Abstract
Fetal growth may be impaired by poor placental function or maternal conditions, each of which can influence the transfer of nutrients and oxygen from the mother to the developing fetus. Large-scale studies of metabolites (metabolomics) are key to understand cellular metabolism and pathophysiology of human conditions. Herein, maternal and cord blood plasma samples were used for NMR-based metabolic fingerprinting and profiling, including analysis of the enrichment of circulating lipid classes and subclasses, as well as the number of sub-fraction particles and their size. Changes in phosphatidylcholines and glycoproteins were prominent in growth-restricted fetuses indicating significant alterations in their abundance and biophysical properties. Lipoprotein profiles showed significantly lower plasma concentrations of cholesterol-intermediate density lipoprotein (IDL), triglycerides-IDL and high-density lipoprotein (HDL) in mothers of growth-restricted fetuses compared to controls (p < 0.05). In contrast, growth-restricted fetuses had significantly higher plasma concentrations of cholesterol and triglycerides transporting lipoproteins [LDL, IDL, and VLDL, (p < 0.005; all)], as well as increased VLDL particle types (large, medium and small). Significant changes in plasma concentrations of formate, histidine, isoleucine and citrate in growth-restricted fetuses were also observed. Comprehensive metabolic profiling reveals that both, mother and fetuses of pregnancies complicated with fetal growth restriction have a substantial disruption in lipid metabolism.
Introduction
Fetal growth restriction (FGR) affects 6–10% of all pregnancies and is defined as the failure to achieve the genetic growth potential, resulting in a given low birthweight1. Growth restricted fetuses have a 5 to 10-fold risk of dying in utero, and higher risk of perinatal morbidity and mortality2,3. In addition, fetuses with growth restriction show metabolic and cardiovascular adaptations that are thought to persist postnatally, with implications for adult disease and repercussions for preventive strategies. A small fraction of the small fetuses diagnosed in utero present as early-onset/severe fetal growth restriction4. However, the majority of clinical instances of fetal smallness occur late in gestation under two main phenotypes, conventionally defined as small for gestational age (SGA) and FGR5,6. While the former is usually associated with near-normal perinatal outcomes and are considered “constitutionally small fetuses”, FGR is characterized by placental maldevelopment and abnormal fetoplacental blood flow7, a higher risk of fetal death3,8–10 and poor perinatal outcomes11,12. It is unclear whether SGA and FGR are different conditions or represent different degrees of severity of the same disease. For instance, despite the clear differences in obstetrical risks, both SGA and FGR show similar features of long-term fetal adaptation to intrauterine undernutrition, including signs of cardiac remodeling13–17, differences in brain cortical development18–20 and microstructure21–23, as well as a higher prevalence of neurodevelopmental delay24–27.
The metabolome of biological fluids represents a sensitive and multifactorial phenotypic signature of disease, providing insights of the interface between the final downstream products of gene expression, the influence of environmental factors and the complex interactions between them28–31. In addition, recent sensitive, rapid, and high-throughput technology advances have provided a solution to measure low concentrated metabolites in human plasma, allowing a comprehensive profiling of metabolic changes in-vivo32–34. Fetal growth may be impaired by poor placental function or maternal conditions, each of which can influence the transfer of nutrients and oxygen from the mother to the developing fetus35. We and others have previously explored the metabolomic profile of fetuses with impaired intrauterine growth36–39. Specifically, a metabolomic analysis of cord blood from early and late-onset FGR revealed significant differences in essentials amino acids as well as an abnormal lipid metabolism in both, early and late-onset FGR; particularly at the expense of unsaturated lipids38. However, the enrichment of circulating lipid classes and subclasses, as well as the number of sub-fraction particles and their size, has not been analyzed in detail. Furthermore, the co-existence of metabolic changes in the maternal compartment has not been explored. A simultaneous and comprehensive characterization of the metabolomic profile of mothers and fetuses with suboptimal fetal growth could provide further insights into the pathophysiological changes underlying these clinical forms of fetal smallness. The objective of this study was to analyze the maternal and cord blood plasma metabolome in pregnancies with two clinical phenotypes of late-onset fetal smallness, SGA and FGR, and to compare them with those from pregnancies with a normal fetal growth.
Results
Clinical characteristics of participants
From October 2014 to March 2016, 80 pregnant women were recruited. Among those, 28 had a normal sonographic estimation of fetal weight that was confirmed at birth [Adequate-for-gestational age fetuses (AGA)] and 52 pregnancies had an antenatal diagnosis of fetal smallness [estimated sonographic fetal weight (EFW) below the 10th centile] that was confirmed at birth (birthweight <10th centile) and were included as cases. According to our clinical protocol, small fetuses were subdivided as follows: those with an EFW <3rd centile (confirmed at birth) and/or abnormal uteroplacental flow defined by an abnormal uterine artery Doppler velocimetry and/or abnormal cerebroplacental ratio (a sonographic Doppler parameter that indicates redistribution of fetal cerebral blood flow) were termed fetal growth restriction (FGR; n = 27), while those with a birthweight between the 3rd and the 9th centile and normal fetoplacental Doppler were considered small-for-gestational age (SGA; n = 25) cases5,40,41.
The maternal sociodemographic and obstetric characteristics are summarized in Table 1. There were no significant differences in maternal age, race, smoking or rate of maternal underweight [pre-gestational body mass index (BMI) <18 kg/m2] or obesity (BMI >30 kg/m2) among the study groups (all p values > 0.05). Furthermore, there were no significant differences between SGA and FGR (p = 0.07) or AGA (p = 0.21) when we analyze pre-gestational BMI as a continuous variable (Table 1).
Table 1.
Clinical characteristics of the study groups.
| Adequate for Gestational Age N = 28 | Small for Gestational Age N = 25 | Fetal Growth Restriction N = 27 | p value | |
|---|---|---|---|---|
| n (%) or median (IQR) | n (%) or median (IQR) | n (%) or median (IQR) | K-wallis | |
| Maternal baseline characteristics | ||||
| Maternal age (years) | 32 (30.5–36.5) | 30 (25–35) | 31.5 (28–34) | 0.16 |
| Maternal race | 0.77 | |||
| White | 23 (82.1) | 19 (79.2) | 24 (92.3) | |
| Latin | 2 (7.1) | 3 (12.5) | 1 (3.8) | |
| Indian/Pakistan | 2 (7.1) | 1 (4.2) | 1 (3.8) | |
| Others | 1 (3.6) | 1 (4.2) | 0 | |
| Pre-gestational maternal BMI (kg/m2) | 0.21 | |||
| < 18.5 | 1 (3.57) | 1 (4.3) | 5 (20) | |
| 18.5–25 | 19 (67.9) | 17 (74) | 16 (64) | |
| 25–30 | 5 (17.9) | 5 (21.7) | 4 (16) | |
| > 30 | 3 (10.7) | 0 | 0 | |
| Smoking | 4 (14.2) | 8 (32) | 5 (18.5) | 0.3 |
| Ultrasound parameters at third trimester evaluation | ||||
| GA at recruitment (weeks) | 32.3 (30–34.8) | 33.8 (31.3–36.4) | 34.4 (32–36) | 0.19 |
| Estimated fetal weight (grams) | 2131 (1477–2615) | 1866 (1406–2134) | 1788 (1567–2040) | 0.24 |
| Estimated fetal weight centile | 50 (39–77) | 5 (3–9) | 3 (1–6) | 0.0001 |
| Mean uterine artery PI (Z-score) | −0.4 (−1.1–0.67) | 0.2 (−0.14–0.78) | 1.05 (−0.86–2.02) | 0.15 |
| Mean uterine artery PI > 95th centile | 1 (4.2) | 0 | 9 (36) | 0.001 |
| Umbilical artery PI (Z-score) | −0.26 (−0.58–0.29) | −0.1 (−0.68–0.4) | 0.44 (−0.1–0.65)* | 0.02 |
| Umbilical artery PI > 95th centile | 0 | 0 | 3 (11.1) | 0.1 |
| Cerebroplacental ratio (Z-score) | −0.16 (−0.7–0.47) | −0.51 (−1.43–0.16) | −1.08 (−1.45–0.11)*¥ | 0.16 |
| Cerebroplacental ratio < p5th centile | 3 (12) | 0 | 5 (18.5) | 0.74 |
| Perinatal outcomes | ||||
| GA at delivery (weeks) | 39.8 (39–40.5) | 39.4 (38.1–40.3) | 37.8 (37.3–39.6)*¥ | 0.002 |
| Birthweight (grams) | 3365 (3060–3575) |
2728¥ (2500–2860)¥ | 2245 (2100–2506)*¥ | 0.0001 |
| Birthweight centile | 42 (35.5–68.5) | 6 (4–9)¥ | 1 (0–2)*¥ | 0.0001 |
| Male gender | 11 (39.3) | 11 (44) | 19 (70.4) | 0.05 |
| Induction of labor | 6 (21.4) | 13 (52) | 18 (66.7) | 0.003 |
| Route of delivery | 0.67 | |||
| Vaginal delivery | 18 (64.3) | 11 (44) | 15 (55.6) | |
| Cesarean section | 9 (32.1) | 12 (48) | 10 (37) | |
| Operative vaginal delivery | 1 (3.6) | 2 (8) | 2 (7.4) | |
| Preeclampsia | 0 | 0 | 3 (11.1) | 0.9 |
BMI: Body mass index; GA: Gestational age; PI: Pulsatility index;
Missing values: Race (2); BMI (4); Uterine artery Doppler velocimetry (6); Umbilical artery Doppler velocimetry (4) and cerebroplacental ratio (4).
*Statistically significant different (p < 0.05), between SGA and FGR. ¥Statistically significant different as compared to AGA.
According to the design of the study, there were no significant differences in the gestational age at recruitment, cases had a significantly lower estimated sonographic fetal weight compared to controls and all patients delivered at term (>37 weeks of gestation). FGR cases had significantly higher rate of abnormal uterine artery Doppler and worse fetoplacental Doppler parameters as compared to controls and SGA. There were no significant differences in the gestational age at delivery between SGA cases and controls (p = 0.15); however, it was significantly lower in FGR cases (p = 0.002). The rate of induction of labor was significantly higher in cases; nevertheless, there were no differences in the route of delivery or rate of preeclampsia among the study groups (Table 1).
1H-NMR spectral fingerprinting in maternal and cord blood spectra
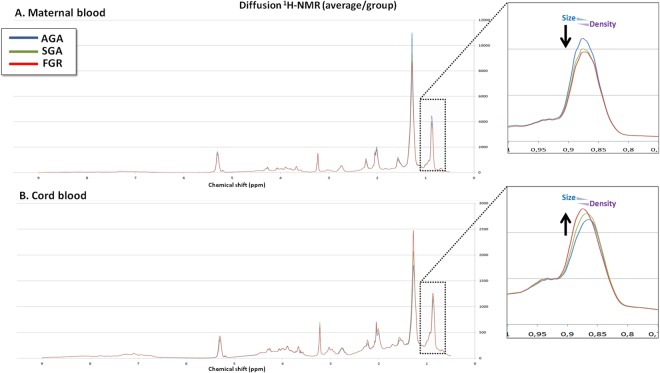
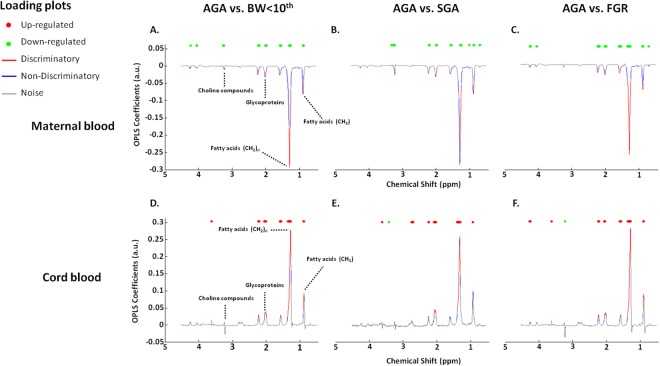
The average Diff spectral vectors generated for maternal and cord blood plasma are shown in Fig. 1. There were differences observed in the spectral vectors among the groups, more evident at 1.29 and 0.89 ppm, suggesting lower relative plasma concentrations of lipids in mothers of small fetuses (both, in SGA and FGR cases), while in cord blood, the relative concentrations of lipids were higher in cases, and mostly in the FGR group. To evaluate the ability of these metabolites in distinguishing small fetuses from controls, we performed non-targeted multivariate discriminant analysis. Fig. 2 represents the loading weight of each spectral point extracted from an OPLS-DA model of the data set (i.e. the most influential regions of the spectrum that are responsible for discrimination between cases and controls). The most discriminatory regions corresponded to lipids, CH2 and CH3, in both, mothers and fetuses [(CH2)n, 1.29 ppm and CH3 at 0.89 ppm)], confirming that these lipids: decrease in mothers of fetuses with FGR and SGA compared to controls, and increase in small fetuses. Furthermore, the approach yielded new findings such as an increase in choline compounds (in this case, most likely phosphatidylcholine) in fetuses (Fig. 2).
Figure 1.
Maternal and cord blood nuclear magnetic resonance profiles among the study groups. The more evident differences in the Diff spectral vectors were observed at 1.29 and 0.89 ppm, suggesting lower relative plasma concentrations of lipids in mothers of small fetuses (both, in SGA and FGR cases), while in cord blood, the relative concentrations of lipids were higher in cases, being greater in FGR compared to SGA cases, as well as compared to controls.
Figure 2.
Loading weight of each spectral variable extracted from an orthogonal partial least square discriminant analysis model (ASCLAN) of the data set among the study population. The color-coded loading plot shows the discriminatory variables, non-discriminatory signal variables and the noise variables, which are indicated by red, blue and grey, respectively. The loading plot shows the most influential regions of the spectrum that are responsible for discrimination between cases and controls.
Metabolic fingerprinting in clinical phenotypes of small fetuses
Based on the non-targeted metabolomics results obtained, quantitative targeted analyses using Liposcale (for lipid profiling, deconvolution of phosphatidylcholines and glycoproteins) and Dolphin (for LMW metabolite profiling) were performed (Fig. S1). The lipoproteins identified and their concentrations (i.e. triglycerides and cholesterol), strongly correlated to the enzymatic colorimetric methods quantifications for the same subjects (Fig. S2). Tables 2 and 3, shows the lipoproteins identified and their concentrations, phosphatidylcholines and glycoprotein properties, as well as LMW metabolites for each clinical phenotype (SGA and FGR) in maternal and cord blood, respectively. The Liposcale analysis showed that mothers of SGA fetuses had significantly lower concentrations of cholesterol-IDL compared to controls (p = 0.02). Similarly, triglycerides transporting lipoproteins, IDL and HDL, were significantly lower in mothers of fetuses with sub-optimal fetal growth compared to AGA mothers (p = 0.01, 0.03 and 0.02, respectively) (Table 2). In terms of lipoproteins properties, there were lower concentrations of large HDL lipoproteins in mothers of FGR fetuses (p = 0.04). Changes in glycoproteins and phosphatidylcholines suggested a disruption in their biophysical properties among study groups. Specifically, the width of one of the phosphatidylcholines peaks was significantly lower in FGR cases (p = 0.02). With regards to LMW metabolites, maternal plasma concentrations of alanine, citrate, 2-oxoisovaleric acid and pyruvate were significantly lower in mothers of small fetuses compared to controls (all p values < 0.05) (Table 2).
Table 2.
Metabolic profiling and targeted metabolomics in maternal blood across the study groups.
| Adequate for Gestational Age N = 28 | Small for Gestational Age N = 25 | Fetal Growth Restriction N = 27 | p values | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | AGA vs. SGA | AGA vs. FGR | Jonckheere-Terpstra test | ||
| Lipids | |||||||
| Cholesterol (mg/dL) | VLDL | 40.5 (30.7–68) | 30.2 (23.9–49.7) | 33.7 (24.3–49.8) | 0.06 | 0.09 | 0.04 |
| IDL | 35.4 (26.2–46.3) | 30.9 (24.1–34.3) | 30 (19.2–37.4) | 0.02 | 0.06 | 0.01 | |
| LDL | 175 (150–217) | 186.7 (170.2–197) | 182.5 (148.6–201.5) | 0.97 | 0.63 | 0.66 | |
| HDL | 78.9 (65.9–96.9) | 80.9 (71–98.6) | 86.5 (77.8–94.1) | 0.75 | 0.24 | 0.89 | |
| Triglycerides (mg/dL) | VLDL | 137.8 (109.8–228) | 116.9 (87.6–163.9) | 117.9 (92.5–170.2) | 0.17 | 0.14 | 0.08 |
| IDL | 30 (25.1–35.8) | 26.1 (21.5–29.6) | 27.4 (20–32.5) | 0.01 | 0.09 | 0.02 | |
| LDL | 42.7 (36.1–52.1) | 38.8 (32.2–42.8) | 39.2 (27.9–44.3) | 0.07 | 0.12 | 0.04 | |
| HDL | 36.8 (32.5–40) | 30.5 (25.6–41.3) | 32.3 (28.7–35.8) | 0.03 | 0.02 | 0.02 | |
| Particle numbers (nmol/L) (umol/L) |
VLDL | ||||||
| Large | 3.2 (2.5–4.8) | 3.1 (2.3–4.1) | 2.9 (2.1–4.1) | 0.3 | 0.14 | 0.08 | |
| Medium | 14.4 (11.3–21) | 12 (8.5–16.7) | 12.9 (10–16.5) | 0.15 | 0.21 | 0.11 | |
| Small | 83.1 (65.2–146) | 66.4 (50–99) | 68 (52.9–104) | 0.14 | 0.08 | 0.05 | |
| LDL | |||||||
| Large | 201 (163–249) | 203 (170–223) | 201 (152–217) | 0.52 | 0.28 | 0.11 | |
| Medium | 520 (468–651) | 527 (486–583) | 526 (411–596) | 0.98 | 0.44 | 0.28 | |
| Small | 621 (490–745) | 591 (514–746.7) | 632 (471–708) | 0.90 | 0.61 | 0.30 | |
| HDL | |||||||
| Large | 0.72 (0.16–1.52) | 0.24 (0.06–0.91) | 0.37 (0.04–0.88) | 0.08 | 0.04 | 0.02 | |
| Medium | 13.1 (10–17.7) | 14 (12.9–17.1) | 14.8 (13.2–16.7) | 0.59 | 0.04 | 0.84 | |
| Small | 34.3 (30.1–39.8) | 33.9 (28.4–38.7) | 35.7 (32–38.4) | 0.72 | 0.49 | 0.30 | |
| Phosphatidylcholines | Area peak 1 | 2 (1.6–2.7) × 106 | 1.8 (1.6–2.2) × 106 | 1.9 (1.2–2.4) × 106 | 0.19 | 0.16 | 0.91 |
| Height peak 1 | 77 (62–101) × 103 | 71 (67–79) × 103 | 76 (51.7–91.7) × 103 | 0.27 | 0.19 | 0.13 | |
| Width peak 1 | 8.71 (8.3–9.1) | 8.32 (7.7–9.2) | 8.13 (7.7–8.8) | 0.12 | 0.02 | 0.01 | |
| Area peak 2 | 0.21 (0.1–0.3) × 106 | 0.36 (0.14–0.5) × 106 | 0.29 (0.1–0.4) × 106 | 0.56 | 0.68 | 0.56 | |
| Height peak 2 | 21 (9.5–28.4) × 103 | 25 (14–35) × 103 | 22 (10.7–31.4) × 103 | 0.70 | 0.90 | 0.47 | |
| Width peak 2 | 3.8 (3.2–4.3) | 4.6 (3.3–4.9) | 4.1 (3.5–4.6) | 0.11 | 0.37 | 0.83 | |
| Area peak 3 | 3.4 (2.3–4.1) × 106 | 2.7 (1.7–4.2) × 106 | 3 (2–4.1) × 106 | 0.42 | 0.89 | 0.44 | |
| Height peak 3 | 164 (105–226) × 103 | 136 (80–198) × 103 | 149 (97–220) × 103 | 0.38 | 0.83 | 0.41 | |
| Width peak 3 | 6.7 (6.4–7.2) | 6.9 (6.5–7.2) | 6.8 (6.5–7.2) | 0.55 | 0.8 | 0.61 | |
| Area peak 4 | 3.3 (2.8–3.9) × 106 | 3.5 (2.7–4) × 106 | 3.3 (3.1–3.9) × 106 | 0.72 | 0.69 | 0.59 | |
| Height peak 4 | 120 (103–151) × 103 | 124 (108–144) × 103 | 123 (111–137) × 103 | 0.88 | 0.99 | 0.48 | |
| Width peak 4 | 8.8 (8.4–9.2) | 9.1 (8.5–9.6) | 8.8 (8.3–9.4) | 0.11 | 0.63 | 0.74 | |
| Glycoproteins | Area peak 1 | 0.47 (0.37–0.54) × 106 | 0.49 (0.35–0.59) × 106 | 0.5 (0.39–0.63) × 106 | 0.83 | 0.18 | 0.89 |
| Height peak 1 | 23.4 (20–25.1) × 103 | 23 (17–25.6) × 103 | 23.5 (21–26.8) × 103 | 0.76 | 0.51 | 0.71 | |
| Width peak 1 | 6.8 (5.7–7.2) | 6.80 (5.68–7.64) | 7.16 (6.5–8.2) | 0.48 | 0.07 | 0.04 | |
| Area peak 2 | 2.8 (2.2–3.7) × 106 | 2.4 (1.9–3.76) × 106 | 2.7 (2.2–3) × 106 | 0.39 | 0.44 | 0.27 | |
| Height peak 2 | 124 (104–157) × 103 | 110 (94–155) × 103 | 123 (111–135) × 103 | 0.38 | 0.48 | 0.70 | |
| Width peak 2 | 7.37 (6.4–8.2) | 6.88 (6.5–8.5) | 7.28 (6.35–7.9) | 0.8 | 0.4 | 0.28 | |
| Area peak 3 | 30 (27–31.6) × 106 | 25.6 (23.2–30.6) × 106 | 26.7 (23.5–30.4) × 106 | 0.09 | 0.08 | 0.03 | |
| Height peak 3 | 280 (256–357) × 103 | 261 (221–341) × 103 | 248 (219–327) × 103 | 0.07 | 0.13 | 0.04 | |
| Width peak 3 | 35 (31–37.6) | 36.3 (32.9–38.8) | 34.3 (32.9–36.8) | 0.24 | 0.53 | 0.66 | |
| Low molecular weight metabolites (mM) | |||||||
| Acetate | 0.05 (0.04–0.08) | 0.06 (0.05–0.12) | 0.07 (0.05–0.09) | 0.07 | 0.19 | 0.07 | |
| Acetone | 0.22 (0.13–0.31) | 0.19 (0.13–0.25) | 0.21 (0.11–0.27) | 0.67 | 0.29 | 0.16 | |
| Alanine | 1.17 (1.02–1.34) | 1.15 (0.99–1.34) | 1.02 (0.89–1.19) | 0.55 | 0.04 | 0.02 | |
| Citrate | 0.69 (0.64–0.74) | 0.64 (0.56–0.69) | 0.56 (0.50–0.68) | 0.04 | 0.01 | 0.002 | |
| Creatine | 0.13 (0.11–0.15) | 0.12 (0.09–0.16) | 0.13 (0.10–0.16) | 0.95 | 0.63 | 0.67 | |
| Creatinine | 0.20 (0.18–0.22) | 0.2 (0.17–0.22) | 0.19 (0.16–0.21) | 0.73 | 0.35 | 0.17 | |
| Formate | 0.09 (0.07–0.11) | 0.1 (0.09–0.12) | 0.09 (0.08–0.13) | 0.17 | 0.48 | 0.78 | |
| Glucose | 5.58 (4.90–6.19) | 5.8 (4.13–6.89) | 5.35 (4.93–6.39) | 0.83 | 0.93 | 0.54 | |
| Glutamine | 1.1 (0.9–1.2) | 1.09 (0.80–1.27) | 1.06 (1.0–1.33) | 0.95 | 0.51 | 0.76 | |
| Glycine | 0.59 (0.48–0.66) | 0.58 (0.51–0.66) | 0.64 (0.51–0.73) | 0.64 | 0.39 | 0.78 | |
| Histidine | 0.24 (0.20–0.28) | 0.20 (0.17–0.27) | 0.22 (0.19–0.24) | 0.33 | 0.17 | 0.11 | |
| Isoleucine | 0.17 (0.15–0.2) | 0.15 (0.12–0.2) | 0.16 (0.13–0.2) | 0.14 | 0.5 | 0.26 | |
| Lactate | 11 (7.57–14) | 11.67 (7.14–15.2) | 11 (7.16–13.5) | 0.98 | 0.61 | 0.32 | |
| Leucine | 0.22 (0.2–0.26) | 0.21 (0.16–0.25) | 0.24 (0.18–0.28) | 0.26 | 0.83 | 0.56 | |
| Mannose | 0.21 (0.19–0.23) | 0.2 (0.16–0.23) | 0.19 (0.17–0.2) | 0.16 | 0.13 | 0.06 | |
| 2-oxoisovaleric acid | 1.02 (0.84–1.27) | 0.77 (0.66–0.94) | 0.78 (0.69–0.94) | 0.0009 | 0.002 | 0.001 | |
| Phenylalanine | 0.28 (0.26–0.3) | 0.3 (0.26–0.32) | 0.3 (0.27–0.35) | 0.22 | 0.1 | 0.04 | |
| Pyruvate | 0.65 (0.43–0.75) | 0.5 (0.34–0.73) | 0.44 (0.35–0.56) | 0.18 | 0.05 | 0.03 | |
| Tyrosine | 0.32 (0.28–0.37) | 0.32 (0.28–0.37) | 0.32 (0.27–0.37) | 0.8 | 0.90 | 0.43 | |
| Valine | 0.52 (0.45–0.57) | 0.51 (0.42–0.59) | 0.52 (0.46–0.57) | 0.58 | 0.81 | 0.57 | |
HDL: High-density lipoprotein; IDL: Intermediate density lipoprotein; IQR: Interquartile range; LDL: Low-density lipoprotein; VLDL: Very low-density lipoprotein. Concentration, size and properties of the peaks are presented as median and interquartile range (IQR).
Table 3.
Metabolic profiling and targeted metabolomics in cord blood across the study groups.
| Adequate for Gestational Age N = 28 | Small for Gestational Age N = 25 | Fetal Growth Restriction N = 27 | p values | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | AGA vs. SGA | AGA vs. FGR | Jonckheere-Terpstra test | ||
| Lipids | |||||||
| Cholesterol (mg/dL) | VLDL | 8.6 (7.47–11.1) | 10.8 (8.4–13.7) | 13.5 (9.2–17.2) | 0.1 | 0.008 | 0.002 |
| IDL | 7.1 (6.4–8.9) | 8.6 (7.2–10.5) | 8.8 (8–10.8) | 0.08 | 0.04 | 0.01 | |
| LDL | 82.1 (79–86) | 80.7 (78.9–86.5) | 80.5 (78.3–86.9) | 0.98 | 0.73 | 0.63 | |
| HDL | 61.9 (58.9–65.1) | 61.6 (58.7–64.2) | 56.5 (53.5–63.3) | 0.81 | 0.06 | 0.97 | |
| Triglycerides (mg/dL) | VLDL | 39.6 (35.7–45.4) | 45.3 (37.8–52.3) | 50.4 (42.2–61.7) | 0.1 | 0.007 | 0.002 |
| IDL | 7.8 (7.1–9.4) | 8.9 (7.9–10.5) | 9.3 (8.6–11.1) | 0.08 | 0.03 | 0.008 | |
| LDL | 6.7 (5.8–8.5) | 8.1 (6.2–9.3) | 7.3 (6.7–9.7) | 0.2 | 0.09 | 0.04 | |
| HDL | 12.4 (11.3–13.4) | 12.7 (12.1–13.1) | 12.3 (11.6–13) | 0.22 | 0.92 | 0.44 | |
| Particle numbers (nmol/L) (umol/L) |
VLDL | ||||||
| Large | 0.9 (0.75–1.1) | 1.1 (0.79–1.32) | 1.23 (1–1.6) | 0.15 | 0.004 | 0.001 | |
| Medium | 4.8 (4.5–5.6) | 5.57 (4.7–5.9) | 6 (5–6.8) | 0.11 | 0.003 | 0.001 | |
| Small | 19.6 (16.5–24.3) | 20.6 (17.1–30.6) | 27.5 (19.4–34.3) | 0.22 | 0.03 | 0.01 | |
| LDL | |||||||
| Large | 82.9 (74–88.7) | 83.7 (78.4–91) | 83.5 (73–90.6) | 0.76 | 0.86 | 0.56 | |
| Medium | 188 (180–202) | 201 (181–209) | 202 (192–213) | 0.13 | 0.04 | 0.02 | |
| Small | 294 (270–315) | 286 (267–329) | 292 (257–317) | 0.55 | 0.67 | 0.74 | |
| HDL | |||||||
| Large | 0.23 (0.14–0.31) | 0.23 (0.18–0.35) | 0.34 (0.26–0.5) | 0.7 | 0.009 | 0.003 | |
| Medium | 10.4 (9.6–11.7) | 10.8 (10.2–11.8) | 9.7 (7.4–11.7) | 0.48 | 0.06 | 0.1 | |
| Small | 19.6 (17.9–20.8) | 18.9 (17.6–22) | 18.9 (17.7–21.1) | 0.78 | 0.59 | 0.72 | |
| Phosphatidylcholines | Area peak 1 | 0.4 (0.32–0.44) × 106 | 0.42 (0.32–0.54) × 106 | 0.51 (0.4–0.58) × 106 | 0.32 | 0.04 | 0.01 |
| Height peak 1 | 14.5 (13.2–16.5) × 103 | 16.5 (13–19) × 103 | 17.6 (15.5–21.4) × 103 | 0.18 | 0.006 | 0.003 | |
| Width peak 1 | 8.9 (8.1–9.6) | 8.6 (8.3–9.2) | 8.5 (8.2–9.4) | 0.34 | 0.37 | 0.86 | |
| Area peak 2 | 0.34 (0.2–0.41) × 106 | 0.41 (0.21–0.5) × 106 | 0.26 (0.15–0.48) × 106 | 0.22 | 0.42 | 0.72 | |
| Height peak 2 | 17 (13.6–20.4) × 103 | 20 (14.4–26) × 103 | 16 (10–24.2) × 103 | 0.09 | 0.88 | 0.36 | |
| Width peak 2 | 6.37 (4.6–7.3) | 6.5 (5.4–6.9) | 5.7 (4.5–7.1) | 0.98 | 0.19 | 0.94 | |
| Area peak 3 | 0.79 (0.34–1.18) × 106 | 1.19 (0.53–1.83) × 106 | 0.66 (0.22–1.56) × 106 | 0.31 | 0.78 | 0.57 | |
| Height peak 3 | 35.1 (21.4–53.2) × 103 | 55.2 (27.4–78.4) × 103 | 31.7 (12.6–59) × 103 | 0.21 | 0.76 | 0.58 | |
| Width peak 3 | 7 (5.8–8.1) | 7.1 (5.3–7.9) | 7.4 (5.8–9.2) | 0.62 | 0.59 | 0.26 | |
| Area peak 4 | 2.1 (1.85–2.41) × 106 | 1.76 (1.1–2.42) × 106 | 1.76 (1.3–2.29) × 106 | 0.12 | 0.05 | 0.03 | |
| Height peak 4 | 76 (65.2–87.8) × 103 | 62.3 (46.3–82.3) × 103 | 64.7 (49.1–81) × 103 | 0.08 | 0.07 | 0.05 | |
| Width peak 4 | 9.3 (8.74–9.6) | 9.1 (8.5–9.5) | 8.9 (8.4–9.2) | 0.26 | 0.07 | 0.04 | |
| Glycoproteins | Area peak 1 | 0.44 (0.36–0.56) × 106 | 0.43 (0.36–0.56) × 106 | 0.46 (0.37–0.56) × 106 | 0.96 | 0.71 | 0.37 |
| Height peak 1 | 13 (11.8–14.4) × 103 | 12.7 (11.4–14.3) × 103 | 13.9 (11.8–16.1) × 103 | 0.53 | 0.21 | 0.14 | |
| Width peak 1 | 11.3 (10.2–12.6) | 10.9 (10–12) | 11 (10.1–12.1) | 0.43 | 0.5 | 0.72 | |
| Area peak 2 | 1.3 (1.2–1.4) × 106 | 1.4 (1.3–1.69) × 106 | 1.6 (1.4–2) × 106 | 0.08 | 0.002 | 0.0004 | |
| Height peak 2 | 52.4 (48–56.2) × 103 | 53 (50–66) × 103 | 59.9 (53.5–70.2) × 103 | 0.17 | 0.009 | 0.003 | |
| Width peak 2 | 8.3 (7.9–8.5) | 8.4 (8.1–8.6) | 8.6 (8.4–9.2) | 0.24 | 0.005 | 0.002 | |
| Area peak 3 | 7.2 (5.6–8.4) × 106 | 7.3 (6.3–8.9) × 106 | 6.7 (5.5–7.7) × 106 | 0.59 | 0.64 | 0.68 | |
| Height peak 3 | 55 (44–64) × 103 | 61.2 (48–71) × 103 | 59 (51.4–70.4) × 103 | 0.15 | 0.19 | 0.1 | |
| Width peak 3 | 46.6 (38–50) | 43.7 (37–49.8) | 38 (33.2–43.9) | 0.18 | 0.002 | 0.0005 | |
| Low molecular weight metabolites (mM) | |||||||
| Acetate | 0.06 (0.05–0.08) | 0.07 (0.06–0.09) | 0.08 (0.07–0.10) | 0.07 | 0.01 | 0.003 | |
| Acetone | 0.12 (0.09–0.23) | 0.12 (0.08–0.21) | 0.1 (0.07–0.17) | 0.69 | 0.24 | 0.87 | |
| Alanine | 1.7 (1.41–1.9) | 1.6 (1.4–1.9) | 1.7 (1.4–1.9) | 0.66 | 0.90 | 0.47 | |
| Citrate | 0.53 (0.5–0.6) | 0.6 (0.5–0.6) | 0.6 (0.4–0.61) | 0.96 | 0.6 | 0.67 | |
| Creatine | 0.16 (0.12–0.2) | 0.17 (0.13–0.2) | 0.18 (0.15–0.25) | 0.72 | 0.25 | 0.12 | |
| Creatinine | 0.17 (0.16–0.2) | 0.18 (0.17–0.2) | 0.18 (0.14–0.22) | 0.34 | 0.78 | 0.33 | |
| Formate | 0.12 (0.1–0.15) | 0.16 (0.12–0.17) | 0.16 (0.11–0.19) | 0.02 | 0.02 | 0.004 | |
| Glucose | 4.25 (3.88–5.1) | 4.42 (3.7–5.03) | 4.6 (3.9–5.38) | 0.84 | 0.33 | 0.2 | |
| Glutamine | 1.35 (1.2–1.5) | 1.39 (1.29–1.6) | 1.42 (1.24–1.56) | 0.42 | 0.53 | 0.26 | |
| Glycine | 1.1 (0.96–1.22) | 1.1 (0.94–1.1) | 1.1 (0.97–1.22) | 0.64 | 0.63 | 0.3 | |
| Histidine | 0.42 (0.39–0.45) | 0.39 (0.34–0.44) | 0.39 (0.35–0.46) | 0.22 | 0.2 | 0.09 | |
| Isoleucine | 0.23 (0.19–0.26) | 0.2 (0.18–0.25) | 0.23 (0.2–0.26) | 0.17 | 0.96 | 0.56 | |
| Lactate | 16.9 (13.2–21.4) | 16.1 (13.5–23.6) | 18.7 (12.2–25.2) | 0.99 | 0.61 | 0.35 | |
| Leucine | 0.35 (0.32–0.42) | 0.35 (0.3–0.4) | 0.36 (0.31–0.42) | 0.53 | 0.98 | 0.52 | |
| Mannose | 0.96 (0.79–1.1) | 0.89 (0.78–0.96) | 0.89 (0.8–1.03) | 0.2 | 0.72 | 0.68 | |
| 2-oxoisovaleric acid | 0.1 (0.08–0.11) | 0.1 (0.08–0.12) | 0.11 (0.09–0.12) | 0.65 | 0.14 | 0.07 | |
| Phenylalanine | 1.46 (1.05–1.64) | 1.20 (0.89–1.5) | 1.26 (0.91–1.42) | 0.75 | 0.13 | 0.23 | |
| Pyruvate | 0.46 (0.44–0.49) | 0.48 (0.46–0.53) | 0.52 (0.44–0.56) | 0.26 | 0.2 | 0.08 | |
| Tyrosine | 0.71 (0.6–0.89) | 0.6 (0.4–0.94) | 0.84 (0.54–0.91) | 0.27 | 0.99 | 0.55 | |
| Valine | 1.12 (0.92–1.32) | 1.1 (0.95–1.35) | 1.18 (0.92–1.41) | 0.99 | 0.74 | 0.4 | |
HDL: High-density lipoprotein; IDL: Intermediate density lipoprotein; IQR: Interquartile range; LDL: Low-density lipoprotein; VLDL: Very low-density lipoprotein. Concentration, size and properties of the peaks are presented as median and interquartile range (IQR).
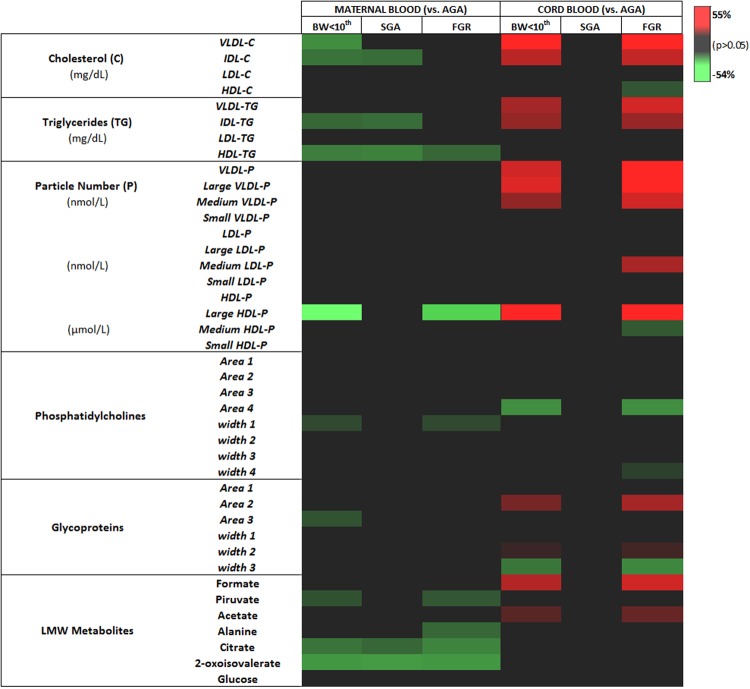
Interestingly, the behavior of triglycerides and cholesterol lipoproteins was the opposite in affected fetuses compared to their mothers. FGR fetuses had significantly higher concentrations of cholesterol-VLDL (+56%), -IDL (+24%) lipoproteins (p = 0.008 and 0.04, respectively), as well as higher concentrations of triglycerides-VLDL (+24%), and -IDL (+18%) compared to controls (p = 0.008 and 0.04, respectively) (Table 3). Importantly, we found a gradient of higher concentrations of cholesterol (VLDL and IDL) and triglycerides (VLDL, IDL and LDL) lipoproteins in SGA and FGR cases compared to controls (Jonckheere–Terpstra test p < 0.05). FGR cases also showed a significant increase in large HDL, medium LDL and all VLDL particle types (large, medium and small: average + 37%) vs. controls (all p values < 0.05). When analyzed as a trend, the Jonckheere-Terpstra test also showed that the increase in particles size and distribution was present in the two clinical phenotypes following a severity ascendant gradient, higher in FGR fetuses. Likewise, changes in phosphatidylcholines and glycoproteins peaks were more prominent in FGR fetuses vs. controls (Table 3). Conversely, among LMW metabolites, formate and acetate showed a significant trend towards increase in cord blood of FGR fetuses (p = 0.02 and 0.01, respectively) (Table 3). No differences were found in the concentrations of glucose between the study groups. Figure. 3 is a heat map constructed to visualize and summarize the significant differences on the maternal and cord blood plasma LMW metabolites, lipid classes, particles and subclasses along with their p-values, and percentage of fold change (among cases and controls).
Figure 3.
Heat map following metabolomic profiling on the annotated and significant (among the three groups) identified in plasma samples of mothers and neonates. A colored heat map of significant LMW metabolites and the Liposcale analysis along with their p-values, and percentage of fold change was obtained to visualize and characterize the differences between adequate for gestational age (AGA), all cases with a birthweight below the 10th centile (BW <10th) and separately small-for-gestational age (SGA) as well as fetal growth restriction (FGR) groups from maternal and umbilical cord blood. Fold change of significant differences (p < 0.05) for each metabolite are on a graded color scale from green (lower value) to black (no differences) to red (high values).
Discussion
Principal findings of the study
In this comprehensive metabolomic study, we expanded previous observations that lipid profiles are disturbed in small fetuses and their mothers. Yet, our innovative approach allowed quantifying the number and size of the particles in the lipoprotein fractions as well as low-molecular-weight metabolites, describing the complex maternal and fetal metabolic response in pregnancies with fetal smallness. However, the study failed to observe any remarkable difference between the two clinical phenotypes of fetal smallness. Thus, SGA and FGR presented similar findings, supporting the notion that both phenotypes might be exposed to different degrees of undernutrition and thus, both might be at risk of metabolic fetal programming. Future studies assessing placenta metabolism might delineate potential mechanisms in both phenotypes.
Why are circulating lipids lower in mothers of growth-restricted fetuses?
Our results describing an overall decrease in circulating lipids in mothers of small fetuses are in line with previous studies42–47. However, this study revealed further details, providing qualitative and quantitative measurements of lipoproteins, their cholesterol and triglyceride concentration, as well as the number of sub-fraction particles and their size. Compared to controls, there was a triglyceride-impoverishment in lipoproteins such as HDL (decrease in large particles) and IDL as well as low concentration of cholesterol in other lipoproteins, namely VLDL and IDL in mothers of both SGA and FGR cases. A maternal metabolic adaptation during pregnancy47–53 is essential to increase availability of cholesterol and triglycerides for fetal development and the synthesis of steroid hormones by the placenta54–56. When maternal plasma cholesterol is low, birthweight is lower than normal. Indeed, term infants of mothers with low total cholesterol weigh are on average 150 g less than those born to mothers with normal cholesterol concentrations57. The first trimester of gestation is considered an anabolic period, in which the mother increases her deposit of fat, through a reduction in the activity of lipoprotein lipase, and increasing in the insulin responsiveness58. Interestingly, this fat accumulation is maintained even under conditions of severe maternal malnutrition59. In the third trimester, a switch to catabolism occurs in order to supply fetal demands and to synthesize large amounts of steroids hormones by the placenta54. The products of an enhanced lipolytic activity reach the liver to be used for triglycerides synthesis and to increase circulating availability of triglycerides by 208% (transported mainly by VLDL), and total, HDL- and LDL-cholesterol in 65, 26 and 64%, respectively50. It seems plausible that in the third trimester of pregnancy, placental insufficiency generates a specific fetal and maternal response that is reflected in the metabolomic profile. Low concentrations of lipids in the maternal circulation may reflect an increased uptake into the placenta. In normal gestation, support of lipids, and particularly essential fatty acids, to the fetus is ensured by increased maternal liver production of VLDL rich in triglycerides (build upon peripheral deposits of adipose tissue)60, peripheral enrichment of HDL and LDL and lipoprotein lipase activities in the placenta61,62. The findings of this and previous studies suggest (1) failure of the normal signaling leading to increased lipids in pregnancy, and/or (2) a reduction in lipid reserve in the mothers.
Why are there higher cord blood lipids in SGA and FGR fetuses?
Our findings confirmed previous studies reporting higher circulating triglycerides and cholesterol lipoproteins in growth-restricted fetuses63–66. Importantly, our metabolic fingerprinting approach allowed to describe that VLDL and IDL were the most deregulated lipoproteins in FGR fetuses, suggesting that VLDL rich in triglycerides may be an alternative fuel mobilized by the growth-restricted fetus. This is a novel finding in humans, since VLDL is mostly synthesized in the fetal liver, implying an altered hepatic synthesis of lipoproteins in adverse intrauterine conditions.
Lipids are fundamental molecules for life. During fetal life, triacylglycerols provide energy for metabolic processes, while fatty acids, cholesterol and phospholipids are required to develop the fetal brain and central nervous system, to build cell membranes, and as a precursor of bile acids and steroid hormones67. Both, the placenta and the fetus have the capacity of denovo cholesterol synthesis. It has been estimated that up to 20% of cholesterol could derive from transplacental passage. Maternal cholesterol-carrying lipoproteins (mainly HDL) are transported across trophoblast and then overpass the endothelial cells of the fetoplacental vasculature, to finally being effluxed into the fetal circulation60. Thus, like in the adult, the liver is the principal source of circulating lipoproteins in the fetus. Previous animal68 and human studies have shown higher concentrations of circulating triglycerides64,69,70, as well as an altered, pro-atherogenic lipid and cholesterol metabolism in growth-restricted fetuses38,71,72. The lipid profile of FGR fetuses described herein is akin to adults with dyslipidemia and atherosclerosis. Large epidemiological as well as experimental evidence have suggested that an adverse intrauterine environment leading to low birthweight may increase the risk for cardiovascular disease later in life73–77. Therefore, important challenges for future research are to ascertain the mechanistic pathways whereby fetal abnormal lipid profile might influence the higher risk of growth restricted fetuses to cardiovascular disease in adulthood.
Changes in low molecular weight metabolites
While major changes were found in lipids, analysis of LMW metabolites revealed lower cord blood concentrations of acetate and formate in late-onset FGR and maternal concentrations of citrate, pyruvate and 2-oxoisovaleric acid in both, SGA and FGR pregnancies. Biophysical abnormalities in phosphatidylcholines and glycoproteins were also detected. Phosphatidylcholine is a major membrane phospholipid, made in mammalian cells from choline via the CDP-choline pathway and has a key role in neuronal differentiation and cell fate determination78. Importantly, phosphatidylcholine biosynthesis is required for normal VLDL secretion from hepatocytes79. Metabolomic analysis showed some differences in comparison with previous studies. For instance, in this study we did not replicate our previous finding of lower concentrations of valine and leucine in late-onset FGR38, nor we could demonstrate differences in other essentials aminoacids in SGA fetuses as reported in other studies36,37,80. Favretto et al. reported up regulation of phenylalanine, tryptophan, and glutamate in FGR fetuses37, while Ivorra et al. reported significant differences cord blood concentrations of five aminoacids in FGR fetuses (proline, glutamine and alanine were reduced, while phenylalanine and citrulline were increased)36. In previous studies, early-onset FGR showed significant decrease in glucose63,81, while no differences have been found in late-onset FGR63. It is known that glucose levels are inversely correlated to the clinical severity of FGR and the capability of transplacental glucose gradient, which might differ between the two phenotypes. Thus, these discrepancies suggest differences in case selection, platforms and protocols used among studies assessing metabolites in placental dyfunction36,37.
Metabolic differences between small-for-gestational age and growth-restricted fetuses
FGR has two main clinical presentations, early-and late-onset disease. Early-onset FGR is highly associated with severe placental insufficiency and chronic fetal hypoxia82,83. In early-onset FGR, the typical scenario is a progressive deterioration of fetal well-being, accompanied with higher rates of preeclampsia and adverse perinatal outcomes, often requiring preterm delivery4. On the other hand, late-onset FGR represents 70–80% of FGR, has less signs of placental disease and the association with preeclampsia is minimal84. Despite a more benign nature as compared with early FGR, and that chronic hypoxia seems to be mild, the increase demands of oxygen and nutrients by vital organs such as brain and heart towards term increase the risk of acute fetal deterioration before labor, as suggested by the high contribution to late-pregnancy mortality, and a high association with intra- partum fetal distress and neonatal acidosis85. One of the aims was to evaluate whether late-onset SGA and FGR are associated with differential metabolomic patterns. Although there is a widely shared assumption that SGA are “constitutionally small”, but otherwise normal, fetuses, more recent studies reported that SGA display similar, albeit milder, neurodevelopmental and cardiovascular changes as those observed in FGR fetuses13,14,25,86,87. In line with this, the present study found that SGA and FGR fetuses had remarkably similar pattern of alterations in maternal and fetal lipid profiles, although there was a gradation of metabolic disruption according to the severity of the disease. These findings support that, at least a proportion of SGA fetuses represent a milder clinical form of true growth restriction not associated with Doppler changes or increased obstetrical risks. It could be hypothesized that SGA fetuses suffer undernutrition and its long-term consequences, but the respiratory function of the placenta is still largely preserved. Consequently, while the clinical distinction between “low risk” SGA and FGR is relevant for obstetrical management, fetal smallness represents a (potential) high-risk situation for long-term quality of life since metabolic adaptations occurs in utero regardless of its clinical prenatal presentation.
Strengths and limitations
The strengths of this study include the prospective design and the inclusion of a well-defined cohort including samples from mothers and fetuses of both, SGA and FGR cases with a relatively large sample size, meaning that the possibility of selection bias was minimized. From a methodological standpoint, the platforms used are highly reproducible and standardized. NMR and mass spectrometry are not mutually exclusive but rather complementary. They have advantages and disadvantages, and the method of choice depends on the aim of the study. One of the main advantages of NMR is its extraordinary reproducibility, which makes this technique a very good option for fingerprinting analysis, such as the study reported herein. Besides identification, we had the interest of quantify specific lipids, therefore we have used NMR spectroscopy since the peak area of a compound in the NMR spectrum is directly related to the concentration of specific nuclei, making quantification of compounds in a complex mixture very precise88,89. On the other hand, NMR is a non-destructive technique that allows samples to be recovered after analysis, which is not trivial considering the incalculable value of these samples. While basal contamination with macromolecules and lipids in the plasma are usually overlooked, herein, were discriminated and quantified and in addition, the resolution of spectral process described (buckets within 0.003 ppm) is significantly higher to that reported before (buckets within 0.05 ppm)36–38. Moreover, the meticulous deconvolution process applied to each metabolite, is by far more rigorous, than the “blinded” quantification previously used36–38.
This is the first time that Liposcale is used in pregnancy. This test was originally developed to rapidly produce a lipoprotein profile of an individual’s plasma as part of cardiovascular disease risk work up90,91. The positive correlations describe between Liposcale and enzymatic colorimetric methods suggests that these variations are biologically relevant. Yet, we acknowledge several limitations. First, our sample size may cover potential associations in other metabolites and hide interaction of confounders (clinical characteristics similar among populations but non-obvious confounders could have affected). Secondly, as we focused in late-onset FGR (by far, the most common clinical presentation), we might have missed the most extreme and severe cases occurring before 32 weeks of gestation, in which fetal metabolic adaptations might be of uttermost relevance. Third, it is likely that our classification has overlapping between SGA and FGR, and for instance it is likely that constitutional small fetuses were included. Fourth, because of the design of the study, we only had few maternal samples of elective cesarean sections (maternal fasting conditions) and thus, non-fasting blood samples were collected. Typically, there is marginal effect of fasting on total cholesterol, HDL-C, and LDL-C, although triglycerides have a tendency to increase approximately 15% in non-fasting vs. fasting samples92–94. Fifth, although questionable, other more difficult to control confounding factors such as differences in the length of labor, time lapse difference between maternal and fetal sampling95 or seasonal variation of lipid profile96 might influence maternal lipid profile in the same form as fetal gender might influence the metabolomic profile of the fetuses included97. Future studies with a larger sample size might include these confounding factors into the analysis.
Conclusion
In summary, metabolic profiling combined with clinical phenotyping demonstrates the potential to enrich stratified medicine research, revealing that mothers of small fetuses present substantial reductions in lipid metabolites, suggesting a failure in the maternal metabolic adaptation to pregnancy. While, both, SGA and growth-restricted fetuses have a substantial increase in lipids, indicating a similar metabolic response to undernutrition, there was a gradation of the metabolic disruption according to the severity of the phenotype.
Materials and Methods
Subjects and sample collection
This study is part of a larger prospective research program on FGR at the Department of Maternal-Fetal Medicine in Hospital Clinic Barcelona, involving maternal, fetal, and perinatal outcomes, as well as short- and long-term postnatal follow-up. A prospective cohort of singleton gestations with antenatal suspicion of fetal smallness and diagnosis established later than 32 weeks of gestation, were prospectively followed; those who delivered neonates with a birthweight below the 10th centile at term (≥37 weeks of gestation) were included as cases (n = 52). According to our clinical protocol, small fetuses were subdivided: those with a birthweight <3rd centile and/or abnormal uterine artery Doppler and/or abnormal cerebroplacental ratio were termed fetal growth restriction (FGR), while those with a birthweight between the 3rd and the 9th centile and normal fetoplacental Doppler were considered small-for-gestational age (SGA) cases. A control group of 28 pregnancies with adequate-for-gestational ages (AGA) fetuses were selected among low-risk pregnancies attending third trimester routine pregnancy care and were included if they delivered term neonates with a normal birthweight (between 20th and 90th centile). Exclusion criteria included multiple gestations, spontaneous preterm labor or delivery, premature rupture of membranes, chromosomal abnormalities or major structural abnormalities. All the patients included in this study were delivered at term and did not receive steroids for fetal lung maturity. The Institutional Research and Ethics Committee approved the study protocol (review board 2014/7154), all parents gave their written informed consent and all experiments were performed in accordance with relevant guidelines and regulations.
Clinical and Ultrasound Data
Maternal, perinatal and neonatal data were prospectively recorded in all patients. Gestational age was calculated on the basis of fetal crown-rump length, measured at 11–13 weeks. Transabdominal ultrasound with Doppler evaluation was performed in both, cases and controls, at recruitment, using 6-4-MHz probes (Siemens Sonoline Antares, Siemens Medical Systems, Malvern, PA, USA) and a Voluson 730 Expert Machine (GE Medical systems, Zipf, Austria). Estimated fetal weight (EFW) was calculated using the Hadlock formula98, and adjusted according to fetal gender and gestational age using local standards99. Feto-placental Doppler included: umbilical artery-pulsatility index (PI), mean uterine artery PI and middle cerebral artery PI. The cerebroplacental ratio was calculated as middle cerebral artery PI/umbilical artery PI100, and defined as abnormal when <5th centile for gestational age100. The mean uterine arteries PI value was considered abnormal when >95th centile101. Cases of FGR were delivered electively between 37 and 38 weeks of gestation, while SGA cases were allowed to deliver up to 40 weeks of gestation5,6,11. Controls were allowed to have a spontaneous onset of labor but, if they reached 41 weeks of gestation, induction of labor was offered.
Biological samples collection and storage
Maternal blood samples were drawn 2–4 hours after delivery (non-fasting conditions but at least eight hours after their last meal and blood sampling). Umbilical vein cord blood samples were obtained from the clamped umbilical cord immediately after delivery of the fetus. All blood samples were collected in EDTA-treated tubes and processed within one hour. Plasma was separated by centrifugation at 3000 rpm for 10 minutes at 4 °C, and stored at −80 °C until further use.
NMR data acquisition
Plasma samples were thawed overnight and prepared for nuclear magnetic resonance (NMR) analyses according to the Bruker-specific metabolomics protocol102. Aliquots of each sample (300 μl) were mixed with 300 μl of sodium phosphate buffer for immediate analysis. High-resolution 1H-NMR spectroscopy data were acquired on a Bruker 600 MHz Spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with an Avance III console and a TCI CryoProbe Prodigy: 1D Nuclear Overhauser Effect SpectroscopY (NOESY), Carr-Purcell-Meiboom-Gill (CPMG), and 2D j-resolved spectroscopy (JRES), all with pre-saturation to suppress the residual water peak, to characterize small molecules such as amino acids and sugars; and 1D Diffusion (Diff, TR/TE, b values, NS, ACQ), to detect larger molecules such as lipoproteins, glycoproteins and choline compounds103,104. All the sequences were run at 37 °C in quantitative conditions (systematic pre-calibration of radio frequency pulses and sample temperature, and same receiver gain adjustment). CPMG and Diff data were preprocessed at the NMR console (TopSpin 3.2, Bruker Biospin, Rheinstetten, Germany) for basic corrections, such as phase correction and exponential line broadening (0.5 Hz for CPMG; 1.0 Hz for Diff).
NMR fingerprinting analysis
For the metabolic fingerprinting analysis, spectral vectors were generated from the CPMG and Diff data by binning (0.003 ppm), alignment (alanine, 1,475 ppm) and region suppression (to minimize the influence of water and EDTA signals, specific to the CPMG data). This was carried out with MestReNova v11.0.3 (Mestrelab Research S.L., Santiago de Compostela, Spain). These spectral vectors were used for automatic spectroscopic data categorization by clustering analysis (ASCLAN), a supervised method to quickly assess discriminatory spectral regions (and indirectly metabolites) in the study groups105. The Diff NMR data was further used for lipoprotein profiling, based on the Liposcale test90,91. This test provides information about the size, lipid concentration (cholesterol and triglycerides) and number of particles for the main classes of lipoproteins [very-low density lipoprotein (VLDL), low-density lipoprotein (LDL), intermediate-density lipoprotein (IDL) and high-density lipoprotein (HDL)], as well as the concentration of particles in their subclasses (large, median, small)90,91. The Diff NMR data was also used to quantify choline compounds (3.3–3.18 ppm) and glycoproteins (2.15-1.9 ppm), based on peak deconvolutions. Details of the method for the Liposcale for lipoprotein characterization and phosphatidylcholines compounds and glycoprotein peak deconvolution are explained in the supplemental material. Finally, CPMG data was used for the profiling of 20 metabolites, based on a new, fully automated version of the software package Dolphin106,107. Signal annotation was based on templates prepared in previous studies with the help of available databases108 and bibliography109–111. Validation of metabolite identification was assisted by STOCSY112 and JRES data.
Statistical analysis
Categorical data are presented as n (%) and continuous data as mean (±SD) or median [interquartile range (IQR)] according to their distribution. To assess the categorical variables, proportions were compared with Fisher’s exact test or the chi-square test. Distributions of continuous variables were examined for normality using the Kolmogorov-Smirnov test. When there was normality of continuous variables, the one-way ANOVA test and unpaired t-tests were used to compare differences. Otherwise, the Kruskal-Wallis one-way analysis of variance and Mann-Whitney U-test were used. Correlation between selected metabolites concentrations by 1H-NMR and enzymatic colorimetric methods were assessed with Pearson coefficients (or Spearman for non-normal distributions). To initially evaluate the ability of metabolites in distinguishing cases from controls, orthogonal partial least square discriminant analysis (OPLS-DA) was performed based on multivariate, supervised, discriminant analysis of spectral vectors – Automatic Spectroscopic data Categorization by cLustering ANalysis (ASCLAN)105. Finally, the Jonckheere–Terpstra test was also used to test if the concentration of metabolites followed a ordered alternative hypothesis across severity groups (controls-SGA-FGR)113,114. Statistical analysis was performed using STATA 14 (Stata Corp LP, 2015, College Station, Texas) and MATLAB (MathWorks Inc, US). A p-value < 0.05 was considered to be statistically significant.
Electronic supplementary material
Acknowledgements
The Biobanks of the Hospital Clínic-IDIBAPS and Hospital Sant Joan de Dèu provided the biological samples used in this project with an appropriate ethical approval. This project has been funded with support of the “Instituto de Salud Carlos III (PI14/00226, INT16/00168 and PI15/00130) integrados en el Plan Nacional de I + D + I”, the “ISCIII-Subdirección General de Evaluación”, the “Fondo Europeo de Desarrollo Regional (FEDER) “Otra manera de hacer Europa” and the Erasmus + Programme of the European Union (Framework Agreement number: 2013-0040). Additionally, this project has received funding from “la Caixa” Foundation; Cerebra Foundation for the Brain Injured Child (Carmarthen, Wales, UK); and AGAUR 2014 SGR grant n° 928. JM was supported by a pre-doctoral governmental “Bolivar Gana con Ciencia” grant from Colombia; while, CP was supported by a Rio Hortega Grant from “Instituto de Salud Carlos III” CM16/00142 and “Fundació Dexeus Mujer”. The funding sources had no involvement in the study design; collection, analysis and interpretation of data or in the writing of this report.
Author Contributions
Jezid Miranda, Eduard Gratacós and Fatima Crispi conceived the hypothesis and study design. Jezid Miranda, Francesca Crovetto, Cristina Paules, María D. Gómez-Roig and Elisenda Eixarch were involved in recruiting the patients, establishing the subject database and sample Biobank. Rui V. Simões, Daniel Cañueto, Miguel A. Pardo-Cea, María L. García-Martín and Nuria Amigó designed the methods for spectrum acquisition and analysis. María L. García-Martín and Rui V. Simões performed the sample preparation, NMR data acquisition and fingerprinting analysis. Jezid Miranda, Rui V. Simões, Miguel A. Pardo-Cea, Nuria Amigó, Rocio Fuertes-Martin, Daniel Cañueto and Nicolau Cañellas analyzed the data. Jezid Miranda, Rui V. Simões and Fatima Crispi, drafted the manuscript. Eduard Gratacós, Fatima Crispi, Ramon Estruch, Monica Domenech and Stefan Hansson revised the manuscript; critically all authors have read and approved the final version of the submitted manuscript.
Data Availability Statement
Clinical information of the patients included, spectrum data and associated protocols are promptly available to readers without undue qualifications in material transfer agreements.
Competing Interests
N. Amigó is stockowner of Biosfer Teslab and has a patent for the NMR method used for lipoprotein characterization. The rest of the authors report no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31832-5.
References
- 1.Lee ACC, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Heal. 2013;1:e26–e36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N. Engl. J. Med. 1999;340:1234–1238. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 3.Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108. doi: 10.1136/bmj.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees C, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE) Ultrasound Obstet. Gynecol. 2013;42:400–408. doi: 10.1002/uog.13190. [DOI] [PubMed] [Google Scholar]
- 5.Figueras F, Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014;36:86–98. doi: 10.1159/000357592. [DOI] [PubMed] [Google Scholar]
- 6.Figueras F, Gratacos E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017;38:48–58. doi: 10.1016/j.bpobgyn.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Oros D, et al. Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late-onset small-for-gestational age fetuses. Ultrasound Obstet. Gynecol. 2011;37:191–195. doi: 10.1002/uog.7738. [DOI] [PubMed] [Google Scholar]
- 8.Flenady V, et al. Stillbirths: Recall to action in high-income countries. Lancet. 2016;387:691–702. doi: 10.1016/S0140-6736(15)01020-X. [DOI] [PubMed] [Google Scholar]
- 9.Moraitis AA, Wood AM, Fleming M, Smith GCS. Birth weight percentile and the risk of term perinatal death. Obstet. Gynecol. 2014;124:274–283. doi: 10.1097/AOG.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 10.Caradeux, J., Martinez-Portilla, R. J., Basuki, T. R., Kiserud, T. & Figueras, F. Risk of fetal death in growth-restricted fetuses with umbilical and/or ductus venosus absent or reversed end-diastolic velocities before 34 weeks of gestation: A systematic review and meta-analysis. American Journal of Obstetrics and Gynecology10.1016/j.ajog.2017.11.566 (2017). [DOI] [PubMed]
- 11.Figueras F, Gratacos E. Stage-based approach to the management of fetal growth restriction. Prenat. Diagn. 2014;34:655–659. doi: 10.1002/pd.4412. [DOI] [PubMed] [Google Scholar]
- 12.Figueras F, Savchev S, Triunfo S, Crovetto F, Gratacos E. An integrated model with classification criteria to predict small-for-gestational-age fetuses at risk of adverse perinatal outcome. Ultrasound Obstet. Gynecol. 2015;45:279–285. doi: 10.1002/uog.14714. [DOI] [PubMed] [Google Scholar]
- 13.Crispi F, et al. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. 2010;121:2427–2436. doi: 10.1161/CIRCULATIONAHA.110.937995. [DOI] [PubMed] [Google Scholar]
- 14.Crispi F, et al. Cardiovascular programming in children born small for gestational age and relationship with prenatal signs of severity. Am. J. Obstet. Gynecol. 2012;207:e1–9. doi: 10.1016/j.ajog.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Cruz, M. et al. Cord Blood Biomarkers of Cardiac Dysfunction and Damage in Term Growth-Restricted Fetuses Classified by Severity Criteria. Fetal Diagnosis and Therapy10.1159/000484315 (2017). [DOI] [PubMed]
- 16.Demicheva E, Crispi F. Long-term follow-up of intrauterine growth restriction: cardiovascular disorders. Fetal Diagn. Ther. 2014;36:143–153. doi: 10.1159/000353633. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-López M, et al. Descriptive analysis of the different phenotypes of cardiac remodeling in fetal growth restriction. Ultrasound Obstet. Gynecol. 2016;109:2079–2088. doi: 10.1002/uog.17365. [DOI] [PubMed] [Google Scholar]
- 18.Egaña-Ugrinovic G, Sanz-Cortes M, Figueras F, Couve-Perez C, Gratacõs E. Fetal MRI insular cortical morphometry and its association with neurobehavior in late-onset small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 2014;44:322–329. doi: 10.1002/uog.13360. [DOI] [PubMed] [Google Scholar]
- 19.Egaña-Ugrinovic G, Sanz-Cortes M, Figueras F, Bargalló N, Gratacós E. Differences in cortical development assessed by fetal MRI in late-onset intrauterine growth restriction. Am. J. Obstet. Gynecol. 2013;209:e1–8. doi: 10.1016/j.ajog.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Simões RV, et al. Brain metabolite alterations in infants born preterm with intrauterine growth restriction: association with structural changes and neurodevelopmental outcome. Am. J. Obstet. Gynecol. 2017;216:62.e1–62.e14. doi: 10.1016/j.ajog.2016.09.089. [DOI] [PubMed] [Google Scholar]
- 21.Eganã-Ugrinovic G, et al. Neurosonographic assessment of the corpus callosum as imaging biomarker of abnormal neurodevelopment in late-onset fetal growth restriction. Fetal Diagn. Ther. 2015;37:281–288. doi: 10.1159/000366160. [DOI] [PubMed] [Google Scholar]
- 22.Egaña-Ugrinovic G, Sanz-Cortés M, Couve-Pérez C, Figueras F, Gratacós E. Corpus callosum differences assessed by fetal MRI in late-onset intrauterine growth restriction and its association with neurobehavior. Prenat. Diagn. 2014;34:843–849. doi: 10.1002/pd.4381. [DOI] [PubMed] [Google Scholar]
- 23.Sanz-Cortes M, Egaña-Ugrinovic G, Zupan R, Figueras F, Gratacos E. Brainstem and cerebellar differences and their association with neurobehavior in term small-for-gestational-age fetuses assessed by fetal MRI. Am. J. Obstet. Gynecol. 2014;210:e1–8. doi: 10.1016/j.ajog.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Figueras F, et al. Small-for-gestational-age fetuses with normal umbilical artery Doppler have suboptimal perinatal and neurodevelopmental outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;136:34–38. doi: 10.1016/j.ejogrb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Eixarch E, et al. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet. Gynecol. 2008;32:894–899. doi: 10.1002/uog.6249. [DOI] [PubMed] [Google Scholar]
- 26.Baschat AA. Neurodevelopment after fetal growth restriction. Fetal Diagnosis and Therapy. 2014;36:136–142. doi: 10.1159/000353631. [DOI] [PubMed] [Google Scholar]
- 27.Gratacos E, Figueras F. Fetal growth restriction as a perinatal and long-term health problem: clinical challenges and opportunities for future (4P) fetal medicine. Fetal diagnosis and therapy. 2014;36:85. doi: 10.1159/000365556. [DOI] [PubMed] [Google Scholar]
- 28.Han X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016;12:668–679. doi: 10.1038/nrendo.2016.98. [DOI] [PubMed] [Google Scholar]
- 29.Holmes E, Wilson ID, Nicholson JK. Metabolic Phenotyping in Health and Disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Bahado-Singh RO, et al. Validation of metabolomic models for prediction of early-onset preeclampsia. Am. J. Obstet. Gynecol. 2015;213:530.e1–530.e10. doi: 10.1016/j.ajog.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Fotiou, M. et al. 1H NMR-based metabolomics reveals the effect of maternal habitual dietary patterns on human amniotic fluid profile. Sci. Rep. 8 (2018). [DOI] [PMC free article] [PubMed]
- 32.Nicholson JK, et al. Metabolic phenotyping in clinical and surgical environments. Nature. 2012;491:384–392. doi: 10.1038/nature11708. [DOI] [PubMed] [Google Scholar]
- 33.Delplancke, T. D. J. et al. Analysis of sequential hair segments reflects changes in the metabolome across the trimesters of pregnancy. Sci. Rep. 8 (2018). [DOI] [PMC free article] [PubMed]
- 34.Marchlewicz, E. H. et al. Lipid metabolism is associated with developmental epigenetic programming. Sci. Rep. 6 (2016). [DOI] [PMC free article] [PubMed]
- 35.Lackman F, Capewell V, Gagnon R, Richardson B. Fetal umbilical cord oxygen values and birth to placental weight ratio in relation to size at birth. Am. J. Obstet. Gynecol. 2001;185:674–682. doi: 10.1067/mob.2001.116686. [DOI] [PubMed] [Google Scholar]
- 36.Ivorra C, et al. Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J. Transl. Med. 2012;10:142. doi: 10.1186/1479-5876-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Favretto D, et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal. Bioanal. Chem. 2012;402:1109–1121. doi: 10.1007/s00216-011-5540-z. [DOI] [PubMed] [Google Scholar]
- 38.Sanz-Cortés M, et al. Metabolomic profile of umbilical cord blood plasma from early and late intrauterine growth restricted (IUGR) neonates with and without signs of brain vasodilation. PLoS One. 2013;8:e80121. doi: 10.1371/journal.pone.0080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horgan RP, et al. Metabolic profiling uncovers a phenotypic signature of small for gestational age in early pregnancy. J. Proteome Res. 2011;10:3660–3673. doi: 10.1021/pr2002897. [DOI] [PubMed] [Google Scholar]
- 40.Gordijn SJ, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016;48:333–339. doi: 10.1002/uog.15884. [DOI] [PubMed] [Google Scholar]
- 41.Figueras, F., Savchev, S., Triunfo, S., Crovetto, F. & Gratacos, E. An integrated model with classification criteria to predict small-for-gestational-age fetuses at risk of adverse perinatal outcome. Ultrasound Obs. Gynecol 279–285, 10.1002/uog.14714 (2015). [DOI] [PubMed]
- 42.Ye K, et al. Maternal serum lipid levels during late pregnancy and neonatal body size. Asia Pac. J. Clin. Nutr. 2015;24:138–43. doi: 10.6133/apjcn.2015.24.1.20. [DOI] [PubMed] [Google Scholar]
- 43.Wadsack C, et al. Intrauterine growth restriction is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition. Am. J. Physiol. Endocrinol. Metab. 2007;292:E476–84. doi: 10.1152/ajpendo.00547.2005. [DOI] [PubMed] [Google Scholar]
- 44.Sattar N, et al. Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. J. Clin. Endocrinol. Metab. 1999;84:128–130. doi: 10.1210/jcem.84.1.5419. [DOI] [PubMed] [Google Scholar]
- 45.Pecks U, et al. The evaluation of the oxidative state of low-density lipoproteins in intrauterine growth restriction and preeclampsia. Hypertens. Pregnancy. 2012;31:156–65. doi: 10.3109/10641955.2010.544805. [DOI] [PubMed] [Google Scholar]
- 46.Munoz A, et al. Relationship of blood rheology to lipoprotein profile during normal pregnancies and those with intrauterine growth retardation. J. Clin. Pathol. 1995;48:571–574. doi: 10.1136/jcp.48.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farias, D. R. et al. Maternal lipids and leptin concentrations are associated with large-for-gestational-age births: A prospective cohort study. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 48.Alvarez JJ, Montelongo A, Iglesias A, Lasunción MF, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J. Lipid Res. 1996;37:299–308. [PubMed] [Google Scholar]
- 49.Wang Q, et al. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence. BMC Med. 2016;14:205. doi: 10.1186/s12916-016-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diareme M, Karkalousos P, Theodoropoulos G, Strouzas S, Lazanas N. Lipid Profile of Healthy Women During Normal Pregnancy. J. Med. Biochem. 2009;28:152–160. doi: 10.2478/v10011-009-0011-1. [DOI] [Google Scholar]
- 51.Bartels Ä, et al. Maternal serum cholesterol levels are elevated from the 1st trimester of pregnancy: a cross-sectional study. J. Obstet. Gynaecol. 2012;32:747–52. doi: 10.3109/01443615.2012.714017. [DOI] [PubMed] [Google Scholar]
- 52.Winkler K, et al. Low density lipoprotein (LDL) subfractions during pregnancy: Accumulation of buoyant LDL with advancing gestation. J. Clin. Endocrinol. Metab. 2000;85:4543–4550. doi: 10.1210/jcem.85.12.7027. [DOI] [PubMed] [Google Scholar]
- 53.Montelongo a, Lasunción Ma, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992;41:1651–1659. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- 54.Herrera E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development–a review. Placenta. 2002;23(Suppl A):S9–S19. doi: 10.1053/plac.2002.0771. [DOI] [PubMed] [Google Scholar]
- 55.Geraghty AA, et al. Maternal blood lipid profile during pregnancy and associations with child adiposity: Findings from the ROLO study. PLoS One. 2016;11:e0161206. doi: 10.1371/journal.pone.0161206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vrijkotte TGM, Algera SJ, Brouwer Ia, van Eijsden M, Twickler MB. Maternal triglyceride levels during early pregnancy are associated with birth weight and postnatal growth. J. Pediatr. 2011;159:736–742.e1. doi: 10.1016/j.jpeds.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Edison RJ, et al. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120:723–33. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 58.Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002;19:43–55. doi: 10.1385/ENDO:19:1:43. [DOI] [PubMed] [Google Scholar]
- 59.Prentice, A. M. & Goldberg, G. R. Energy adaptations in human pregnancy: Limits and long-term consequences. in American Journal of Clinical Nutrition71 (2000). [DOI] [PubMed]
- 60.Woollett LA. Review: Transport of maternal cholesterol to the fetal circulation. Placenta. 2011;32(Suppl 2):S218–21. doi: 10.1016/j.placenta.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tabano S, et al. Placental LPL gene expression is increased in severe intrauterine growth-restricted pregnancies. Pediatr. Res. 2006;59:250–253. doi: 10.1203/01.pdr.0000199441.62045.a1. [DOI] [PubMed] [Google Scholar]
- 62.Gauster M, et al. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J. Clin. Endocrinol. Metab. 2007;92:2256–2263. doi: 10.1210/jc.2006-2403. [DOI] [PubMed] [Google Scholar]
- 63.Sanz-Cortés, M. et al. Metabolomic profile of umbilical cord blood plasma from early and late intrauterine growth restricted (IUGR) neonates with and without signs of brain vasodilation. PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
- 64.Alvino G, et al. Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatr. Res. 2008;64:615–20. doi: 10.1203/PDR.0b013e31818702a2. [DOI] [PubMed] [Google Scholar]
- 65.Hossain MAA, Islam MNN, Shahidullah M, Akhter H. Serum triglyceride level in IUGR babies and its comparison with preterm AGA and term normal babies. Mymensingh Med. J. MMJ. 2006;15:180–182. doi: 10.3329/mmj.v15i2.40. [DOI] [PubMed] [Google Scholar]
- 66.Jones JN, Gercel-Taylor C, Taylor DD. Altered cord serum lipid levels associated with small for gestational age infants. Obs. Gynecol. 1999;93:527–531. doi: 10.1016/s0029-7844(98)00489-x. [DOI] [PubMed] [Google Scholar]
- 67.Carr BR, Simpson ER. Cholesterol synthesis in human fetal tissues. J. Clin. Endocrinol. Metab. 1982;55:447. doi: 10.1210/jcem-55-3-447. [DOI] [PubMed] [Google Scholar]
- 68.Lin G, et al. Metabolomic Analysis Reveals Differences in Umbilical Vein Plasma Metabolites between Normal and Growth-Restricted Fetal Pigs during Late Gestation. J. Nutr. 2012;142:990–998. doi: 10.3945/jn.111.153411. [DOI] [PubMed] [Google Scholar]
- 69.Kim S-M, et al. Cord and maternal sera from small neonates share dysfunctional lipoproteins with proatherogenic properties: Evidence for Barker’s hypothesis. J. Clin. Lipidol. 2017;11:1318–1328. doi: 10.1016/j.jacl.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 70.Nagano N, et al. Insulin-like growth factor-1 and lipoprotein profile in cord blood of preterm small for gestational age infants. J. Dev. Orig. Health Dis. 2013;4:507–12. doi: 10.1017/S2040174413000408. [DOI] [PubMed] [Google Scholar]
- 71.Leduc L, et al. Oxidized low-density lipoproteins in cord blood from neonates with intra-uterine growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;156:46–49. doi: 10.1016/j.ejogrb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Pecks U, et al. Maternal and fetal cord blood lipids in intrauterine growth restriction. J. Perinat. Med. 2012;40:287–296. doi: 10.1515/jpm.2011.135. [DOI] [PubMed] [Google Scholar]
- 73.Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;334:577–580. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 74.Barker DJ. Fetal origins of cardiovascular disease. Ann. Med. 1999;31(Suppl 1):3–6. doi: 10.1080/07853890.1999.11904392. [DOI] [PubMed] [Google Scholar]
- 75.Bateson P, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 76.Li S, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 77.Zhu, W. F., Tang, S. J., Shen, Z., Wang, Y. M. & Liang, L. Growth hormone reverses dyslipidemia in adult offspring after maternal undernutrition. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 78.Paoletti L, Elena C, Domizi P, Banchio C. Role of Phosphatidylcholine during Neuronal differentiation. IUBMB Life. 2011;63:714–720. doi: 10.1002/iub.521. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Vance DE. Thematic Review Series: Glycerolipids. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 80.Liu, J., Chen, X. X., Li, X. W., Fu, W. & Zhang, W. Q. Metabolomic Research on Newborn Infants with Intrauterine Growth Restriction. Med. (United States)95 (2016). [DOI] [PMC free article] [PubMed]
- 81.Soothill RW, Ajayi RA, Nicolaides KN. Fetal biochemistry in growth retardation. Early Hum. Dev. 1992;29:91–97. doi: 10.1016/0378-3782(92)90062-L. [DOI] [PubMed] [Google Scholar]
- 82.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. American Journal of Obstetrics and Gynecology. 2018;218:S745–S761. doi: 10.1016/j.ajog.2017.11.577. [DOI] [PubMed] [Google Scholar]
- 83.Weiner CP, Williamson RA. Evaluation of severe growth retardation using cordocentesis–hematologic and metabolic alterations by etiology. Obstet. Gynecol. 1989;73:225–229. [PubMed] [Google Scholar]
- 84.Savchev S, et al. Evaluation of an optimal gestational age cut-off for the definition of early- and late-onset fetal growth restriction. Fetal Diagn. Ther. 2014;36:99–105. doi: 10.1159/000355525. [DOI] [PubMed] [Google Scholar]
- 85.Figueras F, et al. Diagnosis and surveillance of late-onset fetal growth restriction. American Journal of Obstetrics and Gynecology. 2018;218:S790–S802.e1. doi: 10.1016/j.ajog.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Pérez-Cruz M, et al. Fetal cardiac function in late-onset intrauterine growth restriction vs small-for-gestational age, as defined by estimated fetal weight, cerebroplacental ratio and uterine artery Doppler. Ultrasound Obstet. Gynecol. 2015;46:465–471. doi: 10.1002/uog.14930. [DOI] [PubMed] [Google Scholar]
- 87.Savchev S, et al. Evaluation of an optimal gestational age cut-off for the definition of early-and late-onset fetal growth restriction. Fetal Diagn. Ther. 2014;36:99–105. doi: 10.1159/000355525. [DOI] [PubMed] [Google Scholar]
- 88.Veenstra, T. D.Metabolomics: The final frontier? Genome Medicine4 (2012). [DOI] [PMC free article] [PubMed]
- 89.Nagana Gowda GA, Djukovic D, Bettcher LF, Gu H, Raftery D. NMR-Guided Mass Spectrometry for Absolute Quantitation of Human Blood Metabolites. Anal. Chem. 2018;90:2001–2009. doi: 10.1021/acs.analchem.7b04089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mallol R, et al. Liposcale: a novel advanced lipoprotein test based on 2D diffusion-ordered1H NMR spectroscopy. J. Lipid Res. 2015;56:737–746. doi: 10.1194/jlr.D050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mallol R, et al. Surface fitting of 2D diffusion-edited 1H NMR spectroscopy data for the characterisation of human plasma lipoproteins. Metabolomics. 2011;7:572–582. doi: 10.1007/s11306-011-0273-8. [DOI] [Google Scholar]
- 92.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 94.Oaks BM, et al. Effects of a lipid-based nutrient supplement during pregnancy and lactation on maternal plasma fatty acid status and lipid profile: Results of two randomized controlled trials. Prostaglandins, Leukot. Essent. Fat. Acids. 2017;117:28–35. doi: 10.1016/j.plefa.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walejko, J. M., Chelliah, A., Keller-Wood, M., Gregg, A. & Edison, A. S. Global metabolomics of the placenta reveals distinct metabolic profiles between maternal and fetal placental tissues following delivery in non-labored women. Metabolites8 (2018). [DOI] [PMC free article] [PubMed]
- 96.Ockene IS, et al. Seasonal Variation in Serum Cholesterol Levels: Treatment Implications and Possible Mechanisms. Arch. Intern. Med. 2004;164:863–870. doi: 10.1001/archinte.164.8.863. [DOI] [PubMed] [Google Scholar]
- 97.Pecks U, et al. Fetal gender and gestational age differentially affect PCSK9 levels in intrauterine growth restriction. Lipids Health Dis. 2016;15:1–11. doi: 10.1186/s12944-016-0365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements-A prospective study. Am. J. Obstet. Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 99.Figueras F, et al. Customized birthweight standards for a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;136:20–24. doi: 10.1016/j.ejogrb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 100.Baschat AA, Gembruch U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet. Gynecol. 2003;21:124–127. doi: 10.1002/uog.20. [DOI] [PubMed] [Google Scholar]
- 101.Gómez O, et al. Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet. Gynecol. 2008;32:128–132. doi: 10.1002/uog.5315. [DOI] [PubMed] [Google Scholar]
- 102.Cannet, C. IVDr NMR methods in clinical bodyfluid analysis. IVDr NMR methods in clinical bodyfluid analysis Available at: https://www.bruker.com/fileadmin/user_upload/5-Events/User_Meetings/UM_GERMAN/Downloads/D2_S2_Tools_for_Metabolomics_IVDr_NMR_Methoden_klinischen_Koerperfluessigkeitsanalytik_Claire_Cannet_3.pdf (2016).
- 103.Beckonert O, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 104.Dona AC, et al. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014;86:9887–9894. doi: 10.1021/ac5025039. [DOI] [PubMed] [Google Scholar]
- 105.Zou X, Holmes E, Nicholson JK, Loo RL. Automatic Spectroscopic Data Categorization by Clustering Analysis (ASCLAN): A Data-Driven Approach for Distinguishing Discriminatory Metabolites for Phenotypic Subclasses. Anal. Chem. 2016;88:5670–5679. doi: 10.1021/acs.analchem.5b04020. [DOI] [PubMed] [Google Scholar]
- 106.Gómez J, et al. Dolphin: A tool for automatic targeted metabolite profiling using 1D and 2D 1H-NMR data. Anal. Bioanal. Chem. 2014;406:7967–7976. doi: 10.1007/s00216-014-8225-6. [DOI] [PubMed] [Google Scholar]
- 107.Cañueto, D., Gomez, J., Salek, R. M., X. C. & N. C. rDolphin: a GUI R package for proficient automatic profiling of 1D 1H-NMR spectra of study datasets. Metabolomics (2018). [DOI] [PubMed]
- 108.Wishart DS, et al. HMDB 3.0-The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicholson JK, Foxall PJD, Spraul M, Farrant RD, Lindon JC. 750 MHz 1H and 1H-13C NMR Spectroscopy of Human Blood Plasma. Anal. Chem. 1995;67:793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- 110.Vinaixa M, et al. Metabolomic assessment of the effect of dietary cholesterol in the progressive development of fatty liver disease. J. Proteome Res. 2010;9:2527–2538. doi: 10.1021/pr901203w. [DOI] [PubMed] [Google Scholar]
- 111.Nagana Gowda GA, Gowda YN, Raftery D. Expanding the limits of human blood metabolite quantitation using NMR spectroscopy. Anal. Chem. 2015;87:706–715. doi: 10.1021/ac503651e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cloarec O, et al. Statistical total correlation spectroscopy: An exploratory approach for latent biomarker identification from metabolic 1H NMR data sets. Anal. Chem. 2005;77:1282–1289. doi: 10.1021/ac048630x. [DOI] [PubMed] [Google Scholar]
- 113.Terpstra JT, Magel RC. A new nonparametric test for the ordered alternative problem. J. Nonparametr. Stat. 2003;15:289–301. doi: 10.1080/1048525031000078349. [DOI] [Google Scholar]
- 114.Jonckheere AR. A Distribution-Free k-Sample Test Against Ordered Alternatives. Biometrika. 1954;41:133. doi: 10.1093/biomet/41.1-2.133. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical information of the patients included, spectrum data and associated protocols are promptly available to readers without undue qualifications in material transfer agreements.