Highlights
-
•
Extracranial cerebral artery Doppler imaging show CBF changes during tilt testing.
-
•
Total CBF during tilt testing decreases 6% in healthy volunteers.
-
•
Flow decrease of internal carotid and vertebral arteries during tilting is similar.
Keywords: Cerebral blood flow, Head-up tilt table test, Healthy volunteers, Internal carotid artery blood flow, Vertebral artery blood flow, PetCO2
Abstract
Objectives
Using different techniques, reduction of cerebral blood flow (CBF) during orthostatic stress were demonstrated. One study reported flow reduction of the right internal carotid (ICA) and vertebral (VA) artery during orthostatic stress by Doppler imaging, with different effects on the 2 vessels. Global CBF changes, using this technique, have not been reported. Therefore, flow of the ICA, VA and global CBF were measured during head-up tilt testing.
Methods
33 healthy volunteers underwent tilt testing. At three time points (supine, half way and at the end of the test) Doppler imaging of the ICA and VA was performed, as well as PetCO2 measurements.
Results
Global CBF was significantly reduced by 4.5 ± 2.8% halfway the test and by 6.0 ± 3.4% at the end. All 4 artery flows were significantly reduced during the tilt, without differences between them. Despite small changes in PetCO2 there was a significant relation between de CBF decrease and PetCO2 decrease (p < 0.05).
Conclusions
Orthostatic stress in HV results in a small but significant reduction of CBF by a homogenous reduction in the four cerebral vessels and is modulated by PetCO2 changes.
Significance
CBF changes can be measured during tilt testing using Doppler VA and ICA imaging.
1. Introduction
Positional changes from supine to sitting or standing result in a decrease of cerebral blood flow (CBF) as demonstrated by different techniques (Yoshimoto et al., 1994, Ouchi et al., 2001, Oblak et al., 2002, Alperin et al., 2005, Wang et al., 2010, Bronzwaer et al., 2014). CBF measurements by extracranial Doppler echography of the internal carotid and vertebral arteries (ICA and VA) in the supine position have been described in a number of studies (Schoning et al., 1994, Deane and Markus, 1997, Seidel et al., 1999, Dorfler et al., 2000, Scheel et al., 2000, Yazici et al., 2005, Oktar et al., 2006, Albayrak et al., 2007, Nemati et al., 2009, Nevo et al., 2010, Sato et al., 2012, Liu et al., 2013). Using this technique the decrease in CBF during head up tilt table testing was also demonstrated in one small study (Sato et al., 2012) of healthy volunteers (HV). The authors studied only the right ICA and VA before and during tilt testing and reported a difference in vasculature response to the orthostatic stress of the right ICA and VA: the decrease in right VA blood flow after tilting was not significant from supine, in contrast to the significant decrease of the right ICA blood flow of 9.4% (Sato et al., 2012).
As total CBF changes during tilting have not been reported before, the aim of the study was to measure both left and right ICA and VA blood flows in a large group of HV before and during tilting, allowing assessment of total CBF changes during tilting.
2. Subjects, material and methods
2.1. Subjects
Initially 41 HV underwent head up tilt table testing (HUT). None had signs or symptoms of cerebral- and cardiovascular disease, the electrocardiogram (ECG) and echocardiogram were normal. One HV with a delayed orthostatic hypotension (OH) and profound hypocapnia and 1 with a vasovagal syncope were excluded. Furthermore, 3 HV were excluded because of an insufficient Doppler image quality. In three HV the right or left VA were hypoplastic (2 right and 1 left VA) defined by a supine flow <30 ml/min (Schoning et al., 1994). These HV were also excluded, leaving 33 HV included in this study. Three times after tilting (directly post tilt, at 10 and 20 min post tilt) HV were asked for dizziness/light-headedness. Seven (21%) reported dizziness/light-headedness directly post tilting, at 10 min complaints were present in 3 (9%), and after 20 min these complaints were absent in all. All had a normal heart rate and blood pressure response during HUT, none had a postural orthostatic tachycardia syndrome (POTS) (Task Force for et al., 2009, Freeman et al., 2011). The study has been carried out in accordance with Declaration of Helsinki. All HV gave informed consent, the study was approved by the MEC of the Slotervaart hospital in Amsterdam.
2.2. Methods
HV underwent HUT with 20 min in the supine and 25–30 min in the upright position (70 degrees). They were investigated in the morning, at least 3 h after a light breakfast or in the afternoon 3 h after a light lunch. No formal hydration protocol was applied, but subjects were asked to ingest an ample amount of fluid.
Heart rate (HR), systolic, diastolic and mean blood pressures (SBP, DBP and MAP) were continuously recorded by finger plethysmography using the Nexfin device (BMeye, Amsterdam, NL). Using an independent radio-controlled clock the starting time of HR and BP recording as well as the time of the start of tilting was noted. HR and blood pressures were extracted from the Nexfin device and imported into an Excel spreadsheet. HR and blood pressures at the radio clock corrected echo time intervals (see below) were averaged.
ICA and VA Doppler frames were acquired by one operator (FCV), using a Vivid-I system (GE Healthcare, Hoevelaken, NL) equipped with a 6–13 MHz linear transducer. Flow data of the ICA were obtained ∼1.0–1.5 cm distal to the carotid bifurcation and of the VA at the C3–C5 level. Care was taken that the insonation angle was less than 60 degrees, that the sample volume was positioned in the center of the vessel and that it covered the width of the vessel. High resolution B mode images, color Doppler images and the Doppler velocity spectrum (pulsed wave mode) were recorded in one frame. The order of imaging was fixed: left ICA, left VA, right ICA, and right VA. At least 2 consecutive series of 6 s per artery were recorded. Moreover the recording time of the first and last analyzed artery was noted. These times were corrected to the times of the radio clock.
In the supine position image acquisition started 8 ± 1 min prior to tilting (supine data) and during the upright position 2 series were acquired: one series was started at 13 ± 3 min (mid HUT data) and one at 24 ± 3 min. (end HUT data). Image acquisition lasted 4.0 ± 1.5 min.
During the study period end-tidal PCO2 (PetCO2) was monitored using a Lifesense device (Nonin Medical, Minneapolis USA).
2.3. Data analysis
Calculations of blood flow were performed by one operator (CMCvC). Automated mean blood flow velocities (MFV) were obtained. Vessel diameters were manually traced by CMCvC and FCV independently on B-mode images, from the intima to the opposite intima. Differences in vessel diameter were resolved by consensus. Surface area was calculated as proposed by Sato et al. (2011): the peak systolic and end diastolic diameters were measured, and the mean diameter was calculated as: mean diameter = (peak systolic diameter × 1/3) + (end diastolic diameter × 2/3). Arterial flow was calculated from the mean blood flow velocity × the surface area. Flow in the individual arteries was calculated in 3–6 cardiac cycles and data were averaged. Total CBF was calculated by adding the flow of the 4 arteries.
End tidal CO2 measurements were imported into an Excel spreadsheet and PetCO2 data at the radio clock corrected echo time intervals were averaged.
2.4. Statistical analysis
Data were analyzed using SPSS 21. Distribution of data was tested by the Mann Whitney test. As all data were normally distributed, the data are presented as the mean ± SD. A p value < 0.05 was considered significantly different. HUT mid data and HUT end data were compared to supine date using paired-samples t-tests. Linear regression analysis was performed between the change in PetCO2 and the %CBF change at the end of the study. For reproducibility measurements intraclass correlation coefficients (ICC’s) were calculated using SPSS. Both ICA and VA mean flow velocities, diameters and flows were recalculated for 21 ICA and for 10 VA cycles. For intra-observer variation data were analyzed at least 3 month after the first assessment by CMCvC, for inter-observer variation FCV also performed the measurements.
3. Results
33 healthy volunteers participated. Included were 9 males and 24 females with a mean age of 41 ± 16 years. BMI was 25 ± 5 kg/m2, BSA (Du Bois) 1.87 ± 0.18 m2. No differences were found between morning and afternoon sessions (data not shown).
The ICC’s for intra-observer variation of the ICA flow velocity, diameter and flow were 0.99, 0.78 and 0.86, respectively. For the VA these values were 0.97, 0.91 and 0.92, respectively.
The ICC’s for inter-observer variation of the ICA flow velocity, diameter and flow were 0.99, 0.82 and 0.87, respectively. For the VA these values were 0.97, 0.80 and 0.91, respectively.
Table 1 shows the measurements of hemodynamics, PetCO2 data, and cerebral flow data in the supine position, at 13 min post tilt (mid HUT data) and at end of the tilt period (end HUT). Heart rate significantly increased by mean 16 bpm during tilting, systolic blood pressure decreased mean 8 mmHg at the end of HUT. Diastolic blood pressure and mean arterial pressure did not change. PetCO2 decreased mean 2 mmHg at mid HUT and 1 mmHg at end HUT.
Table 1.
Hemodynamic and cerebral flow measurements during tilt testing.
| Supine | Mid HUT | End HUT | |
|---|---|---|---|
| Heart rate (bpm) | 61 ± 10 | 78 ± 16**** | 77 ± 16**** |
| SBP (mmHg) | 135 ± 14 | 128 ± 15* | 127 ± 15*** |
| DBP (mmHg) | 78 ± 7 | 81 ± 9 ns | 80 ± 8 ns |
| MAP (mmHg) | 100 ± 10 | 97 ± 12 ns | 98 ± 11 ns |
| PetCO2 (mmHg) | 37 ± 3 | 35 ± 3**** | 36 ± 3*** |
| Total CBF (ml/min) | 638 ± 77 | 609 ± 75**** | 599 ± 74**** |
| ICA-L blood flow (ml/min) | 245 ± 47 | 234 ± 50**** | 232 ± 44**** |
| ICA-R blood flow (ml/min) | 237 ± 41 | 225 ± 37**** | 220 ± 41**** |
| VA-L blood flow (ml/min) | 88 ± 27 | 85 ± 28 | 83 ± 28* |
| VA-R blood flow (ml/min) | 68 ± 24 | 65 ± 23** | 64 ± 23** |
| ICA-L flow velocity (cm/s) | 24.7 ± 5.1 | 23.3 ± 5.6 | 24.9 ± 5.3 |
| ICA-R flow velocity (cm/s) | 25.2 ± 5.2 | 25.0 ± 4.8 | 24.2 ± 4.9 |
| VA-L flow velocity (cm/s) | 16.8 ± 3.4 | 17. ± 3.7 | 16.6 ± 4.4 |
| VA-R flow velocity (cm/s) | 15.5 ± 4.3 | 14.7 ± 3.6* | 14.8 ± 3.8 |
| ICA-L diameter (mm) | 4.63 ± 0.46 | 4.47 ± 0.58** | 4.49 ± 0.50* |
| ICA-R diameter (mm) | 4.50 ± 0.50 | 4.40 ± 0.46 | 4.43 ± 0.51 |
| VA-L diameter (mm) | 3.29 ± 0.45 | 3.24 ± 0.54 | 3.25 ± 0.52 |
| VA-R diameter (mm) | 3.06 ± 0.49 | 3.05 ± 0.52 | 3.05 ± 0.50 |
CBF = cerebral blood flow, DBP = diastolic blood pressure, ICA = internal carotid artery, MAP = mean arterial pressure, SBP = systolic blood pressure, VA = vertebral artery.
p < 0.05 versus supine.
p < 0.01 versus supine.
p < 0.005 versus supine.
p < 0.001 versus supine.
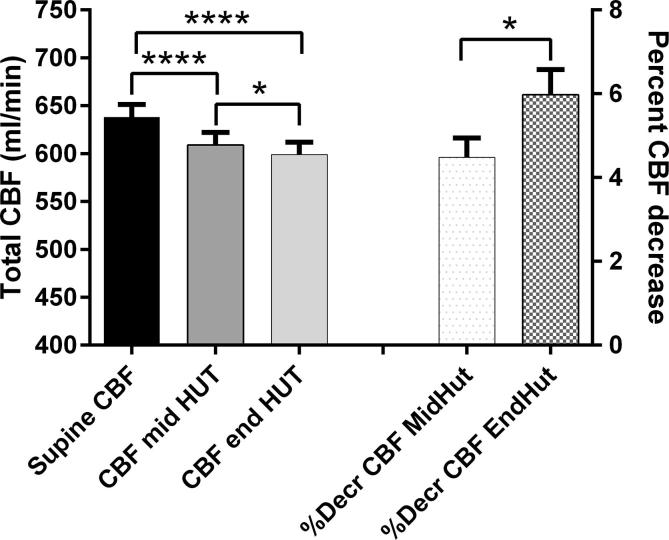
Total CBF decreased significantly from supine 638 ± 77 ml/min to 609 ± 75 ml/min at mid HUT and to 599 ± 74 ml/min at end HUT (both p < 0.001 compared to supine): see Fig. 1. The CBF decrease at end HUT was significantly higher at the end of the tilt compared to mid HUT (p < 0.05). Linear regression showed that there was a weak but significant relation between the decrease of the PetCO2 (PetCO2 base minus end) and the percent decrease of CBF at the end of the study: %decreaseCBFendHUT = 0.52deltaPetCO2 + 5.05 (p < 0.05).
Fig. 1.
Cerebral blood flow (CBF) decrease and percent CBF decrease during head-up tilt table testing (HUT) compared CBF during the supine position. Data are presented as mean + SEM. *: p < 0.05 CBF and %CBF decrease mid versus end HUT. ****: p < 0.001 supine CBF versus CBF mid and end HUT.
The low number of HV (n = 3) with dizziness/light-headedness at the time of mid HUT precluded a CBF change comparison of HV with and without complaints.
Left and right ICA and VA blood flows also decreased significantly during HUT compared to supine. Blood flow velocities of the left and right ICA remained unchanged during HUT compared to supine, as well as the velocity of the left VA. There was a marginal difference in the right VA blood velocity mid HUT versus supine, but not at end HUT versus supine.
The left ICA diameters decreased significantly during HUT, the right ICA diameters also decreased but were not significant. VA diameters did not change from supine to HUT.
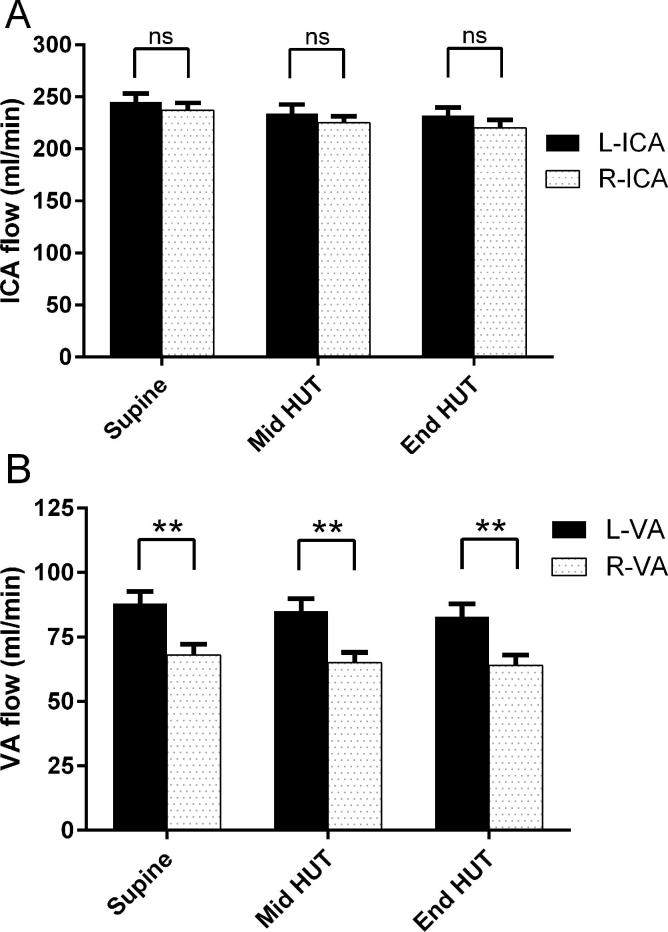
Fig. 2 shows the comparisons between the left and right ICA blood flow and left and right VA blood flow. Left ICA flow was systematically higher than the right ICA flow, but the differences were not significant. Left VA flow was significantly higher than the right VA flow during supine and HUT.
Fig. 2.
Comparison between the left and right internal carotid artery flow (figure A) and between the left and right vertebral artery flow (figure B) during supine position and during head-up tilt table testing (HUT). **: p < 0.01 left versus right VA. ICA = internal carotid artery, VA = vertebral artery.
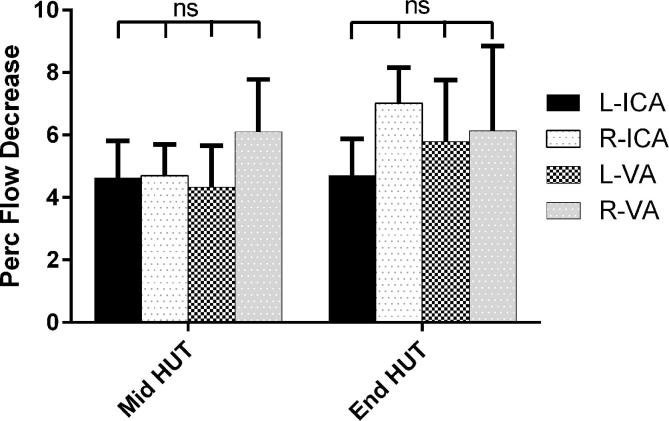
Fig. 3 shows the percent decrease during HUT compared to supine. The decreases in ICA and VA’s during HUT were significant different from zero, and no differences between the 4 vessels were found.
Fig. 3.
Percent decrease of the left and right internal carotid arteries and vertebral arteries flow during HUT, presented as mean + SEM. ICA: internal carotid artery; VA: vertebral artery.
4. Discussion
Almost all of the studies examining cerebral flow during head-up tilt testing in healthy volunteers (in isolation or being the reference of patient studies) were performed with transcranial Doppler (Yoshimoto et al., 1994, Schondorf et al., 2001, Oblak et al., 2002, Wilson et al., 2002, Carey et al., 2003, Razumovsky et al., 2003, Asahina et al., 2006, Gonul et al., 2008, Ocon et al., 2009, Wang et al., 2010, Murrell et al., 2011, Mezei et al., 2013, Riberholt et al., 2013, Yang et al., 2015, Tymko et al., 2016, Viski et al., 2016, Castro et al., 2017). Recently, a small study of 6 HV (Sato et al., 2012) undergoing HUT was published using extracranial Doppler imaging. The authors measured only right ICA and VA flows and found a 9.4% decrease of the ICA blood flow and a non-significant flow decrease of approximately 3% in the right VA. In the current study all 4 cerebral arteries were examined, allowing total CBF calculation. The data show that in healthy volunteers during HUT total CBF decreased by 4.5% halfway the tilt and by 6.0% at the end of the study. The effects of changes in CO2 pressures on CBF have been studied extensively and are important determinants of cerebral flow: (Reivich, 1964, Ainslie and Duffin, 2009, Fierstra et al., 2013, Yoshida et al., 2018). Even in the present study with small changes in CBF and end-tidal CO2 pressure changes of mean 1 mmHg at the end of the study, the decrease of PetCO2 was linearly related to a small but significant decrease of the cerebral vascular flow. This finding strengthens the validity of this technique.
Moreover, flow reduction was significant in all 4 arteries and not significantly different between these arteries (Fig. 2), which is in contrast to a previous extracranial Doppler study (Sato et al., 2012). The difference in VA responsiveness to orthostatic stress between the present study and the abovementioned study was small and possibly, the low number of participants precluded a statistical significance in the latter study.
Using extracranial Doppler techniques a large number of HV studies reported on flow, flow velocity and diameters of the ICA (Schoning et al., 1994, Deane and Markus, 1997, Dorfler et al., 2000, Scheel et al., 2000, Yazici et al., 2005, Krejza et al., 2006, Oktar et al., 2006, Albayrak et al., 2007, Nevo et al., 2010, Sato et al., 2012, Liu et al., 2013) in the supine position. In the present study the supine left and right ICA flows are in line with literature data. In the present study the left ICA flow was not significantly higher than the right ICA flow, in accordance with 2 previous Doppler studies (Yazici et al., 2005, Albayrak et al., 2007). However, one recent study in 228 HV showed that blood flow to the hemisphere, dominant for handedness, was increased compared to the other hemisphere (Jansen van Vuuren, 2017). From this study it may be inferred that left ICA flow was appr. 29% higher in the right-handed subjects. In the present study 28 HV (93%) were right-handed (based on history taking) and in these HV there was also no difference between the left and right ICA flow. The differences are not clear.
ICA diameters were similar to those reported in literature. ICA mean flow velocities were presented in 2 studies (Schoning et al., 1994, Sato et al., 2012) In the present study ICA flow velocities were mean 24.0 and 23.3 cm/s for the left and right ICA, respectively, and were slightly lower than previously reported (Schoning et al., 1994, Sato et al., 2012). This might be explained by the older age in our HV group, as a number of studies found an inverse relation between age and cerebral flow velocities (Oblak et al., 2002, Tegeler et al., 2013, Pase et al., 2014).
In the present study VA flows, velocities and diameters are in line with previous studies (Schoning et al., 1994, Seidel et al., 1999, Dorfler et al., 2000, Scheel et al., 2000, Yazici et al., 2005, Oktar et al., 2006, Albayrak et al., 2007, Nemati et al., 2009, Sato et al., 2012). The right VA flow was significantly lower that the left VA flow. This was also observed in 2 previous studies (Schoning et al., 1994, Albayrak et al., 2007). The mechanism is unclear but a recent study (Jansen van Vuuren, 2017) in right-handed subjects showed that the length of the origin of the brachiocephalic trunk to the end of the right common carotid artery, was longer than on the left side. This longer vessel length resulted in a higher vascular resistance and thus lower flow. If so, distance from the origin of the brachiocephalic trunk to the end of the VA artery may also be longer than on the left side, resulting in a larger resistance over the total vessel length and lower flows.
Using transcranial Doppler, one study (Wang et al., 2010) compared anterior and posterior flow velocities during HUT in men and women. Both the anterior and posterior flow velocity decreased after tilting in both sexes, with a significantly higher posterior flow velocity decrease in women compared to men. For comparison with that study, the flows of the left and right VA of the present study were added and the percent CBF change calculated. There was no significant difference in CBF change between man and women (data not shown). But similar to the study of Wang et al. the number of HV was too small to definitely address this question.
Limitations: the position of sample volumes in the arteries may slightly differ between the three image acquisitions. This may have influenced diameter and flow velocity measurements. Furthermore, vessel diameters were manually drawn, because of lack of automated diameter tracking software. Although consensus determination of the diameters was used, the ICC of diameter calculation was not optimal, in contrast to the automated velocity determination. The use of edge-detection software may lead to more objective and accurate diameter measurements, resulting in less variation of flow measurements. Finally, we did not take into account the menstrual cycle in women, as has been recommended (Thomas et al., 2015). However, cycle dependent alterations in cerebral blood flow velocities have only been described (Krejza et al., 2013) after vasostimulation.
5. Conclusion
During orthostatic stress in healthy volunteers there is a modest decrease in CBF and a similar decrease of blood flow in carotid and vertebral arteries, which is in contrast to a previous study. Moreover, CBF changes are modulated by PetCO2 changes, which further validates this technique. These data may form the basis for patient studies and interventions.
Acknowledgements
We like to thank all healthy volunteers for participating in this study.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declarations of interest
None of the authors have potential conflicts of interest to be disclosed.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cnp.2018.02.004.
Appendix A. Supplementary data
References
- Ainslie P.N., Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Albayrak R., Degirmenci B., Acar M., Haktanir A., Colbay M., Yaman M. Doppler sonography evaluation of flow velocity and volume of the extracranial internal carotid and vertebral arteries in healthy adults. J. Clin. Ultrasound. 2007;35:27–33. doi: 10.1002/jcu.20301. [DOI] [PubMed] [Google Scholar]
- Alperin N., Lee S.H., Sivaramakrishnan A., Hushek S.G. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J. Magn. Reson. Imaging. 2005;22:591–596. doi: 10.1002/jmri.20427. [DOI] [PubMed] [Google Scholar]
- Asahina M., Sato J., Tachibana M., Hattori T. Cerebral blood flow and oxygenation during head-up tilt in patients with multiple system atrophy and healthy control subjects. Parkinsonism Relat. Disord. 2006;12:472–477. doi: 10.1016/j.parkreldis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Bronzwaer A.S., Stok W.J., Westerhof B.E., van Lieshout J.J. Arterial pressure variations as parameters of brain perfusion in response to central blood volume depletion and repletion. Front. Physiol. 2014;5:157. doi: 10.3389/fphys.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B.J., Panerai R.B., Potter J.F. Effect of aging on dynamic cerebral autoregulation during head-up tilt. Stroke. 2003;34:1871–1875. doi: 10.1161/01.STR.0000081981.99908.F3. [DOI] [PubMed] [Google Scholar]
- Castro P., Freitas J., Santos R., Panerai R., Azevedo E. Indexes of cerebral autoregulation do not reflect impairment in syncope: insights from head-up tilt test of vasovagal and autonomic failure subjects. Eur. J. Appl. Physiol. 2017;117:1817–1831. doi: 10.1007/s00421-017-3674-1. [DOI] [PubMed] [Google Scholar]
- Deane C.R., Markus H.S. Colour velocity flow measurement: in vitro validation and application to human carotid arteries. Ultrasound Med. Biol. 1997;23:447–452. doi: 10.1016/s0301-5629(96)00224-4. [DOI] [PubMed] [Google Scholar]
- Dorfler P., Puls I., Schliesser M., Maurer M., Becker G. Measurement of cerebral blood flow volume by extracranial sonography. J. Cereb. Blood Flow Metab. 2000;20:269–271. doi: 10.1097/00004647-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Fierstra J., Sobczyk O., Battisti-Charbonney A., Mandell D.M., Poublanc J., Crawley A.P. Measuring cerebrovascular reactivity: what stimulus to use? J. Physiol. 2013;591:5809–5821. doi: 10.1113/jphysiol.2013.259150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R., Wieling W., Axelrod F.B., Benditt D.G., Benarroch E., Biaggioni I. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Gonul M., Asil T., Balci K., Celik Y., Turgut N., Uzunca I. Changing cerebral blood flow velocity detected by transcranial Doppler ultrasound during head up tilt in patients with multiple sclerosis. Eur. J. Neurol. 2008;15:725–729. doi: 10.1111/j.1468-1331.2008.02179.x. [DOI] [PubMed] [Google Scholar]
- Jansen van Vuuren A., Saling M.M., Ameen O., Naidoo N., Solms M. Hand preference is selectively related to common and internal carotid arterial asymmetry. Laterality. 2017;22:377–398. doi: 10.1080/1357650X.2016.1205596. [DOI] [PubMed] [Google Scholar]
- Krejza J., Arkuszewski M., Kasner S.E., Weigele J., Ustymowicz A., Hurst R.W. Carotid artery diameter in men and women and the relation to body and neck size. Stroke. 2006;37:1103–1105. doi: 10.1161/01.STR.0000206440.48756.f7. [DOI] [PubMed] [Google Scholar]
- Krejza J., Rudzinski W., Arkuszewski M., Onuoha O., Melhem E.R. Cerebrovascular reactivity across the menstrual cycle in young healthy women. Neuroradiol. J. 2013;26:413–419. doi: 10.1177/197140091302600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhu Y.S., Hill C., Armstrong K., Tarumi T., Hodics T. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension. 2013;62:973–979. doi: 10.1161/HYPERTENSIONAHA.113.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezei Z., Olah L., Kardos L., Kovacs R.K., Csiba L., Csepany T. Cerebrovascular hemodynamic changes in multiple sclerosis patients during head-up tilt table test: effect of high-dose intravenous steroid treatment. J. Neurol. 2013;260:2335–2342. doi: 10.1007/s00415-013-6977-0. [DOI] [PubMed] [Google Scholar]
- Murrell C.J., Cotter J.D., George K., Shave R., Wilson L., Thomas K. Cardiorespiratory and cerebrovascular responses to head-up tilt II: influence of age, training status and acute exercise. Exp. Gerontol. 2011;46:1–8. doi: 10.1016/j.exger.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Nemati M., Bavil A.S., Taheri N. Comparison of normal values of Duplex indices of vertebral arteries in young and elderly adults. Cardiovasc. Ultrasound. 2009;7:2. doi: 10.1186/1476-7120-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo O., Soustiel J.F., Thaler I. Maternal cerebral blood flow during normal pregnancy: a cross-sectional study. Am. J. Obstet. Gynecol. 2010;203(475):e1–e6. doi: 10.1016/j.ajog.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Oblak J.P., Zaletel M., Zvan B., Kiauta T., Pogacnik T. The effect of age on cerebrovascular reactivity to cold pressor test and head-up tilt. Acta Neurol. Scand. 2002;106:30–33. doi: 10.1034/j.1600-0404.2002.01186.x. [DOI] [PubMed] [Google Scholar]
- Ocon A.J., Medow M.S., Taneja I., Clarke D., Stewart J.M. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H664–H673. doi: 10.1152/ajpheart.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktar S.O., Yucel C., Karaosmanoglu D., Akkan K., Ozdemir H., Tokgoz N. Blood-flow volume quantification in internal carotid and vertebral arteries: comparison of 3 different ultrasound techniques with phase-contrast MR imaging. AJNR Am. J. Neuroradiol. 2006;27:363–369. [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y., Okada H., Yoshikawa E., Futatsubashi M., Nobezawa S. Absolute changes in regional cerebral blood flow in association with upright posture in humans: an orthostatic PET study. J. Nucl. Med. 2001;42:707–712. [PubMed] [Google Scholar]
- Pase M.P., Grima N.A., Stough C., Scholey A., Pipingas A. Association of pulsatile and mean cerebral blood flow velocity with age and neuropsychological performance. Physiol. Behav. 2014;130:23–27. doi: 10.1016/j.physbeh.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Razumovsky A.Y., DeBusk K., Calkins H., Snader S., Lucas K.E., Vyas P. Cerebral and systemic hemodynamics changes during upright tilt in chronic fatigue syndrome. J. Neuroimaging. 2003;13:57–67. [PubMed] [Google Scholar]
- Reivich M. Arterial Pco2 and Cerebral Hemodynamics. Am. J. Physiol. 1964;206:25–35. doi: 10.1152/ajplegacy.1964.206.1.25. [DOI] [PubMed] [Google Scholar]
- Riberholt C.G., Thorlund J.B., Mehlsen J., Nordenbo A.M. Patients with severe acquired brain injury show increased arousal in tilt-table training. Dan. Med. J. 2013;60:A4739. [PubMed] [Google Scholar]
- Sato K., Fisher J.P., Seifert T., Overgaard M., Secher N.H., Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp. Physiol. 2012;97:1272–1280. doi: 10.1113/expphysiol.2012.064774. [DOI] [PubMed] [Google Scholar]
- Sato K., Ogoh S., Hirasawa A., Oue A., Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J. Physiol. 2011;589:2847–2856. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel P., Ruge C., Schoning M. Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: reference data and the effects of age. Ultrasound Med. Biol. 2000;26:1261–1266. doi: 10.1016/s0301-5629(00)00293-3. [DOI] [PubMed] [Google Scholar]
- Schondorf R., Stein R., Roberts R., Benoit J., Cupples W. Dynamic cerebral autoregulation is preserved in neurally mediated syncope. J. Appl. Physiol. 2001;2001(91):2493–2502. doi: 10.1152/jappl.2001.91.6.2493. [DOI] [PubMed] [Google Scholar]
- Schoning M., Walter J., Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke. 1994;25:17–22. doi: 10.1161/01.str.25.1.17. [DOI] [PubMed] [Google Scholar]
- Seidel E., Eicke B.M., Tettenborn B., Krummenauer F. Reference values for vertebral artery flow volume by duplex sonography in young and elderly adults. Stroke. 1999;30:2692–2696. doi: 10.1161/01.str.30.12.2692. [DOI] [PubMed] [Google Scholar]
- Task Force for the diagnosis, and management of syncope, European Society of Cardiology, European Heart Rhythm Association, Heart Failure Association, Heart Rhythm Society Guidelines for the diagnosis and management of syncope (version 2009) Eur. Heart. J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeler C.H., Crutchfield K., Katsnelson M., Kim J., Tang R., Passmore Griffin L. Transcranial Doppler velocities in a large, healthy population. J. Neuroimaging. 2013;23:466–472. doi: 10.1111/j.1552-6569.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- Thomas K.N., Lewis N.C., Hill B.G., Ainslie P.N. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R707–R720. doi: 10.1152/ajpregu.00211.2015. [DOI] [PubMed] [Google Scholar]
- Tymko M.M., Rickards C.A., Skow R.J., Ingram-Cotton N.C., Howatt M.K., Day T.A. The effects of superimposed tilt and lower body negative pressure on anterior and posterior cerebral circulations. Physiol. Rep. 2016;4:e12957. doi: 10.14814/phy2.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viski S., Orosz M., Czuriga-Kovacs K.R., Magyar M.T., Csiba L., Olah L. The acute effects of alcohol on cerebral hemodynamic changes induced by the head-up tilt test in healthy subjects. J. Neurol. Sci. 2016;368:113–120. doi: 10.1016/j.jns.2016.06.060. [DOI] [PubMed] [Google Scholar]
- Wang Y.J., Chao A.C., Chung C.P., Huang Y.J., Hu H.H. Different cerebral hemodynamic responses between sexes and various vessels in orthostatic stress tests. J. Ultrasound Med. 2010;29:1299–1304. doi: 10.7863/jum.2010.29.9.1299. [DOI] [PubMed] [Google Scholar]
- Wilson T.E., Cui J., Zhang R., Witkowski S., Crandall C.G. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J. Appl. Physiol. 2002;2002(93):85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]
- Yang C., Gao Y., Greaves D.K., Villar R., Beltrame T., Fraser K.S. Prior head-down tilt does not impair the cerebrovascular response to head-up tilt. J. Appl. Physiol. 2015;2015(118):1356–1363. doi: 10.1152/japplphysiol.00871.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici B., Erdogmus B., Tugay A. Cerebral blood flow measurements of the extracranial carotid and vertebral arteries with Doppler ultrasonography in healthy adults. Diagn. Interv. Radiol. 2005;11:195–198. [PubMed] [Google Scholar]
- Yoshida H., Hamner J.W., Ishibashi K., Tan C.O. Relative contributions of systemic hemodynamic variables to cerebral autoregulation during orthostatic stress. J. Appl. Physiol. 2018;2018(124):321–329. doi: 10.1152/japplphysiol.00700.2017. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S., Ueno T., Mayanagi Y., Sekiguchi C., Yumikura S., Miyamoto A. Effect of head up tilt on cerebral circulation. Acta Astronaut. 1994;33:69–76. doi: 10.1016/0094-5765(94)90110-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.