 ± 0.01a | 0.35 ± 0.01b | 0.35 ± 0.02b | 0.37 ± 0.02b |
Values are reported as the mean ± S.D. of replicates (n = 3). Same letter, under the same parameter, indicates the means are not significantly different according to ANOVA and Tukey’s post hoc test (p < 0.05)
TPET transparent polyethylene terephthalate, BPET brown PET, TG transparent glass, BG brown glass
The use of brown packaging allowed a reduction in degradation of pigments in both PET and glass bottles. However, the best solution for protection of pigments appeared to be presence of brown glass, which is a barrier to both light and oxygen, which are both involved in reactions causing degradation of pigments. Another study (Gargouri et al. 2015) carried out on olive oil exposed to light showed a significant decrease in the content of chlorophylls and carotenoids in oil samples. In agreement with our results, Guil-Guerrero and Urda-Romacho (2009) reported that reduction of chlorophylls and carotenoids was higher in TG bottles than BG bottles stored in the dark for 1 year, with chlorophylls remaining constant in the BG bottles, while they were diminished in TG bottles. Caponio et al. (2005) also found that chlorophylls completely disappeared in oils stored in TG bottles and under diffuse light for 12 months at 15 °C in winter and 25 °C in summer.
Chlorophylls play an important role in oxidative stability. The presence of pigments not only determines the green colour of the product that varies from yellow-green to green-gold but also plays an additional function of demonstrating an increased pro-oxidant power in light exposure, and an antioxidant power against auto-oxidation processes when in the dark (Oueslati et al. 2009). In our study, it was not possible to identify any clear correlation between chlorophylls and oxidation, even though their residual presence in all the samples may have contributed to the increase in PV, acidity and K270, despite the EO enrichment and brown or glass packaging.
Total phenols content and free radical scavenging activity
The initial TPC of EVOO was 1036.72 ± 0.2 mgGAE/kg. The TPC of an olive oil is influenced by agronomic and environmental factors, the extraction system and the olive variety. Del Monaco et al. (2015), for example, reported the following TPC of Italian olive oil: 1030 ± 14 mg/kg and 290 ± 35 mg/kg for Lavagnina cultivar (300 m and 50 m of altitude, respectively); 490 ± 18 mg/kg for Taggiasca cultivar; and 2180 ± 69 mg/kg for Gentile di Larino cultivar.
Table 4 shows the changes in the TPC of the oil samples during the accelerated oxidation study. As expected, the TPC decreased during storage in both oils (with and without EO), but enrichment with laurel EO clearly showed a protective effect in original TPC against oxidation with regard to all of the packaging materials, confirming that the measured antiradical activity of EO can play an effective and protective role in olive oil. On average, the TPC of the oil with EO was two time that of the oil without EO at same time and packaging type.
The use of a brown bottle better preserved the phenolic content. This effect was more marked for glass, while for PET, this holds only in not EO enriched samples, suggesting also the involvement of oxygen permeation in the degradation processes of phenols. The role of light and oxygen in TPC degradation was demonstrated in other literature studies (Gargouri et al. 2015), which investigated TPC of olive oil exposed to light and packed in different containers showing that tin container, stainless and dark glass bottles recorded a smaller reduction in TPC compared to clear glass, earthenware jars, PET and PE containers.
Our results agree with the findings of previous workers. Rizzo et al. (2014) also found that the TPC of olive oil stored for 120 days at room temperature (23 ± 2 °C) in transparent PET (clear, green, orange) and opaque PET (white and blue) under different fluorescent lighting conditions decreased, with opaque PET allowing for the highest TPC.
Contrastingly, our results disagree with those of Sousa et al. (2015), who reported that the incorporation of different flavouring agents (garlic, hot chili peppers, laurel, oregano and pepper incorporated dried as were 10 g/L) did not show any protective effect against oxidation of olive oil stored during 3 months at room temperature in the dark which suggests a higher efficiency of essential oil compared to dried herbs. Additionally, Ayadi et al. (2009) observed that enrichment of Tunisian olive oils with selected Tunisian aromatic plants (rosemary, lavender, sage, menthe, basil, lemon and thyme, 5% w/w by maceration of flesh material) did not protect TPC from thermal oxidation (storage in glass bottles at 60 and 130 °C for 55 days and 6 h, respectively). Nevertheless, Khemakhem, et al. (2015) found that maceration of citrus zests in olive oil contributed to the increase in the TPC but that after storage at 60 °C for 40 days, the rate of degradation of TPC was higher than in the oil that was not additionally-flavoured. All of these finding suggest a higher efficiency of essential oil compared to dried herbs or whole fresh aromatic plants.
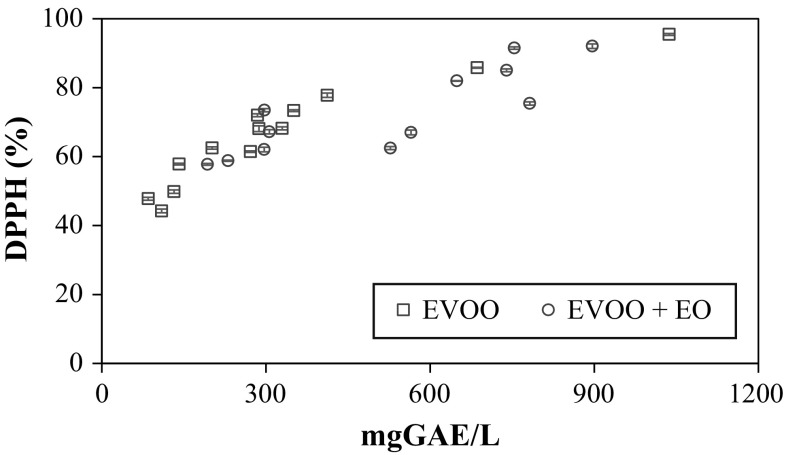
The antioxidant activity of the different oil samples followed essentially the same trend of the TPC. Figure 1 clearly shows the correlation between the TPC and % DPPH independent from EO addition, packaging material and storage time. The graph suggests that an overall, non-specific degradation of the phenolic compounds occurred in the TPC present in the samples, with a slightly lower efficiency of the compounds present in the enriched oil. This result can be attributed to the presence of the bioactive compounds, 1-8 cineol, camphene, sabinene, pinene, linalool methyleugenol terpinen-4-ol, linalyl propanoate and eugenol (Table 1), which are reported to serve as natural inhibitors of oxidation.
Fig. 1.
Correlation between total phenols content (gallic acid equivalents, GAE) and antioxidant activity (DPPH %) of all the extra-virgin olive oil (EVOO) samples of this study, enriched with L. nobilis essential oil (EVOO + EO, 0.01% v/v), packed in bottles of different materials and stored in accelerated photooxidation conditions from 30 to 90 days. Values are reported as means, and the error bars indicate ± S.D. of replicates (n = 3)
Ben Rached et al. (2014) reported that the aromatization of Zalmati olive oil with the EO of Rosmarinus officinalis L. improved its antiradical activity. On the opposite, Baiano et al. (2009) observed that in olive oils enriched with herbs and spices (lemon, oregano, hot pepper, and rosemary) and stored in BG bottles at room temperature for 9 months, the antioxidant potential (according to the β- carotene bleaching assay), significantly decreased compared to unflavoured oil or olive oil with garlic. However, Baiano et al. (2009) added fresh or dried plant material to olives before pressing, which might have been less efficient than the addition of EO to pressed oil.
Conclusion
These results show a significant interaction of addition of essential oil with storage time under fluorescent light and packaging type on the evolution of extra-virgin olive oil in terms of oxidative indices, pigments and total phenols content and antioxidant activity. As expected, adding EO, even though at a very low level (0.01% v/v) and packaging in brown containers (especially glass) enabled maintenance of the highest amount of chlorophylls and carotenoids after 90 days of accelerated photooxidation, such as the highest total phenols content. The last result correlates highly to the antiradical activity of oil. However, EO enrichment and brown packaging could not preserve oxidation indices (PV, K232 and acidity), with the exception of K270, throughout the accelerated oxidation test, probably due to a pro-oxidant effect given by the residual content of chlorophylls. The results suggest that the quality preservation of olive oil can be only partly affected by the addition of EO and that the selection of a packaging with a well-defined barrier for both light and oxygen is necessary to preserve its quality. BPET could be a valid commercial packaging solution for EVOO, but further studies are required to determine the exact correlation between accelerated light exposure and shelf life under normal conditions.
Contributor Information
Aldjia Taoudiat, Email: taoudiatnaimat@gmail.com.
Djamel Djenane, Email: djenane6@yahoo.es.
Zoulikha Ferhat, Email: z.ferhat@ensa.dz.
Giorgia Spigno, Phone: +39 0523599181, Email: giorgia.spigno@unicatt.it.
References
- Adams RP. Identification of essential oil components by gas chromatography/quadruple mass spectroscopy. 4. Carol Stream: Allured Publishing Corporation; 2001. [Google Scholar]
- Asensio CM, Nepote V, Grosso NR. Consumer’s acceptance and quality stability of olive oil flavoured with essential oils of different oregano species. Int J Food Sci Tech. 2013;48:2417–2428. [Google Scholar]
- Ayadi MA, Grati-Kamoun N, Attia H. Physico-chemical change and heat stability of extra virgin olive oils flavoured by selected Tunisian aromatic plants. Food Chem Toxicol. 2009;147:2613–2619. doi: 10.1016/j.fct.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Baiano A, Terracone C, Gambacorta G, La Notte E. Changes in quality indices, phenolic content and antioxidant activity of flavored olive oils during storage. J Am Oil Chem Soc. 2009;86:1083–1092. doi: 10.1007/s11746-009-1446-8. [DOI] [Google Scholar]
- Ben Rached M, Abdallah M, Guerfel M. Compositional quality of Zalmati virgin olive oil: effect of the aromatization process with rosemary essential oils (Rosmarinus officinalis L.) Afr J Agric Res. 2014;9:3276–3282. [Google Scholar]
- Caponio F, Bilancia MT, Pasqualone A, EwaSikorska E, Gomes T. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur Food Res Technol. 2005;221:92–98. doi: 10.1007/s00217-004-1126-8. [DOI] [Google Scholar]
- Caponio F, Paradiso VM, Bilancia MT, Summo C, Pasqualone A, Gomes T. Diacylglycerol isomers in extra virgin olive oil: effect of different storage conditions. Food Chem. 2013;140:772–776. doi: 10.1016/j.foodchem.2012.10.120. [DOI] [PubMed] [Google Scholar]
- Commission Implementing Regulation (EU) No 1348/2013 (2013) Amending Regulation EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Official Journal of the European Union L338/31
- Commission Regulation (EEC) No 2568/91 (1991) on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Official Journal of the European Communities L248/1
- Da Silveira SM, Bittencourt LF, Fronza N, Cunha A, Jr, Neudí Scheuermann G, Werneck Vieira CR. Chemical composition and antibacterial activity of Laurus Nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT- Food Sci Technol. 2014;59:86–93. doi: 10.1016/j.lwt.2014.05.032. [DOI] [Google Scholar]
- Del Monaco G, Officioso A, D’Angelo S, La Cara F, Ionata E, Marcolongo L, Squillaci G, Maurelli L, Morana A. Characterization of extra virgin olive oils produced with typical Italian varieties by their phenolic profile. Food Chem. 2015;184:200–228. doi: 10.1016/j.foodchem.2015.03.071. [DOI] [PubMed] [Google Scholar]
- Del Nobile MA, Ambrosino ML, Sacchi R, Masi P. Design of plastic bottles for packaging of virgin olive oil. J Food Sci. 2003;68:170–175. doi: 10.1111/j.1365-2621.2003.tb14135.x. [DOI] [Google Scholar]
- Djenane D, Beltrán JA, Camo J, Roncalés P. Influence of vacuum-ageing duration of whole beef on retail shelf life of steaks packaged with oregano (Origanum vulgare L.) active film under high O2. J Food Sci Technol. 2016;53:4244–4257. doi: 10.1007/s13197-016-2419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposto S, Taticchi A, Di Maio I, Urbani S, Veneziani G, Selvaggini R, Sordini B, Servili M. Effect of an olive phenolic extract on the quality of vegetable oils during frying. Food Chem. 2015;176:184–192. doi: 10.1016/j.foodchem.2014.12.036. [DOI] [PubMed] [Google Scholar]
- Fadda C, Del Caro A, Sanguinetti AM, Urgeghe P, Vacca V, Arca PP, Piga A. Changes during storage of quality parameters and in vitro antioxidant activity of extra virgin monovarietal oils obtained with two extraction technologies. Food Chem. 2012;134:1542–1548. doi: 10.1016/j.foodchem.2012.03.076. [DOI] [PubMed] [Google Scholar]
- Fernández-Arroyo S, Gómez-Martínez A, Rocamora-Reverte L, Quirantes-Piné R, Segura-Carretero A, Fernández-Gutiérrez A, Ferragut JA. Application of nano LC-ESI-TOF-MS for the metabolomic analysis of phenolic compounds from extra-virgin olive oil in treated colon-cancer cells. J Pharm Biomed Anal. 2012;63:128–134. doi: 10.1016/j.jpba.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Gargouri B, Zribi A, Bouaziz M. Effect of container on quality of Chemlali olive oil during storage. J Food Sci Technol. 2015;52:1948–1959. doi: 10.1007/s13197-014-1273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil-Guerrero JL, Urda-Romacho J. Quality of extra virgin olive oil affected by several packaging variables. Grasas Aceites. 2009;60:125–133. doi: 10.3989/gya.043308. [DOI] [Google Scholar]
- Gutfinger T. Polyphenols in olive oils. J Am Oil Chem Soc. 1981;58:966–968. doi: 10.1007/BF02659771. [DOI] [Google Scholar]
- Kalantzakis G, Blekas G, Pegklidou K, Boskou D. Stability and radical- scavenging activity of heated olive oil and other vegetable oils. Eur J Lipid Sci Technol. 2006;108:329–335. doi: 10.1002/ejlt.200500314. [DOI] [Google Scholar]
- Kanavouras A, Coutelieris FA. Shelf-life predictions for packaged olive oil based on simulations. Food Chem. 2006;96:48–55. doi: 10.1016/j.foodchem.2005.01.055. [DOI] [Google Scholar]
- Khemakhem I, Yaiche C, Ayadi MA, Bouaziz M. Impact of Aromatization by Citrus limetta and Citrus Peels on Olive Oil Quality, Chemical Composition and heat Stability. J Am Oil Chem Soc. 2015;92:701–708. doi: 10.1007/s11746-015-2636-1. [DOI] [Google Scholar]
- Maestri D, Jose M, Labuckas DO, Lamarque AL, Zygadlo J, Guzmán CA. Evaluation of the antioxidant potentiality of anethole, eugenol, thymol and 1,8- cineole in soybean oil. An Asoc Quim Argent. 1997;85:179–187. [Google Scholar]
- Mediouni Ben Jemâa J, Tersim N, Taleb Toudert K, Larbi Khouja M. Insecticidal activities of essential oils from leaves of Laurus nobilis L. from Tunisia, Algeria and Morocco and comparative chemical composition. J Stored Prod Res. 2012;48:97–104. doi: 10.1016/j.jspr.2011.10.003. [DOI] [Google Scholar]
- Minguez-Mosquera MI, Rejano-Navarro L, Gandulrojas B, Sanchez Gomez AH, Garrido-Fernandez J. Color-pigment correlation in virgin olive oil. J Am Oil Chem Soc. 1991;86:332–336. doi: 10.1007/BF02657688. [DOI] [Google Scholar]
- Oueslati I, Anniva C, Daoud D, Tsimidou MZ, Zarrouk M. Virgin olive oil (EVOO) production in Tunisia: the commercial potential of the major olive varieties from the arid Tataouine zone. Food Chem. 2009;112:733–741. doi: 10.1016/j.foodchem.2008.06.041. [DOI] [Google Scholar]
- Park BS, Lee KG, Shibamoto T, Lee SE, Takeoka GR. Antioxidant activity and characterization of volatile constituents of Taheebo (Tabebuia impetiginosa Martius ex DC) J AgricFood Chem. 2003;51:295–300. doi: 10.1021/jf020811h. [DOI] [PubMed] [Google Scholar]
- Piscopo A, Poiana M (2012) Packaging and storage of olive oil. Olive germplasm—the olive cultivation, table olive and olive oil industry in Italy, pp 201–222
- Pristouri G, Badeka A, Kontominas MG. Effect of packaging material headspace, oxygen and light transmission temperature and storage time on quality characteristics of extra virgin olive oil. Food Control. 2010;21:412–418. doi: 10.1016/j.foodcont.2009.06.019. [DOI] [Google Scholar]
- Rizzo V, Torri L, Licciardello F, Piergiovanni L, Muratore G. Quality changes of extra virgin olive oil packaged in coloured polyethylene terephthalate bottles stored under different lighting conditions. Packag Technol Sci. 2014;27:437–448. doi: 10.1002/pts.2044. [DOI] [Google Scholar]
- Sahin F, Güllüce M, Daferera D, Sökmen A, Sökmen M, Polissiou M, Agar G, Özer H. Biological activities of the essential oils and methanol extract of Origanum vulgar ssp. Vulgare in the eastern Anatolia region of Turkey. Food Control. 2004;15:549–557. doi: 10.1016/j.foodcont.2003.08.009. [DOI] [Google Scholar]
- Samaniego-Sanchez C, Oliveras-Lopez MJ, Quesada-Granados JJ, Villalón-Mir M, Lopez-G-Serrana H. Alterations in picual extra virgin olive oils under different storage conditions. Eur J Lipid Sci Technol. 2012;114:194–204. doi: 10.1002/ejlt.201100191. [DOI] [Google Scholar]
- Savarese M, De Marco E, Caporaso N, Sacchi R (2013) Extra virgin olive oil overall quality assessment during prolonged storage in PET containers. In: 1st global virtual conference—section agriculture, vol 1, pp 674–679
- Serrano L, Cruz A, Sousa S, Morais Z. Alterations in monovarietal, blended and aromatized Portuguese virgin olive oils under four storage conditions for 12 months. Eur Food Res Technol. 2016;242(7):1041–1055. doi: 10.1007/s00217-015-2609-5. [DOI] [Google Scholar]
- Sousa A, Casal S, Malheiro R, Lamas H, Bento A, Pereira JA. Aromatized olive oils: influence of flavouring in quality, composition, stability, antioxidants, and antiradical potential. LWT - Food Sci Technol. 2015;60:22–28. doi: 10.1016/j.lwt.2014.08.026. [DOI] [Google Scholar]
- Yildirim G (2009) Effect of storage time on olive oil quality. Master of Science in Food Engineering, Izmir, Institute of Technology