Highlights
-
•
We studied tendon tap reflex in the soleus muscle during Rest, Hand Pull, Teeth Clench, and Jendrassik maneuver.
-
•
Reflex response amplitudes significantly increased during any of the tasks compared with Rest.
-
•
Teeth Clenching alone was sufficient for reflex reinforcement without contracting the arm muscles.
Keywords: Jendrassik maneuver, Achilles tendon stretch reflex, Reinforcement, Presynaptic disinhibition, Gamma activation
Abstract
Objective
For many decades, the Jendrassik maneuver (JM) has been used as a reinforcement for stretch reflexes, although the underlying mechanism of this reinforcement is still not fully understood. Moreover, the term JM has been used for many different muscle contraction strategies as there is no fixed movement for the maneuver in the literature. In this study, we aimed to investigate the effects of clenched hand pull, teeth clenching, and their combined effects to reach standardization.
Methods
Achilles tendon tap reflex responses in the soleus were recorded during rest (R), hand pull (HP), teeth clench (TC), and HP + TC combined, hereafter referred to as the JM.
Results
Reflex response amplitudes significantly increased during JM, HP, and TC in the soleus. HP and JM significantly changed the background activity in the soleus, but TC alone did not.
Conclusion
These results suggest that dominantly presynaptic disinhibitory mechanisms may be responsible for the increase in the tendon tap reflex during HP, TC, and JM.
Significance
Because the findings indicate that HP increases the background activity of the soleus, we suggest that researchers should use only TC during the Jendrassik maneuver to avoid any confounding background activity change.
1. Introduction
The Jendrassik maneuver (JM) has been used to reinforce lower limb reflexes since the 19th century. Despite its common use, the application of the maneuver has not been standardized. While in most research the JM was performed by pulling clenched hands and without teeth clenching (Khanal et al., 2007, Nardone and Schieppati, 2008), other studies included teeth clenching in the maneuver as well (Zehr and Stein, 1999) (Table 1). Although the effects of pulling clenched hands and teeth clenching on both stretch and H-reflex have been examined in separate studies (Boroojerdi et al., 2000, Gregory et al., 2001, Mitsuyama et al., 2017, Sato et al., 2014, Sugawara and Kasai, 2002, Hagbarth et al., 1975, Tuncer et al., 2007), to our knowledge, no study compared the effects of teeth clenching (TC), hand pull (HP), and JM (HP + TC combined) separately in one setup, which is the aim of this study.
Table 1.
Previous methods used to elicit the Jendrassik maneuver.
| Study | Methods |
|||||
|---|---|---|---|---|---|---|
| HC | IF | HG | LLC | ULC | TC | |
| Myriknas et al. (2000) | + | |||||
| Bussel et al. (1978) | + | |||||
| Murthy et al. (1978) | + | + | ||||
| Kawamura and Watanabe (1975) | + | |||||
| Nardone and Schieppati (2008) | + | + | ||||
| Delwaide and Toulouse (1981) | + | + | ||||
| Hagbarth et al. (1975) | + | |||||
| Zehr and Stein (1999) | + | + | + | |||
| Khanal et al. (2007) | + | |||||
| Gregory et al. (2001) | + | + | ||||
| Ribot-Ciscar et al. (2000) | + | |||||
| Zabelis et al. (1998) | + | |||||
HC: Hand clenching; IF: hand pull by trying to pull interlocked fingers apart (similar to our HP protocol); HG: Handgrip; LLC: Lower limb contraction (by different methods); ULC: Upper limb contraction (by different methods); TC: Any limb contraction together with teeth clenching.
The mechanism underlying the JM has been controversial for long. One of the hypothesis was fusimotor activation (Rossi-Durand, 2002, Murthy et al., 1978, Ribot-Ciscar et al., 2000, Burg et al., 1974), which suggested that the JM increases the stretch sensitivity of the muscle spindles through gamma activation. However, the same reinforcing effect was also seen in the H-reflex (Hagbarth et al., 1975, Dowman and Wolpaw, 1988), which is independent of the spindle receptors and hence is presumably not affected by the gamma innervation. Supporting this suggestion, Dowman and Wolpow have found that the JM increased the H-reflex amplitude without changing the background surface electromyography (SEMG) level (Dowman and Wolpaw, 1988).
Another suggested mechanism for the reflex size increase during the JM is a reduced presynaptic inhibition on the spindle primary fiber synapses, i.e., presynaptic disinhibition (Zehr and Stein, 1999, Dowman and Wolpaw, 1988). However, there are some studies claiming against presynaptic disinhibition in the JM facilitation (Gregory et al., 2001, Nardone and Schieppati, 2008). Gregory et al. looked for JM-induced soleus H-reflex facilitation through a quadriceps afferent volley but did not find an increase in the reflex and therefore rejected the presynaptic disinhibitory pathway (Gregory et al., 2001). Nardone et al. used platform rotations to elicit reflex response and obtained short- and medium-latency reflex responses (SLR and MLR, respectively). Although the JM increased SLR, it decreased the second part of MLR strongly. As a result, Nardone et al. accepted that the JM reduces the soleus stretch reflex rather than increase it and argued that the effect on MRL was through a transcortical loop (Gregory et al., 2001).
In our study, we further investigated the mechanism of reflex reinforcement by examining SEMG levels immediately preceding the stimulus (i.e., background muscle activity) and by investigating the summed effects of different reinforcement procedures on Achilles tendon tap reflex.
First, we hypothesize that the stretch reflex response increases in all JM-related muscular activities including HP and TC. The degree of increase in the reflex response may depend upon the type of facilitatory maneuver. Second, we hypothesize that the JM or related muscular activities do not affect the amplitude of the background muscle activity of leg muscles.
2. Methods
2.1. Participants
Ten healthy subjects (5 males: 26 ± 13 years, 178 ± 6 cm, 77 ± 15 kg; 5 females: 26 ± 8 years old, 168 ± 12 cm, 58 ± 13 kg; age range 19–39 years) participated in the experiment. They were free of any symptoms or signs of neurological disease. The subjects received no sedation or drugs. All subjects were informed about the complete experimental procedure and gave written consent prior to the procedure. The procedure for the experiments was accepted by the local Ethics Committee of the Koç University (Decision number: 2015.259.IRB2.096).
2.2. Procedure
The subjects stood upright, barefoot on the platform during the entire procedure. The experiments were performed while standing to control the baseline muscle activity, which determines the size of the reflex response. Moreover, the standing position stabilizes the stiffness of the Achilles tendon so that the effective strength of the tendon-tap reflex stimulus would be similar under different protocols. During the procedure, subjects received calibrated taps to the Achilles tendon from a custom-made reflex hammer (Karacan et al., 2016). In the beginning of each experiment, to normalize the background activity of muscles, the subject stood on tiptoes. This procedure induced a very large SEMG activity on the triceps surae (operational maximal SEMG). After this initial part, the subjects stood upright without tiptoeing for the rest of the experiment. Next, the optimum tap strength for each subject was determined. For this, the tap strength was increased in a steady manner to find the maximum reflex amplitude. Then, the tap intensity was reduced to induce a stretch reflex response that is half of the maximum reflex response for that subject. This level of tap strength was then used throughout the procedure.
There were four separate protocols. For each protocol, tap stimuli were delivered randomly, with the inter-stimulus interval varying from 4 to 8 s. In all procedures, including the rest protocol, the subject was alerted immediately before the tap by saying ‘ready’.
The four protocols were as follows: clenched hand pull (HP), which consists of pulling the hands apart against interlocked fingers; teeth clench (TC), which consisted of biting on an individualized mouth guard with maximal bite force; the JM, which consisted of both clenched hand pull and teeth clench; and rest (R). These protocols were delivered in a randomized manner, and the protocol sequence changed in every subject.
2.3. Data recording
SEMG was used to record the stretch reflex from the right soleus muscle. A light (2.9 g) MEMS piezo linear accelerometer (LIS344ALH, full-scale of ±6G, ECOPACK®) was placed just above the point of tap on the Achilles tendon of the right leg. The skin was shaved and cleaned with alcohol according to the recommendations of the Surface Electromyography for the Non-Invasive Assessment of Muscles (SENIAM) project (Hermens et al., 2000). The Ag/AgCl electrodes (KENDALL® Coviden) with a disc radius of 10 mm were placed on the right soleus 2 cm below the lateral gastrocnemius muscle along the main direction of the muscle fibers (inter-electrode distance 2 cm; bipolar configuration). A ground electrode was placed on the malleolus at the ankle of the same leg.
2.4. Data processing
PowerLAB® data acquisition system (ADInstruments, Oxford, United Kingdom) was used for the SEMG and accelerometer recordings, while LabChart 7® (version 7.3.7, ADInstruments, Oxford, United Kingdom) software was used offline to analyze the data. A 5 Hz high pass filter was used for the accelerometer and a 80–500 Hz band pass filter was used for the SEMG recordings to overcome the mechanical artifact induced by the tap (Sebik et al., 2013). The sampling rate for SEMG and accelerometer data was 10 kHz. The average peak-to-peak (P-P) amplitude of the reflex in SEMG for 10 taps, the integral of rectified background in SEMG for every interval between the taps, and the reflex latencies were determined. Full-wave rectified and smoothed SEMG amplitude obtained from tiptoe was used as a normalization factor (NF) for each subject's background activity.
2.5. Statistical analysis
For every subject, the averages of 10 P-P amplitudes of each of the protocols were taken and normalized against the maximum stretch reflex response for that subject. Shapiro-Wilk test for normal distribution was used. ANOVA and post hoc Bonferroni tests were performed to compare the reflex amplitudes obtained in different protocols. Background EMG activity was calculated in 300 ms of the data starting from 310 ms before the tap until 10 ms before the tap to give us a steady EMG level immediately preceding the stimulus. In addition, the rectified integrals from the tiptoe condition were taken and used as the NF for each subject. ANOVA and post hoc Bonferroni tests were performed to compare the background activity and latencies of each of the four protocols. For the post hoc Bonferroni correction, the significance level was set to p < .01667 for all calculations.
3. Results
We applied stretch reflex protocols during JM, HP, TC, and R in 10 healthy adult subjects. Fig. 1 demonstrates the mean P-P reflex amplitudes of a subject during different protocols for the soleus muscle. The accelerometer measures were compared by ANOVA, and no significant difference was found.
Fig. 1.
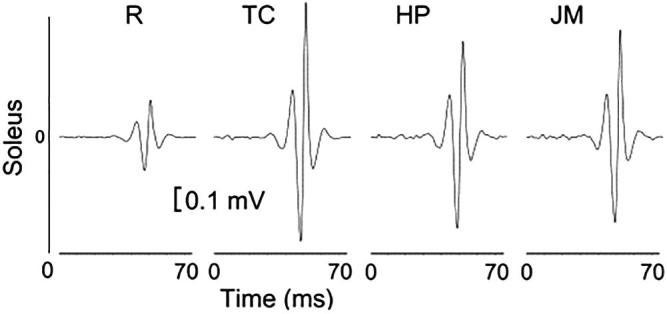
Average stretch reflex amplitudes of one subject. The order of trials was random but shown in an order to illustrate the difference between the rest trials and others. R indicates rest; TC indicates teeth clench, which consists of biting on an individualized mouth guard with maximal force; HP indicates clenched hand pull, which consists of trying to pull the hands apart against interlocked fingers; and JM indicates the Jendrassik maneuver, which consists of both clenched hand pull and teeth clench.
Fig. 2 illustrates the changes in the normalized average stretch reflex amplitudes for each of the experimental protocols. There were significant differences between JM-R, HP-R, and JM-TC. The results showed that all three reinforcement maneuvers increase the reflex amplitude significantly.
Fig. 2.
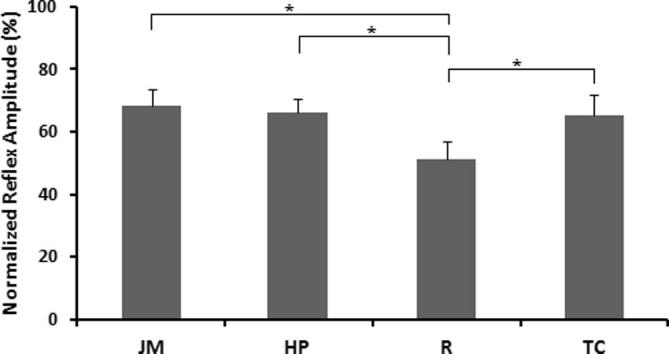
Peak-to-peak reflex responses of the soleus. Comparison of normalized reflex amplitudes of HP (66.20 ± 4.34% MSR), JM (68.40 ± 5.29% MSR), R (51.12 ± 5.72% MSR), and TC (65.16 ± 6.51% MSR) in the soleus. There are significant differences between HP and R, JM and R, and TC and R. *p < 0.01667; **p = 0.017 (on the significance limit). Standard errors are shown. The reflex amplitudes are normalized against the maximum stretch reflex response for that subject. R indicates rest; TC indicates teeth clench, which consists of biting on an individualized mouth guard with maximal force; HP indicates clenched hand pull, which consists of trying to pull the hands apart against interlocked fingers; and JM indicates a specified Jendrassik maneuver, which consists of both clenched hand pull and teeth clench.
Fig. 3 illustrates the changes in normalized SEMG background for the four protocols. There were significant differences between JM-R, HP-R, JM-TC, and HP-TC.
Fig. 3.
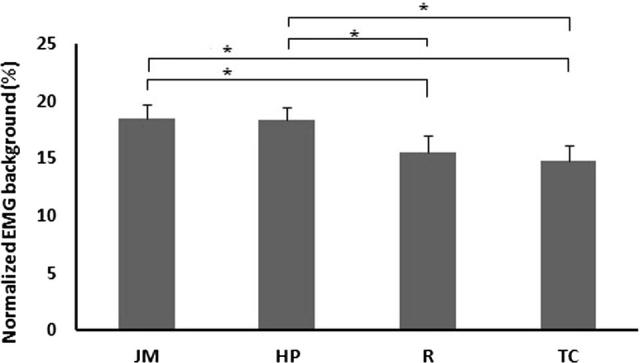
Comparison of the background electromyogram activity. The background SEMG amplitudes of the soleus: JM (17.21 ± 0.64% NF), HP (17.42 ± 0.67% NF), R (14.13 ± 0.48% NF), and TC (13.53 ± 0.61% NF). *p < .01667; standard errors are shown. R indicates rest; TC indicates teeth clench, which consists of biting on an individualized mouth guard with maximal force; HP indicates clenched hand pull, which consists of trying to pull the hands apart against interlocked fingers; and JM indicates a specific Jendrassik maneuver, which consists of both clenched hand pull and teeth clench.
Mean reflex latency for all conditions for the soleus muscle was 35.7 ms. There was no significant difference between the latencies of any of the protocols.
4. Discussion
This study yielded two important findings. First, the effects of JM, HP, and TC were shown on reflex amplitude and background activity in the soleus muscle. Second, the underlying synaptic mechanisms were studied. The results indicated that the reinforcement maneuvers showed their effects through different mechanisms. Possible gamma, postsynaptic, and presynaptic pathways have been explored. We found that in some protocols, the reflex amplitude and background activity increased simultaneously, suggesting a gamma or postsynaptic facilitation, which could also include presynaptic facilitation, whereas in some protocols, only reflex amplitudes increased, while background EMG levels did not change, suggesting a presynaptic disinhibition mechanism.
4.1. Effects of different protocols on reflex amplitude and background activity
4.1.1. Reflex amplitude
In the soleus, JM, HP, and TC all increased the reflex amplitude significantly compared to the rest condition. It was previously established that HP increased both stretch and H-reflex amplitudes in the soleus (Gregory et al., 2001, Zehr and Stein, 1999, Dowman and Wolpaw, 1988). The effect of TC on the stretch reflex response of the soleus was previously not known, although an excitatory effect was reported for H-reflex (Mitsuyama et al., 2017, Tuncer et al., 2007). In the present study, we found that TC has a facilitatory effect on the soleus stretch reflex. The JM also increased the reflex amplitude significantly compared to rest condition. This result was expected as the JM consisted of HP and TC; therefore, the effects of both HP and TC should be combined in the JM. However, the differences between the reflex amplitudes of the JM and HP/TC were not significant. This may be because the level of reflex increase had a limit, which can be reached in each facilitatory procedure.
4.1.2. Background activity
In the soleus, the background SEMG levels during the JM and HP were significantly higher than the SEMG levels during R. TC on the other hand did not change the background SEMG activity significantly. Previous reports have stated no background activity changes accompanying reflex amplitude changes during reinforcement maneuvers (Gregory et al., 2001, Nardone and Schieppati, 2008, Dowman and Wolpaw, 1988). The fact that HP, but not TC, induced a change in the background activity implies that the background activity increase in the JM is through HP. Moreover, there were significant background activity differences between HP/JM and TC, implying that HP has a different pathway from TC to increase the background SEMG activity. Our finding suggests that HP may increase tonic alpha motoneuron activity in the soleus, possibly through tonic gamma activation or postsynaptic activation.
4.2. Possible synaptic mechanisms
To explain the facilitation with the JM, several possible mechanisms should be discussed. Different studies, using microneurography and muscle spindle conditioning, discussed that the main mechanism behind the JM is not the fusimotor activation (Gregory et al., 2001, Hagbarth et al., 1975). The possibility that the facilitation is caused by changes in the excitability of the alpha motoneurons has been denied previously (Gregory et al., 2001, Dowman and Wolpaw, 1988). Such a direct facilitation of motoneurons would be reflected in the background SEMG as more motoneurons would contribute to the background activity (Gregory et al., 2001). Our study showed significant SEMG background activity increase in some conditions (HP and JM), which may explain the reflex amplitude change accompanying it.
Fig. 4 summarizes the possible circuitry that explains the results of this study. In the soleus, the JM and HP increased both reflex amplitude and background activity simultaneously, indicating gamma and/or postsynaptic activation, whereas TC did not lead to any background activity change. As the JM includes both hand pulling and teeth clenching, it can be assumed that gamma/postsynaptic facilitation originates from the related upper limb muscle spindles and not from teeth clenching. Teeth clenching, in contrast, shows its facilitatory effect through presynaptic disinhibition because a reflex amplitude increase was observed without background activity increase.
Fig. 4.
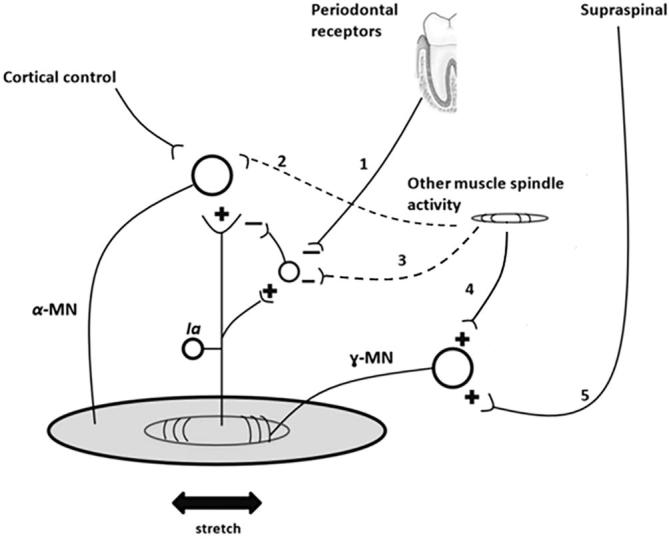
Diagram of possible synaptic pathways that are activated during TC and HP. The effect of TC would originate from the mechanoreceptors in the trigeminal system including the periodontal receptors and jaw muscle spindles, whereas the effect of HP may originate from the spindles of upper limb muscles. The + sign is used to indicate excitatory effect, whereas the – sign is used to indicate inhibitory effect. 1 denotes the neuronal drive from periodontal receptor to the inhibitory interneuron of the 1a afferent neuron. The existence of this pathway is evident in the soleus. 2 denotes the neuronal drive from muscle spindle to the α-motoneuron, which may be present. This pathway may be responsible for the background SEMG activity increase. 3 denotes the neuronal drive from the other muscle spindles to the inhibitory interneuron of the 1a afferent neuron. The existence of this pathway is suspicious in the soleus. 4 denotes the neuronal drive from the other muscle spindles to the γ-motoneuron. 5 denotes the neuronal drive from the supraspinal pathway, which controls the excitation of the γ-motoneuron.
5. Conclusion
This study was designed to explore the intrinsic neuronal pathway of reinforcement maneuvers and introduce a standardized JM methodology so that the same protocol can be used by researchers and a better understanding can be built up on the pathway for the JM. We suggest that researchers use TC as a means of the JM and make sure that the subject does not contract upper limb muscles voluntarily during the maneuver if presynaptic disinhibition is going to be studied. This approach makes sure that the background activity of the muscle does not significantly increase, and hence, any changes in the reflexes can be explained by the presynaptic mechanisms.
6. Limitations of the study
Since the differences between gamma activation and postsynaptic facilitation could not be shown in this study, further research is needed to illustrate the neuronal pathway underlying the facilitatory mechanisms of HP, TC, and JM. We assumed that soleus activation was constant throughout the procedure as the volunteers were upright and their posture did not change noticeably. With this assumption, we could attribute the background activity differences to the different protocols (HP, TC, and JM).
In future studies, to discriminate between gamma and postsynaptic facilitation, a single motor neuron study with set frequency would be beneficial. The synaptic mechanism underlying the JM can be studied further with the help of a computer program to trigger the stimulator only when the unit is firing in a preset frequency range (Türker et al., 1989).
Acknowledgments
Acknowledgements
We acknowledge Ata Berk Demir and Halil Ibrahim Cakar for their contributions during the experiments. We thank Koç University School of Medicine for financing the lab and the PhD students.
Disclosure of interests
The authors declare that they have no conflicts of interest concerning this article.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Boroojerdi B., Battaglia F., Muellbacher W., Cohen L.G. Voluntary teeth clenching facilitates human motor system excitability. Clin. Neurophysiol. 2000;111:988–993. doi: 10.1016/s1388-2457(00)00279-0. [DOI] [PubMed] [Google Scholar]
- Burg D., Szumski A.J., Struppler A., Velho F. Assessment of fusimotor contribution to reflex reinforcement in humans. J. Neurol. Neurosurg. Psychiatry. 1974;37:1012–1021. [Google Scholar]
- Bussel B., Morin C., Pierrot-Deseilligny E. Mechanism of monosynaptic reflex reinforcement during Jendrassik manoeuvre in man. J. Neurol. Neurosurg. Psychiatry. 1978;41:40–44. doi: 10.1136/jnnp.41.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide P.J., Toulouse P. Facilitation of monosynaptic reflexes by voluntary contraction of muscle in remote parts of the body. Brain. 1981;104:701–709. doi: 10.1093/brain/104.4.701. [DOI] [PubMed] [Google Scholar]
- Dowman R., Wolpaw J.R. Jendrassik maneuver facilitates soleus H-reflex without change in average soleus motoneuron pool membrane potential. Exp. Neurol. 1988;101:288–302. doi: 10.1016/0014-4886(88)90012-x. [DOI] [PubMed] [Google Scholar]
- Gregory J.E., Wood S.A., Proske U. An investigation into mechanisms of reflex reinforcement by the Jendrassik manoeuvre. Exp. Brain Res. 2001;138:366–374. doi: 10.1007/s002210100707. [DOI] [PubMed] [Google Scholar]
- Hagbarth K.E., Wallin G., Burke D., Lofstedt L. Effects of the Jendrassik maneuver on muscle spindle activity in man. J. Neurol. Neurosurg. Psychiatry. 1975;38:1143–1153. doi: 10.1136/jnnp.38.12.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens H.J., Freriks B., Disselhorst-Klug C., Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kines. 2000;10:361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Karacan I., Cidem M., Yilmaz G., Sebik O., Cakar H.I., Türker K.S. Tendon reflex is suppressed during whole- body vibration. J. Electromyogr. Kines. 2016;30:191–195. doi: 10.1016/j.jelekin.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Watanabe S. Timing as a prominent factor of the Jendrassik manoeuvre on the H reflex. J. Neurol. Neurosurg. Psychiatry. 1975;38:508–516. doi: 10.1136/jnnp.38.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal S., Sainju R.K., Adhikari P., Thapa R. Eliciting knee jerk–a new method of reinforcement. Nepal Med. Coll. J. 2007;9:278–280. [PubMed] [Google Scholar]
- Mitsuyama A., Takahashi T., Ueno T. Effects of teeth clenching on the soleus H reflex during lower limb muscle fatigue. J. Prosthodont. Res. 2017;61:202–209. doi: 10.1016/j.jpor.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Murthy K.S., Gildenberg P.L., Seeliger-Petersen W. Human muscle afferent responses to tendon taps. I. Characteristics of the waveform recorded with transcutaneous electrodes. J. Neurol. Neurosurg. Psychiatry. 1978;41:220–225. doi: 10.1136/jnnp.41.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myriknas S.E., Beith I.D., Harrison P.J. Stretch reflexes in the rectus abdominis muscle in man. Exp. Physiol. 2000;85:445–450. [PubMed] [Google Scholar]
- Nardone A., Schieppati M. Inhibitory effect of the Jendrassik maneuver on the stretch reflex. Neuroscience. 2008;156:607–617. doi: 10.1016/j.neuroscience.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E., Rossi-Durand C., Roll J.P. Increased muscle spindle sensitivity to movement during reinforcement manoeuvres in relaxed human subjects. J. Physiol. 2000;523:271–282. doi: 10.1111/j.1469-7793.2000.t01-1-00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Durand C. The influence of increased muscle spindle sensitivity on Achilles tendon jerk and H-reflex in relaxed human subjects. Somatosens. Mot. Res. 2002;19:286–295. doi: 10.1080/0899022021000037755. [DOI] [PubMed] [Google Scholar]
- Sato H., Kawano T., Saito M., Toyoda H., Maeda Y., Türker K.S., Kang Y. Teeth clenching reduces arm abduction force. Exp. Brain Res. 2014;232:2281–2291. doi: 10.1007/s00221-014-3919-8. [DOI] [PubMed] [Google Scholar]
- Sebik O., Karacan I., Cidem M., Türker K.S. Rectification of SEMG as a tool to demonstrate synchronous motor unit activity during vibration. J. Electromyogr. Kines. 2013;23:275–284. doi: 10.1016/j.jelekin.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Kasai T. Facilitation of motor evoked potentials and H-reflexes of flexor carpi radialis muscle induced by voluntary teeth clenching. Hum. Mov. Sci. 2002;21:203–212. doi: 10.1016/s0167-9457(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Tuncer M., Tucker K.J., Türker K.S. Influence of tooth clench on the soleus H-reflex. Arch. Oral Biol. 2007;52:374–376. doi: 10.1016/j.archoralbio.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Türker K.S., Seguin J.J., Miles T.S. Modulation of an inhibitory reflex in single motor units in human masseter at different joint angles. Neurosci. Lett. 1989;100:157–163. doi: 10.1016/0304-3940(89)90677-0. [DOI] [PubMed] [Google Scholar]
- Zabelis T.N., Karandreas N.T., Constantinidis T.S., Papageorgiou C.P. The effect of Jendrassik manoeuvre on the latency, amplitude and left-right asymmetry of tendon reflexes. Electromyogr. Clin. Neurophysiol. 1998;38:19–23. [PubMed] [Google Scholar]
- Zehr E.P., Stein R.B. Interaction of the Jendrassik maneuver with segmental presynaptic inhibition. Exp. Brain Res. 1999;124:474–480. doi: 10.1007/s002210050643. [DOI] [PubMed] [Google Scholar]


