Abstract
This work contains original data supporting our research paper “Antibacterial effectiveness meets improved mechanical properties: Manuka Honey/Gellan Gum composite hydrogels for cartilage repair”, Bonifacio et al., in press [1], in which innovative composite hydrogels, based on Gellan Gum/Manuka honey/Halloysite nanotubes were described as biomaterials for cartilage regeneration. Here the composites were further examined by means of Fourier Transform Infrared Spectroscopy, in Attenuated Total Reflectance mode (FT-IR/ATR). Materials devoted to cartilage replacement must possess adequate fluid permeability and lubricating capability, therefore, a deeper investigation on water uptake kinetics of freeze-dried specimens up to 21 days in PBS was carried out. Moreover, since the degradation rate of a biomaterial plays a pivotal role in tissue engineering, weight loss measurements of the prepared hydrogels were performed in simulated synovial fluid, in phosphate buffer solution (PBS) and in lysozyme. Scanning Electron Microscopy images provide insight into the morphology of the freeze-dried samples.
Finally, additional information on Staphylococcus aureus and Staphylococcus epidermidis ability to adhere onto the prepared hydrogel composites in short times were obtained, as well as the chondrogenic potential of the composites assessed by SDS-PAGE followed by Coomassie blue gel staining.
Specifications Table
| Subject area | Material science. Chemistry. |
| More specific subject area | Biomaterials for cartilage regeneration |
| Type of data | Data, table and figures |
| How data was acquired | FT-IR in ATR mode was performed by Perkin-Elmer Spectrum Two (PerkinElmer Inc, Waltham, MA). |
| A TM3030 Hitachi Tabletop scanning electron microscope was employed to obtain the SEM images of the freeze-dried hydrogels. | |
| For microbiological experiments, the optical density was measured by a VICTOR Multilabel Plate Reader spectrophotometer (Perkin Elmer Inc, Waltham, MA). | |
| Chondrogenesis analysis was carried out on protein extracts, separated by Sodium Dodecyl Sulphate - PolyAcrylamide Gel Electrophoresis (SDS-PAGE) and stained with Coomassie blue. | |
| Data format | Analyzed: means ± standard deviations and statistics |
| Experimental factors | FT-IR in ATR mode, SEM analysis and water uptake measurements were carried out on freeze-dried hydrogels. The freeze-drying procedure is the following: as prepared hydrogels were frozen for 24 h at − 20 °C, then freeze-dried at − 55 °C for 48 h with an ALPHA1–2/LDPlus (Martin-Christ, Germany). |
| Weight loss experiments, microbiological test and chondrogenesis analysis were performed on as prepared hydrogels. | |
| Experimental features | For FT-IR/ATR analysis, no further specific sample preparation was required. ATR correction algorithm (included into the Spectrum software) was employed on all the presented spectra. SEM images were obtained on the dehydrated samples without further processing. |
| For swelling and weight loss evaluations, at predetermined time intervals, hydrogels were removed and weighed to determine the water uptake or the weight loss percentage. | |
| Bacterial adhesion was evaluated during the first 90 min of culture onto the prepared hydrogels, measuring the optical density at 600 nm. | |
| Analysis of chondrogenic potential was obtained performing SDS-PAGE followed by Coomassie blue gel staining. | |
| Data source location | Hydrogel characterizations were performed at Department of Chemistry, University of Bari Aldo Moro, Bari (Italy). |
| SEM images were acquired at the School of Engineering, Newcastle University (UK). | |
| Bacterial strains were clinical isolates tested for their multi-drug resistance (MDR) by the Novara Maggiore Hospital, Clinical Microbiology Unit, Novara (Italy). Microbiological experiments were carried out at the Department of Health Sciences, University of Piemonte Orientale (Italy). | |
| Human mesenchymal stem cells (hMSCs) purchased from the American Type Culture Collection (adipose-derived, ATCC-PCS-500-011) were cultured at the Department of Health Sciences, University of Piemonte Orientale “UPO”, Novara (Italy). | |
| Data accessibility | Data are available in this article |
| Related research article | “Antibacterial effectiveness meets improved mechanical properties: Manuka Honey/Gellan Gum composite hydrogels for cartilage repair”, by Bonifacio et al. [1] |
Value of the data
-
•
FT-IR/ATR characterization confirmed the composition of the hydrogel composites after freeze-drying process
-
•
SEM images show the porous architecture of the freeze-dried hydrogels
-
•
The weight loss experiments, performed in three different media over 10 weeks, demonstrated the in vitro stability of the proposed hydrogels, thus allowing stem cells recruiting and differentiation, supporting tissue growth and new matrix deposition.
-
•
Bacterial adhesion evaluation within 90 min allows the direct comparison of the hydrogels’ antibacterial effectiveness, excluding a fast anti-adhesion activity within the early stages of culture.
-
•
The SDS-PAGE followed by Coomassie blue gel staining supported real-time PCR and histological data reported in the main paper.
1. Data
1.1. FT-IR/ATR characterization
FT-IR/ATR measurements of freeze-dried Gellan Gum/Manuka honey/Halloysite nanotubes (GG MH HNT) composite hydrogels, crosslinked with two different ions (i.e., calcium or magnesium), as well as their starting materials, were carried out.
FT-IR/ATR spectra of MH and lyophilized MH resulted quite similar, indicating that water was only partially removed by the process, since it is firmly linked to the sugars (data not shown). Indeed, comparing the weights of honey samples before and after the freeze-drying procedure, the dehydrated hydrogels lost only the 1.4 ± 0.3% of their starting weight.
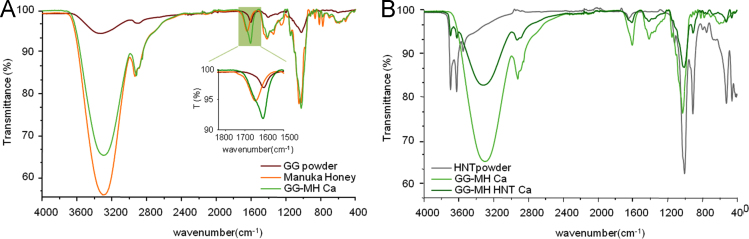
In Fig. 1A, a comparison between GG, freeze-dried MH and GG MH spectra is reported. Since the systems crosslinked with Ca2+ or Mg2+ ions, without HNT, presented similar spectra, for sake of clarity, only the one crosslinked with calcium ions was reported. The spectrum of MH (orange line in Fig. 1A) showed two water bands at 3293 cm−1 (OH stretch) and 1644 cm−1 (OH deformation). Moreover, the band appearing at 1644 cm-1 could also be due to the N-H bending of aminoacids and vitamins [2]. The band from about 1500–750 cm−1 corresponded to the most sensitive absorption region of the honey׳s major components, relevant to honey sugars and organic acids. According to Tulchinsky et al. [3], the spectral region located at 900–750 cm−1 is characteristic of the saccharide configuration. In the range 3290–3320 cm−1, the band relevant to O-H stretching of sugars present in MH and in GG was evident. In GG powder, the bands appearing at 1602 and 1403 cm−1 were due to asymmetric and symmetric stretching of COO- groups, respectively. In the GG MH hydrogel, both the bands at 1602 and 1644 cm−1 were observed (see inset). As far as the GG MH HNT hydrogels were concerned, the presence of HNT was quite evident both for Ca- and Mg-crosslinked systems, even better than in the HNT-containing hydrogels previously reported [4]. The peaks at 3693 and 3622 cm−1 could be associated to the stretching of the inner hydroxyl groups predominantly in a free state (Fig. 1B) [1].
Fig. 1.
FT-IR/ATR characterization. The spectra of the relevant samples are reported in (A) and (B).
1.2. SEM analysis
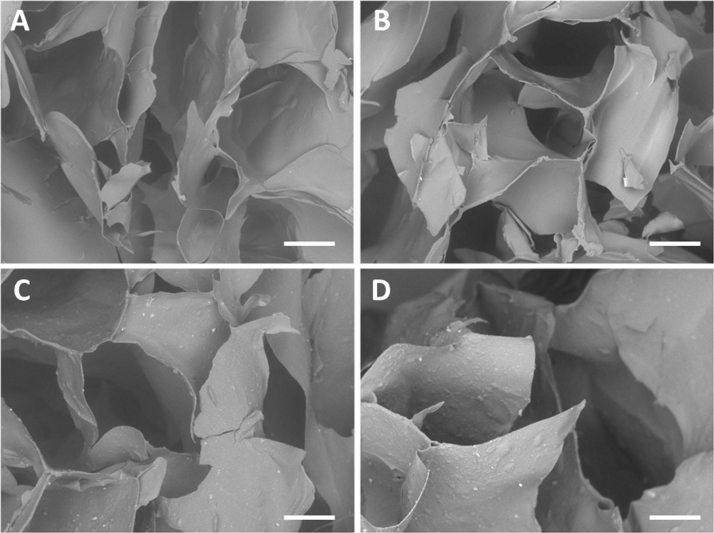
All the samples showed a porous, spongy-like architecture (Fig. 2), characterized by interconnected cavities. Moreover, bright spots could be observed in Fig. 2C and D, ascribable to the presence of HNT in the samples.
Fig. 2.
SEM micrographs of GG-MH based hydrogels, obtained after the freeze-drying process. (A) GG-MH Ca, (B) GG-MH Mg, (C) GG-MH-HNT Ca and (D) GG-MH-HNT Mg. Scale bars: 200 µm.
1.3. Water uptake evaluation
GG-based hydrogels, after a specific freeze-drying and re-hydration, can give rise to spongy-like hydrogels. The experiments reported in this section were performed in order to shed light on the impact of (a) crosslinker cations (Ca2+ or Mg2+), (b) HNT and (c) MH presence on the water uptake properties of the GG-based hydrogels.
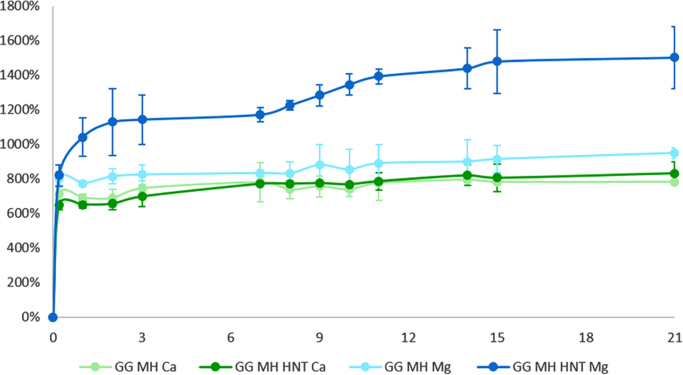
As highlighted in Fig. 3 and Table 1, all the tested polymers swelled very rapidly, because of the presence of large pores typical of freeze-dried systems [1]. In particular, GG MH HNT Mg hydrogels displayed a significantly higher swelling in respect to the other systems, reaching the swelling equilibrium after 15days [1]. Conversely, as far as Ca-crosslinked samples were concerned, HNT presence did not dramatically affect the swelling behavior of the hydrogels [1].
Fig. 3.
Water uptake of the hydrogels. Swelling kinetics up to 21days in PBS of GG MH hydrogels crosslinked with Mg2+ or Ca2+, with and without HNT.
Table 1.
Water content and rewet data of all the investigated hydrogels.
| Sample type | WC% (at 21d) | Rewet % |
|---|---|---|
| GG MH Mg | 950 ± 30 | 42 ± 6 |
| GG MH HNT Mg | 1500 ± 200 | 61 ± 9 |
| GG MH Ca | 780 ± 10 | 33 ± 2 |
| GG MH HNT Ca | 830 ± 70 | 35 ± 4 |
1.4. Weight loss evaluation
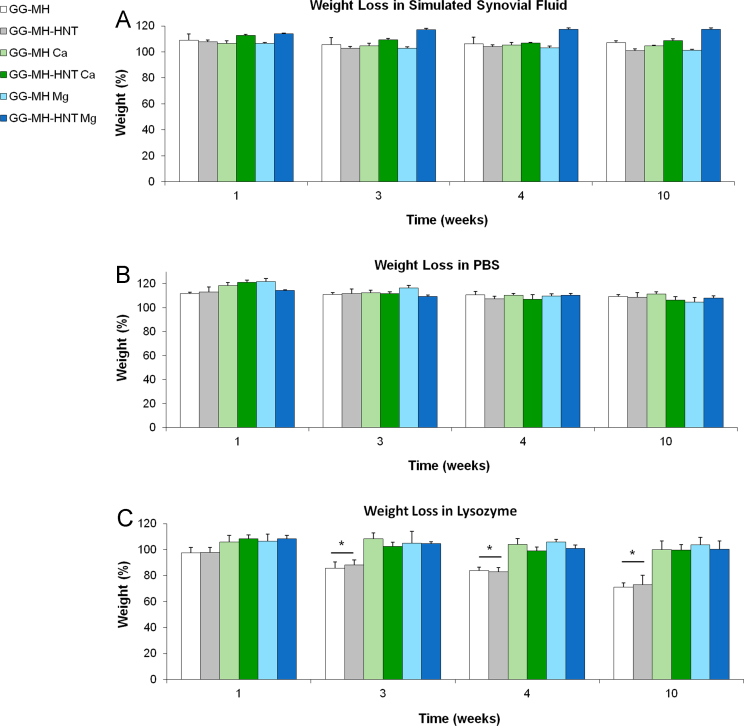
The degradation rate of a biomaterial plays a pivotal role in tissue engineering, since it should occur in parallel with the deposition of new tissue [5]. Therefore, an in vitro study of the weight loss of the prepared hydrogels was performed. Firstly, hydrogels degradation behavior was evaluated in simulated synovial fluid, in order to better mimic cartilage microenvironment. To the best of our knowledge, no previous studies related to hydrogels degradation in this medium were previously carried out. Thus, it was noteworthy to observe that, after 10 weeks in absence of enzymes the weight of all the prepared hydrogels was not significantly altered (Fig. 4A). Similarly, each hydrogel type incubated in PBS was stable up to 10 weeks, without statistically significant weight losses (Fig. 4B), as already reported [6].
Fig. 4.
Weight loss of the hydrogels performed in simulated synovial fluid (A), in PBS (B) and in lysozyme (C). Weight percentages related to the six hydrogel types (with or without HNT, crosslinked with Ca2+, Mg2+ or not cross-linked) were monitored up to 10 weeks.
Lysozyme was used in vitro to mimic the degradation by lysozyme-positive macrophages and it was previously tested in vitro as hydrolytic enzyme able to degrade polysaccharides, including Gellan Gum [7]. As shown in Fig. 4C, only a slight degradation occurred in lysozyme for non-crosslinked hydrogels (after 3, 4 and 10 weeks, p < 0.05). Conversely, the presence of the crosslinking ions improved hydrogels stability, since no significant changes in weight were observed up to 10 weeks for Ca- and Mg-crosslinked hydrogels (Fig. 4C), as already described for other calcium crosslinked hydrogels [8].
1.5. Bacterial adhesion on hydrogels
The turbidity of bacterial solutions was measured in terms of o.d. after the 90 min of adhesion phase to test an eventual fast anti-adhesion activity. However, no significant differences between the tested hydrogels and the control (p > 0.05) were observed, as summarized in Table 2. Indeed, moving from the initial o.d. (T0, standardized to reach 0.001 at 600 nm) to the 90 min o.d., all values were comparable; hence a similar number of bacteria were floating in the supernatants or attached to the specimens’ surfaces. These data were useful to exclude a fast anti-adhesion activity within the first adhesion step.
Table 2.
Bacterial optical density (o.d.) at seeding stage (T0) and after the adhesion phase (90 min). T0 o.d. was standardized to reach 0.001 at 600 nm; after 90 min, all test specimens showed a similar o.d., suggesting no differences in terms of bacterial adhesion interference.
| Sample type |
S. aureus |
S. epidermidis |
||
|---|---|---|---|---|
| T0o.d. | 90 min o.d. | T0o.d. | 90 min o.d. | |
| Control | 0.001 | 0.0039 | 0.001 | 0.0028 |
| GG-MH Mg | 0.001 | 0.0038 | 0.001 | 0.0026 |
| GG-MH-HNT Mg | 0.001 | 0.0039 | 0.001 | 0.0027 |
| GG-MH Ca | 0.001 | 0.0038 | 0.001 | 0.0024 |
| GG-MH-HNT Ca | 0.001 | 0.0038 | 0.001 | 0.0028 |
1.6. Chondrogenesis analysis
The SDS-PAGE followed by Coomassie blue gel staining supported PCR data, as typical bands related to aggrecan and collagen II were detected for all the tested specimens (Fig. 5).
Fig. 5.
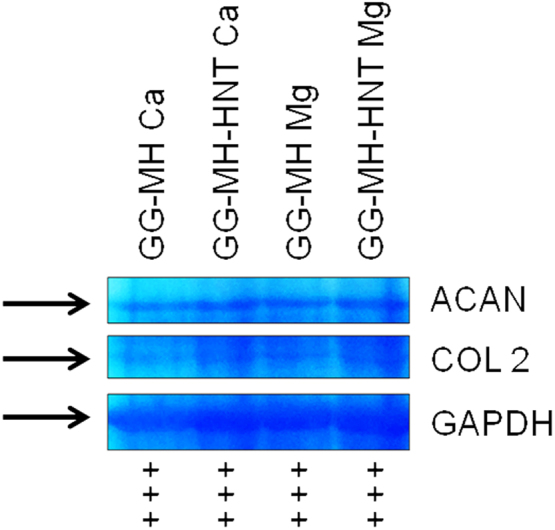
Coomassie blue staining after SDS-PAGE.
2. Experimental design, materials, and methods
2.1. FT-IR/ATR characterization
Dehydrated samples were also analyzed with a Spectrum Two PE instrument (PerkinElmer, USA) equipped with the universal ATR accessory (UATR, Single Reflection Diamond/ZnSe). FT-IR/ATR spectra were acquired from 400 to 4000 cm−1, with a resolution of 4 cm−1. The recorded signals were reported as transmittance percentage.
2.2. SEM analysis
A scanning electron microscope (Hitachi TM3030 Tabletop SEM) was used to study the morphology of freeze-dried gels. The samples were cut into small cylinders (1.5 cm of diameter and 0.4 cm of height), fixed on the aluminium stub using carbon tape and directly observed.
2.3. Water uptake evaluation
Dried polymeric samples were firstly weighed (mid), successively placed in tea bags. The tea bags containing samples were sealed and incubated in PBS at 37 °C to determine the water uptake profile up to 7 days and weighted prior (mi°) and after each time point (mit) (5 h, 1, 2, 3, 7, 8, 9, 10, 11, 14, 15 and 21 d). Moreover, in order to guarantee that the amount of the measured water was only ascribable to the samples swelling, the weight of empty wet tea bags was also considered after each time point (mbt). Therefore, the percentage of water content (WC %) for each sample over time was calculated using Eq. (1) reported below:
| (1) |
Furthermore, the rewet of freeze-dried samples was obtained by ratioing the weight of fully swollen samples (i.e., the weight of 21 days-swollen samples, mi21) to the weight of the as-prepared hydrogels (i.e., before freeze-drying, miap), as shown in Eq. (2):
| (2) |
Each test was performed in triplicate and data were reported as mean ± standard deviation.
2.4. Weight loss evaluation
The degradation behavior of six types of hydrogels (with and without HNT, ionically crosslinked with Ca2+, Mg2+, or not crosslinked) was monitored in vitro in lysozyme, PBS and simulated synovial fluid, the latter was prepared according to Marques et al. [9]. At predetermined time intervals (1, 3, 4 and 10 weeks), hydrogels were removed and weighed to determine the weight percentage. The experiments were performed in triplicate and data were expressed as mean ± standard deviation.
2.5. Bacterial adhesion on hydrogels
The adhesion of Staphylococcus aureus and Staphylococcus epidermidis was evaluated during the first 90 min of culture onto the prepared hydrogels to test an eventual fast anti-adhesion activity. The optical density at 600 nm was evaluated on all the sample types.
2.6. Chondrogenesis analysis
An amount of 25 µg of protein extract was separated onto a 10% SDS-PAGE [10]. Then, the gel was stained by Coomassie blue for 5 min prior to be carefully washed with distilled water. Finally, the gel was immersed ON into a destaining solution.
Acknowledgments
This work was supported by Università degli Studi di Bari "Aldo Moro", Italy. Dr P. Gentile and Dr. A.M. Ferreira are members of the UK EPSRC Centre for Innovative Manufacturing of Medical Devices (MeDe Innovation, EPSRC grant EP/K029592/1). The authors would like to thank Dr. Federica Cassano who contributed to degradation assays.
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.dib.2018.08.155.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Bonifacio M.A., Cometa S., Cochis A., Gentile P., Ferreira A.M., Azzimonti B., Procino G., Ceci E., Rimondini L., De Giglio E. Antibacterial effectiveness meets improved mechanical properties: Manuka Honey/Gellan Gum composite hydrogels for cartilage repair. Carbohydr. Polym. 2018;198:462–472. doi: 10.1016/j.carbpol.2018.06.115. [DOI] [PubMed] [Google Scholar]
- 2.Balaji A., Jaganathan S.K., Ismail A.F., Rajasekar R. Fabrication and hemocompatibility assessment of novel polyurethane-based bio-nanofibrous dressing loaded with honey and Carica papaya extract for the management of burn injuries. Int. J. Nanomed. 2016;11:4339–4355. doi: 10.2147/IJN.S112265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tulchinsky V.M., Zurabiab S.F., Asankozhoev K.A., Kogan G.A., Khorlin A.V. Study of the 325 infrared spectra of oligosaccharides in the region 1000–400 cm−1. Carbohydr. Res. 1976;51:1–8. doi: 10.1016/s0008-6215(00)84031-8. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacio M.A., Gentile P., Ferreira A.M., Cometa S., De Giglio E. Insight into halloysite nanotubes-loaded gellan gum hydrogels for soft tissue engineering applications. Carbohydr. Polym. 2017;163:280–291. doi: 10.1016/j.carbpol.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 5.Spiller K.L., Maher S.A., Lowman A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B: Rev. 2011;17(4):281–299. doi: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu I., Kaonis S., Chen R. A study on degradation behavior of 3D printed gellan gum scaffolds. Procedia CIRP. 2017;65:78–83. [Google Scholar]
- 7.Coutinho D.F., Sant S.V., Shin H., Oliveira J.T., Gomes M.E., Neves N.M., Khademhosseini A., Reis R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials. 2010;31(29):7494–7502. doi: 10.1016/j.biomaterials.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suri S., Banerjee R. in vitro evaluation of in situ gels as short term vitreous substitutes. J. Biomed. Mater. Res. Part A. 2006;79(3):650–664. doi: 10.1002/jbm.a.30917. [DOI] [PubMed] [Google Scholar]
- 9.Marques M.R.C., Loebenberg R., Almukainzi M. Simulated biological fluids with possible applications in dissolution testing. Dissolution Technol. 2011;18(3):15–28. [Google Scholar]
- 10.Vernè E., Ferraris S., Vitale-Brovarone C., Cochis A., Rimondini L. Bioactive glass functionalized with alkaline phosphatase stimulates bone extracellular matrix deposition and calcification in vitro. Appl. Surf. Sci. 2014;313:372–381. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material