Abstract
Chitosan-based coatings and films have been widely studied, demonstrating to be an efficient and eco-friendly approach to extend the shelf life of food products. The effect of incorporating Thymus capitatus essential oil (TCEO) at different concentrations (0.5, 1.0, and 1.5% w/w) on physical, mechanical and antimicrobial properties of chitosan films was studied. The antimicrobial activity of the films was evaluated by agar diffusion method, against 23 spoiling microorganisms isolated from tuna and swordfish (ten Shewanella baltica, one S. morhuae, one S. putrefaciens, two Pseudomonas fluorescens, two P. fragi, five Serratia spp., one Aeromonas molluscorum, and one Acinetobacter radioresistens) and Shewanella putrefaciens ATCC 49138. The films exerted antimicrobial activity against all the tested strain, although not proportional to increasing TCEO concentration. In particular, S. baltica was the most sensitive species and the inhibition was stable after 72 h. In general, TCEO incorporation in chitosan films, significantly (p < 0.05) decreased the water permeability (from 0.577 ± 0.060 gmm/kPahm2 at 61% R.U. for chitosan to 0.487 ± 0.037 gmm/kPahm2 for the film with 1.5% TCEO), the elongation at brake (from 27.322 ± 2.35% for chitosan to 14.695 ± 3.99% for the film with 1.5% TCEO) and increased the tensile strength (from 1.697 ± 0.16% for chitosan to 19.480 ± 2.86% for the film with 1.5% TCEO). Moisture content and water contact angle of the films also showed a similar trend with TCEO introduction, because of crosslinking reaction among the polymer chains and TCEO components. Scanning electron microscopy confirmed structure-properties relationships. These results suggest chitosan films incorporated with TCEO as an alternative treatment to inhibit the growth of degradative bacteria with potential application in the fish industry. The importance of testing more than one strain of the same bacteria species to evaluate the effectiveness of chitosan-essential oils coatings was also demonstrated.
Keywords: Specific spoilage organisms, Chitosan film, Fish, Thymus capitatus essential oil, Antibacterial activity
Introduction
The consumption of fish around the world is high, due to its excellent nutritional properties and flavor (Elsabee and Abdou 2013). Fresh fish has a short shelf-life, for its physical–chemical characteristics, which cause substantial practical problems for its distribution. Thus, improvements in the shelf-life of fish and fish products, could give more choice to the consumers in terms of product availability, and to the producers as regards significant economic incomings by reducing losses attributed to the product spoilage.
Many strategies have been conducted to control spoiling and pathogenic microorganisms, but still remains the need for new sustainable and safe methods; for example, the use of coatings with biodegradable polymers (polysaccharides, lipids, and proteins) having good film-forming and barrier properties is an alternative to synthetic polymers for food packaging (Gómez-Estaca et al. 2010).
In the past years, chitosan-based films have been proposed to reduce microbial contamination in foods, as they are tenacious, enduring and extensible (Jeon et al. 2002). Furthermore, on account of antibacterial activity and non-toxicity, this biopolymer has been used as antimicrobial film, also including antimicrobial components in different applications (Elsabee and Abdou 2013). The research has been focused on the addition of essential oils (EO) to chitosan films. EOs exert strong antibacterial activities (Serio et al. 2010; Paparella et al. 2016), however, their direct use as natural preservatives on foods could be limited because of their volatility and degradability by light and oxygen radicals, thus affecting foods taste and aroma. EOs can be incorporated into edible films and coatings to modify flavor, and odor, besides to boost antimicrobial characteristics (Ballester-Costa et al. 2016).
Thymus capitatus (L.) Hoffmanns et Link (sin. Corydothymus capitatus) known as “Spanish Oregano”, belongs to the Lamiaceae family, common in the Mediterranean region. It is normally used in food, although medicinal applications have also been reported (Tabti et al. 2014) in view of its antibacterial, antifungal, antiviral and antioxidant activities (Bounatirou et al. 2010).
The incorporation of Thymus EO into chitosan films has been proposed, due to the high content of the antimicrobial compounds thymol and carvacrol (Ballester-Costa et al. 2013). Thymus capitatus EO (TCEO), normally used for culinary purposes, has been studied for the inhibition of meat spoilage (Ballester-Costa et al. 2013) and for meat preservation. Thyme EO has been proposed to extend fish shelf life (Ozogul et al. 2017; Kykkidou et al. 2009).
Chitosan coating combined with EOs provides protective effect against food-borne pathogens in meat products (Upadhyay et al. 2015), smoked eel fillets (El-Obeid et al. 2018) and raw chicken fillets (Khanjari et al. 2013), mainly for the inhibitory effect against L. monocytogenes. Chitosan coatings was effective in refrigerated storage of fishery products: the decay of sliced fresh Channa argus was retarded and the shelf life significantly extended by approximately 4–5 days (Yang et al. 2015). Recently, fresh Yellowfin tuna has been preserved using nano-chitosan (Tapilatu et al. 2016), which had a bacteriostatic effect, and somewhat blocked the autolysis process until the end of the storage period, as suggested by the total volatile base nitrogen (TVB-N).
However, in refrigerated fish, mesophilic or psycrotrophic total viable count (TVC) is usually monitored during storage, while this parameter could often underestimate the cell number, being some fastidious microorganisms unable to be detected in commonly used culture media. Among these, Specific Spoilage Organisms (SSOs) such as hydrogen sulfide-producing bacteria, or Gram negatives such as Pseudomonas spp. and Serratia ssp., which are the main responsible of fish microbial spoilage, are included (Serio et al. 2014).
The aims of the present work were: to investigate: (1) the physical–chemical properties and antibacterial activity of chitosan films incorporated with Thymus capitatus essential oil (TCEO) and (2) the potential effect of this film on hindering finfish spoiling, against several Specific Spoilage Organisms (SSO) such as Shewanella baltica, S. morhuae, S. putrefaciens, Pseudomonas fluorescens, P. fragi, Serratia spp., and Aeromonas molluscorum, previously isolated from swordfish and tuna for the antimicrobial evaluation.
Materials and methods
Film preparation
Chitosan-based film was prepared by dissolving chitosan (deacetylation degree of 85%, Mw = 190.000–310.000 Da, Sigma, Milwaukee, WI, USA) to a concentration of 2% (w/w) in aqueous solution of acetic acid (1% v/v). The resultant solution was filtered through a Whatman No. 3 filter paper, then, glycerol (Sigma, Milwaukee, WI, USA) was added at 0.75 mL/g of chitosan as plasticizer and stirred for 30 min. 0.2% Tween 80 (v/v with respect to the EO volume) was added as emulsifier. After 1 h, TCEO from Spain ref W282812 (Sigma, Milwaukee, WI, USA) enhanced with nature-identical substances, was added to reach the final concentration of 0.5, 1.0, and 1.5% (v/v, with respect to the chitosan solution), using an IKA T25-Digital Ultraturrax (Staufen, Germany) at 7000 rpm for two min. After that, solutions (160 mL) were casted on the center of 27 cm × 27 cm glass plates, and dried for 30 h at 25 °C. These concentrations were selected because in previous experiments they showed strong antimicrobial activity against the SSO here studied.
GC–MS analysis of Thymus capitatus essential oil
The composition of Thymus capitatus EO was determined by gas chromatography-mass spectrometry (GC–MS) as previously reported (Delgado et al. 2016). Briefly, the composition was determined in a gas chromatograph spectrometer AT 6890 Series plus (Agilent Technologies, Palo Alto, CA, USA), with a mass selective detector (Agilent Technologies, MSD 5975, Inert XL) (full scan), using DB-5MS fused silica capillary column (60 m, 0.25 mm; 0.25 M, J&W Scientific Inc., Folsom, CA, USA). The temperature program was 10 min at 60 °C, then to 250 °C at 5 °C/min, held for 10 min. Other operating conditions were: carrier gas, helium (99.99%), with a flow rate of 1.1 mL/min; injection volume of 2:1 and split ratio 1:30; 0.1 L of samples were injected manually in split mode. Samples were injected automatically and all the tests were performed in triplicate.
The identification of the components was performed using electron ionization (EI, 70 eV), with mass range from m/z 50–550. The constituents were identified by comparing their RI (retention index) with those provided in the literature, and comparing the mass spectra with those recorded by Adams database (Wiley, 138 and NIST05).
Physical, mechanical, thermal and antimicrobial properties
Mechanical test
The tensile strength (TS), Young Module (YM) and elongation at break (EAB) on the films were measured with a mechanical analyzer (Shimadzu EZ-LX, Japan) according to the ASTM D882–12 (2012). Each film was cut into three dumbbell strips with a size of 165 mm × 30 mm, to obtain a test size of 125 mm × 22 mm. The lower grip was fixed, and the upper grip rose at an extension rate of 12 mm/min with a preload of 1.0 N. Five samples of each film were analyzed.
Surface properties measurements
Surface properties of the films were determined using contact angle measurements and atomic force microscopy. Static water contact angle (WCA) measurements were accomplished using a CAM 200 optical contact angle meter (KSV Instruments Ltd.). For each film synthesized, at least five measurements were made. Five samples of each film were analyzed.
Film solubility in water
Pieces of film of 2 cm2 were cut from each film, dried and weighed to the nearest of 0.0001 g. The solubility in water of the chitosan films was measured from immersion assays under constant agitation in 50 mL of deionized water for 6 h at 25 °C. The initial dry weight and remaining pieces of film after immersion were dried at 110 °C to constant weight (Eq. 1).
| 1 |
Also in this case five samples of each film were analyzed.
Water vapor permeability coefficient (WVP)
Water vapor permeability (WVP) was determined gravimetrically according to Shen and Kamdem (2015) with some modifications. Flawless films were cut into disks with a diameter greater than that of the containers with an aperture of 0.002124 m2. Each vessel was filled with silica gel (0% RH desiccant), leaving an air space of four cm between the bottom of the film and the desiccant. Two desiccators containing saturated solutions of potassium carbonate (42.0% RH) and sodium bromide (61.8% RH) were used and the entire system was placed with five replicates for each treatment. The water vapor transport was determined from the increase in the weight of the permeation cell in a constant transfer state. The weights of the cells were recorded every 12 h for a total of 96 h. The water vapor transmission rate (WVTR) was obtained by dividing the slope (g/h) by the film area (m2). To obtain the value of the WVP, the value of WVTR was taken, multiplied by the film thickness (mm) and divided by the pressure difference (kPa) between the inner and outer surfaces of the film.
Scanning electron microscopy
The microstructure of film samples was studied by scanning electron microscopy (SEM), using the instrument JEOL JSM-6510 LV SEM (Japan Electron Optics, Tokyo, Japan). Samples were prepared using standard techniques as described by Bonilla et al. (2012), mounted on aluminum stubs and sputter-coated with gold (20 nm). Samples were observed using an accelerating voltage of 3 kV for surface analysis of chitosan films and 10 kV for chitosan and chitosan incorporated with TCEO. For surface analysis, magnifications of 170 × were used. For cross-sectional analysis, magnifications of 550 × and 950 × were used.
Antimicrobial activity
The antimicrobial activity of the films was tested on 24 Gram negative strains: Shewanella putrefaciens ATCC 49138 and 23 strains previously isolated from tuna and swordfish and identified (Serio et al. 2014) as Shewanella baltica (n = 10), S. morhuae (n = 1), S. putrefaciens (n = 1), Pseudomonas fluorescens (n = 2), P. fragi (n = 2), Serratia spp. (n = 5), Aeromonas molluscorum (n = 1) and Acinetobacter radioresistens (n = 1). All the strains belonged to the Faculty of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo (Italy). The antibacterial assay was performed by agar diffusion method. Each strain was cultured overnight in Marine Broth (Becton–Dickinson, Milan, Italy), then each bacterial suspension was adjusted to about 1.0 × 107 cells/mL in sterile saline and was spread on Müller Hinton Agar plates (Oxoid-Thermofisher, Rodano, Italy) to reach a final concentration of about 106 cells/plate. The cells concentration was verified onto Tryptone Soy Agar plates (Oxoid-Thermofisher), added with 5 g/L of sodium chloride (Sigma-Aldrich, Milan, Italy).
A square of 10 mm × 10 mm of each film was placed in the middle of each plate, considering the chitosan film (without EO) as control. Ciprofloxacin disks (5 μg, Oxoid Thermofisher) were used as reference. The analysis was performed in triplicate and results were observed after 24, 48, and 72 h of incubation at 25 °C by measuring the inhibition halo around the film samples and the antibiotic disks.
Minimal inhibitory concentration (MIC) of selected TCEO compounds
The antimicrobial activity of carvacrol and thymol (Sigma-Aldrich, Milan, Italy), recognized as among the most abundant compounds detected in TCEO, was assayed against ten strains, including one of the most sensitive and of the most resistant strains of the studied species. The analyses were performed by microdilution according to Chaves-Lopez et al. (2018), with thymol and carvacrol tested concentrations starting from 4.0 mM (corresponding to 1.2 mg mL−1). Positive (without an antimicrobial agent) and negative (chloramphenicol 50 mg mL−1) controls were also included in the assay. The strains were incubated at 25 °C and results were observed after 48 h and confirmed after 72 h. The analyses were performed in duplicate e repeated three times.
Statistical analysis
Bacterial inhibition data, analyses of physical, mechanical, and water vapor permeability properties of the films were subjected to analysis of variance (one-way ANOVA). The averages of the measurements were compared using a Tukey test at p ≤ 0.05. Five different replicates were used for the determination of the film characteristics, while the microbiological analysis were performed in duplicate and repeated three times.
Results and discussion
Identification of volatile components of TCEO
After GC–MS and GC-FID analyses, 25 compounds were recognized in the essential oil (Table 1): 23 monoterpenes (99.7%), one sesquiterpene (0.2%) and one non-identified compound. Monoterpenes have been described to exert acaricidal (Sánchez-Ramos and Castañera 2000) and antimicrobial (Dorman and Deans 2000) effects, while sesquiterpenes might account for antimicrobial and antifungal properties (Grande-Tovar et al. 2016).
Table 1.
Volatile compounds expressed as area percentage, identified in Thymus capitatus essential oil
| Compound | RT | Amount relative (%) | KI | |
|---|---|---|---|---|
| Monoterpenes hydrocarbons | Tricyclene | 17.15 | < 0.1 | 920 |
| α-Thujene | 17.26 | 0.1 | 923 | |
| α-Pinene | 17.65 | 1.5 | 935 | |
| α-Fenchene | 18.43 | 0.3 | 951 | |
| β-Pinene | 19.67 | 0.3 | 981 | |
| β-Myrcene | 20.03 | 2.0 | 991 | |
| p-Mentha-1(7),8-diene | 20.78 | 0.1 | 992 | |
| α-Phellandrene | 20.88 | 0.2 | 1005 | |
| δ-3-Carene | 20.99 | < 0.1 | 1012 | |
| 1,4-Cineole | 21.23 | < 0.1 | 1014 | |
| α-Terpinene | 21.34 | 1.5 | 1018 | |
| p-Cymene | 21.74 | 13.2 | 1026 | |
| Limonene | 21.89 | 0.4 | 1033 | |
| 1,8-Cineole | 22.05 | 0.4 | 1033 | |
| γ-Terpinene | 23.13 | 8.7 | 1064 | |
| N.I. (M+154) | 23.63 | 0.1 | ||
| Monoterpenes oxygenated | Terpinolene | 24.24 | 0.2 | 1078 |
| Linalool | 24.73 | 1.9 | 1100 | |
| Borneol | 27.87 | 0.3 | 1165 | |
| Terpinen-4-ol | 28.15 | 0.6 | 1190 | |
| α-Terpineol | 28.79 | 0.1 | 1200 | |
| Thymol | 32.07 | 6.4 | 1266 | |
| Carvacrol | 32.63 | 59.3 | 1278 | |
| trans-β-Caryophellene | 37.34 | 2.2 | 1424 | |
| Sesquiterpenes oxygenated | Caryophellene oxide | 42.50 | 0.2 | 1581 |
RT, retention time; KI, Kovatz Index relative to C5–C24 n-alkanes on the DB-5 column
Physical properties of the film
In general, thin chitosan TCEO films were obtained with good appearance, attributable to the covalent bonding between TCEO components and the chitosan functional groups. This effect was reported with similar film composition (presence of mono and sesquiterpenes) (Ojagh et al. 2010). The films physical properties are presented in Table 2. With the introduction of 0.5% TCEO, the films had significantly lower (p < 0.05) solubility, moisture content and higher contact angle, with respect to pure chitosan films (control). With increasing TCEO amounts, moisture and solubility decreased significantly, in accordance with previous investigations (Ojagh et al. 2010). Covalent bond formation among chitosan functional groups and EO components decreases the functional groups available for the interaction with water molecules by hydrogen bonds (Hosseini et al. 2009). Solubility is an important parameter, as it can affect water resistance of films, especially in humid environment, due to its biodegradability (Peng et al. 2013). Crosslinking of chitosan films with TCEO resulted in a decrease in the affinity of the natural polymer toward water and produced films with low water solubility, which is beneficial for product integrity and water resistance (Hosseini et al. 2009). On the other side, water contact angle is a measure of the behavior of pure water in contact with the film surface. When a distilled drop of water is placed on the chitosan surface, it forms a spherical cap with a contact angle lower than 90° (Table 2), as a consequence of the hydrophilic nature of chitosan (Altiok et al. 2010). The introduction of TCEO significantly increased the wettability of the films, probably due to the increasing tortuosity of the network. However, with further TCEO increasing, the wetting property of chitosan-TCEO slightly increased, because of the covalent bonds and the hydrophobic character in the forming network, with contact angles higher than for chitosan. It is known that the addition of EO would increase surface hydrophobicity and consequently water contact angles (Ojagh et al. 2010).
Table 2.
Physical properties of chitosan (medium molecular weight) films with Thymus capitatus essential oil
| TCEO (v/v) in film solution (%) | Thickness (mm) | Moisture content (%) | Solubility in water (%) | Water contact angle (°) |
|---|---|---|---|---|
| Control | 0.048 ± 0.002a | 47.78 ± 0.09a | 14.32 ± 0.42a | 43.03 ± 1.70a |
| CS-OEO 0.5 | 0.052 ± 0.005a | 36.81 ± 0.07b | 12.66 ± 0.20b | 72.53 ± 1.23b |
| CS-OEO 1.0 | 0.059 ± 0.001b | 30.91 ± 0.06c | 9.69 ± 0.17c | 77.37 ± 2.00c |
| CS-OEO 1.5 | 0.066 ± 0.002c | 26.29 ± 0.04d | 9.49 ± 0.26c | 86.19 ± 2.11d |
Values correspond to mean ± standard deviation. Different superscript letters in the same column indicate significant differences (p < 0.05)
According to our results, despite the physical properties improved with the increasing of EO, the most interesting concentration of TCEO incorporated into the films should be 1.0%, since it represents a reasonable EO amount and all the physical properties of the films were improved, without affecting the integrity of the films, as confirmed by SEM analysis. An EO excess could deteriorate the film integrity and affect its acceptance by consumers.
Mechanical properties
All the films exhibited significant lower elongation at break, but showed a significant increase in the tensile strength (Table 3). The introduction of TCEO may replace some weak polymer–polymer chain interactions by non-polar hydrophobic interactions with the polymer chain and polar components with polar groups of the chitosan, generating a cross-linker effect which decreased free volume and molecular mobility of the polymer (Ojagh et al. 2010). As mentioned above, the moisture content (Table 2) diminished with TCEO incorporation, determining tensile strength increase. Water is an uncontrollable plasticizer for most hydrocolloids based films, because of its ability to modify the structure of natural polymers, essentially by hydrogen bonding. However, this effect is negligible in our films and insufficient for glycerol alone. The elongation decreasing might also be attributed to the increasing of the pore sizes of the matrix with TCEO introduction, creating possible rupture points (Altiok et al. 2010). It is also possible that the tensile strength increasing and elongation decreasing were caused by the interaction between the chitosan polymer and TCEO, producing a cross-linker effect, causing a sheet-like structure (less molecular mobility and free volume). This morphology means a compact structure, leading to a continuity inside the polymer network and decreasing the elongation, as observed also by Hosseini et al. (2009), using cinnamon oil with chitosan.
Table 3.
Mechanical properties of chitosan films incorporated with Thymus capitatus essential oil
| FILM | TS (MPa)* | YM (MPa)* | EAB (%)* |
|---|---|---|---|
| Control | 1.697 ± 0.16c | 6.419 ± 1.31b | 27.322 ± 2.35a |
| CS-OEO 0.5% | 2.250 ± 0.13c | 10.791 ± 1.53a | 20.467 ± 2.53ab |
| CS-OEO 1.0% | 12.841 ± 1.05b | 4.511 ± 0.37b | 19.393 ± 2.79b |
| CS-OEO 1.5% | 19.480 ± 2.86a | 5.532 ± 0.92b | 14.695 ± 3.99b |
*Values correspond to mean ± standard deviation. Different superscript letters in the same column indicate significant differences (p < 0.05). TS, tensile strength; YM, Young Module; EAB, elongation at break
It is well known that films should improve their mechanical properties, increasing their tension strength but not loosing their elasticity, otherwise, they will not be useful as packaging materials. For that reason, the best amount of EO to include in the films should be 1.0% since it maintains an equilibrium between tension strength and elasticity as confirmed in Table 3. With an excess of 1.5%, films become less elastic and more brittle, while with 0.5%, mechanical properties resemble to the films only with chitosan.
Water vapor permeability
An increase in film permeability, compared to pure chitosan, was observed with the first EO addition (0.5%); however, starting from 1.0% TCEO, a significant permeability decrease was noticed (Table 4). Apparently, small TCEO concentrations decreased the tortuosity of chitosan films, allowing the permeability to increase with small EO concentrations; however, the effect was not proportional to the EO addition and a decrease was observed with the following EO increases (1.0% and 1.5%). This permeability decrease may be caused by the covalent interactions between the network and the EO components, mainly the oxygenated terpenes, generating a crosslinking reaction. It was argued that reducing the availability of hydrophilic groups, the interaction of the matrix with water decreases, producing a more water-resistant film. In addition, the hydrophobic dispersed phase increases the tortuosity factor for mass transfer causing a reduction in permeability (Shen and Kamdem 2015). This effect was also observed in the mechanical properties, where the high degree of contact between chitosan and essential oil weakens the aggregation forces of the chitosan chain, thus diminishing the cohesion of the polymer network, and in general reducing its elongation (Bonilla et al. 2012). Our data suggested that the increase in TCEO concentration to 1.0% and 1.5% reduced the detrimental air diffusion through the film, therefore this effect has to be considered a very favorable characteristic in view of an application as active packaging.
Table 4.
Permeability of Chitosan-TCEO films at different concentrations of essential oil and two relative humidity values
| Permeability (gmm/kPahm2) | ||
|---|---|---|
| FILM | 42.0% R.U. | 61.8% R.U. |
| Control | 0.329 ± 0.056a | 0.577 ± 0.060a |
| CS-OEO 0.5% | 0.402 ± 0.030b | 0.601 ± 0.043a |
| CS-OEO 1.0% | 0.305 ± 0.031a | 0.509 ± 0.027b |
| CS-OEO 1.5% | 0.319 ± 0.021a | 0.487 ± 0.037b |
Values correspond to mean ± standard deviation. Different superscript letters in the same column indicate significant differences (p < 0.05)
Scanning electron microscopy studies
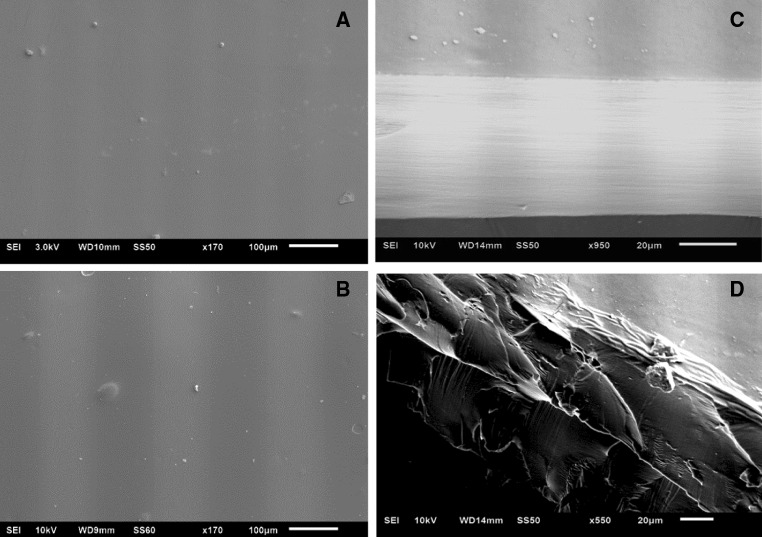
Chitosan films were compact, without cracks or pores, exhibiting a smooth a homogeneous surface and cross-section (Fig. 1a, c). The surface image suggested a sheet-like and dense structure, while the cross-section revealed the sheets stacked in compact layers (Fig. 1b, d). The TCEO incorporated uniformly in the matrix. As already discussed, the covalent bonds between chitosan chains and the EO components, determine a compact, homogeneous sheet-like structure, with a decrease in water permeability, solubility in water and higher tensile strength.
Fig. 1.
Scanning electron microscopy images of chitosan control film (a, b) film containing TCEO 1.0% surfaces; cross-section of chitosan control film (c) and film containing TCEO 1.0% (d). For surface analysis, × 170 magnified and for cross-sectional analysis × 550 and × 950 magnified
Antimicrobial activity
Chitosan is very active against Gram-positive and, to a lesser extent, Gram-negative bacteria. Interactions between chitosan and microbial membranes change their permeability and consequently cause the leakage of intracellular constituents (Schelegueda et al. 2013). Nevertheless, no inhibition in control films was observed (Table 5), confirming results from other authors (Zivanovic et al. 2005), suggesting that the chitosan needs to be dissolved to exert antimicrobial activity; once tightly bound within the film, it could not express antimicrobial effect. Moreover, Coma et al. (2002) suggested that chitosan diffuses very slowly in culture media in agar diffusion method, thus inhibiting only the cells directly in contact with the active sites of chitosan.
Table 5.
Antimicrobial activity of the films (TCEO 0.5, 1.0 and 1.5%) against spoilage bacteria isolated from seafood, as detected in agar-diffusion test after 72 h of incubation at 25 °C, and of ciprofloxacin (CIPR) after 48 h of incubation at 25 °C
| Accession number | Strain | Species | Diameter of inhibition (mm) | ||||
|---|---|---|---|---|---|---|---|
| Control | TCEO 0.5% | TCEO 1.0% | TCEO 1.5% | Ciprofloxacin 5 μg | |||
| JX032781 | SS1 | S. baltica | 10.0 ± 0.0 | 28.1 ± 1.0 | 45.0 ± 1.0** | 41.0 ± 1.0** | 51.0 ± 2.5 |
| JX032785 | SS5 | S. baltica | 10.0 ± 0.0 | 28.2 ± 1.0 | 32.3 ± 1.2** | 32.3 ± 0.8** | 50.8 ± 1.8 |
| JX032804 | SS11 | S. baltica | 10.0 ± 0.0 | 37.3 ± 1.5 | 40.1 ± 0.7* | 37.2 ± 1.1 | 51.4 ± 2.2 |
| JX032806 | SS14 | S. baltica | 10.0 ± 0.0 | 13.2 ± 0.5 | 23.2 ± 0.3** | 21.0 ± 0.4** | 51.3 ± 4.9 |
| JX032789 | ST4 | S. baltica | 10.0 ± 0.0 | 15.4 ± 1.0 | 23.0 ± 1.0** | 23.2 ± 0.3** | 50.6 ± 2.5 |
| JX032790 | ST5 | S. baltica | 10.0 ± 0.0 | 26.0 ± 0.7 | 36.5 ± 0.5** | 30.4 ± 0.5** | 51.4 ± 1.9 |
| JX032792 | ST8 | S. baltica | 10.0 ± 0.0 | 14.2 ± 1.1 | 25.3 ± 0.8** | 26.0 ± 0.0** | 50.0 ± 2.2 |
| JX032793 | ST9 | S. baltica | 10.0 ± 0.0 | 39.0 ± 1.2 | 32.4 ± 1.1** | 40.0 ± 1.0** | 35.0 ± 0.8 |
| JX032794 | ST10 | S. baltica | 10.0 ± 0.0 | 35.1 ± 0.8 | 43.0 ± 1.0** | 35.4 ± 1.1 | 51.0 ± 2.7 |
| JX032795 | ST11 | S. baltica | 10.0 ± 0.0 | 20.3 ± 0.5 | 31.5 ± 0.3** | 30.0 ± 0.1** | 46.0 ± 0.1 |
| JX032787 | ST2 | S. morhuae | 10.0 ± 0.0 | 20.2 ± 0.7 | 28.2 ± 1.1** | 26.2 ± 1.2** | 54.6 ± 3.4 |
| ATCC 49138 | S. putrefaciens | 10.0 ± 0.0 | 22.0 ± 1.0 | 32.0 ± 0.5** | 27.3 ± 0.8** | 46.0 ± 0.2 | |
| JX032786 | SS6 | P. putrefaciens | 10.0 ± 0.0 | 23.2 ± 0.3 | 38.1 ± 0.7** | 38.2 ± 1.2** | 45.3 ± 3.4 |
| JX032797 | SS7 | P. fluorescens | 10.0 ± 0.0 | 16.0 ± 0.5 | 23.4 ± 0.6** | 20.0 ± 0.1** | 49.3 ± 4.1 |
| JX032808 | SS21 | P. fluorescens | 10.0 ± 0.0 | 15.1 ± 0.4 | 20.0 ± 1.0** | 20.3 ± 0.7** | 49.8 ± 3.6 |
| JX032798 | SS10 | P. fragi | 10.0 ± 0.0 | 18.0 ± 0.0 | 17.3 ± 0.7 | 17.4 ± 0.8 | 51.2 ± 1.8 |
| JX032796 | ST19 | P. fragi | 10.0 ± 0.0 | 18.2 ± 0.3 | 19.3 ± 1.2 | 18.0 ± 0.0 | 48.0 ± 2.8 |
| JX032799 | SS9 | Serratia spp. | 10.0 ± 0.0 | 24.0 ± 0.3 | 32.2 ± 0.3** | 28.0 ± 1.5** | 53.0 ± 3.6 |
| JX032800 | SS16 | Serratia spp. | 10.0 ± 0.0 | 18.5 ± 0.5 | 30.3 ± 0.6** | 28.3 ± 1.7** | 38.6 ± 4.2 |
| JX032801 | SS18 | Serratia spp. | 10.0 ± 0.0 | 20.2 ± 0.6 | 27.1 ± 0.8** | 28.2 ± 0.8** | 46.7 ± 3.5 |
| JX032802 | SS19 | Serratia spp. | 10.0 ± 0.0 | 15.0 ± 1.0 | 29.2 ± 1.1** | 26.5 ± 1.5** | 53.2 ± 2.9 |
| JX032803 | SS22 | Serratia spp. | 10.0 ± 0.0 | 16.3 ± 0.7 | 27.4 ± 0.8** | 25.3 ± 0.8** | 53.3 ± 6.6 |
| JX032807 | SS17 | A. molluscorum | 10.0 ± 0.0 | 22.0 ± 1.0 | 32.4 ± 1.1** | 30.0 ± 1.0** | 38.7 ± 3.4 |
| JX032809 | SS23 | Ac. radioresistens | 10.0 ± 0.0 | 18.4 ± 0.4 | 21.0 ± 1.0 | 21.1 ± 2.0 | 52.0 ± 1.6 |
Results are expressed as mean ± standard deviation of three different replicates. Inhibition area (in mm) includes 10 mm film diameter for TCEO and 6 mm diameter for Ciprofloxacin
**p < 0.01, *p < 0.05 according to one-way ANOVA analysis of the means for the TCEO within the same strain, in comparison with TCEO 0.5%
The effect of TCEO against spoiling bacteria is well documented, against Brochotrix thermosphacta, and in less degree on P. aeruginosa (Mith et al. 2014). Antibacterial activity was reported against Serratia marcescens, Shewanella putrefaciens, Pseudomonas fluorescens, Alcaligenes faecalis, Aeromonas hydrophila Pseudomonas fragi, Achromobacter denitrificans, with concentration ranging between 1.87 and 3.75 μL (Ballester-Costa et al. 2013). In our work the addition of Thymus capitatus essential oil to chitosan, contributed to exert antimicrobial activity against all the tested strain. It is noteworthy that the diameter of the halos observed after 24 h was confirmed also after 72 h of incubation, suggesting that the inhibition was stable for at least 3 days. This is a significant result, as the aim of the antimicrobial film development is mainly the shelf-life extension. On the other hand, the inhibition was not increased with the TCEO concentration in the film for 16 out of 24 strains (66.7%), which were inhibited more efficiently by the film containing 1.0% TCEO than by the one with 1.5% TCEO. This result deserves deeper investigations, as EO antimicrobial action generally increases with increasing concentrations (Zivanovic et al. 2005). However, the oil diffusion is related to molecules polarity, therefore the increasing concentration of hydrophobic compounds within the chitosan matrix could affect the migration, and therefore the antimicrobial activity (Oussalah et al. 2006). In addition, EOs chemically interact with chitosan functional groups, and therefore, by increasing the concentration of EOs within the chitosan matrix (Wang et al. 2011), the antimicrobial effect of the film could change as well as physical and mechanical properties. The 1.5% TCEO film had the lowest moisture (26.29 ± 0.04%) and was quite dry with respect to the other films and, most of all, to the control containing only chitosan; this aspect could have influenced the active molecules migration in plates.
The antibacterial activity of the chitosan-TCEO film against S. baltica seemed to be strain-dependent, thus demonstrating the importance of testing more than one strain to evaluate the effectiveness of chitosan films. Shewanella baltica ST9 was inhibited with the same efficacy by the films containing 0.5% and 1.5% TCEO, which were more active than 1.0% TCEO. The most sensitive strains were S. baltica SS1, SS11, and ST10, inhibited by the film containing 1.0% TCEO, with an inhibition halo greater than 40 mm; on the contrary, Pseudomonas fragi SS10 and ST19 were scarcely sensitive, and no significant differences were observed among the effect of the different films. Only for S. baltica ST9 the films showed an antimicrobial activity comparable or greater than 5 μg ciprofloxacin (Table 5). Nevertheless, it must be highlighted that all the strains were susceptible to ciprofloxacin, with a very low MIC (≤ 0.125 μg/mL, unpublished data).
The antimicrobial activity exerted by the films of chitosan and TCEO against the tested fish-spoiling bacteria, belonging to eight distinct species, is a significant result, as Gram negative are usually scarcely sensitive to essential oils alone, because of the presence of lipopolysaccharide. Indeed, the combination of chitosan and EO results more effective, because chitosan specifically interacts with LPS, affecting its structure and increasing the EO entrance in the cytoplasm (Paparella et al. 2016). As reported in Sect. 3.1, the TCEO obtained showed carvacrol, thymol, linalool, p-cymene, γ-terpinene, β-mircene, trans β-caryophyllene as main compounds, that could potentially give a great contribution to the antibacterial effects of the films. When tested individually, carvacrol exerts a stronger antibacterial activity than thymol (Table 6). Except for S. putrefaciens SS6 and P. fluorescens SS7, resulting the most resistant, with MIC of 4.0 and 2.0 mM respectively for thymol and carvacrol, all the other tested strains showed MIC of 2.0 mM for thymol and 1.0 for carvacrol, independently on the sensitivity shown for chitosan and TCEO films. Both the compounds permeabilize the cytoplasmic membrane depolarizing it, and therefore exerting antibacterial effect, thus contributing to the antimicrobial effect first of essential oil, and then of the films.
Table 6.
Minimal inhibitory concentration (MIC) of thymol and carvacrol against selected fish spoiling bacteria, after 48 h of incubation at 25 °C
| Strains | MIC thymol (mM) | MIC carvacrol (mM) |
|---|---|---|
| S. baltica SS1 | 2.0 | 1.0 |
| S. baltica ST4 | 2.0 | 1.0 |
| S. baltica ST9 | 2.0 | 1.0 |
| S. morhuae ST2 | 2.0 | 1.0 |
| S. putrefaciens ATCC 49138 | 2.0 | 1.0 |
| S. putrefaciens SS6 | 4.0 | 2.0 |
| P. fluorescens SS7 | 4.0 | 2.0 |
| P. fragi ST19 | 2.0 | 1.0 |
| Serratia spp. SS9 | 2.0 | 1.0 |
| Serratia spp. SS18 | 2.0 | 1.0 |
Results are expressed as mean of three different replicates. The standard deviation was not reported, as it was 0.0 for all the strains
In this context, Bounatirou et al. (2010) reported the effect of Thymus capitatus EO on Bacillus cereus, Salmonella spp., Listeria innocua, four different strains of Staphylococus aureus and a multi-resistant S. aureus strain. The authors reported high antibacterial activity of the essential oils, compared to synthetic antibacterial compounds. The low antimicrobial activity of single EO components has been often observed, as the mechanism of action of EOs is multi-component, since the whole phytocomplex could simultaneously attack different targets within the cell, with a final result not explainable by a simple additive effect (Tardugno et al. 2018).
The antimicrobial effect of films containing 1% of EO showed not only high antimicrobial activity but also good mechanical properties. Moreover, while the activity of EOs on pathogenic microorganisms is well studied, on the contrary minor attention is paid to spoiling microorganisms, therefore our results increase the knowledge in this field. In this context, S. baltica and S. putrefaciens, together with Serratia spp., P. fluorescens, and P. fragi, are marine fish Specific Spoilage Organisms, responsible for the production of off-odours and off-flavors that determine the rejection of the product. In particular, shewanellas, and to a minor extent also serratias, are strong producers of hydrogen sulphide and most of all of trymethilamine that is an important spoilage index for finfish (Serio et al. 2014). Nevertheless, Pseudomonas spp. and Serratia spp. exert proteolytic activity, produce off-odours and could also be biogenic amines producers, with effects not only on fish spoilage but also on consumers’ safety.
Conclusion
The results showed that Thymus capitatus essential oil (1.10) could be used with chitosan to produce antimicrobial films against spoilage bacteria. Antibacterial activity against important fish SSOs, such as S. baltica and S. putrefaciens, Serratia spp. and P. fluorescens, suggests that the films could potentially be used in fish industries. The introduction of TCEO also increased the tensile strength and decreased the moisture content, water solubility and water permeability, very useful properties in coating applications for shel-life extension of highly perishable foods such as fish and poultry.
Acknowledgements
C.D. Grande acknowledges economic support from the Instituto Colombiano de Investigaciones Cientificas, COLCIENCIAS. C.D.G.T. and he also acknowledges to Doctor Rigoberto C. Advincula and Doctor Joey Mangadlao from Case Western Reserve University for assistance in SEM analysis. Funding was provided by Universidad de San Buenaventura Cali, Università degli Studi di Teramo and Universidad del Atlántico.
Contributor Information
Carlos David Grande-Tovar, Email: carlosgrande@mail.uniatlantico.edu.co.
Johannes Delgado-Ospina, Email: jdelgado1@usbcali.edu.co.
References
- Altiok D, Altiok E, Tihminlioglu F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J Mater Sci-Mater M. 2010;21(7):2227–2236. doi: 10.1007/s10856-010-4065-x. [DOI] [PubMed] [Google Scholar]
- ASTM D882–12 . Standard test method for tensile properties of thin plastic sheeting. West Conshohocken: ASTM International; 2012. [Google Scholar]
- Ballester-Costa C, Sendra E, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind Crop Prod. 2013;50(C):304–311. doi: 10.1016/j.indcrop.2013.07.052. [DOI] [Google Scholar]
- Ballester-Costa C, Sendra E, Fernández-López J, Viuda-Martos M. Evaluation of the antibacterial and antioxidant activities of chitosan edible films incorporated with organic essential oils obtained from four Thymus species. J Food Sci Technol. 2016;53(8):3374–3379. doi: 10.1007/s13197-016-2312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla J, Atarés L, Vargas M, Chiralt A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocolloid. 2012;26:9–16. doi: 10.1016/j.foodhyd.2011.03.015. [DOI] [Google Scholar]
- Bounatirou S, Smiti S, Miguel MG, Faleiro L, Rejeb MN, Neffati M, Costa MM, Figuereido AC, Barroso JS, Pedro LG. Thermal stability of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link.: effect on the chemical composition and the antioxidant and antibacterial activities. Acta Aliment. 2010;39(3):299–307. doi: 10.1556/AAlim.39.2010.3.6. [DOI] [Google Scholar]
- Chaves-Lopez C, Usai D, Donadu MG, Serio A, Gonzalez-Mina RT, Simeoni MC, Molicotti P, Zanetti S, Pinna A, Paparella A. Potential of Borojoa patinoi Cuatrecasas water extract to inhibit nosocomial antibiotic resistant bacteria and cancer cell proliferation in vitro. Food Funct. 2018;9(5):2725–2734. doi: 10.1039/C7FO01542A. [DOI] [PubMed] [Google Scholar]
- Coma V, Martial-Gros A, Garreau S, Copinet A, Salin F, Deschamps A. Edible antimicrobial films based on chitosan matrix. J Food Sci. 2002;67(3):1162–1169. doi: 10.1111/j.1365-2621.2002.tb09470.x. [DOI] [Google Scholar]
- Delgado J, Grande CD, Menjívar JF, Sánchez MS. Relationship between refractive index and thymol concentration in essential oils of Lippia origanoides Kunth. Chil J Agric Anim Sci. 2016;32(2):127–133. doi: 10.4067/S0719-38902016000200006. [DOI] [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- El-Obeid T, Yehia H, Sakkas H, Lambrianidi L, Tsiraki MI, Ioannis N, Savvaidis IN. Shelf-life of smoked eel fillets treated with chitosan or thyme oil. Int J Biol Macromol. 2018;114:578–583. doi: 10.1016/j.ijbiomac.2018.03.125. [DOI] [PubMed] [Google Scholar]
- Elsabee MZ, Abdou ES. Chitosan based edible films and coatings: a review. Mater Sci Eng C-Mater. 2013;33(4):1819–1841. doi: 10.1016/j.msec.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Gómez-Estaca J, López de Lacey A, López-Caballero ME, Gómez-Guillén MC, Montero P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27(7):889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Grande-Tovar CD, Chaves-López C, Viuda-Martos M, Serio A, Delgado-Ospina J, Pérez-Álvarez JA, Ospina N, Paparella A. Sub-lethal concentrations of Colombian Austroeupatorium inulifolium (H.B.K.) essential oil and its effect on fungal growth and the production of enzymes. Ind Crop Prod. 2016;87:315–323. doi: 10.1016/j.indcrop.2016.04.066. [DOI] [Google Scholar]
- Hosseini MH, Razavi SH, Mousavi MA. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J Food Process Preserv. 2009;33(6):727–743. doi: 10.1111/j.1745-4549.2008.00307.x. [DOI] [Google Scholar]
- Jeon YJ, Kamil JY, Shahidi F. Chitosan as an edible invisible film for quality preservation of herring and Atlantic Cod. J Agric Food Chem. 2002;50(18):5167–5178. doi: 10.1021/jf011693l. [DOI] [PubMed] [Google Scholar]
- Khanjari A, Karabagias IK, Kontominas MG. Combined effect of N, O-carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. LWT Food Sci Technol. 2013;53(1):94–99. doi: 10.1016/j.lwt.2013.02.012. [DOI] [Google Scholar]
- Kykkidou S, Giatrakou V, Papavergou A, Kontominas MG, Savvaidis IN. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4°C. Food Chem. 2009;115:169–175. doi: 10.1016/j.foodchem.2008.11.083. [DOI] [Google Scholar]
- Mith H, Duré R, Delcenserie V, Zhiri A, Daube G, Clinquart A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci Nutr. 2014;2(4):403–416. doi: 10.1002/fsn3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010;122(1):161–166. doi: 10.1016/j.foodchem.2010.02.033. [DOI] [Google Scholar]
- Oussalah M, Caillet S, Salmiéri S, Saucier L, Lacroix M. Antimicrobial effects of alginate-based film containing essential oils for the preservation of whole beef muscle. J Food Protect. 2006;69(10):2364–2369. doi: 10.4315/0362-028X-69.10.2364. [DOI] [PubMed] [Google Scholar]
- Ozogul Y, Yuvka I, Ucar Y, Durmus M, Kösker AR, Öz M, Ozogul F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT Food Sci Technol. 2017;75:677–684. doi: 10.1016/j.lwt.2016.10.009. [DOI] [Google Scholar]
- Paparella A, Mazzarrino G, Chaves-López C, Rossi C, Sacchetti G, Guerrieri O, Serio A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016;59:23–31. doi: 10.1016/j.fm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Peng Y, Yin L, Li Y. Combined effects of lemon essential oil and surfactants on physical and structural properties of chitosan films. Int J Food Sci Technol. 2013;48(1):44–50. doi: 10.1111/j.1365-2621.2012.03155.x. [DOI] [Google Scholar]
- Sánchez-Ramos I, Castañera P. Acaricidal activity of natural monoterpenes on Tyrophagus putrescentiae (Schrank), a mite of stored food. J Stored Prod Res. 2000;37(1):93–101. doi: 10.1016/S0022-474X(00)00012-6. [DOI] [PubMed] [Google Scholar]
- Schelegueda LI, Campos MF, Gliemmo CA. Action of chitosan, nisin and sodium lactate on the inhibition and cell membrane damage of Listeria innocua and Shewanella putrefaciens. In: Méndez-Vilas A, editor. Worldwide research efforts in the fighting against microbial pathogens: from basic research to technological developments. Boca Raton: Brown Walker Press; 2013. pp. 3–7. [Google Scholar]
- Serio A, Chiarini M, Tettamanti E, Paparella A. Electronic paramagnetic resonance investigation of the activity of Origanum vulgare L. essential oil on Listeria monocytogenes membrane. Lett Appl Microbiol. 2010;51:149–157. doi: 10.1111/j.1472-765X.2010.02877.x. [DOI] [PubMed] [Google Scholar]
- Serio A, Fusella GC, Chaves-López C, Sacchetti G, Paparella A. A survey on bacteria isolated as hydrogen sulfide-producers from marine fish. Food Control. 2014;39:111–118. doi: 10.1016/j.foodcont.2013.11.003. [DOI] [Google Scholar]
- Shen Z, Kamdem DP. Development and characterization of biodegradable chitosan films containing two essential oils. Int J Biol Macromol. 2015;74:289–296. doi: 10.1016/j.ijbiomac.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Tabti L, Dib M, Gaouar N, Samira B, Tabti B. Antioxidant and antifungal activity of extracts of the aerial parts of Thymus capitatus (L.) Hoffmanns against four phytopathogenic fungi of Citrus sinensis Jundishapur. J Nat Pharm Prod. 2014;9(1):49–54. doi: 10.17795/jjnpp-13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapilatu Y, Nugraheni PS, Ginzel T, Latumahina M, Limmon GV, Budhijanto W. Nano-chitosan utilization for fresh yellowfin tuna preservation. Aquat Procedia. 2016;7(C):285–295. doi: 10.1016/j.aqpro.2016.07.040. [DOI] [Google Scholar]
- Tardugno R, Serio A, Pellati F, D’Amato S, Chaves López C, Bellardi MG, Di Vito M, Savini V, Paparella A, Benvenuti S. Lavandula x intermedia and Lavandula angustifolia essential oils: phytochemical composition and antimicrobial activity against foodborne pathogens. Nat Prod Res. 2018 doi: 10.1080/14786419.2018.1475377. [DOI] [PubMed] [Google Scholar]
- Upadhyay A, Upadhyaya I, Karumathil DP, Yin H, Nair MS, Bhattaram V, Venkitanarayanan K. Control of Listeria monocytogenes on skinless frankfurters by coating with phytochemicals. LWT Food Sci Technol. 2015;63(1):37–42. doi: 10.1016/j.lwt.2015.03.100. [DOI] [Google Scholar]
- Wang L, Liu F, Jiang Y, Chai Z, Li P, Cheng Y, Leng X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J Agric Food Chem. 2011;59(23):12411–12419. doi: 10.1021/jf203165k. [DOI] [PubMed] [Google Scholar]
- Yang F, Hu S, Lu Y, Yang H, Zhao Y, Li L. Effects of coatings of polyethyleneimine and thyme essential oil combined with chitosan on sliced fresh Channa argus during refrigerated storage. J Food Process Eng. 2015;38(3):225–233. doi: 10.1111/jfpe.12155. [DOI] [Google Scholar]
- Zivanovic S, Chi S, Draughon AF. Antimicrobial activity of chitosan films enriched with essential oils. Food Microbiol Saf. 2005;70(1):M45–M51. [Google Scholar]