Abstract
AIM
To study the influence of frontalis muscle flap suspension on ocular surface by analyzing the clinical features and inflammatory cytokines.
METHODS
A prospective, observational case series. Thirty-one eyes of 25 patients with severe congenital blepharoptosis who underwent frontalis muscle flap suspension surgery with at least 6mo of follow-up were included in the study. The main outcome measures were margin reflex distance 1 (MRD1), degree of lagophthalmos, ocular surface disease index (OSDI), fluorescein staining (Fl), tear break-up time (BUT), Schirmer I test, and inflammatory cytokine assay.
RESULTS
The degrees of lagophthalmos significantly increased after surgery. The OSDI scores significantly increased 1wk postoperatively and then decreased 4wk after operation. The Fl scores reflected corneal epithelial defects in sixteen patients at early stage postoperatively. The BUT and Schirmer I test values remained stable and did not show change compared to those before surgery. The inflammatory cytokines in conjunctival epithelial cells (including IL-1β, IL-6, IL-8, TNF-α, and IL-17A) significantly increased 1wk after the surgery (P<0.001), then returned to the normal level at 24wk postoperatively. The levels of inflammatory cytokine IL-1β, IL-6, IL-8, TNF-α, and IL-17A elevated significantly and were positively correlated with OSDI and Fl scores.
CONCLUSION
Frontalis muscle flap suspension surgery results in lagophthalmos in early period of post-operation and relieved after months. The elevation of inflammatory cytokines level may participate in the occurrence of corneal epithelial defects at the early postoperative stage.
Keywords: congenital blepharoptosis, frontalis muscle flap suspension, corneal epithelial defects, inflammation
INTRODUCTION
Congenital blepharoptosis is defined as the congenital maldevelopment of the levator palpebrae superioris, or lid ptosis, present since birth. Congenital blepharoptosis does not simply cause cosmetic problems, but is also usually associated with refractive errors, strabismus, and amblyopia, which may affect the visual quality[1]–[3]. To improve vision, peripheral vision, quality of life activities, and appearance, surgery is recommended for patients with congenital blepharoptosis[4]. In eyelids with poor levator function (less than 4 mm) or for children, the preferred treatment is frontalis suspension surgery, using different materials included fascia lata, alloplastic materials (silicon, polytetrafluoroethylene, non-absorbable sutures), frontalis muscle flap[5]–[8].
Autogenous fascia lata has drawbacks such as the need for a second operative site, scarring on the leg, and difficulty to harvest from children. Along with the application of alloplastic material, there exists the risk of foreign-body reaction and local inflammation. Infection and granuloma formation were found in studies using polytetrafluoroethylene strips and silicone rods[9]–[10]. By direct transpose the frontalis muscles to the tarsal plate to elevate the eyelids, frontalis muscle flap suspension showed an effective and stable treatment outcome in the long term, and meanwhile, avoiding the risk of foreign-body reaction, granuloma[11]–[13]. Hence, frontalis muscle flap suspension is widely performed in patients with severe levator function (less than 4 mm). However, levator muscles and frontalis muscles contract in different ways, and frontalis muscle is controlled by the frontal nerve, which is different from the levator muscle; thus, the frontalis muscle flap suspension cannot completely accord with normal physiological requirements. Patients usually suffer from lagophthalmos, keratoconjunctivitis, conjunctival congestion, and dry eye disease in the early stage after the operation[14]–[16].
Exposure keratopathy and dry eye symptoms result from lagophthalmos were usually treated with eye ointment to prevent from tear film evaporation[16]–[17]. However, varying degrees of dry eye symptoms and keratitis were found in our postoperative patients even treated with copious lubrication. Thus, we were wondering whether there is another factor contribute to these dye eye related symptoms and signs, for example, ocular surface inflammation. The aim of the present study was to investigate the influence of frontalis muscle flap suspension on ocular surface by analyzing the clinical features and inflammatory cytokines after frontalis muscle flap suspension.
SUBJECTS AND METHODS
Patient Characteristics
This prospective serial case study included 25 patients with severe congenital blepharoptosis who underwent unilateral or bilateral frontalis muscle flap suspension surgery during the period from June 2013 to March 2016. Fourteen males and 11 females with a mean (±SD) age of 15.2±8.7y (range, 5-38y) were recruited (Table 1). Nineteen patients exhibited unilateral blepharoptosis, and 6 patients exhibited bilateral cases. All the recruited patients showed severe ptosis with poor levator muscle function (less than 4 mm) in the affected eyelids. Patients with ocular surface diseases such as lacrimal duct obstruction, ectropion, entropion, blepharitis, trichiasis, keratitis, dry eye disease, and lagophthalmos were excluded. DEWS's standard was followed as the diagnosis criterion for dry eye[18]. Further exclusion criteria were evidence of situations that may result in ocular surface change, such as negative Bell's phenomenon (the eye does not roll upward on attempted eye closure), history of previous eyelid surgery, and wearing contact lenses. The study was approved by the Ethics Committee of the Zhongshan Ophthalmic Center, Sun Yat-sen University and registration number is 2013MEKY015. Informed consent for the study was obtained at the time of enrollment. The study adhered to the tenets of the Declaration of Helsinki.
Table 1. Demographic and clinical characteristics of the patients.
| Characteristics | Data |
| No. of Patients | 25 (31 eyes) |
| Unilateral | 19 (19 eyes) |
| Bilateral | 6 (12 eyes) |
| Male (%) | 14 (56%) |
| Age (y) | 15.2±8.7 |
| MRD1 pre-op (mm) | 0.6±1.0 |
MRD1: Mean margin reflex distance 1.
Surgical Techniques
All the patients received frontalis muscle flap suspension surgery by the same senior oculoplastic surgeon (Lu R) under general anesthesia. Briefly, the operation steps are shown in Figure 1. After surgeries, tobramycin eye drops and ointment (Tobrex, Alcon, USA) were applied until 1mo after the surgery. There are no anti-inflammatory ingredients in these drops, which may affect the levels of inflammatory mediators.
Figure 1. Procedure of frontalis muscle flap suspension surgery.
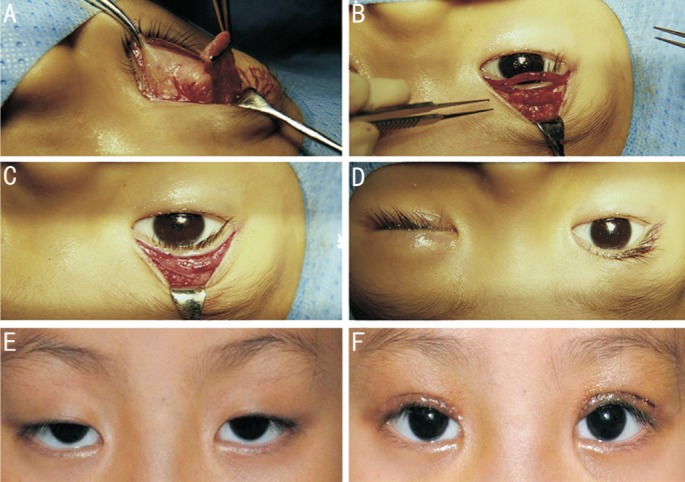
A: A 15×20 mm quadrangular frontalis muscle flap was dissected and pulled down; B: The free flap border was then attached to the tarsal plate with three 5-0 silk sutures and the redundant muscle was trimmed; C: Reset of the orbicularis oculi muscle; D: The eyelid level was adjusted according to the marking before anesthesia and the skin was closed; E, F: An example figure of a patient before and 1wk after the surgery.
Assessments
Preoperative and postoperative assessments included mean margin reflex distance 1 (MRD1), degree of lagophthalmos, Ocular Surface Disease Index (OSDI), tear break-up time (BUT), Schirmer I test, fluorescein staining (Fl), and inflammatory cytokine [interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, and IL-17A] assay by real-time polymerase chain reaction (PCR). All the measurements were performed and completed by two doctors. All the tests were taken before and postoperatively 1, 4, 12, and 24wk.
The MRD1 refers to the distance between the corneal light reflex and the upper eyelid margin while fixating on a penlight held directly in front of the patient. Postoperative MRD1<2.0 mm and >4.5 mm defined as undercorrection and overcorrection[19]. The degree of lagophthalmos before and after the surgery was assessed using a ruler with millimeter accuracy. The subjects were seated in the examining chair and were instructed to close eyes gently, followed by measuring the distance between upper and lower eyelids with the ruler as the degrees of lagophthalmos. Each patient's subjective reports were evaluated after objective observation using a validated Chinese-translated version of the OSDI questionnaire. In some younger children who may not easily understand the questions, communication with the help of parents was needed to obtain precise results. The Fl used fluorescein strips that are wetted with a normal saline solution. Punctate staining was recorded using a standardized grading system of 0-3 for each of the five areas using a previously reported method[20]. The BUT was evaluated using a commercially available fluorescein test strip, and was rubbed onto the lower lateral palpebral conjunctiva. The subjects were instructed to blink three times, and then opened their eyes without blinking. The BUT was measured in seconds from the moment the subjects opened their eyes without blinking. Results were obtained three times per eye, and the average for both eyes was recorded. The Schirmer I test was performed with anesthesia to measure basic tear production. The subjects were seated in the examining chair with the room lights dimmed. The test was accomplished by placing 1 drop of topical anesthetic drops (Alcaine, Alcon, USA) in each inferior conjunctival sac, followed 1min after by gentle blotting of the inferior tear meniscus and blotting of the palpebral conjunctiva of the everted lower eyelid with a sterile cotton applicator. A Schirmer strip (filter paper) was placed on the lower eyelid margin in the lower conjunctival fornix, at the junction of the middle and temporal one third of the lower eyelid margin, sufficiently distant from the cornea to avoid pain and reflex lacrimation. The distance that the tears traveled on the filter paper was measured after 5min.
In inflammatory cytokines assay by real-time PCR, the sterile cellulose acetate filter membranes (Pall Life Sciences, USA) were cut to size of 6 mm×9 mm and placed on the nasal and/or temporal conjunctiva of the affected eyes for 5s to obtain the specimens. The specimens were then immediately soaked into a vial with 350 µL of lysis buffer, and stored at -80°C before carrying out total RNA extraction. Total RNA was extracted with an RNeasy Micro Plus Kit (Qiagen, USA) according to the manufacturer's instructions, quantified with a NanoDrop spectrophotometer, and stored at -80°C. The first strand cDNA was synthesized by reverse transcriptase from 500 ng of total RNA using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara Bio Inc., Japan). Quantitative real-time PCR was performed using the LightCycler 480 real-time PCR system (Roche, Switzerland) with 20 µL reaction volume containing 5 µL of cDNA, 1 µL gene expression assay, and 10 µL of LightCycler 480SYBR Green I Master (Roche, Switzerland). IL-1β, IL-6, IL-8, TNF-α, and IL-17A, which is the most common inflammatory cytokine on ocular surfaces, were selected as the target cytokines.
Statistical Analysis
The Wilcoxon matched-pairs, signed-rank test was used to compare the differences between the preoperative and postoperative degree of lagophthalmos, OSDI scores, BUT, Schirmer I test, Fl scores, and inflammatory cytokine assay data. The Spearman correlation test was used to make a correlation analysis between the measurements. P<0.05 were considered statistically significant. All statistical tests were performed using GraphPad Prism 5.0 software (Graph-Pad Prism, Inc., San Diego, CA, USA; http://www.graphpad.com).
RESULTS
All the patients showed different degrees of lagophthalmos postoperatively. There were no complications, including undercorrection, overcorrection, and wound infection; but one patient developed mild exposure keratitis that was successfully managed with ocular lubricants and resolved by 3mo. All the patients' eyelid positions returned to normal levels (the upper eyelid covers superior corneal limbus by 1-2 mm) postoperatively. An example of appearance improvement is shown in Figure 1.
Clinical Assessments
The MRD1 was significantly increased after frontalis muscle flap suspension surgery [0.6±1.0 (-1 to 2) preoperatively, 3.4±0.7 (2 to 5) at 1wk, 3.3±0.6 (2 to 5) at 4wk, 3.2±0.5 (2 to 4) at 12wk, 3.3±0.5 (2 to 4) at 24wk postoperation, P<0.001]. The degrees of lagophthalmos were significantly increased after frontalis muscle flap suspension surgery (0 preoperatively, 3.0 to 4.0 at 1wk, 1.0 to 3.5 at 4wk, 1.0 to 3.0 at 12wk, 0.5 to 2.0 at 24wk postoperation, P<0.001). The OSDI score was significantly increased at 1wk postoperatively (2.3 to 10.0 preoperatively, 7.5 to 20.8 at 1wk postoperation, P<0.001) but subsequently decreased to normal level (2.8 to 15.3 at 4wk, 2.8 to 12.5 at 12wk, 2.3 to 10.0 at 24wk postoperation, P>0.05). None of the patients had corneal epithelium damage before the surgery; after the surgery, superficial punctate keratitis was noted in sixteen patients, which was resolved by the last follow-up. The Fl score reflected the degree of superficial punctate keratitis (0 preoperatively; 0 to 9 at 1wk, P<0.001; 0 to 7 at 4wk, 0 to 3 at 12wk, P<0.05; 0 at 24wk postoperation). The BUT was not significantly changed after frontalis muscle flap suspension surgery (15.7±1.7s preoperatively vs 14.9±1.6s at 1wk, 15.6±1.3s at 4wk, 15.6±1.6s at 12wk, 15.8±1.4s at 24wk postoperation, P>0.05), and Schirmer I test values were also not significantly changed after the surgery (17.8±1.5 mm preoperatively vs 18.8±2.2 mm at 1wk, 18.4±2.2 mm at 4wk, 17.3±2.7 mm at 12wk, 17.7±2.6 mm at 24wk postoperation, P>0.05; Figure 2). Figure 3 shows that the OSDI score was significantly correlated to the Fl score (r=0.572, P<0.05) 1wk postoperatively. There was no correlation between other clinical examination results.
Figure 2. Graph showing the comparison of MRD1, degree of lagophthalmos, OSDI, Fl scores, BUT, and Schirmer I test scores pre- and 1, 4, 12, and 24wk postoperatively.
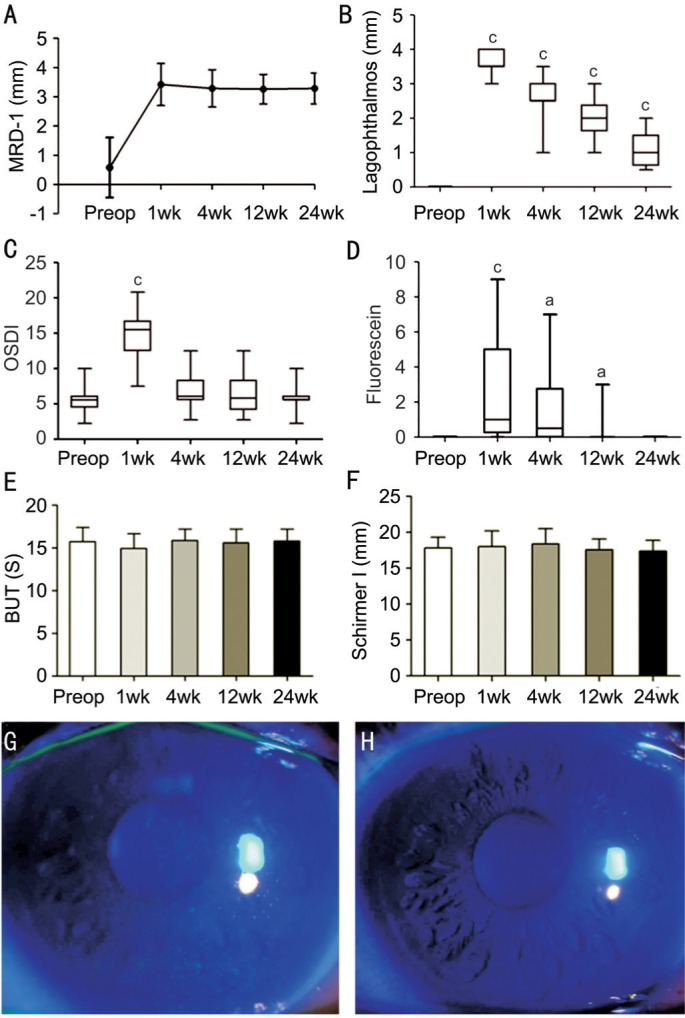
A: The MRD1 improved significantly after the surgery and maintained for at least 24wk; B: The degree of lagophthalmos obviously increased at the early stage after the surgery, but recovered gradually; C: The OSDI score increased significantly at 1wk postoperatively, but returned to the normal level at 4wk; D: Fl score increased significantly at 1wk postoperatively, but completely recovered at 24wk; E: BUT before and after the surgery showed no significant differences; F: Schirmer I test score before and after the surgery showed no significant differences; G, H: The corneal fluorescein staining of a patient at 1 and 24wk after surgery. aP<0.05, bP<0.01, cP<0.001.
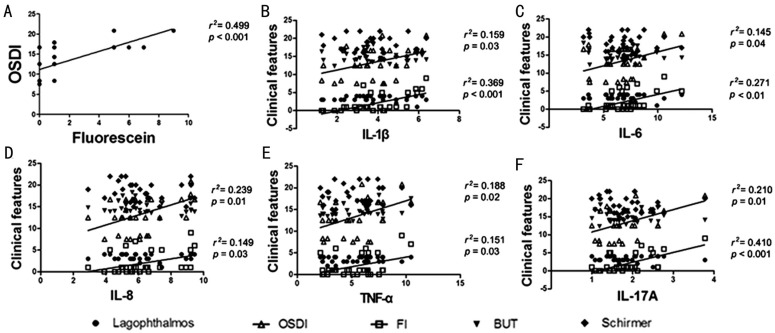
Figure 3. The graph showing the correlation between inflammatory cytokines and clinical features.
A: The OSDI score was significantly correlated to the Fl score; B: The levels of IL-1β were significantly correlated to OSDI score and Fl score; C: The levels of IL-6 were significantly correlated to OSDI score and Fl score; D: The levels of IL-8 were significantly correlated to OSDI score and Fl score; E: The levels of TNF-α were significantly correlated to OSDI score and Fl score; F: The levels of IL-17A were significantly correlated to OSDI score and Fl score.
Inflammatory Cytokines Test
The inflammatory cytokines including IL-1β, IL-6, IL-8, TNF-α, and IL-17A in the conjunctival epithelial cells were significantly increased at 1wk postoperatively (P<0.001) and showed a trend of gradual decline with time. At 24wk postoperatively, the levels of these inflammatory cytokines returned to the normal range (Figure 4). After the surgery, the levels of inflammatory factors IL-1β, IL-6, IL-8, TNF-α, and IL-17A were significantly correlated to the OSDI score and Fl score (P<0.05) as shown in Figure 3. The degree of lagophthalmos, BUT, and Schirmer I test had no correlation to inflammatory factors (P>0.05).
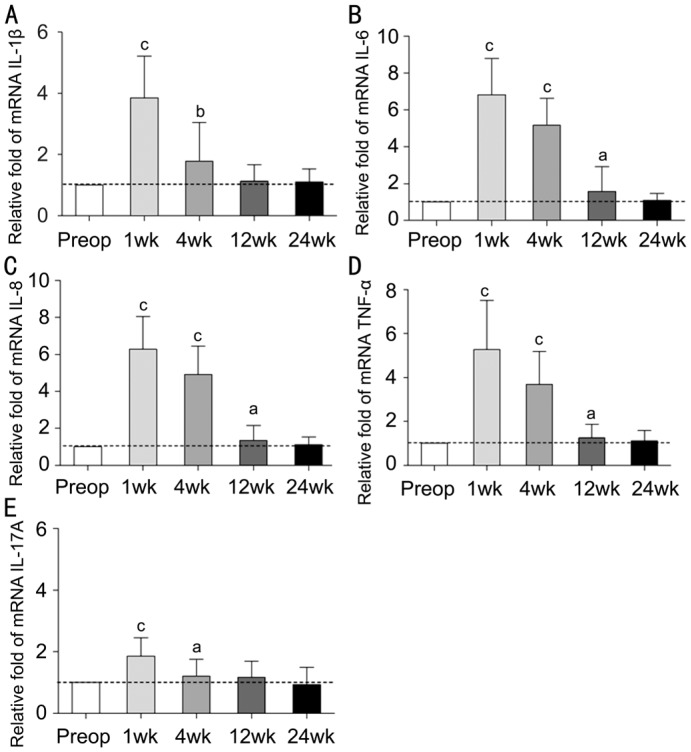
Figure 4. Bar graph showing the inflammatory cytokines change pre- and postoperatively 1, 4, 12, and 24wk.
A: The level of IL-β was increased significantly at 1 and 4wk postoperatively, but returned to the normal level at 12wk postoperation; B: The level of IL-6 was increased significantly at 1, 4, and 12wk postoperatively, but returned to the normal level at 24wk postoperation; C: The level of IL-8 was increased significantly at 1, 4, and 12wk postoperatively, but returned to the normal level at 24wk postoperation; D: The level of TNF-α was increased significantly at 1, 4 and 12wk postoperatively, but returned to the normal level at 24wk postoperation; E: The level of IL-17A was increased significantly at 1 and 4wk postoperatively, but returned to the normal level at 12wk postoperation. aP<0.05, bP<0.01, cP<0.001.
DISCUSSION
According to our results, frontalis muscle flap suspension surgery is a good choice for blepharoptosis patients with poor levator function because of the effectiveness and stability. In our study, the mean MRD1 recovered to a normal value after the surgery and remained stable until 24wk postoperatively, which proved that the appearance problem was resolved effectively, although it slightly declined at 4wk postoperatively than at 1wk postoperatively (P>0.05). This procedure also avoids the need for a linking structure with the risk of foreign-body reaction, absorption, granuloma formation, and late exposure as well as the need for a second incision.
An incomplete blink and lagophthalmos frequently occurs after frontalis muscle flap suspension surgery, which may lead to corneal exposure and excessive evaporation of the tear film. Hence, we have evaluated the degree of lagophthalmos, tear film and lacrimal secretion function after frontalis muscle flap suspension surgery. In present study, we found that all the patients presented varying degrees of lagophthalmos postoperatively, which showed a trend of gradual decline with time; this finding is consistent with Kumar et al's[21] results. The reason for postoperative lagophthalmos might be the different contraction direction of the frontalis muscle and levator muscle and the swelling of the frontalis muscle at a postoperative early stage, or shortage of the distance between tarsus and frontalis muscle.
In the evaluation of dry eye related symptoms and signs, we found that the OSDI and Fl scores significantly increased after surgery, and the correlation between OSDI and Fl scores showed that the corneal epithelium damage may lead to the increase in OSDI. A 10-year follow-up study of 892 patients showed that dry eye symptoms and chemosis following blepharoplasty were 26.5% and 26.3%, respectively[16]. Kim et al[22] found that upper eyelid blepharoplasty, or blepharoptosis repair, may cause a decrease in corneal sensation and an increase in tear production in the early postoperative period. Different from the increased tear production in Kim et al's[22] study, Watanabe et al[23] found that long-term tear volume was decreased after blepharoptosis surgery. They measured tear volume by video meniscometry, which could measure finer changes in tear volume. Li et al[24] found that frontalis suspension affect tear film break-up time, winking frequency and eyelid closure much more than levator advancement. Shao et al[25] demonstrated that the Schirmer test values were significantly decreased 1wk postoperatively, but returned to baseline by 3mo. Intriguingly, no obvious change in the BUT and Schirmer I test values was observed in our study. In general, the frontalis muscle flap suspension surgery would not affect the lacrimal gland and the accessory lacrimal gland, which control the secretion of tears. The study of McKinney and Zukowski[26] also showed that the blepharoplasty surgery is not a risk factor for dry eye. In addition, tobramycin ointment was applied every night to protect the ocular surface from air exposure in our study, and the Fl showed no observable correlation with lagophthalmos. On the other hand, recent studies showed that released inflammatory factors and lymphocytes infiltration could be one of the key factors of ocular surface inflammations[27]. Hence, we proposed that parts of the ocular surface changes in OSDI and Fl scores might be rooted in the accumulation of inflammatory factors.
Sonoda et al[28], de Paiva and Pflugfelder[29] found that the increase in inflammatory factors including IL-1α, IL-1β, IL-6, and TNF-α were related to proliferation, keratinization, and neovascularization of conjunctival epithelial cells. In the present study, we found that the levels of IL-1β, IL-6, IL-8, TNF-α, and IL-17A were significantly increased after surgery. There are studies demonstrated ocular surface inflammation after intraocular and extraocular surgeries[30]–[31]. As one part of the integrated ocular surface system, eyelid's inflammation caused by temporary injury of the tarsus and frontalis muscle in frontalis muscle flap suspension surgery may transfer to conjunctival sac, but the specific mechanism needs further research[32]. From another aspect, the immediate lagophthalmos and damage to the ocular surface epithelium may result in ocular surface stress, which then causes the release of inflammatory cytokines. Some studies showed that the levels of IL-1β, IL-6, IL-8, and TNF-α of the conjunctival epithelium was significantly elevated in dry eye patients, paralleled with the severity of dry eye disease[33]–[35]. These inflammatory cytokines appear to initiate an inflammation cascade on the ocular surface. Hence, we need to pay extra attention to the patients with autoimmune disease like rheumatoid arthritis and systemic lupus erythematosus who are susceptible to dry eye, to avoid postoperative inflammation become the last straw that overloads the camel.
In the present study, besides the level of IL-1β, IL-6, IL-8, and TNF-α, the level of IL-17A dramatically increased after surgery. IL-17, which mediates the production of inflammatory cytokine, has been proven to play an important role in adaptive and innate immunity[36]. The increasing IL-17A level indicated that the immunological changes may be involved in the symptoms and damage of the ocular surface after the surgery. IL-17A could significantly stimulate the production of TNF-α, IL-6, and IL-1β[37]. IL-1β is a strong inducer of many inflammatory factors such as IL-6, IL-8, and TNF-α. Hence, the upregulated IL-17A and IL-1β expression is usually correlated with elevated levels of IL-6 and TNF-α[38].
Functions of these inflammatory factors may suggest the effect of the elevated inflammatory factors on the ocular surface condition at the early postoperative stage. Kang et al[39] reported that IL-17 was correlated with corneal fluorescein staining in patients with dry eye with systemic inflammatory disease. Yoon et al's[40] study also showed that IL-6 and Fl scores were positively correlated. In our study, a positive correlation was observed between levels of inflammatory factors and OSDI scores or Fl scores. It indicates that the increase in IL-1β, IL-6, IL-8, TNF-α, and IL-17A related to the symptoms and corneal epithelial defects.
The limitations of this study are that sampling errors might result from the limited sample size. Additionally, the study compared the preoperative and postoperative conditions instead of the comparison with a control group with an anti-inflammation application. Further studies may consider the setup of a control group with the use of postoperative anti-inflammatory agents to investigate a more appropriate postoperative treatment for blepharoptosis patients.
Our findings suggest that frontalis flap suspension would correct severe blepharoptosis effectively and the lagophthalmos occurs in early period of post-operation but relieve after months. The elevation of inflammatory factor levels related to the corneal epithelial defect at the early postoperative stage.
Acknowledgments
Foundation: Supported by the National Natural Science Foundation of China (No.81670823).
Conflicts of Interest: Li K, None; Zhang XC, None; Cai XX, None; Quan YD, None; Lu R, None.
REFERENCES
- 1.El Essawy R, Elsada MA. Clinical and demographic characteristics of ptosis in children: a national tertiary hospital study. Eur J Ophthalmol. 2013;23(3):356–360. doi: 10.5301/ejo.5000239. [DOI] [PubMed] [Google Scholar]
- 2.Griepentrog GJ, Diehl N, Mohney BG. Amblyopia in childhood eyelid ptosis. Am J Ophthalmol. 2013;155(6):1125–1128.e1. doi: 10.1016/j.ajo.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griepentrog GJ, Mohney BG. Strabismus in childhood eyelid ptosis. Am J Ophthalmol. 2014;158(1):208–210.e1. doi: 10.1016/j.ajo.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill KV, Bradley EA, Meyer DR, Custer PL, Holck DE, Marcet MM, Mawn LA. Functional indications for upper eyelid ptosis and blepharoplasty surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(12):2510–2517. doi: 10.1016/j.ophtha.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu Y, Nagasao T, Shido H, Fujii T, Kato T, Aoki M, Takada K, Kishi K. Intra-eyebrow frontalis suspension using inverted Y-shaped short autogenous fascia lata for blepharoptosis with poor levator function. J Plast Reconstr Aesthet Surg. 2015;68(1):49–55. doi: 10.1016/j.bjps.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 6.Kokubo K, Katori N, Hayashi K, Kasai K, Kamisasanuki T, Sueoka K, Maegawa J. Frontalis suspension with an expanded polytetrafluoroethylene sheet for congenital ptosis repair. J Plast Reconstr Aesthet Surg. 2016;69(5):673–678. doi: 10.1016/j.bjps.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Shah KP, Mukherjee B. Efficacy of frontalis suspension with silicone rods in ptosis patients with poor Bell's phenomenon. Taiwan J Ophthalmol. 2017;7(3):143–148. doi: 10.4103/tjo.tjo_36_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldey SH, Baylis HI, Goldberg RA, Shorr N. Frontalis muscle flap advancement for correction of blepharoptosis. Ophthalmic Plast Reconstr Surg. 2000;16(2):83–93. doi: 10.1097/00002341-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Wasserman BN, Sprunger DT, Helveston EM. Comparison of materials used in frontalis suspension. Arch Ophthalmol. 2001;119(5):687–691. doi: 10.1001/archopht.119.5.687. [DOI] [PubMed] [Google Scholar]
- 10.Chung HW, Seah LL. Cosmetic and functional outcomes of frontalis suspension surgery using autologous fascia lata or silicone rods in pediatric congenital ptosis. Clin Ophthalmol. 2016;10:1779–1783. doi: 10.2147/OPTH.S113814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou M, Jin R, Li Q, Duan Y, Huang L, Yu D. Frontalis muscle flap advancement for correction of severe ptosis under general anesthesia: modified surgical design with 162 cases in China. Aesthetic Plast Surg. 2014;38(3):503–509. doi: 10.1007/s00266-014-0297-3. [DOI] [PubMed] [Google Scholar]
- 12.Hou D, Li G, Fang L, Li B. Frontalis muscle flap suspension for the correction of congenital blepharoptosis in early age children. PLoS One. 2013;8(1):e53185. doi: 10.1371/journal.pone.0053185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medel R, Vasquez L, Wolley Dod C. Early frontalis flap surgery as first option to correct congenital ptosis with poor levator function. Orbit. 2014;33(3):164–168. doi: 10.3109/01676830.2014.881396. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Kim YD. Surgical treatment of unilateral severe simple congenital ptosis. Taiwan J Ophthalmol. 2018;8(1):3–8. doi: 10.4103/tjo.tjo_70_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokhtarzadeh A, Bradley EA. Safety and long-term outcomes of congenital ptosis surgery: a population-based study. J Pediatr Ophthalmol Strabismus. 2016;53(4):212–217. doi: 10.3928/01913913-20160511-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prischmann J, Sufyan A, Ting JY, Ruffin C, Perkins SW. Dry eye symptoms and chemosis following blepharoplasty: a 10-year retrospective review of 892 cases in a single-surgeon series. JAMA Facial Plast Surg. 2013;15(1):39–46. doi: 10.1001/2013.jamafacial.1. [DOI] [PubMed] [Google Scholar]
- 17.Demirci H, Frueh BR. Palpebral spring in the management of lagophthalmos and exposure keratopathy secondary to facial nerve palsy. Ophthalmic Plast Reconstr Surg. 2009;25(4):270–275. doi: 10.1097/IOP.0b013e3181ab6f08. [DOI] [PubMed] [Google Scholar]
- 18.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 19.McCulley TJ, Kersten RC, Kulwin DR, Feuer WJ. Outcome and influencing factors of external levator palpebrae superioris aponeurosis advancement for blepharoptosis. Ophthalmic Plast Reconstr Surg. 2003;19(5):388–393. doi: 10.1097/01.IOP.0000087071.78407.9A. [DOI] [PubMed] [Google Scholar]
- 20.Lu R, Huang R, Li K, Zhang X, Yang H, Quan Y, Li Q. The influence of benign essential blepharospasm on dry eye disease and ocular inflammation. Am J Ophthalmol. 2014;157(3):591–597.e1-2. doi: 10.1016/j.ajo.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Chaudhuri Z, Chauhan D. Clinical evaluation of refractive changes following brow suspension surgery in pediatric patients with congenital blepharoptosis. Ophthalmic Surg Lasers Imaging. 2005;36(3):217–227. [PubMed] [Google Scholar]
- 22.Kim HH, De Paiva CS, Yen MT. Effects of upper eyelid blepharoplasty on ocular surface sensation and tear production. Can J Ophthalmol. 2007;42(5):739–742. doi: 10.3129/i07-141. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe A, Selva D, Kakizaki H, Oka Y, Yokoi N, Wakimasu K, Kimura N, Kinoshita S. Long-term tear volume changes after blepharoptosis surgery and blepharoplasty. Invest Ophthalmol Vis Sci. 2014;56(1):54–58. doi: 10.1167/iovs.14-15632. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Lin H, Zheng Y, Lin X, Xu Y. Study of the difference of ocular surface change and restoration after two correction surgeries of congenital ptosis. Yan Ke Xue Bao. 2007;23(4):219–226. [PubMed] [Google Scholar]
- 25.Shao C, Fu Y, Lu L, Chen J, Shen Q, Zhu H, Fan X. Dynamic changes of tear fluid after cosmetic transcutaneous lower blepharoplasty measured by optical coherence tomography. Am J Ophthalmol. 2014;158(1):55–63.e1. doi: 10.1016/j.ajo.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 26.McKinney P, Zukowski ML. The value of tear film breakup and Schirmer's tests in preoperative blepharoplasty evaluation. Plast Reconstr Surg. 1989;84(4):572–576. discussion 577. [PubMed] [Google Scholar]
- 27.Brignole F, Pisella PJ, Goldschild M, De Saint Jean M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41(6):1356–1363. [PubMed] [Google Scholar]
- 28.Sonoda S, Uchino E, Nakao K, Sakamoto T. Inflammatory cytokine of basal and reflex tears analysed by multicytokine assay. Br J Ophthalmol. 2006;90(1):120–122. doi: 10.1136/bjo.2005.076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008;71(6 Suppl):89–95. doi: 10.1590/s0004-27492008000700017. [DOI] [PubMed] [Google Scholar]
- 30.Weber M, Kodjikian L, Kruse FE, Zagorski Z, Allaire CM. Efficacy and safety of indomethacin 0.1% eye drops compared with ketorolac 0.5% eye drops in the management of ocular inflammation after cataract surgery. Acta Ophthalmol. 2013;91(1):e15–e21. doi: 10.1111/j.1755-3768.2012.02520.x. [DOI] [PubMed] [Google Scholar]
- 31.Salman IA. Comparison of the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% following strabismus surgery. Eurasian J Med. 2016;48(3):186–188. doi: 10.5152/eurasianjmed.2016.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 33.El Annan J, Goyal S, Zhang Q, Freeman GJ, Sharpe AH, Dana R. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye-associated corneal inflammation. Invest Ophthalmol Vis Sci. 2010;51(7):3418–3423. doi: 10.1167/iovs.09-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 35.Stern ME, Schaumburg CS, Siemasko KF, Gao J, Wheeler LA, Grupe DA, De Paiva CS, Calder VL, Calonge M, Niederkorn JY, Pflugfelder SC. Autoantibodies contribute to the immunopathogenesis of experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53(4):2062–2075. doi: 10.1167/iovs.11-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129(3):311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bian F, Qi H, Ma P, Zhang L, Yoon KC, Pflugfelder SC, Li DQ. An immunoprotective privilege of corneal epithelial stem cells against Th17 inflammatory stress by producing glial cell-derived neurotrophic factor. Stem Cells. 2010;28(12):2172–2181. doi: 10.1002/stem.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19(3):201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 39.Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci. 2011;26(7):938–944. doi: 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26(4):431–437. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]