Abstract
AIM
To compare three initial monthly intravitreal ranibizumab (IVR) injections followed by pro re nata (PRN) dosing with one initial monthly IVR injections followed by PRN dosing for macular edema (ME) secondary to branch retinal vein occlusion (BRVO).
METHODS
Forty-two eyes of 42 patients who had IVR injections for BRVO were retrospectively studied. Eighteen eyes received 1 initial IVR injection (1+PRN group) and 24 eyes received 3 monthly IVR injections (3+PRN). At 1, 3, 6 and 12mo; spectral-domain optical coherence tomography (SD-OCT) was performed. Central macular thickness (CMT), the integrity of the external limiting membrane (ELM), the presence of subretinal fluid, cyst size, the presence of inner segment/outer segment (IS/OS) defect were determined.
RESULTS
At baseline the mean CMT was 521.3±153.2 µm in the 3+PRN group while it was 438.1±162.4 µm in 1+PRN group. At the final visit, mean CMT was 278.3±87.8 µm in the 3+PRN group and 285.2±74.2 µm in the 1+PRN group (P=0.079). The changes in CMT over the entire study period were also comparable in both groups (243±160 µm in the 3+PRN group, and 152.9±175.3 µm in the 1+PRN group; P=0.090). At baseline, best-corrected visual acuity (BCVA) was 0.92±0.60 logarithm of the minimal angle of resolution (logMAR) in the 3+PRN group, while it was 0.72±0.46 logMAR in the 1+PRN group. Final BCVA was 0.42±0.55 logMAR in the 3+PRN group and 0.38±0.50 logMAR in the 1+PRN group (P=0.979). Additionally, the BCVA changes from baseline to final visit were not significantly different (-0.50±0.45 logMAR in the 3+PRN group, and -0.33±0.39 logMAR in the 1+PRN group; P=0.255).
CONCLUSION
No significant differences in the anatomical or functional results are found between 3+PRN and 1+PRN regimens in the patients receiving ranibizumab for ME secondary to BRVO. Intact IS/OS and baseline BCVA are good predictor of the visual gain, while baseline CMT is a good predictor of the anatomical gain.
Keywords: branch retinal vein occlusion, ranibizumab, macular edema, therapy, predictive factors
INTRODUCTION
Retinal vein occlusion (RVO) is the second most frequent retinal vascular disorder after diabetic retinopathy. RVO is divided into central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO). Vascular compression during arteriovenous passages, degenerative changes in venous walls and hypercoagulability are the underlying pathophysiology of BRVO. Retinal ischemia after vascular occlusion can cause an increase the amount of vascular endothelial growth factor (VEGF) which can increase vascular permeability and cause macular edema (ME)[1]–[2]. ME is the main cause of vision loss[3]–[8]. There are some treatment modalities for ME such as intravitreal dexamethasone implants, laser treatment, and intravitreal injections of anti-VEGF agents[9]–[11].
Ranibizumab 0.5 mg which is an anti-VEGF agent was approved in June 2010 for the treatment of ME due to BRVO and CRVO in the United States, based on the 6mo-results of two phase III, randomized, double-masked, 12mo, controlled study-BRAVO. In this study, six sequential monthly intravitreal ranibizumab (IVR) injections followed by pro re nata (PRN) regimen improved best-corrected visual acuity (BCVA) compared with imitation[12]–[13]. Yet the requirement of multiple intravitreal anti-VEGF agent injections for initial treatment of ME after BRVO is not well figured out and they may increase the risk of systemic or ocular complications[14]–[15].
In the real-life clinical practice, decreasing the number of anti-VEGF injection of the initial phase of treatment might be effective. In our study, we treated ME due to BRVO with two groups: 1 and 3 monthly initial IVR injections followed by PRN regimen. We compared them with central macular thickness (CMT), BCVA changes from pre-injection to final visit. There are not enough real-life studies about anti-VEGF treatment in ME due to BRVO. So we aimed to compare PRN treatment outcomes following three consecutive doses and single dose treatment in our clinic.
SUBJECTS AND METHODS
Patients
Forty-two eyes of 42 patients (27 men and 15 women) with treatment-naive acute ME due to BRVO were studied. Symptom durations of the patients were less than 2mo before the examination. All of the patients who had intravitreal injections of ranibizumab (0.5 mg/0.05 mL) at Okmeydani Research & Traning Hospital between June 2014 and December 2016 were retrospectively studied. Patients with CMT>300 µm in optical coherence tomography (OCT) were treated. Twenty four eyes received 3 monthly IVR injections (3+PRN group) and 18 eyes received one initial IVR injections (1+PRN group). The patients who have minimum 12mo follow up period were inclueded in this study. None of the patients had macular grid laser photocoagulation. It is known that RVO leads retinal non-perfusion. Peripheral retinal non-perfusion (PRNP) was found in the 7 eyes of 3+PRN group and 5 eyes of 1+PRN group with fluorescein angiography. These PRNP areas were out of macula and away at least two disc diameters from optic nerve head. Fluorescein angiography-guided argon laser scatter photocoagulation was performed to PRNP areas one week after first injection similar to WAVE study protocol[16]. The effects of argon laser scatter photocoagulation were compared with “no laser” patients. Patients with a history of cerebral infarction, anti-VEGF therapy, dexamethasone therapy, vitrectomy, uveitis, glaucoma or other vitreoretinal diseases were excluded. This retrospective study was conducted in accordance with the Declaration of Helsinki. All necessary authorizations were obtained from the Institutional Review Board of Okmeydani Research & Traning Hospital, Istanbul, Turkey.
Ophthalmic Examinations
All of the patients had standard ophthalmic examinations before treatment and post treatment (1, 2, 3, 6, 12mo and final visit). The examinations included slit-lamb microscopy, BCVA, tonometry, spectral-domain optical coherence tomography (SD-OCT), indirect ophthalmoscopy. BCVA was measured with Snellen chart, and the decimal visual acuity was converted to the logarithm of the minimal angle of resolution (logMAR) units for the statistical analyses.
Optical Coherence Tomography Measurement
OCT acquisition was performed on the SD-OCT (Cirrus HD-OCT; Carl Zeiss Meditec). The morphologic features of ME, CMT, the presence of subretinal fluid, the integrity of external limiting membrane (ELM) and ellipsoid zone, cyst size, the presence of inner segment/outer segment (IS/OS) defect were assessed and analyzed with SD-OCT by two experienced ophthalmologists. The average value of each parameter was considered for statistical analyzes.
Statistical Analysis
Statistical analyses were performed using the SPSS software version 15. Descriptive analyses were presented using means and standard deviations for normally distributed variables. Student's t-test and Mann-Whitney U test were used to compare the parameters between the groups. When investigating the effect of the two different treatment regimens on the change in BCVA and CMT over time, repeated measures analysis of variance (ANOVA) was used. When investigating the visual and anatomical gain by different regimens (1+PRN and 3+PRN) the effect of all predictors were investigated by using both simple and multiple regression analysis (with enter method). A P value of 0.05 was considered statistically significant.
RESULTS
Forty-two eyes of 42 patients with ME due to BRVO were examined in this retrospective cohort study. All of the patients have a minimum 12mo follow-up time. Twenty-four eyes received 3 monthly IVR injections (3+PRN group), 18 eyes received 1 initial IVR injection (1+PRN group). Both two groups were similar in terms of age, gender and the duration of the interval between the onset of the RVO and initiation of the treatment (P=0.601, P=0.783, P=0.169 respectively). Only one (4.2%) patient in the 3+PRN group showed a cataract progression and one (4.2%) had to be started anti-glaucomatous treatment. The baseline characteristics of the patients were summarized in Table 1.
Table 1. Baseline characteristic of the patients.
| Parameters | 3+PRN group (n=24) | 1+PRN group (n=18) | P |
| Age (y) | 60.2±10.2 | 58.6±8.9 | 0.601 |
| Gender, male | 15 (62.5) | 12 (66.7) | 0.783 |
| Follow-up period (mo) | 14.5±2.5 | 14.8±2.6 | 0.980 |
| No. of injections | 4.2±1.3 | 2.8±1.6 | 0.004a |
| Cataract development | 1 (4.2) | 0 (0.0) | 1.00 |
| Anti-glacomatous theraphy | 1 (4.2) | 0 (0.0) | 1.00 |
| Argon laser scatter photocoagulation | 7 (29.2) | 5 (27.8) | 0.921 |
| Baseline CMT (µm) | 521.3±153.2 | 438.1±162.4 | 0.098 |
| Final CMT (µm) | 278.3±87.8 | 285.2±74.2 | 0.079 |
| Baseline BCVA (logMAR) | 0.92±0.60 | 0.72±0.46 | 0.354 |
| Final BCVA (logMAR) | 0.42±0.55 | 0.38±0.50 | 0.979 |
| Duration of symptoms (d) | 23.79±7.15 | 20.78±6.54 | 0.169 |
| Subretinal fluid | 9 (37.5) | 5 (27.7) | 0.508 |
| Integrity of ELM (yes/no) | 13/11 | 11/7 | 0.327 |
| Integrity of IS/OS (yes/no) | 15/9 | 9/9 | 0.418 |
CMT: Central macular thickness; BCVA: Best-corrected visual acuity; ELM: External limiting membrane; IS/OS: Inner segment/outer segment. aStatistically significant.
mean±SD, n (%)
Final CMT was 278.3±87.8 µm in the 3+PRN group and 285.2±74.2 µm in the 1+PRN group (P=0.079). Additionally, the CMT changes from baseline to final visit were not significantly different (243±160 µm in the 3+PRN group, and 152.9±175.3 µm in the 1+PRN group; P=0.090). The trend in CMT changes over time was also similar in both groups (Figure 1). When the change in CMT was investigated, initial CMT was adjusted in one-way ANCOVA for the confounding effect and there was not a statically significant difference between groups (P=0.585).
Figure 1. Changes in CMT from baseline.
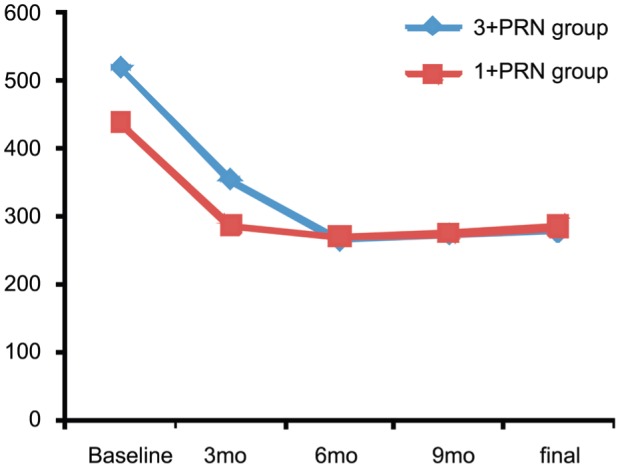
CMT was decreased statistically significant in both groups (P<0.001). However, there is no difference between the two groups (P=0.090).
Final BCVA was 0.42±0.55 logMAR in the 3+PRN group and 0.38±0.50 logMAR in the 1+PRN group (P=0.979). Additionally, the BCVA changes from baseline to final visit were not significantly different (-0.50±0.45 logMAR in the 3+PRN group, and -0.33±0.39 logMAR in the 1+PRN group; P=0.255). The trend in BCVA changes over time was also similar in both groups (Figure 2). When the change in BCVA was investigated, initial CMT was adjusted in one-way ANOVA for the confounding effect and there was not a statically significant difference between groups (P=0.693).
Figure 2. Changes in logMAR BCVA from baseline.
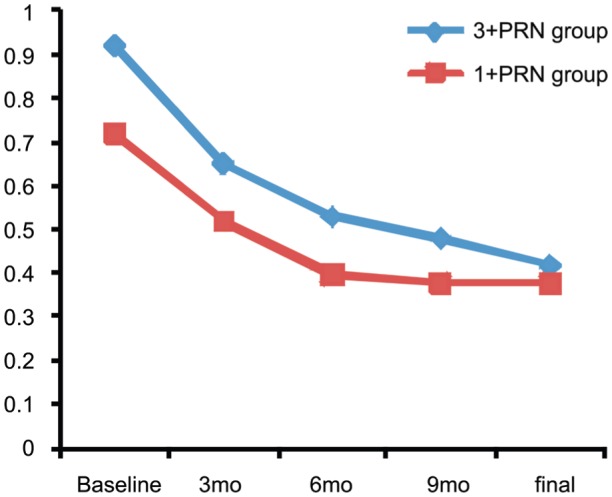
BCVA was improved statistically significant in both groups (P<0.001). However, there is no difference between the groups (P=0.255).
We investigated by using simple regression analysis whether argon laser scatter photocoagulation to PRNP had an effect at final functional and anatomical outcomes. We found that it did not have any effect (P=0.552 and P=0.685). Argon laser scatter photocoagulation did not effect also number of injections (P=0.193).
The predictor factors of the visual and anatomical gain were also investigated in the entire study group. When investigating the changes in BCVA and CMT by different regimens (1+PRN and 3+PRN) the effect of all predictors were investigated by using both simple and multiple regression analysis (with enter method). The results of regression analysis were shown in Tables 2, 3. Multiple linear regression analysis with enter method revealed that baseline CMT was the most valuable predictive factor for the change in CMT (Beta coefficient=0.938, P<0.001). In addition, baseline BCVA was found to be the most important predictive factor for the change in BCVA (Beta coefficient=-0.351, P=0.003). Integrity of IS/OS was found to correlate with visual and anatomical gain with simple regression anaylsis (Beta coefficient=-0.286, P=0.034 and Beta coefficent=176.681, P<0.001 respectively).
Table 2. Predictive factors for visual gain.
| Predictive factors | Simple regression analysis |
Multiple regression analysis |
||
| Beta coefficient | P | Beta coefficient | P | |
| Subretinal fluid | -0.71 | 0.234 | 0.286 | 0.175 |
| Intact ELM | 0.132 | 0.332 | -0.081 | 0.699 |
| Cystoid spaces | 0.000 | 0.770 | 0.114 | 0.487 |
| Initial CMT | -0.001 | 0.017 | -0.279 | 0.151 |
| Initial BCVA | -0.351 | 0.003 | -0.393 | 0.043 |
| Status of LFK | -0.090 | 0.552 | -0.086 | 0.596 |
| Integrity of IS/OS | -0.286 | 0.034 | -0.215 | 0.354 |
CMT: Central macular thickness; BCVA: Best-corrected visual acuity; ELM: External limiting membrane; IS/OS: Inner segment/outer segment; LFK: Laser photocoagulation. Visual gain was defined as BCVA (logMAR) at 12mo minus BCVA (logMAR) at baseline.
Table 3. Predictive factors for anatomical gain.
| Predictive factors | Simple regression analysis |
Multiple regression analysis |
||
| Beta coefficient | P | Beta coefficient | P | |
| Subretinal fluid | 187.964 | 0.000 | 0.035 | 0.744 |
| Cystoid spaces | 0.350 | 0.113 | 0.21 | 0.790 |
| Initial CMT | 0.938 | 0.000 | 0.759 | 0.000 |
| Initial BCVA | 178.392 | 0.000 | 0.167 | 0.091 |
| Status of LFK | -24.133 | 0.685 | -0.057 | 0.488 |
| Integrity of IS/OS | 176.681 | 0.000 | 0.031 | 0.768 |
CMT: Central macular thickness; BCVA: Best-corrected visual acuity; LFK: Laser photocoagulation; IS/OS: Inner segment/outer segment. Anatomical gain was defined as CMT at baseline minus CMT at 12mo.
DISCUSSION
In this study, the patients initially received one or three monthly IVR for ME. At 6mo and final visit, there was no significant difference in CMT or BCVA change from baseline between the 2 regimen groups. We believe that these two treatment regimens may achieve similar results. We attributed this finding to the difference in intravitreal VEGF levels of the patients. Considering the natural pathophysiology of BRVO, ischemia induces VEGF secretion. Since the amount of ischemia is not standard in all the patients, the VEGF levels will be variable. In current literature, there is only one study comparing the single injections with 3 monthly injections of IVR in the treatment of ME due to BRVO. Miwa et al[17] confirmed that there is no significant difference between one and three monthly IVR injections as in our study.
There is one study which compares one or three monthly injections with bevacizumab. Ito et al[18] reported that the single injection of bevacizumab's and three monthly injections results were similar. There were not any significant differences in visual outcomes and CMT findings.
In the BRAVO trial, patients received six monthly IVR in the first 6mo. According to their retreatment criteria, additional injections were performed in the follow-up (6+PRN regimen)[12]–[13],[19]. Surely, it is difficult to compare the BRAVO trial and the current study due to different inclusion criteria. But the real-life studies are overgrowing every single day in the literature which reveal different results compared to randomized clinical trials.
In a recent study, Osaka et al[20] studied patients with ME secondary to CRVO. Twenty nine eyes of the pateins were treated with 3+PRN regimen, while 20 eyes were treated 1+PRN regimen. They followed the patents for twelve month. At final visit, they reported that 1+PRN regimen achieved visual outcomes similar to those of 3+PRN regimen with fewer injections.
In brief, we believe these three treatment regimens (1+PRN, 3+PRN, 6+PRN) can achieve similar results, especially in good responding patients to the first anti-VEGF injection. We hypothesis that the response to first anti-VEGF injection helps on forecasting the VEGF concentaritions as well the prognosis of the patients.
We also investigated the predictor factors of the change in BCVA and CMT in the entire group. We found that baseline CMT was significantly associated with the anatomical gain. Similar to our results, Ach et al[21] reported that the initial CMT was a predictive factor for short and long-term responses to anti-VEGF treatment.
The multiple regression analyses showed that the pretreatment BCVA had the highest correlation with visual gain. Additional, the statistical analysis showed that the BCVA improvement at 12-month follow-up was better in the eyes with intact photoreceptor IS/OS layer. Similar to our conclusion, Shin et al[22] also found that integrity of IS/OS is significantly correlated with visual gain.
In the WAVE[16] and RELATE[23] studies, the researcher wanted to investigate the effect of peripheral laser application on treatment burden and visual outcomes. But these two studies were different in some aspects. First, in WAVE study peripheral laser application was performed after first injections, while in RELATE study it was done after two consecutive injections. Secondly, inclusion criteria were different between those the trials. In WAVE study, patients had ischemic RVO and ME poorly responsive to anti-VEGF and they investigated whether those patients could benefit after argon laser scatter photocoagulation. But in RELATE study, at baseline 41% of patients were treatment naive and perfusion status was not considered as an inclusion criteria. Despite these differences both studies concluded that peripheral laser photocoagulation did not effect number of injections and visual outcomes in RVO patients under ongoing anti-VEGF treatment. In our study we performed argon laser scatter photocoagulation after first injections similar to WAVE study. But our patients were treatment naive and perfusion status was not an inclusion criteria similar to RELATE study. Although our study was different from these studies and had fewer patients we found that argon laser scatter photocoagulation did not affect treatment burden and visual outcomes in BRVO patients with ongoing anti-VEGF treatment.
In conclusion, our results suggest that both 3+PRN IVR and 1+PRN IVR regimens may achieve similar results in one-year follow-up. Another result is that baseline SD-OCT characteristics can be helpful in predicting the final visual outcome after IVR injection in patients with ME secondary to BRVO. Especially the integrity of IS/OS and initial BCVA are significantly correlated with visual gain. And finally, peripheral photocoagulation did not impact treatment burden, visual and anatomical outcomes in patients with ME due to BRVO.
Acknowledgments
Conflicts of Interest: Bayat AH, None; Çakır A, None; Özturan ŞG, None; Bölükbaşı S, None; Erden B, None; Elçioğlu MN, None.
REFERENCES
- 1.Jaulim A, Ahmed B, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33(5):901–910. doi: 10.1097/IAE.0b013e3182870c15. [DOI] [PubMed] [Google Scholar]
- 2.Son BK, Kwak HW, Kim ES, Yu SY. Comparison of ranibizumab and bevacizumab for macular edema associated with branch retinal vein occlusion. Korean J Ophthalmol. 2017;31(3):209–216. doi: 10.3341/kjo.2015.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz N, Pinilla I, Casas P, Garcia-Martin E, Idoipe M, Fuertes I, Cascante M, Cristobal Bescos JA. Ranibizumab for macular edema following branch retinal vein occlusion. Acta Ophthalmologica. 2010;246(88) [Google Scholar]
- 4.Moon BG, Cho AR, Kim YN, Kim JG. Predictors of refractory macular edema after branch retinal vein occlusion following intravitreal bevacizumab. Retina. 2018;38(6):1166–1174. doi: 10.1097/IAE.0000000000001674. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology. 2009;116(6):1188–1194. doi: 10.1016/j.ophtha.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25. doi: 10.1016/j.preteyeres.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, Nguyen HP, Wang JJ, Wong TY. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094–1101. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion. N Engl J Med. 2010;363(22):2135–2144. doi: 10.1056/NEJMcp1003934. [DOI] [PubMed] [Google Scholar]
- 9.Mylonas G, Sacu S, Dunavoelgyi R, Matt G, Blum R, Buehl W, Pruente C, Schmidt-Erfurth U, Macula Study Group Response of retinal sensivity to ranibizumab treatmant of macular edema after acute branch retinal vein occlusion. Retina. 2013;33(6):1220–1226. doi: 10.1097/IAE.0b013e3182794b06. [DOI] [PubMed] [Google Scholar]
- 10.Korobelnik JF, Kodjikian L, Delcourt C, Gualino V, Leaback R, Pinchinat S, Velard ME. Two-year, prospective, multicenter study of the use of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in France. Graefes Arch Clin Exp Ophthalmol. 2016;254(12):2307–2318. doi: 10.1007/s00417-016-3394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamei M, Terasaki H, Yoshimura N, Shiraga F, Ogura Y, Grotzfeld AS, Pilz S, Ishibashi T. Short-term efficacy and safeyt of ranibizumab for macular oedema secondary to retinal vein occlusin in Japanese patients. Acta Ophthalmol. 2017;95(1):e29–e35. doi: 10.1111/aos.13196. [DOI] [PubMed] [Google Scholar]
- 12.Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG, BRAVO Investigators Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102–1112. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, Murahashi WY, Rubio RG. Sustained benefits from ranibizumab for macular edema following central vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041–2049. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Rayess N, Rahimy E, Shah CP, Wolfe JD, Chen E, DeCross FC, Storey P, Garg SJ, Hsu J. Incidence and clinical features of post-injection endophthalmitis according to diagnosis. Br J Ophthalmol. 2016;100(8):1058–1061. doi: 10.1136/bjophthalmol-2015-307707. [DOI] [PubMed] [Google Scholar]
- 15.Kida T, Tsujikawa A, Muraoka Y, Harion S, Osaka R, Murakami T, Ooto S, Suzuma K, Maroishita S, Fukumoto M, Suzuki H, Ikeda T. Cotton wool spots after anti-vascular endothelial growth factor therapy for macular edema associated with central retinal vein occlusion. Ophthalmologica. 2016;235(2):106–113. doi: 10.1159/000443622. [DOI] [PubMed] [Google Scholar]
- 16.Wykoff CC, Ou WC, Wang R, Brown DM, Cone C, Zamora D, Le RT, Sagong M, Wang K, Sadda SR, WAVE Study Group Peripheral laser for recalcitrant macular edema owing to retinal vein occlusion: the WAVE trial. Ophthalmology. 2017;124(6):919–921. doi: 10.1016/j.ophtha.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Miwa Y, Muraoka Y, Osaka R, Ooto S, Murakami T, Suzuma K, Takahashi A, Iida Y, Yoshimura N, Tsujikawa A. Ranıbızumab for macular edema after branch retınal veın occlusıon one ınitial ınjection versus three monthly ınjections. Retina. 2017;37(4):702–709. doi: 10.1097/IAE.0000000000001224. [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Saishin Y, Sawada O, Kakinoki M, Miyake T, Sawada T, Kawamura H, Ohji M. Comparison of single injection and three monthly injections of intravitreal bevacizumab for macular edema associated with branch retinal vein occlusion. Clin Ophthalmol. 2015;9:175–180. doi: 10.2147/OPTH.S76261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varma R, Bressler NM, Suñer I, Lee P, Dolan CM, Ward J, Colman S, Rubio RG, BRAVO and CRUISE Study Groups Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials. Ophthalmology. 2012;119(10):2108–2118. doi: 10.1016/j.ophtha.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Osaka R, Muraoka Y, Miwa Y, Manabe K, Kobayashi M, Takasago Y, Ooto S, Murakami T, Suzuma K, Iida Y, Tsujikawa A. Anti vascular endothelial growth factor therapy for macular edema following central retinal vein occlusion: 1 initial injection versus 3 monthly injections. Ophthalmologica. 2018;239(1):27–35. doi: 10.1159/000479049. [DOI] [PubMed] [Google Scholar]
- 21.Ach T, Hoeh AE, Schaal KB, Scheuerle AF, Dithmar S. Predictive factors for changes in macular edema in intravitreal bevacizumab therapy of retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2010;248(2):155–159. doi: 10.1007/s00417-009-1167-6. [DOI] [PubMed] [Google Scholar]
- 22.Shin HJ, Chung H, Kim HC. Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol. 2011;89(1):35–40. doi: 10.1111/j.1755-3768.2010.02063.x. [DOI] [PubMed] [Google Scholar]
- 23.Campochiaro PA, Hafiz G, Mir TA, Scott AW, Solomon S, Zimmer-Galler I, Sodhi A, Duh E, Ying H, Wenick A, Shah SM, Do DV, Nguyen QD, Kherani S, Sophie R. Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: the RELATE trial. Ophthalmology. 2015;122(7):1426–1437. doi: 10.1016/j.ophtha.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


