Highlights
-
•
CTS is an association of symptoms and signs, assumed to be caused by median neuropathy at the wrist (MNW).
-
•
NCS are currently the best way to document the severity of MNW and contribute to CTS diagnosis.
-
•
NCS can assist the choice of appropriate treatment for CTS and should be performed before any invasive treatment.
-
•
Repeat studies should be used for follow-up of conservatively managed patients and those with uncertain diagnoses.
-
•
Needle EMG is not obligatory and is performed when indicated for differential diagnosis or lesion localization.
Keywords: Carpal tunnel syndrome, Median neuropathy at the wrist, Nerve conduction studies, Needle EMG, Surgical decompression, Local injection of corticosteroids, Conservative management
Abstract
This paper summarises the views of four experts on the place of neurophysiological testing (EDX) in patients presenting with possible carpal tunnel syndrome, in guiding their treatment, and in reevaluations. This is not meant to be a position paper or a literature review, and heterogeneous viewpoints are presented. Nerve conduction studies should be performed in patients presenting with possible carpal tunnel syndrome to assist diagnosis, and may need to be repeated at intervals in those managed conservatively. There is evidence that local corticosteroid injection is safe and effective for many patients, thereby avoiding or deferring surgical decompression. All patients should undergo EDX studies before any invasive procedure for CTS (injection or surgery). Needle EMG studies are not obligatory, but may be needed in those with severe disease and those in whom an alternate or concomitant diagnosis is suspected.
1. Introduction
Carpal tunnel syndrome is perhaps the commonest cause of referral for neurophysiological testing, herein referred to as electrodiagnostic testing or EDX. There are many misconceptions about the condition, its underlying pathophysiology, patient demographics and optimal treatment. Views differ between different craft groups, and it is relevant for the readers of this journal that a recent Editorial asserted that, in carpal tunnel syndrome, nerve conduction studies may be an “unnecessary evil” (Fowler, 2017).
Triggered by the comprehensive systematic review performed by the American Orthopedic Association (AAOS, 2016), and in the light of the Editorial by Fowler (2017), the Editors and Editorial Board commissioned Commentaries on these issues. This document presents the views of the authors, based on individual experience and literature. It is not a systematic review of the field and, while the authors agree on the general principles and conclusions, this is not a consensus document. The opinions expressed below are not designed to constitute yet-another set of “Guidelines”. In discussing issues, the authors assume that “best-practice” is followed in the choice of test and how to perform them, and refer readers to the AANEM Guidelines (see Sonoo’s commentary, and Anon, 1993a, Jablecki et al., 1993, Jablecki et al., 2002). The authors were tasked to discuss the value of nerve conduction studies (NCS) in CTS. It is beyond the scope of this task to consider the value of ultrasound which (i) is a valuable complementary tool, (ii) assesses anatomy not function, and (iii) is less valuable for assessing functional severity.
In the Opinions below, we argue that there are three main reasons for NCS in CTS: (i) to make a diagnosis of median neuropathy at the wrist (MNW), thereby supporting the clinical diagnosis of CTS, (ii) to assess the severity of the MNW because that can help guide treatment decisions, and (iii) to detect abnormalities suggesting other conditions, if relevant.
2. Masahiro Sonoo: Review of existing guidelines and diagnostic criteria, and the role of nerve conduction study in CTS evaluation
Several neurological disorders to which EDX tests make significant contributions have widely-approved diagnostic criteria, such as amyotrophic lateral sclerosis (ALS) (de Carvalho et al., 2008, Brooks et al., 2009) or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) (Van den Bergh et al., 2010). These criteria are usually composed of sub-criteria regarding clinical symptoms and signs, EDX tests, other supportive laboratory tests, and exclusion of other disorders. Although some typical patients may be diagnosed with sufficient certainty from clinical signs alone, no one will openly argue “needle EMG is unnecessary for the diagnosis of ALS” or “nerve conduction studies (NCS) are useless for the diagnosis of CIDP”. In contrast to these disorders, however, there is a widespread view among surgeons that EDX tests are not necessary at all for CTS diagnosis (Glowacki et al., 1996, Graham, 2008, Zyluk and Szlosser, 2013, Fowler, 2017). Another difference is that widely-accepted diagnostic criteria considering both clinical and EDX parameters are still lacking for CTS. In my commentary, I will first review existing guidelines and diagnostic criteria for CTS, and then discuss the proper role of EDX, specifically NCS, in CTS diagnosis.
2.1. Existing guidelines
The earliest comprehensive CTS guidelines were those by American Academy of Neurology (AAN) in 1993 (Anon, 1993a), which have not been updated so far. According to them, EDX tests are required for diagnosis when diagnosis is uncertain, and are recommended for all cases in order to classify severity.
A practice parameter for EDX by the American Association of Electrodiagnostic Medicine (AAEM; presently the American Association of Neuromuscular and Electrodiagnostic Medicine, AANEM) and other societies was published associated with the above clinical guidelines (Anon, 1993b), with a full literature review (Jablecki et al., 1993). It gave one of three grades of recommendation for each NCS technique. This practice parameter was updated in 2002 (Jablecki et al., 2002). Notably, this guideline recommends as “practice standard” that some of the sensitive comparative or short-segment tests should be conducted when routine tests are normal.
In 2009, the AAOS published the first version of their guidelines (Keith et al., 2009). There, EDX tests were recommended as an option (“may obtain”) to differentiate between diagnoses, and were strongly recommended (“should obtain”) when surgical management was being considered. The AAOS guidelines updated in 2016 will be discussed later.
2.2. Existing diagnostic criteria
There are very few published diagnostic criteria for CTS. The AAN guidelines (Anon, 1993a) gave “diagnostic criteria”, although these were just a list of symptoms and signs, and they only said that “the likelihood of CTS increases with the number of standard symptoms and provocative factors listed below”. Rempel et al. proposed criteria for the classification of CTS to be used in epidemiologic studies (Rempel et al., 1998). These were consensus criteria developed through discussion by 12 experts, and hence they are expert opinion. However, these are the only existing criteria that considered both clinical symptoms/signs and EDX tests. Witt et al. proposed criteria solely based on clinical symptoms and signs, also expert opinion (Witt et al., 2004).
Graham et al. developed the most sophisticated criteria based solely on clinical symptoms and signs (Graham et al., 2006). They first selected 8 criteria out of 57 candidates by the Delphi method, and created 28 = 256 virtual case histories containing every possible combination of the 8 criteria. Then, 16 experts judged whether each case had CTS or not. Through logistic regression analyses, they constructed criteria composed of 6 items with a weight for each (CTS-6). Although using complicated procedures, it is essentially an expert opinion not based on real patients.
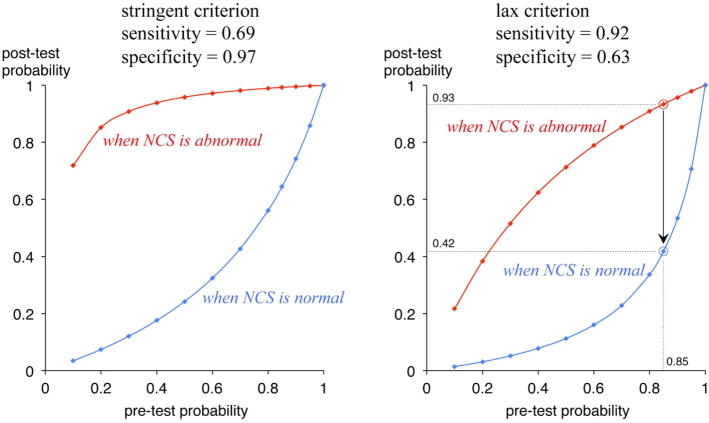
Graham subsequently argued that addition of EDX tests to CTS-6 did not increase the probability of diagnosis (Graham, 2008). His main argument was that the “average” change in probability after an NCS test was around zero and therefore NCS were unnecessary for most patients. However, the presented data clearly demonstrate that the post-test probability of the “individual” case changes greatly depending on the NCS results (Fig. 1). For example, when the pre-test probability was as high as 0.85, the post-test probability using the lax criterion would be 0.93 if NCS were abnormal, but 0.42 if NCS were normal, clearly indicating that NCS contribute greatly to the diagnosis even when the pre-test probability is sufficiently high. This is a natural consequence from the sufficiently good sensitivity/specificity of NCS adopted in his study, 69%/97% or 92%/63% (Jackson and Clifford, 1989, Stevens, 1987). Therefore, their conclusion that NCS add little is misleading.
Fig. 1.
Calculated pre-test and post-test probability using two studies (Jackson et al., 1989; Stevens, 1987) adopted by Graham (2008) as stringent and lax criteria. Using the lax criterion, a patient with the pre-test probability of 0.85 will keep the post-test probability of 0.93 if NCS is abnormal, but the post-test probability will decline to 0.42 if NCS is normal. The results exactly agree with Fig. 1 in Graham (2008) using the stringent criterion.
2.3. Lack of gold standard and the argument of new AAOS guideline
The lack of a gold standard is the fundamental problem in the diagnosis of CTS (Rempel et al., 1998, Kilmer and Davis, 2002). There are three candidate diagnostic measures that could be a criterion standard: clinical symptoms and signs, EDX tests, and surgical outcome. The lack of gold standard means that none of these are perfect, and both false negatives and false positives are present for each. What is important here is that in order to evaluate sensitivity and specificity, i.e., to identify false negatives and false positives, the reference standard must be outside the relevant modality. For example, to judge diagnostic yields of EDX studies, the diagnosis of CTS should be based on clinical criteria (Jablecki et al., 1993). However, the unsolved problem is that the outside reference standard itself may also have false negatives or false positives.
Regarding clinical symptoms and signs, CTS is a clinical syndrome and a subject with no symptoms, i.e., no pain, paraesthesiae or loss of skilled hand movement, cannot be called CTS. In this sense, the sensitivity of the “presence of any of the above symptoms” must be 100%, with very low specificity. The sensitivity and specificity of individual symptoms and signs have been investigated using EDX tests as the reference standard, giving various results and generally only moderate diagnostic power for each criterion (de Krom, 1990, Katz et al., 1990, Kuhlman and Hennessey, 1997, Bland, 2000b, D'Arcy and McGee, 2000, Hansen et al., 2004).
False positive EDX tests definitely occur. The specificity values presented in the AAEM practice parameter, 97% to 99% (Jablecki et al., 2002), seem excessive. Up to 20% false positives among general population or hand workers have been reported (Nathan et al., 1994, Atroshi et al., 1999). Most of these “false positives” are thought to have asymptomatic median neuropathy at the wrist (MNW). Regarding false negative EDX tests, I agree with the opinion that false negative rate must be sufficiently low, although not zero, if one uses sensitive comparison methods (Dawson et al., 1999).
Surgical outcome is another candidate for a reference standard, although this is not applicable to cases managed conservatively. Furthermore, placebo effect and surgical complications may constitute false positives and false negatives, respectively. The fact that the outcome is often evaluated by the surgeon who conducted surgery is another limitation of this parameter (Bland, 2001).
The revised AAOS guideline (AAOS, 2016) appears, at first glance, to mention only hand-held NCS and neglect most other studies. However, careful inspection reveals that EDX studies are scored as moderate evidence, together with diagnostic questionnaires such as CTS-6, under the heading of “diagnostic scales”. Furthermore, I completely agree with their conclusion, presented under the heading of Future Research, that “Establishing consensus on a reference standard for the diagnosis for CTS is the most important research goal in this area” (p. 188 of AAOS, 2016).
The lack of a gold standard means that no-one can say that a patient has CTS or not with 100% accuracy. In this situation, considering as many imperfect parameters that can contribute to diagnosis as possible is a reasonable and scientific way. In this situation, the “diagnostic criteria” cannot be more than the expert opinion, inherently. However, there must be two important points that one should adhere to when making such diagnostic criteria as an expert opinion. First, we should seek the best reference standard possible, as exactly argued by the revised AAOS guideline (AAOS, 2016). Secondly, we should be unbiased when selecting candidates of diagnostic measures. NCS, which previous studies suggested sufficiently high sensitivity and specificity, should naturally be considered as a good candidate for this purpose.
2.4. Practical use of NCS
Most experts will agree that a typical CTS patient can be diagnosed only from clinical symptoms and signs with high certainty. Even in such cases, to confirm the diagnosis objectively using NCS must be useful. Rarely, a patient I diagnosed with CTS clinically may unexpectedly show normal NCS results. This is not at all frequent, contrary to the suggestion from opponents of EDX tests, probably because I routinely use sensitive comparison tests including the ring-finger method. In such a rare case, I reconsider the diagnosis. The patient may prove to have just a conversion disorder, easily influenced by suggestion during initial history taking and neurological examinations. Very rarely, I come to the conclusion that the patient has true EDX-negative CTS. Even in such cases, sensitive NCS tests often show a right-left difference within the normal range in accordance with the dominance of symptoms.
It was reasonable that the first version of the AAOS guideline strongly recommended EDX when surgical management was considered (Keith et al., 2009). Dawson et al. also recommended that EDX tests should be performed before every operation, to confirm the diagnosis in order to prevent unnecessary operations, and to serve as a baseline in determining the postoperative state of the nerve, should the operation fail to relieve symptoms (Dawson et al., 1999). Johnson compared CTS surgery without EDX tests to treating pneumonia without a chest x-ray and a sputum culture (Johnson, 1993). We should freely utilize two powerful diagnostic measures, clinical symptoms/signs and EDX tests, in order to confirm the diagnosis, especially before an invasive intervention is attempted.
Are NCS painful? Needle EMG is indeed painful and we should avoid this if possible: I almost never perform needle EMG of the abductor pollicis brevis muscle for the evaluation of CTS. I conduct needle EMG only when the diagnosis is uncertain and the suspicion of other disorders, typically cervical radiculopathy, strongly remains. In extremely severe cases with an absent compound muscle action potential (CMAP), needle EMG of proximal median muscles may be necessary to localize the lesion. However, this is very rare if the recording from the second lumbrical muscle is attempted (Boonyapisit et al., 2001). NCS are less uncomfortable than blood sampling for most people (Alshaikh et al., 2016). No one would try to diagnose diabetes without knowing the blood sugar level because blood sampling is painful.
I suspect that the fierce debate over EDX is rather specific to USA, where the medical fee for EDX is very high. In Japan the medical fee is reasonable, or too low, at least for NCS. Hand surgeons are usually willing to obtain NCS data before operation. The problem is rather the paucity of EDX experts or good technicians around them. To enhance the usage and improve the quality of EDX are important goals in our country, probably also in other countries.
3. Daniel Menkes: The role of electrodiagnostic testing in the diagnosis of carpal tunnel syndrome
The American Academy of Orthopedic Surgeons published an article that discussed the management of carpal tunnel syndrome (CTS) based on the evidence published in the medical literature (AAOS, 2016). This article repeatedly stated that “electrodiagnostic tests [were used] as the reference standard,” citing the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM, formerly known as the American Association of Electrodiagnostic Medicine, AAEM), and other electrodiagnostic methods (Jablecki et al., 2002). Restated, nerve conduction studies with needle electromyography were used as the reference standard for the diagnosis of carpal tunnel syndrome (CTS). It is against this reference that all other methods were compared. On this basis, the AAOS recommendations were that, with the exception of thenar atrophy, individual physical diagnosis signs were not useful in establishing the diagnosis of CTS. Electrodiagnostic studies (EDX), rather than other clinical methodologies, were determined to be the most sensitive and specific. The only other electrodiagnostic test commented upon in this publication was the use of hand-held nerve conduction study devices for which this review found limited evidence to support their use. Despite this comprehensive literature review, Fowler took a contrary position questioning the need for EDX (Fowler, 2017). The proper response requires an overview of the issues raised by Dr. Fowler, followed by an evaluation of the evidence. Fowler’s main objections may be summarized as follows; EDX cannot define a case of median neuropathy at the wrist (MNW), patients with CTS improve with surgery even with normal EDX, that EDX studies are painful, and that clinical neurophysiologists perform studies out of habit, for financial reward or both. Each of these statements will be refuted.
Much of the literature does not distinguish between CTS and MNW but this is an important distinction. MNW refers to median nerve dysfunction at the transverse carpal ligament whereas CTS refers to a collection of symptoms and signs that may occur independently of MNW. Thus, it is possible to have an asymptomatic MNW wherein there is significant median nerve dysfunction as detected by EDX. This commonly occurs in the setting of an underlying polyneuropathy as “sick nerves are more prone to compression” (Fowler and Scadding, 2013). Thus, asymptomatic patients may have clinical and electrodiagnostic evidence of median nerve dysfunction resulting from compression at the transverse carpal ligament. Failure to address this issue may lead to irreversible axon loss. Conversely, it is possible to have all the symptoms of CTS with minimal or even no electrophysiological evidence of a MNW. It is important to define a disease entity such that a comparison of treatment modalities can be conducted. The AAOS guidelines utilize EDX as the gold standard for establishing a diagnosis of MNW. It is unclear how Dr. Fowler defines a case of MNW. Dr. Fowler invokes the memory of US Supreme Court Justice Potter Stewart when he implies that he cannot define CTS but he knows it when he sees it. Medical science requires objectivity to distinguish those affected by a disease and those that are not. The AAOS concurs that the diagnosis of MNW is established through the proper application of EDX studies. Dr. Fowler does not propose an alternative means of assessing MNW much less cite evidence-based literature to support his position. He needs to propose a definition of CTS that can be reproduced in standard clinical practice.
His second objection is that patients with CTS will improve even in the absence of EDX abnormalities. He cites the study by Glowacki et al. as evidence of the non-utility of EDX in CTS (Glowacki et al., 1996). However, this study has a number of significant flaws. First, the issue of defining CTS versus MNW is at issue in this manuscript as well. Secondly, statistics can only be performed when all measurements are independent. It is improper to use two hands in the same patient and automatically assume that these are truly independent measurements (Sainani, 2010). Finally, one should question whether or not symptomatic resolution reflects a physiological or a placebo effect. Arthroscopic surgery for knee osteoarthritis was once considered the standard of care as many patients reported pain relief. However, Moseley et al. determined that the pain relief outcomes after arthroscopic lavage or arthroscopic debridement were no better than those after a placebo procedure (Moseley et al., 2002). In the absence of a similar controlled trial for CTS with non-diagnostic EDX studies, a placebo effect of surgery cannot be excluded. It is conceivable that sham surgery might have a statistically similar success rate in patients with CTS and non-diagnostic EDX. If this placebo effect is proven, then surgery should be reserved for patients with MNW, as defined by EDX, who have significant EDX abnormalities or who demonstrate worsening on sequential EDX despite conservative treatment. Indeed, the AAOS guideline recommends a broad spectrum approach to the treatment of MNW from non-medical (immobilization) to medications (oral, phonopheresis, and injectable) to surgical release. In the absence of data from the suggested sham surgery trial for CTS without MNW, the concept of proportionate response would dictate that cases of CTS without definitive EDX evidence of MNW should receive less aggressive treatments whereas surgery should be reserved for patients in whom there is unequivocal EDX evidence of MNW.
Fowler did not specifically address his approach to asymptomatic patients with EDX evidence of a MNW. Compression neuropathies initially result in myelin dysfunction as the myelin recedes from the compression site (Ochoa et al., 1972). If the compression progresses, then secondary axon loss results (Ochoa and Marotte, 1973). Once the compression is relieved, the nerve remyelinates but the repair does not duplicate the premorbid state (Bonnaud-Toulze and Raine, 1980). Thus, EDX may never return to normal. However, the purpose of EDX is to identify MNW and monitor its progression such that a properly timed intervention may be undertaken.
The appended table proposes a classification system for MNW and the recommended treatments based on the concept of proportionate response. The author opines that interventions should be based on the degree of median nerve dysfunction as defined by EDX.
In his third objection, Fowler incorrectly states that EDX are performed for financial gain. Reimbursement for EDX varies between countries but it is not the lucrative procedure that Dr. Fowler perceives. Even within the United States, there was a significant reduction in reimbursement for EDX in 2013 and yet the number of studies performed by neurologists and physiatrists has hardly changed (Callaghan et al., 2016). Continuing to perform needed studies despite a reduction in reimbursement is a profound refutation of an underlying financial motivation. EDX studies continue to be ordered in cases of CTS because this is the current gold standard for identifying evidence of median nerve compression. Until other diagnostic methods are demonstrated to be superior, EDX should always be obtained whenever there is a clinical suspicion of a MNW or in patients with an underlying polyneuropathy who are at increased risk for developing a MNW, (e.g. diabetic polyneuropathy).
Fowler also implies that EDX are painful as well as unnecessary. EDX are generally well tolerated by most individuals, especially the nerve conduction studies, which are non-invasive. Electromyography is less invasive than phlebotomy (Alshaikh et al., 2016), as the needle gauge is smaller. Needle electromyography is also the only means by which motor axon loss can be definitively established. While there is no universal agreement on when needle electromyography should be performed (Werner and Andary, 2011), it allows for the assessment of alternative diagnoses such as a proximal median nerve lesion or a cervical radiculopathy. The author performs these studies whenever the referring physician requests that alternative diagnoses be excluded or when abnormalities of median motor conduction studies are identified. This allows for a proposed classification of MNW as depicted in Table 1.
Table 1.
Suggested Management Principles.
| CTS severity | Electrodiagnostic findings | Intervention |
|---|---|---|
| CTS without MNW | None | Symptomatic treatment |
| Mild | Abnormal comparison studies/median sensory nerve abnormalities | Symptomatic treatment |
| Moderate | Prolonged distal motor latency to the abductor pollicis brevis with normal APB CMAP amplitude | Injections/surgery with progression |
| Severe | Above plus either reduced median to APB CMAP amplitude and/or abnormal needle EMG in the thenar muscles | Surgery if not contraindicated |
In conclusion, the AAOS concurs with the AANEM and other clinical neurophysiologists that EDX serve an essential role in the evaluation of MNW. I opine that EDX are far more sensitive and specific than any other clinical methodology for determining the presence of a MNW. In the absence of a definitive study demonstrating the efficacy of surgery on patients with CTS and normal or minimally abnormal EDX studies, conservative treatment should always precede surgical intervention. However, surgical intervention should be considered when there is EDX evidence of an interval progression of a MNW despite the use of conservative measures. More advanced cases of MNW should be considered for surgical decompression in an attempt to mitigate axon loss. For asymptomatic patients with EDX of MNW, our surgical colleagues should consider a median nerve release when there is evidence of significant axon loss as defined by EDX studies.
4. Jeremy Bland: What is the purpose of nerve conduction studies in CTS?
It is not only the latest AAOS guidelines for CTS (AAOS, 2016) and editorializing individual surgeons (Fowler, 2017) addressed by Dr. Menkes that have recently questioned the role of NCS in the management of CTS, nor is the controversy confined to the USA as suggested by Dr. Sonoo. Recent draft guidelines from the British Orthopaedic Association have also suggested that there is no need for laboratory confirmation of the diagnosis before surgery, and there is a steady stream of papers purporting to show that carpal tunnel surgery without prior investigation is effective (Zyluk and Szlosser, 2013) and that NCS results have no predictive value for the outcome of surgery (Concannon et al., 1997). Many hand surgeons are frustrated by being told by neurophysiologists or insurers that they should order NCS on their CTS patients. They know from personal experience that when they have confidently diagnosed CTS the vast majority of patients are delighted with the outcome of surgery. This personal experience is an accurate reflection of the usual outcome of surgery and recent surveys suggest that many surgeons operate without arranging NCS (Lane et al., 2014, Sears et al., 2017). We therefore have a conflict of opinion between surgeons who see no value in NCS as part of their routine management of CTS patients and neurophysiologists who believe that their tests do add clinical value and should be carried out in every patient with suspected CTS before surgery.
A principal cause of misunderstanding is a simplistic view of NCS for CTS as a ‘diagnostic’ test – i.e., one which tries to define whether the patient has the disease. This view has given rise to numerous studies attempting to find the ‘El Dorado’ of the perfect discriminatory nerve conduction method for CTS (Rosenbaum, 1999), while the fact that no such test exists allows skeptical surgeons to quote false negative rates of 30% (Atroshi et al., 2003), and false positive rates of 46% (Redmond and Rivner, 1988), as evidence of diagnostic unreliability. Use of NCS for diagnosis has been well addressed by my co-authors. Here I encourage the use of NCS as simply another tool to be used in the assessment of the patient, and I will quantify their contribution to clinical decision making.
First, NCS can detect other pathology. Dr. Fowler asks what is the “number needed to treat” (NNT) for NCS testing in patients with “classic CTS” to reveal an unexpected diagnosis that affects patient care (Bland and Fowler, 2017). I know of no published study which adequately addresses this question, partly because of the difficulty in defining “classic CTS”. However in 21,000 referrals to my own clinic for suspected CTS, 7% of patients with normal tests for CTS had neurophysiological evidence of another neurological problem which could explain the symptoms and 5% of the patients with evidence of CTS also had evidence of another neurological disorder which would either influence the outcome of treatment for CTS or require treatment on its own merits, suggesting an NNT of about 8.
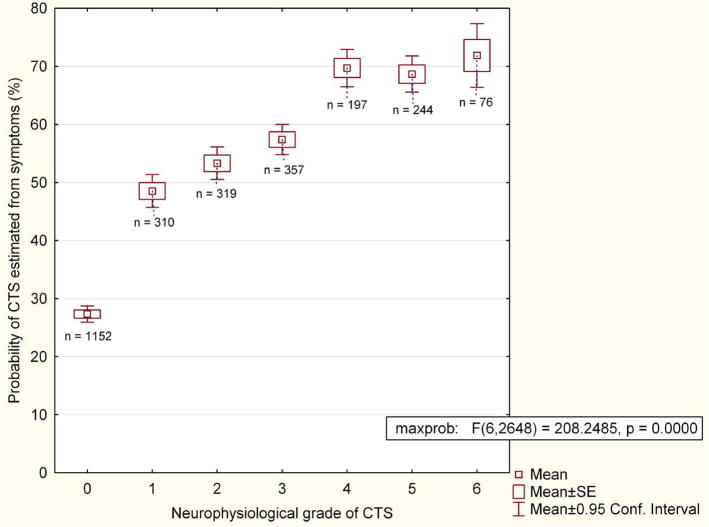
Secondly, NCS provide a quantitative measure of the physiological function of the median nerve which may be used to guide treatment and for prognosis. This is a continuum ranging from the pristine conduction of the 15 year old normal nerve to the total absence of measurable function. There is no true boundary level of function below which patients should be considered ‘abnormal’ and normal limits are simply statistical constructs based on the range of function measured in an asymptomatic healthy population. Some individuals who fall outside our ‘normal’ range will have median nerve pathology without symptoms (MNW), others will simply represent one extreme of the range of normality. Despite the lack of clear separation between normal and abnormal subjects there is a strong relationship between median nerve function as measured by NCS and the probability that individuals will present with the clinical syndrome of CTS (Fig. 2).
Fig. 2.
The probability of CTS estimated from symptoms vs neurophysiological severity of CTS in 2,695 subjects with hand symptoms. The probability of CTS (vertical axis) is derived from the algorithms used on www.carpal-tunnel.net. The higher the score, the more typical of CTS is the patient’s presentation. (As the degree of NCS abnormality increases it becomes more likely that a clinician will recognize the problem as CTS.) The neurophysiological severity was assessed as in Bland (2000a).
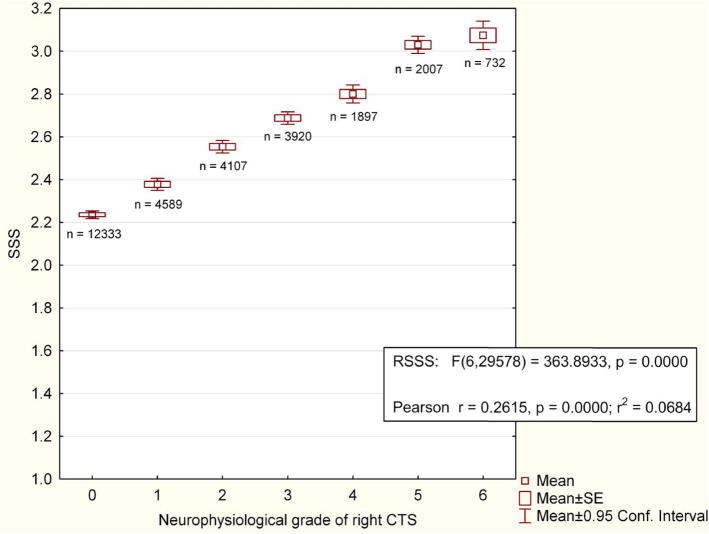
Another source of puzzlement is the existence of patients with subjectively severe symptoms of CTS but little or no NCS abnormality, or the converse situation of very poor nerve function but few symptoms, contributing to the surgeon’s feeling that the tests have no relation to reality. Dr. Burke has explained the reasons for the limited correlation between symptom severity and NCS but despite claims to the contrary (Mondelli et al., 2000), there is a highly significant, though weak, correlation between neurophysiological and subjective severity (Fig. 3).
Fig. 3.
Relationship between neurophysiological severity of CTS, assessed as in Bland (2000a), and subjective severity of symptoms in 29,594 tests in patients referred with possible CTS (some patients tested on more than one occasion). SSS = Boston/Levine symptom severity score for right hand at the time of test.
If every patient presenting with clinical CTS eventually required surgery, as is believed by some surgeons, then the most cost-effective approach is to operate immediately. Surgery however is not inevitable. The natural history of untreated CTS has been inadequately studied but there is evidence that some patients may improve without any specific intervention (Padua et al., 2001), and some improve with splints alone (Gerritsen et al., 2002). Local corticosteroid injection is unquestionably effective in the short to medium term (Marshall et al., 2007) but this review is outdated and some long-term studies now suggest that a significant proportion of patients who are treated non-surgically remain well for 5–8 years (Jenkins et al., 2012, Evers et al., 2017, Hameso and Bland, 2017). Surgery also has significant long-term morbidity (Bland, 2007), several orders of magnitude greater than the complication rate for corticosteroid injection which has a serious complication rate <0.1% (Kaile and Bland, 2018). There are thus several non-surgical options and many patients prefer to avoid surgery.
Discussion of the role of NCS in choosing treatment is inseparable from considerations of prognosis. NCS are useful primarily because they help us to predict what will happen following intervention. The literature on this topic is extensive with some reports showing that more severe NCS changes are associated with poorer outcomes (Iida et al., 2008, Ezquerra-Hernando et al., 2014), some that more severe NCS changes are associated with better outcomes (Atroshi et al., 1998, Straub, 1999), and some finding no association with outcome at all (Concannon et al., 1997, Watchmaker and Watchmaker, 2017). The contradictory results are explained by the non-linear relationship between NCS severity and surgical outcome (Fig. 4) and by many studies being under-powered. A study of milder patients finds that more severe NCS results correlate with a better prognosis while one of more severe patients finds the opposite. Collapsing the NCS severity categories into fewer grades concatenates patients with good and poor prognoses, such that the average outcome is the same in each group.
Fig. 4.
Relationship between pre-operative neurophysiological grade, assessed as in Bland (2000a), and surgical outcome. Overall subjective opinion of the effect on symptoms of surgery for 7,410 routine NHS carpal tunnel decompressions. Patient opinions were collected 3 months to 2 years after surgery. Number of cases in each group is indicated in the bars.
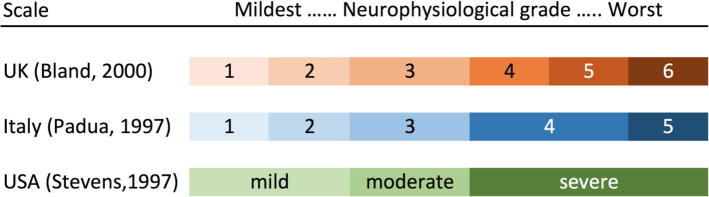
A physiological severity scale for CTS must allow fine gradations. Mild/moderate/severe does not convey the subtlety of NCS measurement and there is no agreement on what these terms mean when they are used in the conclusion of a report. Individual neurophysiological measurements are poorly suited to this task. The sensory nerve action potential becomes unrecordable as CTS becomes more severe while the distal motor latency is both insensitive to early CTS and also becomes unrecordable at the most severe extreme. Neurophysiologists should not expect busy surgeons to be intimately familiar with the nuances of electrophysiological data. Therefore a simple but formal grading scheme is required. Three popular schemes and their relationships to each other are shown in Fig. 5.
Fig. 5.
Popular grading schemes for CTS. For details of the grading criteria based on NCS, see (Padua et al., 1997, Stevens, 1997, Bland, 2000a).
Two ‘one-size fits all’ approaches to treatment have been suggested; Treat everyone conservatively (splints and/or steroids), and then operate on those who do not respond, or operate on everyone. The first, though immediately appealing and widely used in the UK, runs the risk that patients in grades 4–5 at presentation will deteriorate into groups with a poorer prognosis during a cycle of conservative treatment. The second results in patients who may not need it having surgery. I suggest a third strategy based on measuring the severity of CTS, graded as in Fig. 5. Patients in grades 1 and 2 are treated by splinting and local corticosteroid injection initially. Should they fail to respond or relapse and deteriorate to grade 3, their chances of a good result from surgery will be better than they would have been had they proceeded direct to surgery. Patients in grades 4–6 should proceed directly to surgery, though grade 6 patients should be warned that their prospects of complete recovery are less good. Grade 3 patients may be offered a choice of medical or surgical treatment.
Recent guidelines suggest choosing initial treatment on clinical grounds, patients with thenar weakness and wasting being offered surgery while those without can be treated conservatively. However, waiting for thenar atrophy, which is strongly correlated with grade 5 and 6 NCS, before operating is a recipe for poor results. In the absence of thenar weakness, i.e., in grades 0–4, it is impossible to reliably determine the neurophysiological grade using clinical features and the only way to make a rational choice of treatment is to carry out the NCS. Once conservative treatment has been tried in the milder patients the situation on relapse of symptoms is different and it is then entirely reasonable to proceed to surgery whatever the NCS show, though it should be noted that a complete failure to respond to steroid injection is a poor prognostic sign for surgery in itself (Edgell et al., 2003, de Miranda et al., 2015).
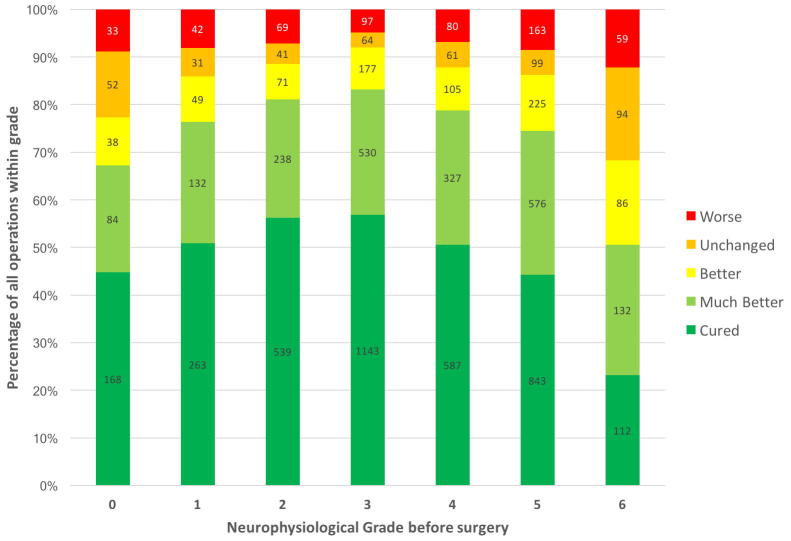
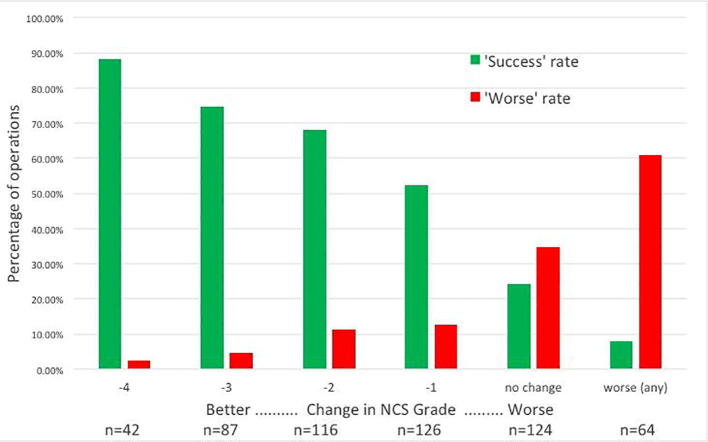
Thirdly, when the outcome of surgery is unsatisfactory NCS can assist in determining the reason for failure. Surgeons are often perplexed by the fact that NCS can be ‘abnormal’ after they have ‘solved’ the problem with the scalpel and the patient is asymptomatic. Dr. Burke has covered the reasons for this. NCS are not however insensitive to changes produced by treatment. Successful carpal tunnel surgery is strongly associated with improvement in the NCS and satisfactory success rates of 70–90% are associated with at least a two grade improvement – Fig. 6; see also (Seror, 1992). Failure of the post-operative studies to show this improvement in comparison with timely pre-operative studies which showed grade 5 or less CTS is very suggestive of inadequate decompression.
Fig. 6.
Change in neurophysiological grade from before to after surgery in 559 operations vs outcome of surgery. The neurophysiological severity was assessed as in Bland (2000a). Green bars show the percentage of patients with a given improvement in NCS results reporting that symptoms are completely cured or much improved. Red bars show the percentage of patients with a given change in NCS who report themselves worse after surgery.
5. David Burke: Comments on CTS and the AAOS guidelines
The AAOS Guidelines are based on analysis of the strength of evidence in the literature, and this is important in guiding evidence-based practice. However it is not easy to publish papers on such a common condition unless one can point to something truly “novel” about the report. We should keep in mind that this could bias published reports in favour of unusual cases and studies of aetiology, such as the literature on workplace injury, designed to establish a role for manual labour and repetitive wrist movement.
The Guidelines indicate that there is strong evidence for an association between thenar wasting and carpal tunnel syndrome, and this is not surprising in the context of a patient presenting with symptoms suggestive of carpal tunnel syndrome. However neurologists should be concerned if there is thenar wasting without those symptoms. In any case the diagnosis of carpal tunnel syndrome should be made long before wasting (or fixed sensory loss) develop because this suggests a severe compression neuropathy, and the results of decompression are likely to be incomplete. In the Guidelines, the recommendations on demographics, symptoms, physical signs and provocative manoeuvres are based on each feature considered in isolation: the Authors of the Guidelines emphasise this by underlining “alone” in “…because alone, each has a poor or weak association…”. However clinicians rely not on isolated signs and symptoms but on the whole picture, and the lack of strong evidence for a symptom or sign by itself does not negate the value of that feature in context.
5.1. Distribution of symptoms
Virtually all clinicians would agree that a young female woken at night by numbness in the median nerve distribution, dysaesthesiae and/or pain in the upper limb, relieved by shaking the wrist is likely to have carpal tunnel syndrome. Not infrequently I encounter practitioners who expect the “positive” symptoms of carpal tunnel syndrome to conform to a median nerve distribution. However the site where pain is experienced is a notoriously poor localising symptom (in contrast to tenderness or “negative” symptoms), and it has long been recognized that proximal discomfort occurs in CTS. There are many references confirming an extra-median distribution of symptoms, including pain in shoulder and upper arm (Loong, 1977, Torebjörk et al., 1984, Jackson and Clifford, 1989, Stevens et al., 1999, Nora et al., 2004, Seror, 2005, Zanette et al., 2007). There are reports of patients successfully treated by carpal tunnel decompression, who reported pain extending from the neck to the hand (Crymble, 1968) and with shoulder pain (Kummell and Zazanis, 1973). It is common for patients to say that their troubles involve the whole of the palmar aspect of the hand and fingers, even the numbness. Sensory disturbance involved digit 5 in 39% patients with CTS and pain was felt in digit 5 in 11% (Clark et al., 2011), leading the authors to conclude that “An atypical distribution of symptoms is a common occurrence and should not discourage diagnosis of CTS”. Similarly Zanette et al. report that “extramedian pattern and proximal pain were found in 33.3% and 37.5% of patients (Zanette et al., 2010). While patients with symptoms restricted to a median distribution and those with extra-median distribution may respond equally well to surgery (Claes et al., 2014), there is evidence that those with paraesthesiae only in the median nerve distribution have more severe deficits than those who report paraesthesiae in a glove distribution (Caliandro et al., 2006). With regard to mechanisms and treatment, it is of interest that Zanette et al. found evidence consistent with “central sensitization”, and concluded that their findings may explain “the persistence of sensory symptoms after median nerve surgical release and the presence of non-anatomical sensory patterns in neuropathic pain” (Zanette et al., 2010). The possibility of “central sensitization” in CTS is unproven, but it raises questions whether absence of a satisfactory response to decompression necessarily means that the diagnosis was suspect and the surgery unnecessary (i.e., a positive response to decompression may not satisfy Dr. Sonoo’s requirements for a “gold standard”).
Discomfort following driving long distances is, in my experience, also common (because that involves two manoeuvres each of which increase pressure in the carpal tunnel – contraction of the forearm flexor muscles (whose tendons pass through the carpal tunnel), and wrist extension (which increases pressure more than wrist flexion - even though Phalen’s test involves prolonged wrist flexion). Prolonged wrist extension can produce conduction block even in previously healthy subjects, though more readily in patients with CTS. The manoeuvre produces a focal compressive lesion of the median nerve, analogous to the peroneal nerve lesion produced by crossing one’s legs, and, in both normal subjects and patients, the changes in axonal excitability are those of nerve compression (Kiernan et al., 1999). While exercise should be encouraged, I stress that push-ups, exercising by lifting weights or dumbbells, or sitting, placing the hands flat on the floor and lifting the body from the floor using the hands are exercises that involve two manoeuvres that increase pressure in the carpal tunnel.
5.2. Carpal tunnel syndrome is a syndrome
Given this, practitioners should report nerve conduction studies appropriately. First, nerve conduction studies cannot establish “carpal tunnel syndrome”, as pointed out by Dr. Menkes, and I am reluctant to apply terms such as “false positive” and “false negative” to NCS in CTS. The authors agree that NCS are designed to demonstrate MNW not CTS, i.e., to demonstrate the underlying lesion responsible for the symptoms and signs of CTS. They support the diagnosis of CTS, but cannot make it. Secondly, the defined abnormalities of nerve conduction need not be symptomatic, just as radiological evidence of cervical spondylosis does not mean that symptoms are due to that abnormality. In this respect, many patients have similar (though commonly less severe) abnormalities of nerve conduction on their other, asymptomatic side. While nerve conduction abnormalities commonly improve following successful decompression surgery, they often do not normalise, as also noted by Dr. Menkes. It can therefore be difficult to attribute a nerve conduction abnormality to recurrence of nerve compression in a patient who had previously undergone decompression surgery, particularly if preoperative studies were not performed. This is one of a number of reasons why NCS should be undertaken in all patients with suspect carpal tunnel syndrome prior to surgery. Before they go under the knife, most patients want to know that the surgeon is indeed operating on a documented abnormality rather than an undocumented clinical diagnosis. Not to undertake nerve conduction studies prior to surgery is bordering on clinical arrogance; all practitioners will have seen patients who underwent decompression for carpal tunnel syndrome but probably never had the condition.
There are patients in whom I think the diagnosis is probably carpal tunnel syndrome but in whom the nerve conduction studies are not diagnostic according to the criteria that I use. In my view this is not CTS without MNW: it is CTS without definable MNW using available tests. Nevertheless it is rare for the studies to be pristine: there are commonly discrepancies that do not quite reach the required level for diagnosis, and I request to see the patient again for repeat studies in 6–12 months, unless the problems resolve.
5.3. Why are patients commonly asymptomatic and without deficits when tested?
In CTS, NCS demonstrate focal abnormalities in large myelinated axons of the median nerve. At the time of testing, patients are commonly not symptomatic, the numbness and dysaesthesiae having subsided shortly after waking. The yield of NCS in these patients is remarkably high, and is not improved further by studying patients when they have their distressing symptoms. Readers should keep in mind two issues. First, we do not study axons of small calibre responsible for pain, perhaps the most distressing symptom. Secondly, in those fibres that we can study, large myelinated axons, we look for conduction slowing across the carpal tunnel (with or without attenuation of the compound action potential). We artificially synchronise all axons to discharge together and conduction slowing of 0.5–1.0 ms across the carpal tunnel may be “diagnostic”. In terms of function, however, conduction slowing of this degree is immaterial provided that a sufficient number of axons can conduct. With large myelinated cutaneous sensory axons, the delay will have no effect on the perception of touch which requires temporal and spatial summation of the activity of a number of asynchronously discharging axons. Similarly force production is not affected by a motor conduction delay of a few milliseconds, given the asynchrony of discharge of motor axons and that the twitch force of individual motor units lags some 100 ms after EMG. For normal function, the important issue is the number of conducting axons, but here we need to consider that loss of amplitude/area of the evoked nerve volley could be due to phase cancellation in a more dispersed volley than normal, and this is particularly so for sensory potentials. Accordingly that there is a significant correlation between conduction abnormalities and symptomatology (Fig. 3) is somewhat remarkable, a “tribute” to the value of NCS. When numbness persists during the day, the sensory potential is always small, and I am then more likely to recommend invasive management.
5.4. When should we contemplate median nerve decompression?
The symptoms of carpal tunnel syndrome can resolve with time, and management should be directed to (i) alleviating discomfort, and (ii) preserving nerve function. I agree with Dr. Menkes and Dr. Bland that the greater the pre-existing nerve damage, the greater the need for decompression, and am happy for conservative approaches to be tried in those with less abnormal nerve conduction findings. Dr. Bland’s extensive experience with local injection of corticosteroids is impressive and influential: they are safe, effective and can result in improved ultrasound and nerve conduction findings (Seror, 1992, Cartwright et al., 2011). Others have concluded “Over the short term, local steroid injection is better than surgical decompression for the symptomatic relief of CTS. At 1 year, local steroid injection is as effective as surgical decompression for the symptomatic relief of CTS” (Ly-Pen et al., 2005, Andreu et al., 2014), but the same group reported a randomized trial of patients treated by local corticosteroid injection or by decompression, and found that only surgery resulted in an improvement of the neurophysiological parameters at 12-months follow-up (Andreu et al., 2014), a finding that differs from that of Cartwright et al. If the patient can tolerate the discomfort and the amplitudes of sensory potentials are preserved, I am content to see if the condition will resolve without external interference.
6. Summary and recommendations
-
•
Carpal tunnel syndrome [CTS] is a syndrome that is often but not invariably caused by a demonstrable median neuropathy at the wrist (MNW). Thus, there are patients with CTS with normal EDX and asymptomatic patients with MNW as detected by EDX.
-
•
NCS provide a reliable measure to document MNW and therefore contribute to the diagnosis of CTS.
-
•
Patients with CTS frequently report symptoms outside the median nerve territory, whereas MNW will result in EDX abnormalities confined to the median nerve.
-
•
Nerve conduction studies can be negative or manifest minimal abnormalities. Follow-up studies at a suitable interval are advised in patients with mild or equivocal findings. Such patients should be treated conservatively. However, these patients should be tested on relapse to ensure that progression is not occurring.
-
•
Every patient with suspected CTS should undergo NCS before invasive treatment (e.g., corticosteroid injection or surgery). Local corticosteroid injection can be as effective as decompression in alleviating symptoms, and there is evidence that EDX and ultrasound abnormalities lessen in the 12 months following injection. Whether they are as effective as surgical decompression in the long-term (>12 months) is unknown.
-
•
NCS severity may be used to assist choice of initial treatment, but should not dictate it absolutely. Patient factors such as the degree of discomfort and social factors such as the needs of employment are important considerations.
-
•
Needle EMG is not obligatory in all cases of CTS but may be performed when clinically indicated for differential diagnosis or lesion localization. Indications for needle EMG also include establishing how acute and complete is the abnormality, or that the abnormality is indeed at the carpal tunnel. The latter might require EMG of muscles outside the thenar eminence depending on the differential diagnosis.
-
•
Patients with unsatisfactory surgical outcomes at 6 weeks to 3 months should undergo repeat testing and the results compared with the pre-operative studies.
-
•
Lacking a gold standard for diagnosis, clinicians should use all reasonable diagnostic measures including symptoms/signs and NCS to increase diagnostic accuracy. The claim that NCS are not necessary at all for the diagnosis or management of CTS is unsubstantiated. NCS can also document the state of neural function before an invasive procedure allowing for a post-intervention comparison when required.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- American Academy of Orthopaedic Surgeons, Management of carpal tunnel syndrome: Evidence based clinical practice guideline, www.aaos.org/ctsguideline, February 29, 2016. [DOI] [PubMed]
- Alshaikh N.M., Martinez J.P., Pitt M.C. Perception of pain during electromyography in children: A prospective study. Muscle Nerve. 2016;54:422–426. doi: 10.1002/mus.25069. [DOI] [PubMed] [Google Scholar]
- Andreu J.L., Ly-Pen D., Millan I., de Blas G., Sanchez-Olaso A. Local injection versus surgery in carpal tunnel syndrome: neurophysiologic outcomes of a randomized clinical trial. Clin. Neurophysiol. 2014;125:1479–1484. doi: 10.1016/j.clinph.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Anon Practice parameter for carpal tunnel syndrome. Neurology. 1993;43:2406–2409. [PubMed] [Google Scholar]
- Anon Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: Summary statement. Muscle Nerve. 1993;13:1390–1391. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]
- Atroshi I., Gummesson C., Johnsson R., Ornstein E. Diagnostic properties of nerve conduction tests in population-based carpal tunnel syndrome. BMC Musculoskelet. Disord. 2003;4:9. doi: 10.1186/1471-2474-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atroshi I., Gummesson C., Johnsson R., Ornstein E., Ranstam J., Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- Atroshi I., Johnsson R., Ornstein E. Patient satisfaction and return to work after endoscopic carpal tunnel surgery. J. Hand Surg. Am. 1998;23:58–65. doi: 10.1016/S0363-5023(98)80090-7. [DOI] [PubMed] [Google Scholar]
- Bland J.D.P. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23:1280–1283. doi: 10.1002/1097-4598(200008)23:8<1280::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bland J.D. The value of the history in the diagnosis of carpal tunnel syndrome. J. Hand Surg. Br. 2000;25:445–450. doi: 10.1054/jhsb.2000.0452. [DOI] [PubMed] [Google Scholar]
- Bland J.D.P. Do nerve conduction studies predict the outcome of carpal tunnel decompression? Muscle Nerve. 2001;24:935–940. doi: 10.1002/mus.1091. [DOI] [PubMed] [Google Scholar]
- Bland J.D.P. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36:167–171. doi: 10.1002/mus.20802. [DOI] [PubMed] [Google Scholar]
- Bland J.D.P., Fowler J.R. Nerve conduction studies for carpal tunnel syndrome: gold standard or unnecessary evil? Orthopedics. 2017;40:141–142. doi: 10.3928/01477447-20170419-01. [DOI] [PubMed] [Google Scholar]
- Bonnaud-Toulze E., Raine C.S. Remodelling during remyelination in the peripheral nervous system. Neuropathol. Appl. Neurobiol. 1980;6:279–290. doi: 10.1111/j.1365-2990.1980.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Boonyapisit K., Katirji B., Shapiro B., Preston D.C. Lumbrical and interossei recording in severe carpal tunnel syndrome. Muscle Nerve. 2001;25:102–105. doi: 10.1002/mus.10002. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2009;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Caliandro P., La Torre G., Aprile I., Pazzaglia C., Commodari I., Tonali P. Distribution of paresthesias in Carpal Tunnel Syndrome reflects the degree of nerve damage at wrist. Clin. Neurophysiol. 2006;117:228–231. doi: 10.1016/j.clinph.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Callaghan B.C., Burke J.F., Skolarus L.E., Jacobson R.D., De Lott L.B., Kerber K.A. Medicare's reimbursement reduction for nerve conduction studies: effect on use and payments. JAMA Intern. Med. 2016;176:697–699. doi: 10.1001/jamainternmed.2016.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright M.S., White D.L., Demar S., Wiesler E.R., Sarlikiotis T., Chloros G.D. Median nerve changes following steroid injection for carpal tunnel syndrome. Muscle Nerve. 2011;44:25–29. doi: 10.1002/mus.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes F., Kasius K.M., Meulstee J., Grotenhuis J.A., Verhagen W.I. Treatment outcome in carpal tunnel syndrome: does distribution of sensory symptoms matter? J. Neurol. Sci. 2014;344:143–148. doi: 10.1016/j.jns.2014.06.044. [DOI] [PubMed] [Google Scholar]
- Clark D., Amirfeyz R., Leslie I., Bannister G. Often atypical? The distribution of sensory disturbance in carpal tunnel syndrome. Ann. R. Coll. Surg. Engl. 2011;93:470–473. doi: 10.1308/003588411X586191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon M.J., Gainor B., Petroski G.F., Puckett C.L. The predictive value of electrodiagnostic studies in carpal tunnel syndrome. Plast. Reconstr. Surg. 1997;100:1452–1458. doi: 10.1097/00006534-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Crymble B. Brachial neuralgia and the carpal tunnel syndrome. Brit. Med. J. 1968;iii:470–471. doi: 10.1136/bmj.3.5616.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcy C.A., McGee S. Does this patient have carpal tunnel syndrome? JAMA. 2000;283:3110–3117. doi: 10.1001/jama.283.23.3110. [DOI] [PubMed] [Google Scholar]
- Dawson D.M., Hallett M., Wilbourn A.J. 3 ed. Lipincott-Raven; Philedelphia, Pensylvania, USA: 1999. Entrapment Neuropathies. [Google Scholar]
- de Carvalho M., Dengler R., Eisen A., England J.D., Kaji R., Kimura J. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- de Krom M.C.T.F.M. Knipschild PG, Kester ADM, Spaans F. Efficacy of provocative tests for diagnosis of carpal tunnel syndrome. Lancet. 1990;335:393–395. doi: 10.1016/0140-6736(90)90218-t. [DOI] [PubMed] [Google Scholar]
- de Miranda G.V., Fernandes C.H., Raduan J.N., Meirelles L.M., Dos Santos J.B., Faloppa F. Corticoid injection as a predictive factor of results of carpal tunnel release. Acta Ortop. Bras. 2015;23:76–80. doi: 10.1590/1413-78522015230200943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell S.E., McCabe S.J., Breidenbach W.C., LaJoie A.S., Abell T.D. Predicting the outcome of carpal tunnel release. J. Hand Surg. Am. 2003;28:255–261. doi: 10.1053/jhsu.2003.50031. [DOI] [PubMed] [Google Scholar]
- Evers S., Bryan A.J., Sanders T.L., Gunderson T., Gelfman R., Amadio P.C. Corticosteroid injections for carpal tunnel syndrome: long-term follow-up in a population-based cohort. Plast. Reconstr. Surg. 2017;140:338–347. doi: 10.1097/PRS.0000000000003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquerra-Hernando L., Gomez-Vallejo J., Corella-Abenia E., Albareda-Albareda J. Factores pronosticos en la cirugia del sindrome del tunel carpiano. Acta Orthop. Mex. 2014;28:160–163. [PubMed] [Google Scholar]
- Fowler J.R. Nerve conduction studies for carpal tunnel syndrome: gold standard or unnecessary evil? Orthopedics. 2017;40:141–142. doi: 10.3928/01477447-20170419-01. [DOI] [PubMed] [Google Scholar]
- Fowler T.J., Scadding J.W. 3rd Edition. CRC Press; Boca Raton, FL: 2013. Clinical Neurology. [Google Scholar]
- Gerritsen A.A.M., de, Vet HCW, Scholten R.J.P.M., Bertelsmann F.W., de Krom M.C.T.F.M., Bouter L.M. Splinting vs Surgery in the treatment of carpal tunnel syndrome: a randomised controlled trial. JAMA. 2002;288:1245–1251. doi: 10.1001/jama.288.10.1245. [DOI] [PubMed] [Google Scholar]
- Glowacki K.A., Breen C.J., Sachar K., Weiss A.P. Electrodiagnostic testing and carpal tunnel release outcome. J. Hand Surg. Am. 1996;21:117–122. doi: 10.1016/S0266-7681(96)80025-8. [DOI] [PubMed] [Google Scholar]
- Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J. Bone Joint Surg. Am. 2008;90:2587–2593. doi: 10.2106/JBJS.G.01362. [DOI] [PubMed] [Google Scholar]
- Graham B., Regehr G., Naglie G., Wright J.G. Development and validation of diagnostic criteria for carpal tunnel syndrome. J. Hand Surg. Am. 2006;31:919–924. [PubMed] [Google Scholar]
- Hameso A., Bland J.D. Prevalence of decompression surgery in patients with carpal tunnel syndrome 8 years after initial treatment with a local corticosteroid injection. J. Hand Surg. Eur. 2017;42:275–280. doi: 10.1177/1753193416671102. [DOI] [PubMed] [Google Scholar]
- Hansen P.A., Micklesen P., Robinson L.R. Clinical utility of the flick maneuver in diagnosing carpal tunnel syndrome. Am. J. Phys. Med. Rehabil. 2004;83:363–367. doi: 10.1097/01.phm.0000124439.14757.99. [DOI] [PubMed] [Google Scholar]
- Iida J.-i., Hirabayashi H., Nakase H., Sakaki T. Carpal tunnel syndrome: electrophysiological grading and surgical results by minimum incision open carpal tunnel release. Neurol. Med. Chir. (Tokyo) 2008;48:554–559. doi: 10.2176/nmc.48.554. [DOI] [PubMed] [Google Scholar]
- Jablecki C.K., Andary M.T., Floeter M.K., Miller R.G., Quartly C.A., Vennix M.J. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome. Neurology. 2002;58:1589–1592. doi: 10.1212/wnl.58.11.1589. [DOI] [PubMed] [Google Scholar]
- Jablecki C.K., Andary M.T., So Y.T., Wilkins D.E., Williams F.H. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve. 1993;16:1392–1414. doi: 10.1002/mus.880161220. [DOI] [PubMed] [Google Scholar]
- Jackson D.A., Clifford J.C. Electrodiagnosis of mild carpal tunnel syndrome. Arch. Phys. Med. Rehabil. 1989;70:199–204. [PubMed] [Google Scholar]
- Jenkins P.J., Duckworth A.D., Watts A.C., McEachan J.E. Corticosteroid injection for carpal tunnel syndrome: a 5-year survivorship analysis. Hand. 2012;7:151–156. doi: 10.1007/s11552-012-9390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.W. Diagnosis of carpal tunnel syndrome The gold standard. Am. J. Phys. Med. Rehabil. 1993;72:1. doi: 10.1097/00002060-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Kaile E., Bland J.D.P. Safety of corticosteroid injection for carpal tunnel syndrome. J. Hand Surg. Eur. 2018;43:296–302. doi: 10.1177/1753193417734426. [DOI] [PubMed] [Google Scholar]
- Katz J.N., Larson M.G., Sabra A., Krarup C., Stirrat C.R., Sethi R. The carpal tunnel syndrome: diagnostic utility of the history and physical examination findings. Ann. Int. Med. 1990;112:321–327. doi: 10.7326/0003-4819-112-5-321. [DOI] [PubMed] [Google Scholar]
- Keith M.W., Masear V., Chung K.C., Maupin K., Andary M., Amadio P.C. American academy of orthopaedic surgeons clinical practice guideline on diagnosis of carpal tunnel syndrome. J Bone Joint. Surg. Am. 2009;91:2478–2479. doi: 10.2106/JBJS.I.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan M.C., Mogyoros I., Burke D. Conduction block in carpal tunnel syndrome. Brain. 1999;122:933–941. doi: 10.1093/brain/122.5.933. [DOI] [PubMed] [Google Scholar]
- Kilmer D.D., Davis B.A. Electrodiagnosis in carpal tunnel syndrome. Hand Clin. 2002;18:243–255. doi: 10.1016/s0749-0712(01)00009-9. [DOI] [PubMed] [Google Scholar]
- Kuhlman K.A., Hennessey W.J. Sensitivity and specificity of carpal tunnel syndrome signs. Am. J. Phys. Med. Rehabil. 1997;76:451–457. doi: 10.1097/00002060-199711000-00004. [DOI] [PubMed] [Google Scholar]
- Kummell B.M., Zazanis G.A. Shoulder pain as the presenting complaint in carpal tunnel syndrome. Clin. Orthop. 1973;92:227–230. doi: 10.1097/00003086-197305000-00019. [DOI] [PubMed] [Google Scholar]
- Lane L.B., Starecki M., Olson A., Kohn N. Carpal tunnel syndrome diagnosis and treatment: a survey of members of the american society for surgery of the hand. J. Hand Surg. Am. 2014;39:2181–2187. doi: 10.1016/j.jhsa.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Loong S.C. The carpal tunnel syndrome: a clinical and electrophysiological study in 250 patients. Proc. Aust. Assoc. Neurol. 1977;14:51–65. [PubMed] [Google Scholar]
- Ly-Pen D., Andreu J.-L., de Blas G., Sanchez-Olaso A., Millan I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled clinical trial. Arthritis Rheum. 2005;52:612–619. doi: 10.1002/art.20767. [DOI] [PubMed] [Google Scholar]
- Marshall S., Tardiff G., Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2007;(2):CD001554. doi: 10.1002/14651858.CD001554.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli M., Reale F., Sicurelli F., Padua L. Relationship between the self-administered Boston questionnaire and electrophysiological findings in follow-up of surgically-treated carpal tunnel syndrome. J. Hand Surg. Br. 2000;25:128–134. doi: 10.1054/jhsb.2000.0361. [DOI] [PubMed] [Google Scholar]
- Moseley J.B., O’Malley K., Petersen N.J., Menke T.J., Brody B.A., Kuykendall D.H. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- Nathan P.A., Takigawa K., Keniston R.C., Meadows K.D., Lockwood R.S. Slowing of sensory conduction of the median nerve and carpal tunnel syndrome in Japanese and American industrial workers. J. Hand Surg. Br. 1994;19:30–34. doi: 10.1016/0266-7681(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Nora D.B., Becker J., Ehlers J.A., Gomes I. Clinical features of 1039 patients with neurophysiological diagnosis of carpal tunnel syndrome. Clin. Neurol. Neurosurg. 2004;107:64–69. doi: 10.1016/j.clineuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Ochoa J., Fowler T.J., Gilliatt R.W. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J. Anat. 1972;113:433–455. [PMC free article] [PubMed] [Google Scholar]
- Ochoa J., Marotte L.R. The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J. Neurol. Sci. 1973;19:491–495. doi: 10.1016/0022-510x(73)90045-2. [DOI] [PubMed] [Google Scholar]
- Padua L., Monaco M.L., Gregori B., Valente E.M., Padua R., Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol. Scand. 1997;96:211–217. doi: 10.1111/j.1600-0404.1997.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Padua L., Padua R., Aprile I., Pasqualetti P., Tonali P. Multiperspective follow-up of untreated carpal tunnel syndrome: a multicenter study. Neurology. 2001;56:1459–1466. doi: 10.1212/wnl.56.11.1459. [DOI] [PubMed] [Google Scholar]
- Redmond M., Rivner H. False positive electrodiagnostic tests in carpal tunnel syndrome. Muscle Nerve. 1988;11:511–517. doi: 10.1002/mus.880110515. [DOI] [PubMed] [Google Scholar]
- Rempel D., Evanoff B., Amadio P.C., de Krom M., Franklin G., Franzblau A. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am. J. Public Health. 1998;88:1447–1451. doi: 10.2105/ajph.88.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum R. Carpal tunnel syndrome and the myth of El dorado. Muscle Nerve. 1999;22:1165–1167. doi: 10.1002/(sici)1097-4598(199909)22:9<1165::aid-mus1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Sainani K. The importance of accounting for correlated observations. PM R. 2010;2:858–861. doi: 10.1016/j.pmrj.2010.07.482. [DOI] [PubMed] [Google Scholar]
- Sears E.D., Lu Y.T., Wood S.M., Nasser J.S., Hayward R.A., Chung K.C. Diagnostic testing requested before surgical evaluation for carpal tunnel syndrome. J. Hand Surg. Am. 2017;42:623–629. doi: 10.1016/j.jhsa.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seror P. Nerve conduction studies after treatment for carpal tunnel syndrome. J. Hand Surg. Br. 1992;17:641–645. doi: 10.1016/0266-7681(92)90191-4. [DOI] [PubMed] [Google Scholar]
- Seror P. Symptoms of thoracic outlet syndrome in women with carpal tunnel syndrome. Clin. Neurophysiol. 2005;116:2324–2329. doi: 10.1016/j.clinph.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Stevens J.C. The electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1987;10:99–113. doi: 10.1002/mus.880100202. [DOI] [PubMed] [Google Scholar]
- Stevens J.C. The electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1997;20:1477–1486. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Stevens J.C., Smith B.E., Weaver A.L., Bosch E.P., Deen H.G.J., Wilkens J.A. Symptoms of 100 patients with electromyographically verified carpal tunnel syndrome. Muscle Nerve. 1999;22:1448–1456. doi: 10.1002/(sici)1097-4598(199910)22:10<1448::aid-mus17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Straub T.A. Endoscopic carpal tunnel release: a prospective analysis of factors associated with unsatisfactory results. Arthroscopy. 1999;15:269–274. doi: 10.1016/s0749-8063(99)70033-2. [DOI] [PubMed] [Google Scholar]
- Torebjörk H.E., Ochoa J.L., Schady W. Referred pain from intraneural stimulation of muscle fascicles in the median nerve. Pain. 1984;18:145–156. doi: 10.1016/0304-3959(84)90882-0. [DOI] [PubMed] [Google Scholar]
- Van den Bergh P.Y., Hadden R.D., Bouche P., Cornblath D.R., Hahn A., Illa I. European federation of neurological societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the european federation of neurological societies and the peripheral nerve society – first revision. Eur. J. Neurol. 2010;17:356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- Watchmaker, J.D., Watchmaker, G.P., 2017. Independent variables affecting outcome of carpal tunnel surgery Hand (N Y). https://doi/org/10.1177/1558944717703739 Apr 1:1558944717703739 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Werner R.A., Andary M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve. 2011;44:597–607. doi: 10.1002/mus.22208. [DOI] [PubMed] [Google Scholar]
- Witt J.C., Hentz J.G., Stevens J.C. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve. 2004;29:515–522. doi: 10.1002/mus.20019. [DOI] [PubMed] [Google Scholar]
- Zanette G., Cacciatori C., Tamburin S. Central sensitization in carpal tunnel syndrome with extraterritorial spread of sensory symptoms. Pain. 2010;148:227–236. doi: 10.1016/j.pain.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Zanette G., Marani S., Tamburin S. Proximal pain in patients with carpal tunnel syndrome: a clinical-neurophysiological study. J. Peripher. Nerve Syst. 2007;12:91–97. doi: 10.1111/j.1529-8027.2007.00127.x. [DOI] [PubMed] [Google Scholar]
- Zyluk A., Szlosser Z. The results of carpal tunnel release for carpal tunnel syndrome diagnosed on clinical grounds, with or without electrophysiological investigations: a randomized study. J. Hand Surg. Eur. 2013;38:44–49. doi: 10.1177/1753193412445162. [DOI] [PubMed] [Google Scholar]