Abstract
Potentilla atrosanguinea, native to Himalayan region, is well known for its curative effects in traditional medicinal system. An ultra performance liquid chromatography–diode array detection method for the quantification of constituents of root part of P. atrosanguinea has been developed along with antioxidant activity evaluation. A simple and sensitive quantification method developed for seven compounds however only four compounds; p-coumaric acid (4), rutin (7), tiliroside (14) and kaempferol (16) were quantified as others were in lesser amount. Syringic acid and quercetin were found in trace amount whereas chlorogenic acid was absent in the ethanol extract of roots of P. atrosanguinea. Total polyphenolic and flavonoid contents were determined to be 21.75 mg of gallic acid equivalent and 8.57 mg of quercetin equivalent per gram of dry plant material, respectively. Antioxidant activity of extract was assessed using three assays; 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and ferric reducing antioxidant power (FRAP). The IC50 values; 35.75 μg/ml and 30.35 μg/ml by DPPH and ABTS assays for ethanolic extract showed excellent free radical scavenging potential of its root part. The ferric reducing ability (FRAP) value, 26.67 mg of ascorbic acid per gram also indicated its higher antioxidant potential.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3383-8) contains supplementary material, which is available to authorized users.
Keywords: Potentilla atrosanguinea, Rosaceae, UPLC–DAD, Polyphenols, Flavonoids, Antioxidant activity
Introduction
Potentilla atrosanguinea Lodd. is one of the about 500 species of Potentilla genus belongs to family Rosaceae, grown in Himalayan region. It is commonly known as larsu (Sharma et al. 2004) and dori (Rana and Samant 2011). The leaf part of P. atrosanguinea has been reported to be used as herbal tea while its roots are used as food which has a taste similar to sweet potatoes, parsnips or chestnuts (Kalia et al. 2008). Traditionally, Potentilla species used as medicines are mainly present in the different parts of Asia, North America and Europe (Tomczyk and Latté 2009). Potentilla species have ornamental as well as ethnomedicinal uses, the whole plant of most of the species are well documented in oriental systems of medicines. Several traditional uses of Potentilla species have been reported. Different species used for the treatment of various diseases such as cancers, viral infections, ulcers, fever, cough, tooth ache, diabetes, haemorrhage and enterobiasis (Tomczyk and Latté 2009). The medicinal uses of extracts of some Potentilla species have been mentioned in Chinese medicinal system as antirheumatic, detoxifiers and also used for the treatment of hepatitis, diarrhoea, scabies etc. (Xue et al. 2005, 2006).
A number of chemical constituents such as phenolic compounds, triterpenoids, flavonoids, condensed tannins and hydrolysable tannins have been reported from different Potentilla species. Tannins have been explained as important constituents of aerial and underground parts of Potentilla species which were responsible for most of their pharmacological activities such as astringent, anti-microbial, anti-inflammatory and immunomodulating (Tomczyk and Latté 2009). However limited data reports are available regarding the chemical constituents of P. atrosanguinea. In one of the study, flavonoids from flowers of some of the Potentilla species had been isolated by paper chromatography (Harborne and Nash 1984). Flavonol glycosides were identified in P. atrosanguinea Loddiges ex D. Don where as chalcones and flavanones were found absent (Harborne and Nash 1984). A HPLC quantification method had been developed which demonstrated the presence of phenolic compounds and potent antioxidant potential of P. atrosanguinea Lodd. var. argyrophylla (Kalia et al. 2008). Superoxide dismutase (SOD), a hyper-thermostable enzyme, has been isolated from P. atrosanguinea which was further engineered through the mutating single amino acid to increases its thermal stability two times (Kumar et al. 2012).
Polyphenols were also reported in two varieties of P. atrosanguinea cv. Hort. Hybr. and P. atrosanguinea cv. Scarlett (Świeżewska and Chojnacki 1989). Triterpenic acids have also been quantified by HPTLC method in P. atrosanguinea G. Lodd. ex D. Don (Świeboda et al. 2014). Most of methods reported for the identification and quantification of chemical constituents of Potentilla species are based on HPLC, GC–MS and HPTLC. However in recent years, for the analysis and quantification of natural chemical constituents, UPLC analysis methods have been used more comparatively to other quantification methods because of their higher sensitivity and less analysis time. A HPLC quantitative analysis method followed by UPLC qualitative method has been developed for the identification of polyphenols and tannins present in commercially available herbal products of P. tormentilla (Fecka et al. 2015). However there is no report regarding the UPLC method development and quantification of P. atrosanguinea chemical constituents in the literature best to our knowledge.
In the present study, a new method has been developed for UPLC quantification of chemical constituents of root part of P. atrosanguinea. The developed method was validated for four compounds; p-coumaric acid (4), rutin (7), tiliroside (14) and kaempferol (16) simultaneously. In addition, tentative identification of eight major compounds of root parts of P. atrosaguinea by the same method was carried out using mass fragmentation pattern. Furthermore total phenolic content, total flavonoid content and antioxidant activity of root extracts were evaluated.
Materials and methods
Material
P. atrosanguinea plant material was collected in the month of August 2013, from Lahaul & Spiti district of Himachal Pradesh, India, situated at an elevation of 4590 m from sea level. Plant material was identified by the taxonomist Dr. Brij Lal and voucher specimen (voucher number-PLP-16515) deposited in herbarium of the CSIR-IHBT, Palampur, India.
Chemicals
Chlorogenic acid, syringic acid and quercetin were purchased from sigma Aldrich, India. p-Coumaric acid (4), rutin (7), tiliroside (14) and kaempferol (16) were isolated from root part of P. atrosanguinea and characterized by comparison of MS, 1D- and 2D-NMR spectral data. DPPH, ABTS, ascorbic acid, potassium persulphate and gallic acid were purchased from Sigma Aldrich. Ferric chloride and potassium acetate were purchased from Hi-media Lab, Mumbai and Ranbaxy, New Delhi respectively. All the analytical grade solvents; methanol, water and formic acid were purchased from J. T. Baker (Avantor Performance Material Inc., Center Valley, PA, USA). All other chemicals and solvents used were of analytical grade.
Extraction and isolation
The dried underground part (1.5 kg) of P. atrosanguinea was percolated with 95% EtOH at room temperature (3.5 L × 1, followed by 1.5 L × 7, 95: 5, v/v). The ethanol extracts were combined and evaporated to dryness under vacuum at 45 °C to obtain residue (67 g). Part of this extract (57 g) was suspended in distilled water followed by successive partitions with CHCl3 (500 ml × 3, 8 g), EtOAc (500 ml × 3, 11.6 g) and n-butanol (500 ml × 3, 20 g). The CHCl3 and EtOAc extracts were combined on the basis of similar TLC profiling. By repeated column chromatography using silica gel (230–400 mesh size) and reverse phase silica gel (C18) leads to isolation of four compounds; (4), (7), (14) and (16). NMR data of isolated molecules was recorded in CD3OD solvent on NMR Bruker 600 MHz for their characterization using TMS as an internal standard.
Standard preparation
Stock solutions of individual standard compounds p-coumaric acid (4), rutin (7), tiliroside (14), kaempferol (16), syringic acid, quercetin and chlorogenic acid of 1 mg/ml were prepared in methanol.
Sample preparation
Microwave assisted extraction was performed in convection microwave (IFB) using 3.5 g powdered plant material and 100 ml extraction solvent (ethanol) at 1400 W power for 5 min. The extract was filtered, concentrated under reduced pressure and lyophilized. The extract was dissolved in methanol to get a final concentration of 10 mg/ml. The resulted stock solution was filtered through a Whatmann PTFE syringe filter of 0.2 μm and for analysis, 1 μl of the sample was injected into UPLC–DAD system.
UPLC conditions
The UPLC analysis was carried out on a Waters ACQUITY Ultra High Performance LC system (Water, Milford, MA, USA) equipped with an autosampler, a binary solvent delivery pump, PDA detector and Masslynx v4.1 software. UPLC BEH C18 column (Waters Acquity), 2.1 mm × 100 mm, 1.7 μm particle size with column temperature 30 °C was used. For UPLC analysis, mobile phase consisted of 0.05% formic acid in water (A) and methanol (B) was used. The gradient programme was: 0–0.50 min, 90%; 2.0–7.0 min, 75%; 12.0–14.0 min, 30%; 15.0–18.0 min, again 90% of A. The injection volume was 1 μl and PDA detection wave length was set at 265 nm.
Mass spectrometry conditions
MS/MS was performed on Q-TOF mass spectrometer equipped with an ESI source (Micromass, Manchester, UK) and Masslynx v4.1 software. Data acquisition for UPLC analysis was carried out using positive ion mode over a mass range of m/z 100–2000. Positive as well as negative ion ESI–MS analysis was carried out by direct infusion using a syringe pump with a flow rate of 0.25 ml min−1. The MS parameters were set as, cone voltage 22 V; capillary voltage 3.1 kV; desolvation temperature 200 °C. The desolvation gas flow and cone gas were set 500 L/h and 50 L/h respectively. The source temperature, scan time and interscan delay were set at 80 °C, 1.0 s and 0.1 s respectively.
Method validation
The validation of method includes determination of linearity, LOD, LOQ, selectivity, precision and recovery. The calibration curves were obtained by plotting the nominal standard concentration (x) versus the peak area (y) of the analytes and linearity was determined by the analysis of a series of standard solutions of 4, 7, 14 and 16. Compounds were identified in the sample by comparing their retention time and UV λmax with standards. For each standard, the diluted standard solutions were injected for the evaluation of LOD and LOQ till signal to noise ratio (S/N) for LOD was 3:1 and for LOQ, 10:1. The repeatability and reproducibility of the developed method were ascertained by measuring the intra-day and inter-day variability. A sample was analyzed three times per day and three consecutive days for evaluating intra-day and inter-day variability. The precision for intra-day and inter-day was expressed as percentage of relative standard deviation (% RSD). The accuracy of the developed method was assessed by recovery study. For this standard solutions with three different concentration levels were added to the samples and these samples were analyzed by the developed method after extraction.
Total phenolic content
Total phenolic content (TPC) in the ethanolic extract of root part of P. atrosanguinea was determined by Folin–Ciocalteu’s reagent (Walia et al. 2016). Gallic acid (1 mg/ml, prepared in 80% aqueous ethanol) was used as a standard for the preparation of standard curve. Sequential dilutions of aqueous gallic acid; 20, 40, 60, 80, 100 μg/ml were prepared. Stock solution of sample, 2 mg/ml was prepared in ethanol. Each sample 100 μl, was mixed with 1.0 ml 35% Na2CO3 and 0.5 ml of Folin–Ciocalteu’s reagent in a volumetric flask of 25 ml and distilled water was added to make total volume up to 25 ml. Absorbance of the sample was measured at 730 nm using UV–visible spectrophotometer (UV 2450 Shimadzu spectrophotometer) after incubating at room temperature for 30 min. TPC of root part of P. atrosanguinea was expressed as mg of gallic acid equivalent (GAE) per gram of dry plant material. TPC of sample was determined in triplicate.
Total flavonoid content
Total flavonoid content (TFC) in root extract was measured using method described by Walia et al. (2016). Stock solutions; sample (2 mg/ml) and standard quercetin (1 mg/ml) were prepared in ethanol and 80% of aqueous ethanol, respectively. Different concentrations; 20, 40, 60, 80 and 100 μg/ml of standard were prepared. 1.5 ml ethanol, 0.1 ml 10% aluminium chloride and 0.1 ml potassium acetate were mixed to each 100 μl of sample, after that total volume was made up to 5 ml. After 30 min incubation at room temperature, absorbance was measured at 415 nm using UV–visible spectrophotometer. TFC of sample was expressed as quercetin equivalent (QE) per gram of dry plant material. For the TFC determination, assay was performed in triplicates.
Evaluation of antioxidant activity
Free radical-scavenging activity using DPPH radical
The DPPH radical-scavenging activity was determined by the procedure based on previously reported method proposed by Kalia et al. (2008) with slight modifications. Different concentrations of ascorbic acid standard (5, 10, 15, 20, 25 μg/ml) and sample (20, 40, 60, 80, 100 μg/ml) were prepared in ethanol. 100 μl of each sample was mixed with 1.9 ml of 0.1 mM DPPH solution prepared in ethanol. Absorbance was recorded at 517 nm after the 30 min incubation at room temperature in dark using UV–Vis spectrophotometer. Results of DPPH assay were expressed as IC50, which indicates the sample concentration required to inhibit 50% DPPH free radicals. Assay was performed in triplicates. The percentage inhibition of DPPH free radical (I%) was calculated as follows:
where Ac is the absorbance of control and As is the absorbance of extract. By plotting a graph between percentage inhibition (I%) and concentrations of the extract.
Free radical scavenging ability using ABTS
The free radical scavenging activity by ABTS was measured according to Kalia et al. (2008). 7 mM ABTS solution prepared in water was mixed to 2.45 mM potassium persulphate solution and kept the mixture at room temperature in dark for 12–16 h to produce ABTS free radical. The absorbance of resulted ABTS+ solution was set at 0.700 ± 0.02 at 734 nm by diluting with ethanol. Different concentrations; 5, 10, 15, 20 and 25 μg/ml of standard ascorbic acid were prepared in ethanol. Sample was also diluted to different concentrations; 20, 40, 60, 80, 100 μg/ml in ethanol. 2 ml of ABTS+ solution was added to 100 μl of each sample, incubated for 4 min and absorbance was measured at 734 nm using UV–Vis spectrophotometer. Assay was performed in triplicates. ABTS+ inhibition activity of ethanolic extract was expressed as IC50, which indicates the sample concentration required to scavenge 50% ABTS+. The percentage inhibition of ABTS free radical (I%) was measured as follows:
where Ac represents the absorbance of control and As shows the absorbance of extract. For the calculation of the IC50 of the extracts, a graph was plotted between inhibition percentages of the concentrations.
Ferric-reducing antioxidant power (FRAP) assay
FRAP assay was carried out using the method based on Walia et al. (2016) and Benzie and Strain (1996). The capability of antioxidant to reduce tripyridyltriazine complex of Fe3+ i.e. (Fe3+/TPTZ) into blue colored complex Fe2+/TPTZ is measured. 0.3 M acetate buffer (pH = 3.6), 10 mmol of TPTZ solution 40 mmol of HCl and 20 mmol of ferric chloride hexahydrate solutions in the ratio 10: 1: 1 (v/v/v) were mixed and heated at 37 °C to prepare FRAP reagent. Stock solution; 1 mg/ml of sample (ethanolic extract) and different standard (ascorbic acid) concentrations; 5, 10, 15, 20, 25 μg/ml were prepared in ethanol. To each sample (100 μl), 1.5 mL of FRAP solution was added and incubated for 4 min. The absorbance was measured at 593 nm using UV–Vis spectrophotometer. FRAP assay measurements performed in triplicates.
Results and discussion
Isolation and identification of compounds
Four compounds were isolated from combined ethyl acetate and chloroform extract by column chromatography. These compounds were identified as p-coumaric acid (4), rutin (7), tiliroside (14) and kaempferol (16) by comparing their spectral data with literature (Agnihotri et al. 2008; Kazuma et al. 2003; Iwashina and Kokubugata 2012; Vega et al. 2007). NMR data has been provided in supplementary information for all the isolated molecules (Suppl. Figures 1–4).
Optimization of UPLC conditions
The chromatographic conditions were optimized for the better resolution with standard mixture and sample solutions. Different proportions of ACN, methanol and water were tested; 0.05% formic acid in water and methanol was optimized for the better separation and good peak shape. The column temperature was set at 30 °C to achieve better base line resolution after the optimization of the different temperatures 25, 30, 35 and 40 °C. Seven compounds chlorogenic acid (retention time, tR: 3.98), syringic acid (tR: 5.30), p-coumaric acid (4; tR: 7.52), rutin (7; tR: 10.46), quercetin (tR: 11.86), tiliroside (14; tR: 12.04) and kaempferol (16; tR: 12.56) were separated. Figure 1 shows the chromatograms of standards and sample by comparing retention times and UV λmax of standard, compounds in the sample were identified.
Fig. 1.
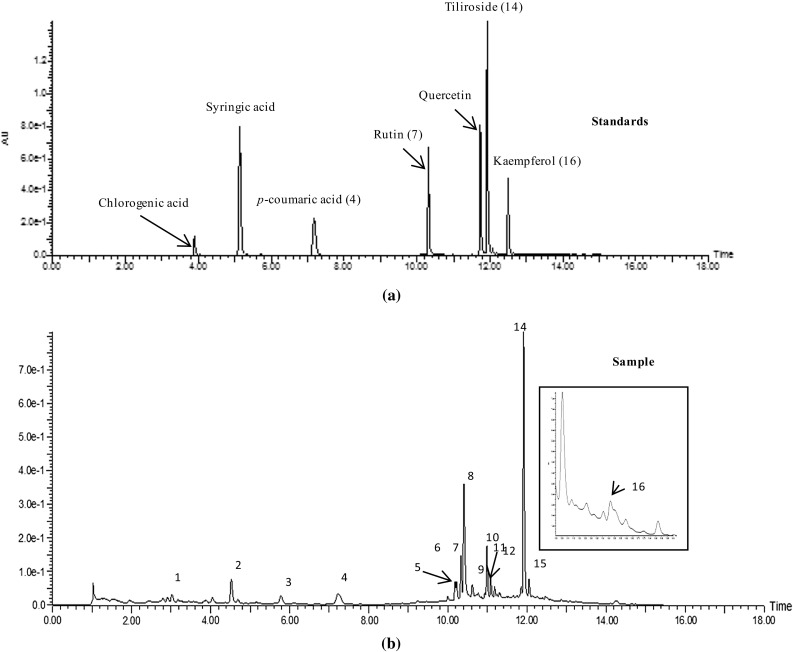
UPLC chromatogram of standards (a) and sample (b)
Validation of parameters
A fast and sensitive UPLC–DAD method was developed for the simultaneous quantification of seven compounds in root extract of P. atrosanguinea. The calibration curves were prepared by injecting standard solutions of ten concentration levels keeping injection volume constant in the range of 0.195–100 μg/ml for all the standards (4, 7 and 14); for 16, 0.195–50 μg/ml) and good linearity of method was showed regression coefficients (R2) ranging from 0.997 to 0.994 (Table 1). The LODs and LOQs for the compounds 4, 7 and 14 were 0.012 and 0.04 μg/ml respectively. For the compound 16, LOD and LOQ were 0.048 and 0.14 μg/ml respectively. The intra- and inter-day precisions; expressed as I% relative standard deviation (% RSD), obtained in the range of 0.32–0.59 μg/ml and 1.2–1.8 μg/ml, respectively. Recovery of each standard was determined by spiking the sample with three different concentrations (Suppl. Table 1); I% recovery was obtained in the range of 95.4–102.0% having I% RSD ranging from 1.24 to 2.5% for the quantified compounds. These results showed excellent recovery having all the values within acceptable limits; which indicates the extraction of phenolic compounds was nearly complete during sample processing. The overall validation results showed good linearity, accuracy, precision and sensitivity of developed UPLC–DAD method, therefore this could be used for the quantification analysis of other phenolic bearing plants.
Table 1.
Method validation data
| Compounds | Regression equation | Linearity (μg/mL) | R 2 | LOD (μg/mL) | LOQ (μg/mL) | Intraday Precision (n = 6) | Interday Precision (n = 3) |
|---|---|---|---|---|---|---|---|
| 4 | y = 237.7x + 254.6 | 0.195–100 | 0.997 | 0.012 | 0.04 | 0.42 | 1.8 |
| 7 | y = 196.9x + 204.3 | 0.195–100 | 0.997 | 0.012 | 0.04 | 0.57 | 1.6 |
| 14 | y = 232.8x + 255.4 | 0.195–100 | 0.996 | 0.012 | 0.04 | 0.32 | 1.7 |
| 16 | y = 236.5x + 87.92 | 0.195–50 | 0.998 | 0.048 | 0.14 | 0.59 | 1.2 |
UPLC–DAD–ESI–MS–MS analysis
The retention times, MS/MS data and UV λmax of four identified compounds were compared with their standards and literature. A positive ion mode ESI–MS–MS was used for the identification of compounds. The LC–MS–MS, their mass fragmentations and UV λmax of quantified compounds were presented in Suppl. Table 2. Compound 4, p-coumaric acid has molecular ion peak at m/z 165 [M + H]+ in the mass spectra and its fragmentation at m/z 147 [M + H–H2O]+ showed the loss of a water molecule (Wang et al. 2012). In mass spectra of compound 7, rutin; there is a loss of rhamnose and sugar units shown by fragmentations at m/z 465 [M + H–rhm]+ and 303 [M + H–glu]+ respectively (Cuyckens and Claeys 2004). From mass spectra of compound 14, tiliroside, mass fragmentations at m/z 309 [M + H–coumaroylglucoside]+ indicates the loss of coumaroylglucoside unit and at m/z 147 showed a characteristics fragment of coumaroyl unit (Felipe et al. 2014). The mass spectra of compound 16, kaempferol showed the fragmentations at m/z 259 [M + H–CO]+ indicates loss of a carbon monoxide unit, 241 [M + H–H2O–CO]+ loss of water molecule and a carbon monoxide unit, 213 [M + H–H2O–2CO]+ loss of water molecule with two molecules of carbon monoxide units (Tsimogiannis et al. 2007).
In addition to these four quantified molecules (4, 7, 14 and 16); eight compounds were also tentatively identified from P. atrosanguinea root extract by mass fragmentations and UV λmax (Suppl. Table 3). Mainly derivatives of quercetin, kaempferol and rhamnetin were identified tentatively. Karanjin (2) was observed at tR 4.52 and m/z 293 [M + H]+ with UV λmax 215, 276 and 354 (Perumalsamy et al. 2015; Yi et al. 2015); at tR 5.78 rhamnetin-O-diglucuronide (3) was observed at m/z 669 with fragmentations at 493 [M + H–glucuronide]+ and 317 [M + H–two units of glucuronide]+ by elimination of one and two units of glucuronide, respectively, UV λmax: 201, 253 and 353 (Schieber et al. 2002). Quercetin glucuronide (6) was observed at tR 10.22, having m/z 479 [M + H]+ with fragment at m/z 303 [M + H–glucuronide]+ with UV λmax 256 and 344 (Iwashina et al. 2012) whereas at tR 10.41 with m/z 475, major fragmentation at m/z 301 showed quercetin derivative (8) by negative mass fragmentation pattern. At tR 10.99, kaempferol derivative (10) with m/z 918 [M + H]+ with fragmentations at 617, 471, 287 and UV λmax 264, 346. Another kaempferol derivative (12) at tR 11.18 was observed i.e. kaempferol-3-O-glucosyl rhamnosyl-(p-coumaroyl) hexoside having m/z at 903 [M + H]+ with fragmentation at 287 (Duo et al. 2007) and UV λmax 268 and 338. At tR 11.09, rhamnetin derivative (11) was observed at m/z 984 with fragmentations at 623, 491, 315 identified by negative mass fragmentation pattern with UV λmax 203, 253, 354 (Schieber et al. 2002). Another compound; quercetin glucuronide (13) was observed at tR 11.30 with m/z 479 [M + H]+ with fragmentation at m/z 303 [M + H–glucuronide]+ (Iwashina et al. 2012).
Quantification of constituents
The polyphenol and flavonoid compounds in the 80% aqueous ethanol extract are presented in Table 2 and their structures are given in Suppl. Figure 5. Tiliroside (14) was present in higher amount (0.76 mg/g of dry plant material) in the sample. The other identified compounds were kaempferol (16), followed by p-coumaric acid (4) and rutin (7). However chlorogenic acid was found absent in the sample, whereas syringic acid and quercetin were found in trace amount in the sample. In the previous study, HPLC quantification of aerial part of P. atrosanguinea showed the presence of phenolic compounds; chlorogenic acid, (+)-catechin, caffeic acid, p-coumaric acid and quercetin (Kalia et al. 2008).
Table 2.
Amount of quantified compounds presents in root part of P. atrosanguinea
| Compound | Amount (mg/g of plant material) |
|---|---|
| Chlorogenic acid | Absent |
| Syringic acid | Traces |
| p-Coumaric acid (4) | 0.13 |
| Rutin (7) | 0.14 |
| Quercetin | Traces |
| Tiliroside (14) | 0.9 |
| Kaempferol (16) | 0.032 |
Total polyphenol and flavonoid content
Total polyphenol content was 21.75 mg of GAE/g of dry plant material. However polyphenol content of aerial part was slightly higher, 26.7 ± 0.11 to 30.7 ± 0.05 mg of GAE/g for dry plant material (Kalia et al. 2008). Total flavonoid content of P. atrosanguinea root part was 8.57 mg of QE/g of dry plant material whereas content of aerial part was reported to be higher, 16.8 ± 0.07 to 20.8 ± 0.02 mg of QE/g of dry plant material (Kalia et al. 2008). The total polyphenol and flavonoid content in root part of P. atrosanguinea are presented in Table 3. The therapeutic effects of Potentilla species are well explicated and most of these could be described by the high content of polyphenols (Tomczyk et al. 2010). In addition, one report showed that the phenolic content of plants used as herbal tea helps in lowering coronary heart diseases and risk of atherosclerosis (Shahidi and Wanasundara 1992). Jaitak et al. (2010) has evaluated the total phenolic content of different extract/fractions (methanol, ethyl acetate, butanol and water) of root part of P. fulgens (another species of Potentilla from Himalayan region). They also showed that high phenolic content which has been varied from 20.61 ± 0.38 to 33.28 ± 0.11 mg GAE/g of dry plant material.
Table 3.
Total polyphenol and flavonoid content of P. atrosanguinea root extract
| Sample | Total polyphenol contenta | Total flavonoid contentb |
|---|---|---|
| PA-RT | 21.75 | 8.57 |
aData expressed as mg of GAE/g of dry plant material
bData expressed as mg of QE/g of dry plant material
Antioxidant activity
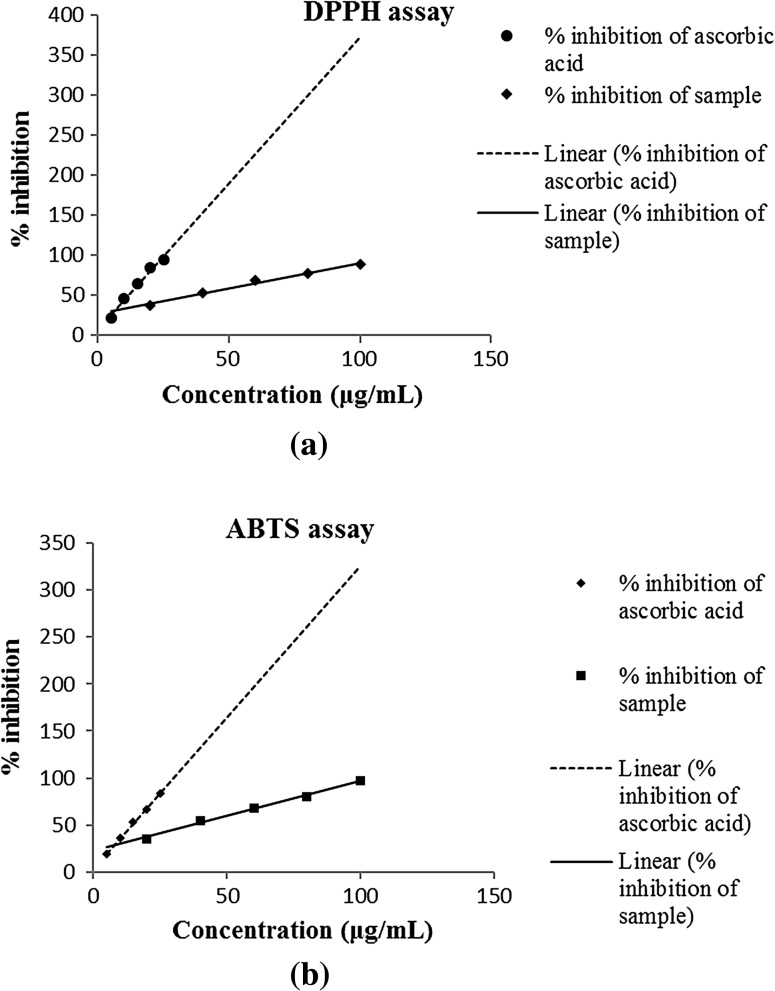
DPPH assay showed, IC50 for standard ascorbic acid and sample were 11.63 and 35.79 μg/ml, respectively (Table 4). ABTS assay also showed similar results as IC50 was 14.52 μg/ml for ascorbic acid and 30.35 μg/ml for sample. The free radical scavenging by sample measured by DPPH and ABTS assays is shown in Fig. 2. Antioxidant activity measured by FRAP assay was 18.1 mg of ascorbic acid per gram. Our results showed slightly less antioxidant ability of root part as compared earlier for its aerial part (Kalia et al. 2008). Natural antioxidants are of vast importance due to their usage in food supplements and treatment of oxidative stress-related diseases (Kumar et al. 2016). Antioxidant activity of different extract/fractions of root part of P. fulgens also revealed the high scavenging potential. DPPH and ABTS assays showed variation from 0.60 ± 0.04 to 2.41 ± 0.05 and 0.67 ± 0.07 to 2.54 ± 0.06 respectively mM trolox equivalent/mg of extract. Ferric reducing ability was found to vary from 0.72 ± 0.0 to 3.57 ± 0.05 mM trolox equivalent/mg of extract by Jaitak et al. (2010).
Table 4.
Antioxidant activity of P. atrosanguinea root extract
| Sample | DPPH (IC50 in μg/mL) | ABTS (IC50 in μg/mL) | FRAP (mg of ascorbic acid per gram) |
|---|---|---|---|
| Ascorbic acid | 11.63 | 14.52 | – |
| PA-RT | 35.79 | 30.35 | 18.1 |
Fig. 2.
Antioxidant activity of root extract a DPPH assay; b ABTS assay
Conclusion
A new simple and sensitive UPLC-PDA method has been developed for the separation and quantification of seven phenolic compounds of root extract of P. atrosanguinea. This method could be applied for the routine qualitative and quantitative analysis of phenolic constituents in any plant species or their part. The results showed analysis depicted considerable amount of phenolic content and excellent scavenging potential of root extract of P. atrosanguinea that can be used as a source of natural oxidants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors gratefully acknowledge Dr Sanjay Kumar, the Director, CSIR-IHBT, Palampur, India for providing encouragement and support during the course of work. Authors would also like to thanks CSIR, New Delhi, India for funding BSC-106 and BSC-209 projects under which this work was carried out.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Agnihotri VK, ElSohly HN, Khan SI, Smillie TJ, Khan IA, Walker LA. Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry. 2008;69:2061–2066. doi: 10.1016/j.phytochem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as measurement of “antioxidant power”: The Frap assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- Duo J, Lee VSY, Tzen JTC, Lee MR. Identification and composition of phenolic compounds in the preparation of oolong tea manufactured by semifermentation and drying processes. J Agric Food Chem. 2007;55:7462–7468. doi: 10.1021/jf0718603. [DOI] [PubMed] [Google Scholar]
- Fecka I, Kucharska AZ, Kowalczyk A. Quantification of tannins and related polyphenols in commercial products of tormentil (Potentilla tormentilla) Phytochem Anal. 2015;26:353–366. doi: 10.1002/pca.2570. [DOI] [PubMed] [Google Scholar]
- Felipe DF, Brambilla LZS, Porto C, Pilau EJ, Cortez DAG. Phytochemical analysis of Pfaffia glomerata inflorescences by LC-ESI-MS/MS. Molecules. 2014;19:15720–15734. doi: 10.3390/molecules191015720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB, Nash RJ. Flavonoid pigments responsible for ultraviolet patterning in petals of the genus Potentilla. Biochem Syst Ecol. 1984;12:315–318. doi: 10.1016/0305-1978(84)90055-3. [DOI] [Google Scholar]
- Iwashina T, Kokubugata G. Flavone and flavonol glycosides from the leaves of Triumfetta procumbens in Ryukyu Islands. Bull Natl Mus Nat Sci Ser B. 2012;38:63–67. [Google Scholar]
- Iwashina T, Smirnov SV, Damdinsuren O, Kondo K. Flavonoids from Reaumuria soongarica (Tamaricaceae) in Mongolia. Bull Natl Mus Nat Sci Ser B. 2012;38:189–195. [Google Scholar]
- Jaitak V, Sharma K, Kalia K, Kumar N, Singh HP, Kaul VK, Singh B. Antioxidant activity of Potentilla fulgens: an alpine plant of western Himalaya. J Food Compos Anal. 2010;23:142–147. doi: 10.1016/j.jfca.2009.02.013. [DOI] [Google Scholar]
- Kalia K, Sharma K, Singh HP, Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J Agric Food Chem. 2008;56:10129–10134. doi: 10.1021/jf802188b. [DOI] [PubMed] [Google Scholar]
- Kazuma K, Noda N, Suzuki M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry. 2003;62:229–237. doi: 10.1016/S0031-9422(02)00486-7. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dutt S, Bagler G, Ahuja PS, Kumar S. Engineering a thermo-stable superoxide dismutase functional at sub-zero to >50 °C, which also tolerates autoclaving. Sci Rep. 2012;2:387. doi: 10.1038/srep00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kumar P, Koundal R, Agnihotri VK. Antioxidant properties and UPLC-MS/MS profiling of phenolics in jacquemont’s hazelnut kernals (Corylus jacquemontii) and its byproducts from western Himalaya. J Food Sci Technol. 2016;53:3522–3531. doi: 10.1007/s13197-016-2329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumalsamy H, Jin Jang M, Kim JR, Kadarkarai M, Ahn YJ. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasit Vectors. 2015;8:237. doi: 10.1186/s13071-015-0848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana MS, Samant SS. Diversity, indigenous uses and conservation status of medicinal plants in Manali wildlife sanctuary, North western Himalaya. Indian J Tradit Knowl. 2011;10:439–459. [Google Scholar]
- Schieber A, Keller P, Streker P, Klaiber I, Carle R. Detection of isorhamnetin glycosides in extracts of apple (Malus domestic acv. “Brettacher”) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem Anal. 2002;13:87–94. doi: 10.1002/pca.630. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Sharma PK, Chauhan NS, Lal B. Observations on the traditional phytotherapy among the inhabitants of Parvati valley in western Himalaya, India. J Ethnopharmacol. 2004;92:162–176. doi: 10.1016/j.jep.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Świeboda R, Jóźwiak A, Jóźwiak G, Waksmundzka-Hajnos M. Thin layer chromatography and chemometric studies of selected Potentilla species. Am J Anal Chem. 2014;5:1109–1120. doi: 10.4236/ajac.2014.516118. [DOI] [Google Scholar]
- Świeżewska E, Chojnacki T. The occurrence of unique, long-chain polyprenols in the leaves of Potentilla species. Acta Biochim Pol. 1989;36:143–158. [PubMed] [Google Scholar]
- Tomczyk M, Latté KP. Potentilla—A review of its phytochemical and pharmacological profile. J Ethnopharmacol. 2009;122:184–204. doi: 10.1016/j.jep.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Tomczyk M, Pleszcyńska M, Wiater A. Variation in total polyphenolics contents of aerial parts of Potentilla species and their anticarcinogenic activity. Molecules. 2010;15:4639–4651. doi: 10.3390/molecules15074639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. Characterization of flavonoid subgroups and hydroxyl substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega MRG, Esteves-Souza A, Vieira IJC, Mathias L, Braz-Filho R, Echevarria A. Flavonoids from Annona dioica leaves and their effects in Ehrilich Carcinoma cells, DNA-topoisomerase I and II. J Braz Chem Soc. 2007;18:1554–1559. doi: 10.1590/S0103-50532007000800016. [DOI] [Google Scholar]
- Walia M, Kumar S, Agnihotri VK. UPLC-PDA quantification of chemical constituents of two different varieties (golden and royal) of apple leaves and their antioxidant activity. J Sci Food Agric. 2016;96:1440–1450. doi: 10.1002/jsfa.7239. [DOI] [PubMed] [Google Scholar]
- Wang J, Yue YD, Tang F, Sun J. Screening and analysis of the potential bioactive components in rabbit plasma after oral administration of hot-water extracts from leaves of Bambusa textilis McClure. Molecules. 2012;17:8872–8885. doi: 10.3390/molecules17088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue PF, Luo G, Zeng WZ, Zhao YY, Liang H. Secondary metabolites from Potentilla multifida L. (Rosaceae) Biochem Syst Ecol. 2005;33:725–728. doi: 10.1016/j.bse.2004.12.012. [DOI] [Google Scholar]
- Xue PF, Zhao YY, Wang B, Liang H. Secondary metabolites from Potentilla discolour Bunge (Rosaceae) Biochem Syst Ecol. 2006;34:825–828. doi: 10.1016/j.bse.2006.07.003. [DOI] [Google Scholar]
- Yi D, Wang Z, Yi L. Development and validation of an LC-MS method for determination of karanjin in rat plasma: application to preclinical pharmacokinetics. J Chromatogr Sci. 2015;53:456–461. doi: 10.1093/chromsci/bmu064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.