Abstract
Artificial intelligence is a general term that means to accomplish a task mainly by a computer, with the least human beings participation, and it is widely accepted as the invention of robots. With the development of this new technology, artificial intelligence has been one of the most influential information technology revolutions. We searched these English-language studies relative to ophthalmology published on PubMed and Springer databases. The application of artificial intelligence in ophthalmology mainly concentrates on the diseases with a high incidence, such as diabetic retinopathy, age-related macular degeneration, glaucoma, retinopathy of prematurity, age-related or congenital cataract and few with retinal vein occlusion. According to the above studies, we conclude that the sensitivity of detection and accuracy for proliferative diabetic retinopathy ranged from 75% to 91.7%, for non-proliferative diabetic retinopathy ranged from 75% to 94.7%, for age-related macular degeneration it ranged from 75% to 100%, for retinopathy of prematurity ranged over 95%, for retinal vein occlusion just one study reported ranged over 97%, for glaucoma ranged 63.7% to 93.1%, and for cataract it achieved a more than 70% similarity against clinical grading.
Keywords: artificial intelligence, deep learning, machine learning, images processing, ophthalmology
INTRODUCTION
Artificial intelligence (AI) is a general term that means to accomplish a task mainly by a computer, with minimal human beings involved[1]. In other words, the purpose of AI is to make computers mimic the way of our thinking, and improve our work efficiency in the modern fast-pace life. It has become one of the most influential information technology revolutions[2]. Great progress has been made in theoretical research and its application as far as we can see. AI is widely accepted as the appearance of many robots in difference fields, especially in bioinformatics. Combined with medicine, some robot-assisted surgery has been conducted successfully. It makes doctor's work more precisely and effectively. Nowadays, AI-assisted medical screening and diagnosis based on images are emerging[3]–[5]. As we all hear, melanoma, a skin cancer could be diagnosed with a computer algorithm based on macro images captured by a common camera[6]. In the field of ophthalmology, especially in the blind-causing diseases, it mainly attributes to medical imaging identification and auxiliary diagnosis.
The application of this technology of AI mainly depends on machine learning[7], which is represented by mathematical algorithms and models formed through lots of input experience.
SUBJECTS AND METHODS
We searched these English-language studies relative to ophthalmology published on PubMed and Springer databases. Later we gave a classification and statistic. Its application mainly concentrates on the diseases with a high incidence, such as diabetic retinopathy (DR), age-related macular degeneration (AMD), glaucoma, retinopathy of prematurity (ROP), age-related or congenital cataract and few with retinal vein occlusion (RVO).
Principle of Artificial Intelligence
The AI devices mainly fall into two major categories[8] -the machine learning techniques[9] and the natural language processing methods. But so far, the former is the auxiliary screening and diagnostic technique what we often talk about[10].
Machine learning provides techniques or algorithms that can automatically build a model of complex relationships by processing the input available data and generalizing a performance standard[7]. And it can be briefly described as enabling computers make successful predictions or judgments by repeatedly learning existing representative materials. To be able to form an accurate model, machine learning often requires a large number of training data. And most of them need to be labeled its features in advance by relative authoritative experts. Besides, some other data are used to verify the established algorithm. That means the processes mainly include two parts, training set and validation set. Therefore, an important step is to collect a lot of representative training examples. Some experts mark the easy-identify and distinctive features, and input the computer to make it recognize and remember. It is crucial that the feature selection or extraction requires much experience.
There are mainly two deep learning models, including convolutional neural network (CNN) and massive-training artificial neural network (MTANN)[11]. They are powerful tools for identifying and classifying images. To our knowledge, CNN and MTANN both have many layers. The major differences are that convolutional operations are underwent within the network in CNN, whereas in MTANN they are outside the network. After an iterative process, the last convolution layer is connected with the whole. What's more, CNN needs much more images than the latter. CNN has been successfully used in many fields, such as, large-scale image classification[12], scene labeling[13] and so on.
RESULTS
Some current studies based on machine learning have achieved a satisfactory preliminary outcome. For example, the image identification of non-proliferative diabetic retinopathy (NPDR), proliferative diabetic retinopathy (PDR) and AMD attracts most of the attention. The diagnostic sensitivity for AMD ranged from 75% to 100%. Similarly, the sensitivity of detection and accuracy for PDR ranged from 75% to 91.7% and for NPDR, ranged from 75% to 94.7%. The average rate of diagnosis for these diseases can reach 91.3%[14]. Also, Ting et al's[15] study aims to develop and evaluate the deep learning system for DR, AMD and glaucoma, based on the fundus images of multiethnic populations. Compared with professional graders, they conclude that their system can achieve a relative high sensitivity and specificity.
Diabetic Retinopathy and Artificial Intelligence
DR is the leading cause of blindness in the working-age people[16], which mainly affects the retinal microvasculature, leading to progressive damage[17]. With more and more people affected, DR is gradually deemed to the global public health problem[18]. Therefore, the large scale screening of DR is needed urgently to detect potentially threatening changes at early stage which will benefit for treatment and management. As we all know, early intervention is the most cost-effective choice[19].
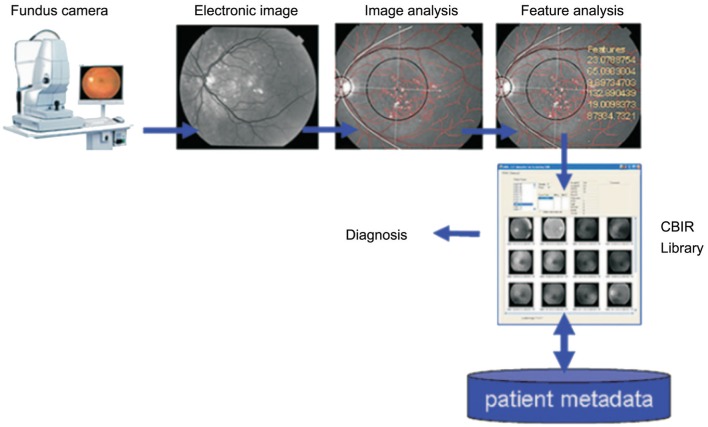
The automatic identification of DR has attracted a lot of attention, with studies conducting microaneurysm, hemorrhage, exudation, cotton-wool spot and neovascularization detection, and even further classify stages. Most of them use the fundus images as input. The process can be partly represented by Figure 1[14]. The computers receive many images labeled with diagnostic lesions, extract their characteristics and finally build a model. And then, it can identify the new input images and give a judgement. Wong et al[20] propose a method based on microaneurysms and hemorrhages to by a three-layer feed forward neural network to classify the DR stages. Imani et al[21] form a technique to detected the exudation and blood vessel by morphological component analysis (MCA), and Pavle used the CNN. Yazid et al[22] put forward that they identified the hard exudation and optic disc based on inverse surface thresholding. Some reports say that they use a Lattice Neural Network with Dendritic Processing (LNNDP) or enhancement techniques to detect blood vessels in retinal images[23]–[24]. Akyol et al[25] detect the optic disc of fundus images automatically by using keypoint detection, texture analysis, and visual dictionary techniques. Niemeijer et al[26] fast detect the optic disc by a method combined k-nearest neighbour (kNN) and cues. They report that the sensitivity of automatic DR screening ranges from 75%-94.7%, also the specificity, accuracy is comparable and promising.
Figure 1. A fundus image is submitted to locate anatomic structures and lesions followed by feature extraction and analysis. The features are an index for searching the library to compare with similar images from database. It can also combine the patient's clinical metadata.
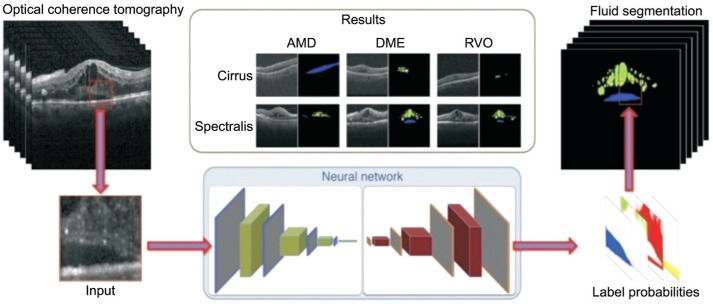
Furthermore, there will be a few studies involved with multimodal data to verify a disease more precisely. For instance, combining macular optical coherence tomography (OCT) with fundus image indentify macular edema, which is the sign of timely treatment. After all, a study has reported an algorithm can detect and quantify subretinal or intraretinal fluid based on OCT images, just described as Figure 2[27].
Figure 2. Illustration of the automated detection of macular fluid in OCT.
The intraretinal cystoid fluid is marked in green, subretinal fluid is marked blue. AMD: Age-related macular degeneration; DME: Diabetic macular edema; RVO: Retinal vein occlusion.
Apart from the above automatic detection and identification of DR, the study of the evaluation of deep learning models for DR grades. They reported the errors of deep learning models mainly concentrated on missing the microaneurysm and artifacts. For the moderate or worse DR, the sensitivity of deep learning models is about 97.1%, compared with the ophthalmologists' 83.3%. Maybe the quality of input images is responsible for the minimal lesions missing, they think[28].
Age-related Macular Degeneration and Artificial Intelligence
AMD is a chronic and irreversible macular disease characterized by drusen, retinal pigment changes, choroidal neovascularization, hemorrhage, exudation and even geographic atrophy[29]. It is one of the leading causes of central vision loss in people aged over 50[30]. With the social population aging and the severity of this disease, it's necessary to perform AMD screening regularly. Automatic AMD diagnosis may obviously reduce the work load of clinicians and improve efficiency.
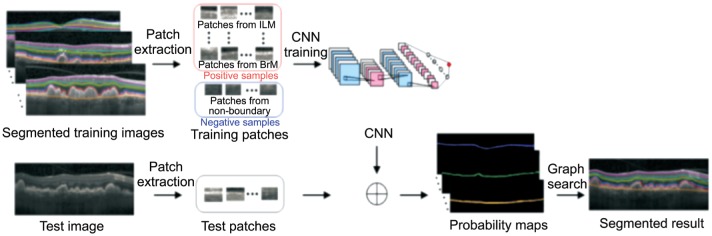
Many studies have reported their preliminary results. Most of them use fundus images as input original materials, and extract features of early, intermediate and late AMD to distinguish from the healthy images[31]. They can obtain a sensitivity ranging from 87% to 100%, also with a relatively high accuracy[32]. They think taking fundus photo as input is cheaper than OCT examination. But also, there exist researches combined spectral domain OCT with deep learning about AMD, including the macular fluid quantity of neovascular AMD (nAMD) just like Figure 2 and the retinal layers segmentation of dry AMD like Figure 3[33]. After an iteration training, the training and validation accuracy are both 100%[34]. They believe that other macular diseases will obtain the same effective results.
Figure 3. Outline of the algorithm to segment the retinal layers of dry AMD.
As we all know, intravitreal injection of anti-VEGF drugs is the first-line therapy for nAMD[35] and the follow-up observation is also very important. Bogunovic et al[36] utilize an algorithm to observe the treatment responders using OCT images. Some researchers combine the machine learning with OCT images to observe and predict the possibility of retreatment[37]. The model they built achieves a comparable performance for predicting the low and almost 50% better performance in predicting the high retreatment requires.
Retinal Vein Occlusion and Artificial Intelligence
RVO has an estimated prevalence ranging from 0.3% to 2.1%[38]–[40] in different individuals, which is one of the most common blind-causing diseases, ranking after DR[41]. We think, the direct reason of RVO may be that sclerotic retinal artery compress the retinal vein and block the blood return of terminal arborizations. Further, it causes superficial hemorrhage, exudation, and retinal edema. If any lesion involves macular, it will lead to vision acuity decreased significantly, or even blindness. Its risk factors mainly are people with old age and vascular sclerosis[42]–[44], such as hypertension, arteriosclerosis or cardiovascular disease. Thus, the early diagnosis of RVO is crucial for vision recovery.
Automatic diagnosis will benefit both patients and ophthalmologists, if it is widely used. At present, the machine learning in RVO is relatively rare. A team reported that they utilized CNN combined with patch-based and image-based vote methods to recognize the fundus image of branch retinal vein occlusion automatically. They received a high accuracy over 97%[45]. It's encouraging for the following researches.
Retinopathy of Prematurity and Artificial Intelligence
ROP is a leading cause of childhood blindness all over the world[46]–[47] and it is largely treatable with appropriate and timely diagnosis. Clinical studies have shown that ROP with plus disease or retinopathy in zone one stage 3 even without plus disease requires timely treatment to prevent blindness, and infants with pre-plus disease require close observation[48]. Repeated screening and follow-up of ROP will consume a lot of manpower and energy. So the application of AI in ROP screening may improve the efficiency of ROP care.
Many studies have tried the automatic identification of ROP. Most of them focused on two-level classification (plus or not plus disease)[49]–[52]. They achieved a promising result. An report says that they could distinguish the plus disease with a 95% accuracy, which is comparable to experts' diagnosis, much more precise than non-experts[53]. And Ataer-Cansizoglu et al's[54] study took advantages of tortuosity and dilation features from arteries and veins to distinguish not plus or pre-plus or plus disease. They classify the ROP more specifically and benefitial for the treatment.
Anterior Segment Diseases and Artificial Intelligence
Maybe, cataract and glaucoma are very common diseases in ophthalmology[55]–[56]. It is not surprising that there are some reports about the application of machine learning in anterior segment diseases[57]–[61]. Cataracts are a clouding of the lens and the leading cause of blindness all over the world[55]. The automatic recognition will be cost-effective.
Gao et al[57] have reported that they proposed a system automatically grade the severity of nuclear cataracts by slit-lamp images. First, they find the lens region of interest and then CNN filters randomly select image patches generating local representations by an iteration process with random weights. Their system achieved a more than 70% similarity against clinical grading. Other like the research of Liu et al[58], they mainly focus on the identification of pediatric cataracts. They achieve an exceptional accuracy and sensitivity in lens classification and density. Also, it can automatically grade a cataract by lens OCT[59].
Glaucoma is a disease that mainly damages the optic nerve, which can cause irreversible blindness[56],[60]. Although glaucoma may not be cured, the processing can be slow down by reasonable treatment[61]. Thus, early detection of glaucoma is highly needed. The detection of glaucoma mainly depends on the intraocular pressure, thickness of retinal nerve fiber, optic nerve and visual field examination[62]–[63].
Omodaka et al[64] developed a machine learning algorithm to classify the optic disc of open-angle glaucoma and reached a accuracy of 87.8%. Their algorithm based on the quantitative parameters mainly from the optic disc OCT examination. Many studies have tried to apply the machine learning in glaucoma identification. The machine usually assesses the cup disc ratio[65]–[66] in the fundus images, the visual field[67] or the thickness of retinal nerve fiber examined by OCT[68]. The accuracy of early diagnosis ranges from 63.7% to 93.1% depending on the input images.
DISCUSSION
AI-assisted automated screening and diagnosis of the common diseases in ophthalmology may eventually help maximize the doctors' role at the clinic. Outside the clinic, AI platforms offer the patients more medical opportunities and reduce obstacles to access for an eye care where an ophthalmologist is not available. To some extent, new technologies based on AI may reduce social inequalities[69]. Looking further into the future, AI-assisted system shows the potential to relieve the overburdened healthcare system's problems.
In general, the process of automatically detect a disease mainly include three steps[11],[70]. Firstly, it's necessary to collect a large amount of images, and relative experts have to label the characteristic lesions. It is fundamental but very crucial. Secondly, computers extract the features of a disease through a particular program based on the input of marked images. Finally, a given image can be distinguished from other kind of disease by statistical feature of target lesions.
According to these studies, some algorithms have been preliminarily formed, such as DR, ROP, AMD, RVO, glaucoma, cataract and so on. However, with so many present reports, there is seldom one realized a 100% accuracy and sensitivity. That is to say, not every image can be identified precisely or not be missed. Not only does it depend on the computer technique, but the quality of input images[71]–[72]. The main factors caused poor quality of posterior or anterior segment images may be the patient's head or eyeball movement, undilated pupil, frequent blinking, opaque refractive medium and poor fixation[73]–[74]. Besides, the marking process by experts is also quite important. It's the foundation of computer learning. Thus, the annotators must be trained for a uniform standard.
Besides that, there may exist some other limitations about deep learning[11],[75]. First, forming an algorithm needs a lot of computational cost and training experience. That means AI may be just useful for the diseases with a high morbidity. For rare diseases, it may not be available. Second, the computer recognizes a structure or a feature mechanically, so AI could not completely identify a disease separated from our intervention. A small portion of feature and variation that look like unusual will be missed. We infer that AI can pick out the majority of people with a kind of disease, not all of them. Third, to some extent, this work is complicated. The characteristics of a disease and parameters of an algorithm differ from tasks to tasks. Finally, if the relationship between input and expected output materials is complex, the machine will probably not build a model. What's more important is that it may cause a mistake. Nguyen et al[76] described the process how the neural networks lead to a wrong classification.
From this perspective, AI can really efficiently conduct a task, but a certain degree of human intervention is essential during the process.
In conclusion, AI has been widely studied in ophthalmological image processing, mainly based on the fundus photographs. Indeed, it achieves a promising accuracy comparable with clinical experts. However, more efforts should be made to explore the neural network to assist our work.
Acknowledgments
Conflicts of Interest: Du XL, None; Li WB, None; Hu BJ, None.
REFERENCES
- 1.Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69S:S36–S40. doi: 10.1016/j.metabol.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Obermeyer Z, Emanuel EJ. Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med. 2016;375(13):1216–1219. doi: 10.1056/NEJMp1606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doi K. Computer-aided diagnosis in medical imaging historical review, current status and future potential. Comput Med Imaging Graph. 2007;31(4-5):198–211. doi: 10.1016/j.compmedimag.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doi K. Diagnostic imaging over the last 50 years research and development in medical imaging science and technology. Phys Med Biol. 2006;51(13):R5–27. doi: 10.1088/0031-9155/51/13/R02. [DOI] [PubMed] [Google Scholar]
- 5.Giger ML. Machine learning in medical imaging. J Am Coll Radiol. 2018;15(3PtB):512–520. doi: 10.1016/j.jacr.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Gautam D, Ahmed M, Meena YK, Ul HA. Machine learning-based diagnosis of melanoma using macro images. Int J Numer Method Biomed Eng. 2018;34(5):e2953. doi: 10.1002/cnm.2953. [DOI] [PubMed] [Google Scholar]
- 7.Lee A, Taylor P, Kalpathy-Cramer J, Tufail A. Machine learning has arrived. Ophthalmology. 2017;124(12):1726–1728. doi: 10.1016/j.ophtha.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 8.Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, Wang Y, Dong Q, Shen H, Wang Y. Artificial intelligence in healthcare past, present and future. Stroke and Vascular Neurology. 2017;2(4):230–243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darcy AM, Louie AK, Roberts LW. Machine learning and the profession of medicine. JAMA. 2016;315(6):551–552. doi: 10.1001/jama.2015.18421. [DOI] [PubMed] [Google Scholar]
- 10.Murff HJ, FitzHenry F, Matheny ME, Gentry N, Kotter KL, Crimin K, Dittus RS, Rosen AK, Elkin PL, Brown SH, Speroff T. Automated identification of postoperative complications within an electronic medical record using natural language processing. JAMA. 2011;306(8):848–855. doi: 10.1001/jama.2011.1204. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K. Overview of deep learning in medical imaging. Radiol Phys Technol. 2017;10(3):257–273. doi: 10.1007/s12194-017-0406-5. [DOI] [PubMed] [Google Scholar]
- 12.Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. Communications of the ACM. 2017;60(6):84–90. [Google Scholar]
- 13.Farabet C, Couprie C, Najman L, Lecun Y. Learning hierarchical features for scene labeling. IEEE Trans Pattern Anal Mach Intell. 2013;35(8):1915–1929. doi: 10.1109/TPAMI.2012.231. [DOI] [PubMed] [Google Scholar]
- 14.Chaum E, Karnowski TP, Govindasamy VP, Abdelrahman M, Tobin KW. Automated diagnosis of retinopathy by content-based image retrieval. Retina. 2008;28(10):1463–1477. doi: 10.1097/IAE.0b013e31818356dd. [DOI] [PubMed] [Google Scholar]
- 15.Ting DSW, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–2223. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocur I, Resnikoff S. Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol. 2002;86(7):716–722. doi: 10.1136/bjo.86.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jyothi S, Chowdhury H, Elagouz M, Sivaprasad S. Intravitreal bevacizumab (Avastin) for age-related macular degeneration a critical analysis of literature. Eye (Lond) 2010;24(5):816–824. doi: 10.1038/eye.2009.219. [DOI] [PubMed] [Google Scholar]
- 18.Taylor HR, Keeffe JE. World blindness a 21st century perspective. Br J Ophthalmol. 2001;85(3):261–266. doi: 10.1136/bjo.85.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bek T, Erlandsen M. Visual prognosis after panretinal photocoagulation for proliferative diabetic retinopathy. Acta Ophthalmol Scand. 2006;84(1):16–20. doi: 10.1111/j.1600-0420.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 20.Wong LY, Acharya R, Venkatesh YV, Chee C, Min LC. Identification of different stages of diabetic retinopathy using retinal optical images. Information Sciences. 2008;178:106–121. [Google Scholar]
- 21.Imani E, Pourreza HR, Banaee T. Fully automated diabetic retinopathy screening using morphological component analysis. Comput Med Imaging Graph. 2015;43:78–88. doi: 10.1016/j.compmedimag.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Yazid H, Arof H, Isa HM. Automated identification of exudates and optic disc based on inverse surface thresholding. J Med Syst. 2012;36(3):1997–2004. doi: 10.1007/s10916-011-9659-4. [DOI] [PubMed] [Google Scholar]
- 23.Vega R, Sanchez-Ante G, Falcon-Morales LE, Sossa H, Guevara E. Retinal vessel extraction using Lattice Neural Networks with Dendritic Processing. Comput Biol Med. 2015;58:20–30. doi: 10.1016/j.compbiomed.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Mirsharif Q, Tajeripour F, Pourreza H. Automated characterization of blood vessels as arteries and veins in retinal images. Comput Med Imaging Graph. 2013;37(7-8):607–617. doi: 10.1016/j.compmedimag.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Akyol K, Şen B, Bayır Ş. Automatic detection of optic disc in retinal image by using keypoint detection, texture analysis, and visual dictionary techniques. Comput Math Methods Med. 2016;2016:6814791. doi: 10.1155/2016/6814791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niemeijer M, Abràmoff MD, van Ginneken B. Fast detection of the optic disc and fovea in color fundus photographs. Med Image Anal. 2009;13(6):859–870. doi: 10.1016/j.media.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlegl T, Waldstein SM, Bogunovic H, et al. Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology. 2018;125(4):549–558. doi: 10.1016/j.ophtha.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Krause J, Gulshan V, Rahimy E, Karth P, Widner K, Corrado GS, Peng L, Webster DR. Grader variability and the importance of reference standards for evaluating machine learning models for diabetic retinopathy. Ophthalmology. 2018;125(8):1264–1272. doi: 10.1016/j.ophtha.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou CF, Cotch MF, Vitale S, Zhang X, Klein R, Friedman DS, Klein BE, Saaddine JB. Age-related eye diseases and visual impairment among U.S. adults. Am J Prev Med. 2013;45(1):29–35. doi: 10.1016/j.amepre.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mookiah MR, Acharya UR, Fujita H, Koh JE, Tan JH, Noronha K, Bhandary SV, Chua CK, Lim CM, Laude A, Tong L. Local configuration pattern features for age-related macular degeneration characterization and classification. Comput Biol Med. 2015;63:208–218. doi: 10.1016/j.compbiomed.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Burlina P, Pacheco KD, Joshi N, Freund DE, Bressler NM. Comparing humans and deep learning performance for grading AMD: a study in using universal deep features and transfer learning for automated AMD analysis. Comput Biol Med. 2017;82:80–86. doi: 10.1016/j.compbiomed.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang L, Cunefare D, Wang C, Guymer RH, Li S, Farsiu S. Automatic segmentation of nine retinal layer boundaries in OCT images of non-exudative AMD patients using deep learning and graph search. Biomed Opt Express. 2017;8(5):2732–2744. doi: 10.1364/BOE.8.002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treder M, Lauermann JL, Eter N. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graefes Arch Clin Exp Ophthalmol. 2018;256(2):259–265. doi: 10.1007/s00417-017-3850-3. [DOI] [PubMed] [Google Scholar]
- 35.Wong TY, Liew G, Mitchell P. Clinical update new treatments for age-related macular degeneration. Lancet. 2007;370(9583):204–206. doi: 10.1016/S0140-6736(07)61104-0. [DOI] [PubMed] [Google Scholar]
- 36.Bogunovic H, Waldstein SM, Schlegl T, Lang G, Sadeqhipour A, Liu X, Gerendas BS, Osbome A, Schmidt-Erfurth U. Prediction of Anti-VEGF treatment requirements in neovascular amd using a machine learning approach. Invest Ophthalmol Vis Sci. 2017;58(7):3240–3248. doi: 10.1167/iovs.16-21053. [DOI] [PubMed] [Google Scholar]
- 37.Kvannli L, Krohn J. Switching from pro re nata to treat-and-extend regimen improves visual acuity in patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2017;95(7):678–682. doi: 10.1111/aos.13356. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(10):1243–1247. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 39.Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005;112(4):540–547. doi: 10.1016/j.ophtha.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Yasuda M, Kiyohara Y, Arakawa S, et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population the Hisayama study. Invest Ophthalmol Vis Sci. 2010;51(6):3205–3209. doi: 10.1167/iovs.09-4453. [DOI] [PubMed] [Google Scholar]
- 41.Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheunq N, Kowalski JW, Nquyen HP, Wang JJ, Wong TY. Natural history of branch retinal vein occlusion an evidence-based systematic review. Ophthalmology. 2010;117(6):1094–1101.e5. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 42.Lim LL, Cheung N, Wang JJ, Islam FM, Mitchell P, Saw SM, Aung T, Wong TY. Prevalence and risk factors of retinal vein occlusion in an Asian population. Br J Ophthalmol. 2008;92(10):1316–1319. doi: 10.1136/bjo.2008.140640. [DOI] [PubMed] [Google Scholar]
- 43.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. discussion 141–143. [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Xu L, Jonas JB. Vein occlusion in Chinese subjects. Ophthalmology. 2007;114(9):1795–1796. doi: 10.1016/j.ophtha.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Zhao RQ, Chen ZH, Chi ZR. Convolutional neural networks for branch retinal vein. Conf Proc IEEE. 2015 [Google Scholar]
- 46.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020-the right to sight. Bull World Health Organ. 2001;79(3):227–232. [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert C, Fielder A, Gordillo L, Quinn G, Semiqlia R, Visintin P, Zin A, International NO-ROP Group Characteristics of infants with severe ROP in countries with low, moderate, and high levels of development implications for screening programs. Pediatrics. 2005;115(5):e518–e525. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 48.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 49.Ataer-Cansizoglu E, Kalpathy-Cramer J, You S, Keck K, Erdogmus D, Chiang MF. Analysis of underlying causes of inter-expert disagreement in retinopathy of prematurity diagnosis. Application of machine learning principles. Methods Inf Med. 2015;54(1):93–102. doi: 10.3414/ME13-01-0081. [DOI] [PubMed] [Google Scholar]
- 50.Ataer-Cansizoglu E, Bolon-Canedo V, Campbell JP, Bozkurt A, Erdogmus D, Kalpathy-Cramer J, Patel S, Jonas K, Chan RV, Ostmo S, Chiang MF, i-ROP Research Consortium Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity performance of the “i-ROP” system and image features associated with expert diagnosis. Transl Vis Sci Technol. 2015;4(6):5. doi: 10.1167/tvst.4.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolón-Canedo V, Ataer-Cansizoglu E, Erdogmus D, Kalpathy-Cramer J, Fontenla-Romero O, Alonso-Betanzos A, Chiang MF. Dealing with inter-expert variability in retinopathy of prematurity: a machine learning approach. Comput Methods Programs Biomed. 2015;122(1):1–15. doi: 10.1016/j.cmpb.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell JP, Ataer-Cansizoglu E, Bolon-Canedo V, et al. Expert diagnosis of plus disease in retinopathy of prematurity from computer-based image analysis. JAMA Ophthalmol. 2016;134(6):651–657. doi: 10.1001/jamaophthalmol.2016.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelman R, Jiang L, Du YE, Martinez-Perez ME, Flynn JT, Chiang MF. Plus disease in retinopathy of prematurity pilot study of computer-based and expert diagnosis. J AAPOS. 2007;11(6):532–540. doi: 10.1016/j.jaapos.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ataer-Cansizoglu E, Akcakaya M, Orhan U, Erdogmus D. Manifold learning by preserving distance orders. Pattern Recognit Lett. 2014;38:120–131. doi: 10.1016/j.patrec.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tandon R. Re: Waltz et al.: Clinical outcomes of TECNIS toric intraocular lens implantation after cataract removal in patients with corneal astigmatism (Ophthalmology 2015;12:239-47) Ophthalmology. 2016;123(1):e4. doi: 10.1016/j.ophtha.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Murthy GV, Gupta SK, Bachani D, Jose R, John N. Current estimates of blindness in India. Br J Ophthalmol. 2005;89(3):257–260. doi: 10.1136/bjo.2004.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao X, Lin S, Wong TY. Automatic feature learning to grade nuclear cataracts based on deep learning. IEEE Trans Biomed Eng. 2015;62(11):2693–2701. doi: 10.1109/TBME.2015.2444389. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Jiang J, Zhang K, Long E, Cui J, Zhu M, An Y, Zhang J, Liu Z, Lin Z, Chen J, Cao Q, Li J, Wu X, Wang D, Lin H. Localization and diagnosis framework for pediatric cataracts based on slit-lamp images using deep features of a convolutional neural network. PLoS One. 2017;12(3):e0168606. doi: 10.1371/journal.pone.0168606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillner M, Eppig T, Langenbucher A. Automatic intraocular lens segmentation and detection in optical coherence tomography images. Z Med Phys. 2014;24(2):104–111. doi: 10.1016/j.zemedi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Damms T, Dannheim F. Sensitivity and specificity of optic disc parameters in chronic glaucoma. Invest Ophthalmol Vis Sci. 1993;34(7):2246–2250. [PubMed] [Google Scholar]
- 61.Ohnell H, Heijl A, Brenner L, Anderson H, Bengtsson B. Structural and functional progression in the early manifest glaucoma trial. Ophthalmology. 2016;123(6):1173–1180. doi: 10.1016/j.ophtha.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118(9):1766–1773. doi: 10.1016/j.ophtha.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oddone F, Lucenteforte E, Michelessi M, Rizzo S, Donati S, Parravan M, Virqili G. Macular versus retinal nerve fiber layer parameters for diagnosing manifest glaucoma a systematic review of diagnostic accuracy studies. Ophthalmology. 2016;123(5):939–949. doi: 10.1016/j.ophtha.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 64.Omodaka K, An G, Tsuda S, Shiga Y, Takada N, Kikawa T, Takahashi H, Yokota H, Akiba M, Nakazawa T. Classification of optic disc shape in glaucoma using machine learning based on quantified ocular parameters. PLoS One. 2017;12(12):e0190012. doi: 10.1371/journal.pone.0190012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Xianyu, Xu Yanwu, Wong Damon Wing Kee, Wong Tien Yin, Liu Jiang. Glaucoma detection based on deep convolutional neural network. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:715–718. doi: 10.1109/EMBC.2015.7318462. [DOI] [PubMed] [Google Scholar]
- 66.Cerentini A, Welfer D, Cordeiro d'Ornellas M, Pereira Haygert CJ, Dotto GN. Automatic identification of glaucoma using deep learning methods. Stud Health Technol Inform. 2017;245:318–321. [PubMed] [Google Scholar]
- 67.Asaoka R, Murata H, Iwase A, Araie M. Detecting Preperimetric glaucoma with standard automated perimetry using a deep learning classifier. Ophthalmology. 2016;123:1974–1980. doi: 10.1016/j.ophtha.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 68.Muhammad H, Fuchs TJ, De Cuir N, De Moraes CG, Blumberg DM, Liebmann JM, Ritch R, Hood DC. Hybrid deep learning on single wide-field optical coherence tomography scans accurately classifies glaucoma suspects. J Glaucoma. 2017;26(12):1086–1094. doi: 10.1097/IJG.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heidary F, Gharebaghi R. Ideas to assist the underprivileged dispossessed individuals. Med Hypothesis Discov Innov Ophthalmol. 2012;1(3):43–44. [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JG, Jun S, Cho YW, Lee H, Kim GB, Seo JB, Kim N. Deep learning in medical imaging general overview. Korean J Radiol. 2017;18(4):570–584. doi: 10.3348/kjr.2017.18.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sevik U, Kose C, Berber T, Erdol H. Identification of suitable fundus images using automated quality assessment methods. J Biomed Opt. 2014;19(4):046006. doi: 10.1117/1.JBO.19.4.046006. [DOI] [PubMed] [Google Scholar]
- 72.Paulus J, Meier J, Bock R, Hornegger J, Michelson G. Automated quality assessment of retinal fundus photos. Int J Comput Assist Radiol Surg. 2010;5(6):557–564. doi: 10.1007/s11548-010-0479-7. [DOI] [PubMed] [Google Scholar]
- 73.Kose C, Sevik U, Ikibas C, Erdol H. Simple methods for segmentation and measurement of diabetic retinopathy lesions in retinal fundus images. Comput Methods Programs Biomed. 2012;107(2):274–293. doi: 10.1016/j.cmpb.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 74.Fleming AD, Philip S, Goatman KA, Olson JA, Sharp PF. Automated assessment of diabetic retinal image quality based on clarity and field definition. Invest Ophthalmol Vis Sci. 2006;47(3):1120–1125. doi: 10.1167/iovs.05-1155. [DOI] [PubMed] [Google Scholar]
- 75.Bengio Y, Courville A, Vincent P. Representation learning a review and new perspectives. IEEE Trans Pattern Anal Mach Intell. 2013;35(8):1798–1828. doi: 10.1109/TPAMI.2013.50. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen A, Yosinski J, Clune J. Deep neural networks are easily fooled: high confidence predictions for unrecognizable images. In Computer Vision and Pattern Recognition(CVPR'15), IEEE. 2015 [Google Scholar]