Highlights
-
•
The active recording electrode site influences the CMAP waveform of the trapezius muscle (TM).
-
•
CMAP becomes high by placement of the active recording electrode 2 cm behind the belly of the TM.
-
•
Volume conduction from the supraspinatus muscle affects the CMAP waveform of the TM.
Abbreviations: ALS, amyotrophic lateral sclerosis; CMAP, compound muscle action potential; CoV, coefficient of variation; CSA, cervical spondylotic amyotrophy; NCS, nerve conduction study; SBMA, spinal–bulbar muscular atrophy; SD, standard deviation; TM, trapezius muscle
Keywords: Active recording electrode, Compound muscle action potential, Nerve conduction study, Supraspinatus muscle, Trapezius muscle, Volume conduction
Abstract
Objective
We investigated how the active electrode placement site influences compound muscle action potential (CMAP) configuration of the upper trapezius muscle (TM).
Methods
A nerve conduction study of the accessory nerve was performed, and the CMAPs obtained with two different placement sites, i.e., placement of the active recording electrode on the belly of the upper TM (CMAP-A) and placement of the electrode 2 cm behind the belly (CMAP-B), were compared. CMAPs were also obtained with the active recording electrode placed in the supraspinous fossa (CMAP-C).
Results
All CMAPs were recorded from 21 healthy volunteers. The mean peak-to-peak amplitude of CMAP-B was 3.4 mV higher than that of CMAP-A (11.0 ± 4.0 mV vs. 14.4 ± 4.9 mV; P < 0.01). The mean peak-to-peak amplitude of CMAP-C was 10.3 ± 5.0 mV.
Conclusions
CMAP of the upper TM was always higher when the active recording electrode was placed 2 cm behind the belly of the muscle.
Significance
When stimulating the accessory nerve, a current spread occurs to the C5 spinal nerve root and another CMAP originating from the supraspinatus muscle occurs in the supraspinous fossa. The volume conduction from the supraspinatus muscle affects the active recording electrode on the TM, resulting in an increase in CMAP amplitude.
1. Introduction
Compound muscle action potential (CMAP) of the upper trapezius muscle (TM) is routinely used in a nerve conduction study (NCS) and a repetitive nerve stimulation test. A repetitive nerve stimulation test on the upper TM is frequently used for diagnosis of neuromuscular transmission disorders, especially myasthenia gravis (Costa et al., 2004, Misra et al., 2006, Amandusson et al., 2017, Bou Ali et al., 2017), and for diagnosis of motor neuron diseases including amyotrophic lateral sclerosis (ALS) (Killian et al., 1994, Iwanami et al., 2011, Hatanaka et al., 2017, Zheng et al., 2017) and spinal–bulbar muscular atrophy (SBMA) (Inoue et al., 2009, Kim et al., 2013). Although a repetitive nerve stimulation test can show decremental responses in stimulation at a low frequency of 3 or 5 Hz, it has not yet been considered as a standard test for diagnosis of ALS or SBMA unlike myasthenia gravis.
For upper TM recording, the active recording electrode is placed on the belly of the muscle and the reference electrode is placed on the acromion (Ogawa et al., 2013, Seror et al., 2017). In our experience, when the active recording electrode is placed several centimeters behind the belly of the muscle, the CMAP has high amplitude and a steep rise (short rise time) (Fig. 1). Because the CMAP configuration varies depending on the position of the active recording electrode, if the position of the active recording electrode is not exactly constant, electrophysiological parameters may be different even for the same examiner or on the same day. The origin of the difference in CMAP configuration has not been established. The aims of this study were to determine how the placement site of the active recording electrode influences the CMAP configuration of the upper TM and to clarify the origin of the difference in CMAP configuration. Although there are various possible explanations, we hypothesized that the volume conduction from the supraspinatus muscle accounts for the difference in the CMAP configuration.
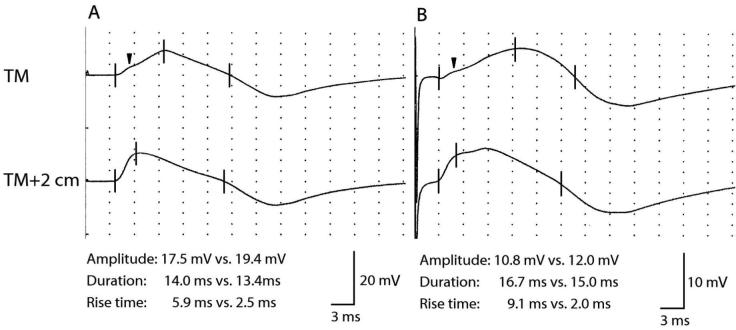
Fig. 1.
CMAP waveforms that varied depending on the position of the active recording electrode. (A) CMAPs obtained in a 24-year-old man. (B) CMAPs obtained in a 48-year-old man. The upper CMAPs (TM) were obtained by placing the active recording electrode on the belly of the upper TM, and the lower CMAPs (TM + 2 cm) were obtained by placing the active recording electrode 2 cm behind the belly of the upper TM. The three vertical lines indicate the onset latency, the latency of the first major deflection, and the latency of the return to baseline, respectively. “Amplitude” represents peak-to-peak amplitude (mV). “Duration” represents negative duration (ms). “The black arrow” in the upper CMAPs (TM) indicates a slight deflection originating from the supraspinatus muscle.
2. Methods
2.1. Subjects
The study subjects were 21 healthy volunteers. Individuals with a previous history of cervical spondylosis, myelopathy, plexopathy, peripheral neuropathy, or diabetes mellitus were excluded. All subjects signed an informed consent form before evaluation. The Ethics Committee of Kawasaki Medical School and Hospital approved this study.
2.2. CMAP response recorded from the upper TM: two different procedures
All NCSs were performed with an electromyography machine (Neuropack MEB-2216; Nihon Kohden, Tokyo, Japan). The bandpass filter was set at 10 Hz-3 kHz. Subjects lay in a supine position with a relaxed posture. Skin temperature was measured at the shoulder surface and was maintained at ≥32 °C. The skin of the shoulder was cleaned with alcohol to decrease impedance.
The accessory NCS was performed on one side or both sides. The spinal accessory nerve was stimulated with a bar electrode at the posterior border of the sternomastoid muscle between the mastoid and the clavicle (Ogawa et al., 2013, Seror et al., 2017). Stimuli were rectangular electrical pulses of 0.2 ms in duration. Before a maneuver, an appropriate stimulation point with the lowest threshold was determined. Supramaximal stimulation was assured by increasing the stimulus intensity by 30–40% beyond the intensity at which a CMAP increased continuously up to the maximum. Ag–AgCl cup electrodes were used for recording CMAPs. CMAPs of the upper TM were simultaneously recorded at two different placement sites of the active recording electrode: placement of the active recording electrode on the belly of the upper TM (CMAP-A) and placement of the electrode 2 cm behind the belly (CMAP-B) (Fig. 2). In both cases, the reference electrode was shared and placed on the acromion. A ground electrode was placed over the shoulder between the stimulating and recording electrodes.
Fig. 2.
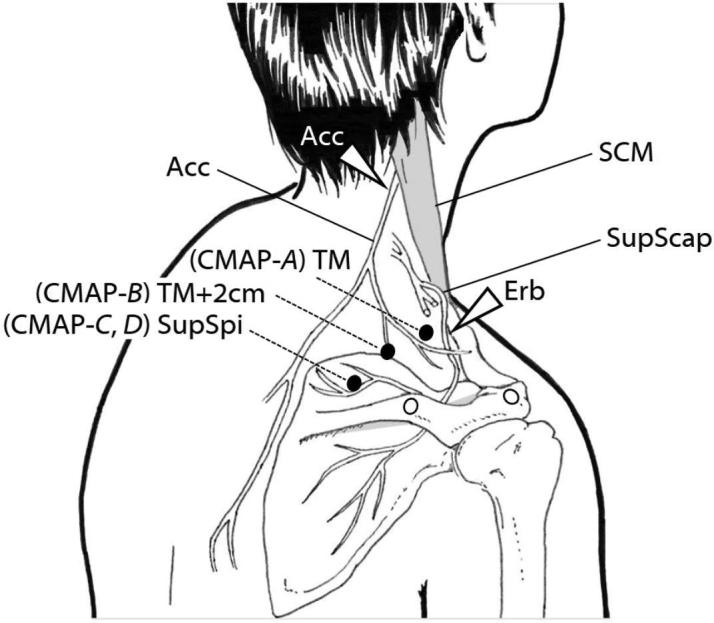
Technique for NCS recording of CMAPs of the upper TM and supraspinatus muscle. ACC, accessory nerve; Erb, Erb’s point in the supraclavicular fossa: SCM, sternomastoid muscle; SupScap, suprascapular nerve; SupSpi, supraspinatus muscle; TM, trapezius muscle. “The white arrow” indicates the stimulation point. “The black circle” indicates the active recording electrode, and “the white circle” indicates the reference electrode.
Conduction parameters including onset latency, peak-to-peak amplitude, baseline-to-peak amplitude, duration of CMAPs, and rise time were measured. The rise time was indicated by steepness of the slope of CMAPs and was defined as the interval between the onset latency and the latency of the first major deflection (Fig. 1). The latency of the first major deflection was not always the same as the peak latency and often preceded the peak latency. The conduction parameters in the two different procedures were compared (CMAP-A vs. B).
2.3. CMAP response recorded from the supraspinatus muscle: two different procedures
As mentioned in the Introduction, we hypothesized that the origin of the difference in CMAP configuration regarding the high amplitude and steep rise in CMAP is the volume conduction from the supraspinatus muscle. We therefore attempted to record CMAP (which we termed CMAP-C) of the supraspinatus muscle in the supraspinous fossa simultaneously with stimulation of the spinal accessory nerve and recording CMAP-A and B. For supraspinatus recording, the active recording electrode was placed on the belly of the muscle and the reference electrode was placed 3 cm lateral to it (Fig. 2). To confirm that CMAP-C mainly originated from the supraspinatus muscle and did not originate from the upper TM, the waveform was compared with CMAP (which we termed CMAP-D) obtained by routine suprascapular NCS. In the suprascapular NCS, the suprascapular nerve was stimulated in the supraclavicular fossa (Erb’s point) and the recording electrodes were placed in the supraspinous fossa as in the case of CMAP-C. Conduction parameters including peak-to-peak amplitude, baseline-to-peak amplitude, duration of CMAPs, and rise time in two different procedures were compared (CMAP-C vs. D).
2.4. Combination of two CMAP responses recorded from different sites
We speculated that combining a CMAP that originated from the supraspinatus muscle with a CMAP that originated from the true upper TM might produce a high and steep waveform such as CMAP-B. CMAP-C and CMAP-A were therefore combined using a wave editing function, and the waveform (which we termed CMAP-E) was superimposed over CMAP-B for comparison. Conduction parameters including onset latency, peak-to-peak amplitude, baseline-to-peak amplitude, duration of CMAPs, and rise time were compared for CMAP-B and -E.
2.5. Statistical analysis
Statistical analysis using the Wilcoxon signed-rank test was carried out to compare values obtained by the different methods (CMAP-A vs. B, CMAP-C vs. D, and CMAP-B vs. E). For descriptive statistics, mean, standard deviation (SD), and coefficient of variation (CoV) were calculated. P-values <0.05 were considered statistically significant.
3. Results
3.1. Results for CMAP of the upper TM: comparison of two procedures
All CMAPs of the upper TM were recorded from the 21 healthy volunteers (17 men and 4 women) with a mean age of 33.5 ± 12.0 (range, 20–57) years. Fourteen subjects were examined on both sides, and 7 subjects were examined on one side; a total of 35 CMAPs were recorded. Maximal stimulation was usually achieved at about 6–10 mA. To ensure supramaximal stimulation, the stimulation intensity was increased usually by less than 14 mA. In CMAP-A, the peak-to-peak amplitude was 5.0–20.1 mV and the baseline-to-peak amplitude was 2.8–11.2 mV. In CMAP-B, the peak-to-peak amplitude was 7.2–25.2 mV and the baseline-to-peak amplitude was 3.4–13.1 mV. The peak-to-peak and baseline-to-peak amplitudes of CMAP-B were always higher than those of CMAP-A (both P < 0.01). The rise times of CMAP-B were always shorter than those of CMAP-A (P < 0.01), but there was little difference (<2.0 ms) in 8 cases (22.9%). The NCS results for the conduction parameters are summarized in Table 1.
Table 1.
Values of CMAP recorded from the upper TM: two different procedures.
| n = 35 | (CMAP-A) TM |
(CMAP-B) TM + 2 cm |
P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | CoV | Mean | SD | CoV | ||
| onset latency (ms) | 2.6 | 0.4 | 0.15 | 2.6 | 0.4 | 0.15 | 0.73 |
| peak-to-peak amplitude (mV) | 11.0 | 4.0 | 0.36 | 14.4 | 4.9 | 0.34 | <0.01 |
| baseline-to-peak amplitude (mV) | 6.2 | 2.4 | 0.39 | 8.2 | 2.8 | 0.34 | <0.01 |
| duration (ms) | 14.5 | 1.6 | 0.11 | 13.1 | 1.3 | 0.1 | <0.01 |
| rise time (ms) | 6.3 | 2.8 | 0.44 | 2.1 | 0.4 | 0.19 | <0.01 |
CMAP, compound muscle action potential; CoV, coefficient of variation; SD, standard deviation; TM, trapezius muscle.
3.2. Results for CMAP of the supraspinatus muscle: comparison of two procedures
All CMAPs of the supraspinatus muscle were also recorded from the 21 healthy volunteers (total of 35 CMAPs). In CMAP-C, the peak-to-peak amplitude was 4.7–20.6 mV and the baseline-to-peak amplitude was 2.7–12.5 mV. In CMAP-D, the peak-to-peak amplitude was 4.6–20.8 mV and the baseline-to-peak amplitude was 2.3–12.3 mV. There was no statistically significant difference in peak-to-peak amplitude, baseline-to-peak amplitude, duration of CMAPs, or rise time when the two procedures were compared. The NCS results for the conduction parameters are summarized in Table 2. The waveforms of CMAP-C and -D were identical, and both waveforms were the same response from the same muscle. Thus, CMAP-C was regarded as the response mainly originating from the supraspinatus muscle.
Table 2.
Values of CMAP recorded from the supraspinatus muscle: two different procedures.
| n = 35 | (CMAP-C) SupSpi (Acc) |
(CMAP-D) SupSpi (Erb) |
P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | CoV | Mean | SD | CoV | ||
| onset latency (ms) | 2.8 | 0.4 | 0.14 | 1.9 | 0.2 | 0.11 | <0.01 |
| peak-to-peak amplitude (mV) | 10.3 | 5.0 | 0.49 | 10.3 | 4.8 | 0.47 | 0.99 |
| baseline-to-peak amplitude (mV) | 5.5 | 2.6 | 0.47 | 5.7 | 2.6 | 0.46 | 0.64 |
| duration (ms) | 11.3 | 1.4 | 0.12 | 11.1 | 1.6 | 0.14 | 0.42 |
| rise time (ms) | 2.0 | 0.7 | 0.35 | 2.0 | 0.7 | 0.35 | 0.99 |
Acc, accessory nerve; CMAP, compound muscle action potential; CoV, coefficient of variation; Erb, Erb’s point in the supraclavicular fossa; SD, standard deviation; SupSpi, supraspinatus muscle.
3.3. Results for the combination of two CMAPs recorded from different sites: comparison with CMAP-B
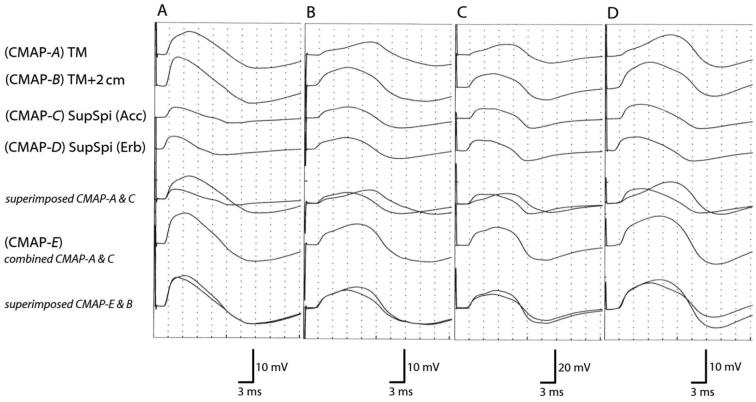
In CMAP-E, the peak-to-peak amplitude was 9.9–32.1 mV and the baseline-to-peak amplitude was 5.2–16.9 mV. The peak-to-peak and baseline-to-peak amplitudes of CMAP-E were always higher than those of CMAP-B (P < 0.01 and P = 0.04, respectively). However, there was no statistically significant difference in rise time or duration of CMAPs when CMAP-E and CMAP-B were compared. The NCS results for the conduction parameters are summarized in Table 3. Four typical cases are shown in Fig. 3. When CMAP-E was superimposed over CMAP-B, the waveforms of CMAP-E were almost consistent with those of CMAP-B except for amplitude. CMAP-E and -B were considered common responses. As the attenuation of electric potential during volume conduction was not considered, the combination of two CMAPs from separate sites caused quite larger amplitude.
Table 3.
Values of a combination of two CMAPs: comparison with CMAP recorded from the upper TM.
| n = 35 | (CMAP-E) Combination |
(CMAP-B) TM + 2 cm |
P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | CoV | Mean | SD | CoV | ||
| onset latency (ms) | 2.7 | 0.5 | 0.19 | 2.6 | 0.4 | 0.15 | 0.36 |
| peak-to-peak amplitude (mV) | 18.5 | 6.7 | 0.36 | 14.4 | 4.9 | 0.34 | <0.01 |
| baseline-to-peak amplitude (mV) | 10.0 | 3.7 | 0.37 | 8.2 | 2.8 | 0.34 | 0.04 |
| duration (ms) | 13.0 | 1.3 | 0.1 | 13.1 | 1.3 | 0.1 | 0.87 |
| rise time (ms) | 2.0 | 0.4 | 0.2 | 2.1 | 0.4 | 0.19 | 0.36 |
CMAP, compound muscle action potential; CoV, coefficient of variation; SD, standard deviation; TM, trapezius muscle.
Fig. 3.
Four typical cases of CMAPs of the upper TM and supraspinatus muscle. (A) CMAPs obtained in a 28-year-old man. (B) CMAPs obtained in a 33-year-old man. (C) CMAPs obtained in a 46-year-old man. (D) CMAPs obtained in a 53-year-old woman. (CMAP-A) TM: CMAPs were obtained by placing the active recording electrode on the belly of the upper TM. (CMAP-B) TM + 2 cm: CMAPs were obtained by placing the active recording electrode 2 cm behind the belly of the upper TM. (CMAP-C) SupSpi (Acc): CMAPs were obtained by placing the active recording electrode on the belly of the supraspinatus muscle with stimulation of the spinal accessory nerve. (CMAP-D) SupSpi (Erb): CMAPs were obtained by placing the active recording electrode on the belly of the supraspinatus muscle with stimulation of Erb's point in the supraclavicular fossa. (CMAP-E) combined CMAP-A and C.
4. Discussion
This study demonstrated that the placement site of the active recording electrode considerably influences the CMAP configuration of the upper TM. The results obtained in the upper TM of healthy subjects showed that the mean peak-to-peak amplitude was 3.4 mV higher and mean baseline-to-peak amplitude was 2.0 mV higher when the active electrode was placed 2 cm behind the belly of the muscle. In addition, at that placement site, mean rise time was 4.2 ms shorter and the slope of CMAP was steeper.
The differences in the CMAP configuration would most likely be due to the volume conduction from the supraspinatus muscle. When stimulating the spinal accessory nerve, a current spread occurs to the C5 spinal nerve root and CMAP of the supraspinatus muscle would occur in the supraspinous fossa. In this study, the current spread occurred even with low stimulation intensity at about 6–7 mA. Because maximal stimulation was usually achieved by higher stimulation intensity than by lower stimulation intensity, it was considered that the current spread could not be excluded in almost all cases. The volume conduction from the supraspinatus muscle affects the active recording electrode on the upper TM, thus resulting in the differences in CMAP configuration. When the amplitude scale was adjusted to a larger calibration, even when the active recording electrode was placed on the belly of the muscle, a slight deflection originating from the supraspinatus muscle was sometimes observed in the slope of the waveform (Fig. 1). When the active recording electrode was placed 2 cm behind the belly of the muscle, as the distance from the supraspinatus muscle was closer, this deflection became conspicuous, and the amplitude became higher and the slope of the waveform became steeper.
In proximal cervical spondylotic amyotrophy (CSA), the C5 and C6 anterior roots are predominantly impaired (Imajo et al., 2012), but, in our experience, proximal CSA may cause slightly low CMAP amplitude in the upper TM even though the muscle may well be spared. Because the TM is innervated by the spinal accessory nerve that arises from the C1 to C4 spinal nerve (Oh, 2003), it cannot be explained by atrophy of the upper TM. Considering the results of this study, it may be explained by atrophy of the supraspinatus muscle. This finding is most useful when taken in conjunction with needle electromyography, and it may become an opportunity for diagnosis of a C5 anterior root lesion.
Ogawa et al. (2013) introduced a new maneuver for repetitive nerve stimulation testing in the upper TM. To reduce pseudofacilitation, the shoulder of a supine subject was elevated passively and was held firmly by the examiner. Although we routinely use this maneuver, the amplitude of CMAP sometimes varied depending on the position of the shoulder. This may be because the active recording electrode moves forward with the skin when the shoulder is elevated. As the distance from the supraspinatus muscle is separated, the amplitude of CMAP tends to be lower.
In this study, we found a previously unreported pitfall when recording CMAP of the upper TM. From the point of view of reproducibility, it is important to place the active recording electrode at the same position because the amplitude of CMAP greatly varies depending on the position of the active recording electrode. For accurate evaluation of the course of a disease, it is necessary to record the precise position of the active recording electrode in the chart at the first examination and to place the active recording electrode at the same position at the time of re-examination. Movement of the position of the active recording electrode not only in the vertical direction but also in the horizontal direction will affect the shape of CMAP of the upper TM, and it is therefore important to record the position from the acromion.
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
None of the authors have potential conflicts of interest or financial interest to be disclosed.
References
- Amandusson Å., Elf K., Grindlund M.E., Punga A.R. Diagnostic utility of repetitive nerve stimulation in a large cohort of patients with myasthenia gravis. J. Clin. Neurophysiol. 2017;34:400–407. doi: 10.1097/WNP.0000000000000398. [DOI] [PubMed] [Google Scholar]
- Bou Ali H., Salort-Campana E., Grapperon A.M., Gallard J., Franques J., Sevy A., Delmont E., Verschueren A., Pouget J., Attarian S. New strategy for improving the diagnostic sensitivity of repetitive nerve stimulation in myasthenia gravis. Muscle Nerve. 2017;55:532–538. doi: 10.1002/mus.25374. [DOI] [PubMed] [Google Scholar]
- Costa J., Evangelista T., Conceição I., de Carvalho M. Repetitive nerve stimulation in myasthenia gravis–relative sensitivity of different muscles. Clin. Neurophysiol. 2004;115:2776–2782. doi: 10.1016/j.clinph.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Hatanaka Y., Higashihara M., Chiba T., Miyaji Y., Kawamura Y., Sonoo M. Utility of repetitive nerve stimulation test for ALS diagnosis. Clin. Neurophysiol. 2017;128:823–829. doi: 10.1016/j.clinph.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Imajo Y., Kato Y., Kanchiku T., Suzuki H., Yoshida Y., Funaba M., Taguchi T. Prediction of surgical outcome for proximal-type cervical spondylotic amyotrophy novel mode of assessment using compound action potentials of deltoid and biceps brachii and central motor conduction time. Spine. 2012;37:1444–1449. doi: 10.1097/BRS.0b013e31826e2ead. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hemmi S., Miyaishi M., Kutoku Y., Murakami T., Kurokawa K., Sunada Y. Muscular fatigue and decremental response to repetitive nerve stimulation in X-linked spinobulbar muscular atrophy. Eur. J. Neurol. 2009;16:76–80. doi: 10.1111/j.1468-1331.2008.02349.x. [DOI] [PubMed] [Google Scholar]
- Iwanami T., Sonoo M., Hatanaka Y., Hokkoku K., Oishi C., Shimizu T. Decremental responses to repetitive nerve stimulation (RNS) in motor neuron disease. Clin. Neurophysiol. 2011;122:2530–2536. doi: 10.1016/j.clinph.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Killian J.M., Wilfong A.A., Burnett L., Appel S.H., Boland D. Decremental motor responses to repetitive nerve stimulation in ALS. Muscle Nerve. 1994;17:747–754. doi: 10.1002/mus.880170708. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Park K.D., Kim S.M., Sunwoo I.N. Decremental responses to repetitive nerve stimulation in x-linked bulbospinal muscular atrophy. J. Clin. Neurol. 2013;9:32–35. doi: 10.3988/jcn.2013.9.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra U.K., Kalita J., Srivastava A. A study of diagnostic yield, technical ease and patient discomfort of low rate repetitive nerve stimulation test in patients with myasthenia gravis. Electromyogr. Clin. Neurophysiol. 2006;46:337–341. [PubMed] [Google Scholar]
- Ogawa G., Sonoo M., Hatanaka Y., Kaida K., Kamakura K. A new maneuver for repetitive nerve stimulation testing in the trapezius muscle. Muscle Nerve. 2013;47:668–672. doi: 10.1002/mus.23664. [DOI] [PubMed] [Google Scholar]
- Oh S.J. third ed. Williams & Wilkins; Baltimore: 2003. Clinical Electromyography. Nerve Conduction Studies; p. 611. [Google Scholar]
- Seror P., Stojkovic T., Lefevre-Colau M.M., Lenglet T. Diagnosis of unilateral trapezius muscle palsy: 54 Cases. Muscle Nerve. 2017;56:215–223. doi: 10.1002/mus.25481. [DOI] [PubMed] [Google Scholar]
- Zheng C., Jin X., Zhu Y., Lu F., Jiang J., Xia X. Repetitive nerve stimulation as a diagnostic aid for distinguishing cervical spondylotic amyotrophy from amyotrophic lateral sclerosis. Eur. Spine J. 2017;26:1929–1936. doi: 10.1007/s00586-017-5060-4. [DOI] [PubMed] [Google Scholar]